Abstract
Heme oxygenase (HO) catalyzes the rate-limiting step in heme degradation to produce carbon monoxide (CO), iron, and biliverdin. Biliverdin is subsequently converted to bilirubin by its reductase, and iron is recycled for heme synthesis. The inducible HO isoform, HO-1, is involved in the protection of multiple tissues and organs. The mechanism of protective actions of HO-1 has not been completely elucidated, but recent evidence suggests that one or more of heme metabolites can mediate the protective effects of HO-1. Particularly, CO mimics the antioxidant, anti-inflammatory, anti-apoptotic and antiproliferative actions of HO-1. Many of these effects of CO depend on the production of cyclic guanosine monophosphate (cGMP), and the modulation of mitogen-activated protein kinase (MAPK) pathways. The transcription factors, including nuclear factor E2-related factor-2 (Nrf2), and their upstream kinases, including MAPK pathway, play an important regulatory role in HO-1 expression by dietary antioxidants and drugs. This review attempts to concisely summarize the molecular and biochemical characteristics of HO-1, with a discussion on the mechanisms of signal transduction and gene regulation that mediate the induction of HO-1 by dietary antioxidants and drugs. In addition, the cytoprotective roles of HO-1 shall be discussed from the perspective of each of the metabolic by-products.
Keywords: heme oxygenase, antioxidant, anti-inflammation, anti-apoptosis, antiproliferation
Introduction
Heme, a complex of ferrous iron and protoporphyrin IX, has a variety of biological functions, including oxygen binding, electron transport, and chemical catalysis [1]. Various types of proteins contain this molecule as their prosthetic group. When these proteins turn over, the heme is not salvaged but degraded by the enzyme heme oxygenase (HO) [2]. HO catalyzes the first and rate-limiting step in the oxidative degradation of free heme to produce equimolar quantities of carbon monoxide (CO), ferrous iron, and biliverdin (BV) [3]. BV is subsequently converted to bilirubin (BR) by a BV reductase, and iron is recycled for heme synthesis. Two genetically distinct isozymes of HO have been characterized: an inducible form, heme oxygenase-1 (HO-1), and a constitutively expressed form, heme oxygenase-2 (HO-2). Although both HO-1 and HO-2 catalyze the identical biochemical reaction, they differ in genetic origin, in primary structure, in molecular weight, and in their substrate and kinetic parameters [4]. Moreover, HO-1, once expressed, can metabolize high amounts of free heme to produce high concentrations of its enzymatic by-products that may affect various biological events. HO-1 can be expressed primarily by its substrate, free heme, and also by a wide variety of stimuli, inducing hypoxia, hyperoxia, pro-inflammatory cytokines, nitric oxide (NO), heavy metals, ultraviolet radiation, heat shock, shear stress, and hydrogen peroxide [4, 5]. This adaptive response of HO-1 to these cytotoxic stimuli implies that HO-1, besides its role in heme degradation, may function as a critical cytoprotective molecule.
This review attempts to concisely summarize the molecular and biochemical characteristics of HO-1, with a discussion on the important mechanisms of signal transduction and gene regulation that mediate the induction of HO-1 particularly by dietary antioxidants and drugs. In addition, the integrative survival response evoked by HO-1 shall be discussed from the perspective of each of the metabolic by-products.
HO-1 Deficiency
Essential lessons from the analysis of HO-1-deficient human case and mice indicate that HO-1 is involved in the protection of multiple tissues and organs and that cytoprotection afforded by HO-1 is associated with its antioxidant, anti-inflammatory, anti-apoptotic and antiproliferative effects.
Mice
Poss and Tonegawa [6, 7] have first reported that HO-1-deficient mice are extremely sensitive to oxidative injury, thus establishing that HO-1 is an important cytoprotective system. The HO-1-deficient mice exhibited a decreased birth rate, growth retardation, anemia, tissue iron deposition, hepatosplenomegaly, lymphadenopathy, leukocytosis, and glomerulonephritis [8]. The HO-1-deficient mice developed a chronic inflammatory state that progresses with age [9].
Human
Yachie and colleagues [10–12] have reported the first human case of HO-1 deficiency in a 6-yr-old boy. The child suffered from similar symptoms as the HO-1-deficient mice, including growth failure, anemia, increased iron binding capacity, tissue iron deposition, lymphadenopathy, leukocytosis, and increased sensitivity to oxidant injury [8]. The HO-1-deficient human case died of an inflammatory syndrome [8, 11].
HO-1 Expression
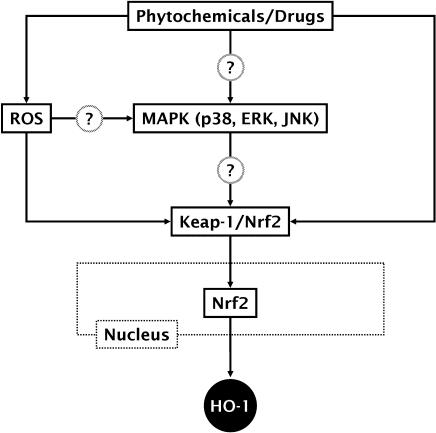
A number of intracellular signaling molecules and their downstream transcription factors have been identified to involve HO-1 expression [13]. Among them, the mitogen-activated protein kinase (MAPK), as one of upstream pathways, and the nuclear factor E2-related factor 2 (Nrf2), as one of downstream transcription factors, are well described to be more important in HO-1 expression by non-toxic phytochemicals and drugs [13, 14], and thus briefly discussed below (Fig. 1).
Fig. 1.
Signaling pathways leading to HO-1 expression by phytochemicals and drugs through MAPK-dependent Nrf2 activation. Besides MAPK, protein kinase C (PKC), phosphatidylinositol 3-kinase (PI3K), and RNA-dependant protein kinase-like endoplasmic reticulum kinase (PERK), depending on cell types and agent properties, have been also implicated in Nrf2 activation. Other transcription factors that also regulate HO-1 expression include heat-shock factor (HSF), nuclear factor-erythroid 2 (NF-E2), activator protein-1 (AP-1), and nuclear factor-κB (NF-κB). Upon external stimuli, the active forms of these transcription factors translocate to the nucleus where they bind to the specific DNA sequence leading to the transcription of HO-1 gene. Some phytochemicals directly activate Nrf2 nuclear translocation or indirectly through ROS generation.
MAPK pathway
The MAPK super-family comprises three primary signaling cascades: the extracellular signal regulated kinase (ERK), the c-Jun NH2-terminal kinase or stress-activated kinase (JNK/SAPK), and the p38 MAPK. Recent studies have focused on the roles of MAPK pathways in HO-1 expression in diverse cell types in response to various stimuli [14]. For examples, curcumin stimulates HO-1 expression through p38 MAPK pathway [15–18]. α-Lipoic acid induces HO-1 expression through ERK pathway [19]. Paclitaxel induces the expression of HO-1 via JNK pathway [20]. Interestingly, the inhibition of ERK, JNK, or p38 MAPK activation individually is insufficient to completely abolish HO-1 expression by oxidized low density lipoprotein; however, inhibition of these MAPK pathways in combination results in a significantly greater attenuation of HO-1 expression [21], suggesting that these MAPK pathways act in concert to regulate HO-1 expression.
Nrf2 activation
Nrf2 is a member of the basic leucine zipper family of transcription factors. Under normal physiological conditions, Nrf2 resides in the cytoplasm bound to its inhibitor protein, Keap-1, an actin-binding protein. On stimulation, Nrf2 is released from Keap-1 and rapidly translocates into the nucleus, where it binds to a DNA sequence in target genes [22]. The important role of Nrf2 in the stress-dependent expression of HO-1 has been highlighted by the finding that HO-1 is less inducible in Nrf2-deficient mice [23]. Moreover, transduction of vascular smooth muscle cells with Nrf2-expressing adenovirus increased cytoprotective HO-1 expression [24].
Integrative Survival Response Evoked by HO-1 and Heme Metabolites
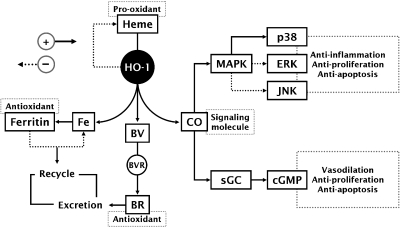
Although the protective effects of HO-1 have been confirmed in a number of experimental models, the mechanism of its action has not been completely elucidated. Degradation of the pro-oxidant heme by HO-1 appears to first aid in tissue protection; however, recent evidence suggests that one or more of the by-products of its heme catabolism (CO, free iron or BV/BR) mediates the cytoprotective effects of HO-1 [4] (Fig. 2).
Fig. 2.
Potential signaling pathways activated by HO-1 and heme metabolites leading to tissue protection. HO-1 reduces the pro-oxidant heme through enzymatic reaction. Iron released from this reaction triggers pathways involving the activation of the iron regulatory protein, leading to its sequestration and recycle. BR and BV potentially contribute to cellular antioxidant balance prior to their excretion. CO activates sGC, resulting in production of cGMP. CO also modulates MAPK pathways, thereby leading to anti-inflammatory, anti-apoptotic, and antiproliferative effects. The solid and dotted arrow lines shows either an increase or activation and either a decrease or inactivation, respectively.
Heme degradation
Heme is inherently dangerous when released from intracellular heme-containing proteins [1]. It offers toxic effects to tissues and organs through oxidative stress by generating reactive oxygen species (ROS) via Fenton reaction [25], which may promote membrane lipid peroxidation and inflammation. Thus, heme catabolism by HO-1 is the first important antioxidant mechanism for survival from the redox environment induced by free heme. It should be noted that the insufficient catabolism of free heme by HO-1 may induce disturbances of the iron-reuse system, causing iron-deficiency anemia as already evidenced in HO-1-deficient human and mice [8].
CO
Although the gaseous CO is classically thought of as a toxic molecule and cellular asphyxiate, recent studies have revealed that CO has profound effects on the intracellular signaling processes, culminating in anti-inflammatory, antiproliferative, and anti-apoptotic effects (excellently reviewed in Ref. 4). The pleiotropic effects of CO may involve two general mechanisms; CO can activate soluble guanylyl cyclase (sGC), with the resultant production of cyclic guanine monophosphate (cGMP), and CO can modulate MAPK pathway. CO, like NO, binds to the heme moiety of sGC, leading to the stimulation of sGC and subsequent elevation of cGMP levels that causes protective vascular relaxation [26]. Other cGMP-mediated effects of CO include the inhibition of platelet aggregation [27] and vascular smooth muscle proliferation [26], and the protection of pancreatic β cells from apoptosis [28]. Most interestingly, CO induces a general down-regulation of pro-inflammatory cytokine production through MAPK-dependent pathway, leading to anti-inflammatory tissue protection [29, 30]. Also, both anti-apoptotic and antiproliferative actions of CO involve the MAPK pathway [31, 32].
Iron and ferritin
Although ferrous iron, an extremely pro-oxidative molecule, is released during the breakdown of free heme by HO-1, the iron is rapidly removed by ferritin, a ubiquitously existing intracellular protein that is able to effectively sequester intracellular iron and, hence, limit its pro-oxidant capacity. Thus, it is not surprising that ferritin expression is enhanced in conjunction with HO-1 expression [33]. Ferritin synthesized as a consequence of HO-1 activity has been implicated as a cytoprotective molecule in a number of experimental models [34].
BV/BR
In cellular system, the soluble greenish pigment BV is rapidly converted by BV reductase into the hydrophobic yellowish pigment BR. BR is now recognized as being a potent antioxidant manufactured by the body, because BR can directly scavenge ROS and reduce ROS production [35]. By virtue of its ROS-scavenging capacity, BR confers cardiovascular, hepatic, and neural protection [35]. Moreover, it is becoming clear that low plasma concentrations of BR are associated with a low incidence of cardiovascular disorders in humans (e.g., atherosclerosis and ischemic heart disease) [36].
HO-1 Inducer
As HO-1 expression is widely recognized as an effective cellular strategy to counteract a variety of stressful events [4], the induction of HO-1 by pharmacological modulators may represent a novel target for therapeutic intervention. Particularly, the identification of non-cytotoxic HO-1 inducers may maximize the intrinsic protective potential of cells.
Phytochemicals
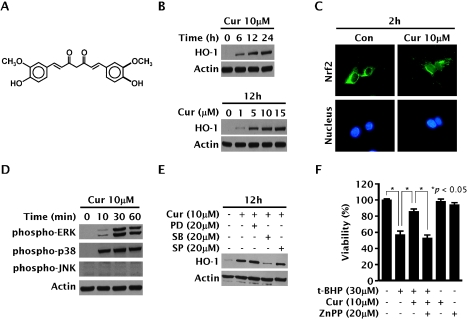
Several antioxidants from plant origins and drugs have been reported to induce HO-1 expression in various types of cells [37]. Among them, curcumin (Fig. 3A), a polyphenolic compound isolated from the rhizome of Curcuma longa Linn (Zingiberaceae), is well characterized as a non-cytotoxic HO-1 inducer in endothelial cells [16], astrocytes [38], macrophages [39], and vascular smooth muscle cells [40]. In the liver HepG2 cells, curcumin also induced HO-1 expression in a time- and dose-dependent manner (Fig. 3B). Curcumin activated Nrf2 nuclear translocation (Fig. 3C), which is presumably an upstream step of HO-1 expression. The experiments designed to determine a possible role of MAPK pathways in HO-1 expression by curcumin showed that curcumin activates ERK and p38 MAPK pathways (Fig. 3D), but not JNK pathway. Additionally, the use of specific inhibitors for the MAPK pathways confirmed the involvement of p38 MAPK pathway, but not of ERK or JNK pathways, in HO-1 expression (Fig. 3E). Interestingly, pre-incubation for 12 h with curcumin resulted in an enhanced cellular resistance to the potent oxidant t-butylhydroperoxide; this cytoprotective effect was considerably attenuated by zinc protoporphyrin IX, an inhibitor of HO activity (Fig. 3F).
Fig. 3.
Cytoprotective HO-1 expression by curcumin through MAPK-dependent Nrf2 activation in HepG2 cells. (A) Structure of curcumin. (B, C, D and E) Cells were treated with indicated concentrations of curcumin for indicated time periods in the absence or presence of inhibitors for MAPK pathways, and Western blotting analysis for HO-1 expression and phosphorylation of ERK, p38 MAPK and JNK and fluorescence microscopic examination for Nrf2-nuclear translocation by using green-fluorescence dye conjugated to Nrf2 antibody were performed as previously described [40]. (F) Cells were pre-incubated for 12 h with 10 µM of curcumin, and exposed for 6 h to 30 µM of ter-butylhydroperoxide in the absence or presence of 20 µM of zinc protoporphyrin IX.
CO
Surprisingly, CO can induce HO-1 expression, raising a possibility that CO may exert full effects not only through the first pathway associated with its original ability but also through the second pathway associated with HO-1 expression that, in addition to CO-mediated effects, may provide other effects. In this regard, CO may serve as an effective HO-1 inducer. Sawle and coworkers [41] originally demonstrated that a CO-releasing molecule (CORM) increased HO-1 expression and HO activity. However, they did not explore the cellular mechanism for this effect of CO on HO-1 expression, as well as its physiological significance. Later, Lee and coworkers [42] confirmed that both CO gas and CORM induced HO-1 expression via Nrf2-dependent pathway in HepG2 cells and primary rat hepatocytes. This is further confirmed by Kim and coworkers [43]. They demonstrated that exogenous CO activated Nrf2 through the phosphorylation of protein kinase R-like endoplasmic reticulum kinase (PERK), resulting in HO-1 expression. CO-induced activation of PERK was followed by the phosphorylation of eukaryotic translation initiation factor 2 and the expression of activating transcription factor 4. However, CO failed to induce X-box binding protein-1 (Xbp-1) expression and activating transcription factor 6 (ATF6) cleavage. Instead, CO prevented Xbp-1 expression and ATF6 cleavage induced by endoplasmic reticulum (ER) stress inducers such as tunicamycin. They also demonstrated that CO rendered endothelial cells resistant to ER stress, which is potentially therapeutic in vascular diseases associated with ER stress. CO prevented endothelial apoptosis triggered by ER stress inducers through the suppression of C/EBP homologous protein (CHOP) expression, which was associated with its activation of p38 MAPK. The endogenous CO produced from HO-1 was also cytoprotective against ER stress through CHOP suppression.
Conclusion
Growing evidence supports HO-1 as an ‘omnipotent’ protein that, when expressed in a variety of disease states, exerts numerous activities that offer tissue protection. The precise underlying mechanisms for HO-1-based protection are not yet completely understood, but appear to involve the effects of by-products of HO-1 activity on intracellular signaling pathways. Overall, the ultimate goal of most research in HO-1 is to find a therapeutic use, and there could be several approaches to this issue. Transfer of HO-1 gene and administration of the reaction products of HO-1 have been attempted in animals with promising results, but not in human studies, probably due to potentially injurious effects. However, it is worth mentioning that several dietary antioxidants, of which safety is well characterized in humans, can induce HO-1 expression in a variety of cell types, and this may be a mechanism for their beneficial effects in several diseases. Further studies are required to investigate the effects of other dietary antioxidants and examine the signaling pathways involved in HO-1 expression.
Acknowledgement
This work was supported by Wonkwang University (2006).
Abbreviations
- HO-1
heme oxygenase-1
- BR
bilirubin
- BV
biliverdin
- NO
nitric oxide
- Nrf2
nuclear factor E2-related factor 2
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal regulated kinase
- JNK
c-Jun NH2-terminal kinase
- ROS
reactive oxygen species
- sGC
soluble guanylyl cyclase
- cGMP
cyclic guanine monophosphate
- CORM
CO-releasing molecule
- PERK
protein kinase R-like endoplasmic reticulum kinase
- Xbp-1
X-box binding protein-1
- ATF 6
activating transcription factor 6
- ER
endoplasmic reticulum
- CHOP
C/EBP homologous protein
References
- 1.Furuyama K., Kaneko K., Vargas V. Heme as a magnificent molecule with multiple missions: heme determines its own fate and governs cellular homeostasis. Tohoku J. Exp. Med. 2007;213:1–16. doi: 10.1620/tjem.213.1. [DOI] [PubMed] [Google Scholar]
- 2.Kikuchi G., Yoshida T., Noguchi M. Heme oxygenase and heme degradation. Biochem. Biophys. Res. Commun. 2005;338:558–567. doi: 10.1016/j.bbrc.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Naito Y., Takagi T., Yoshikawa T. Heme oxygenase-1: a new therapeutic target for inflammatory bowel disease. Aliment Pharmacol. Ther. 2004;20:177–184. doi: 10.1111/j.1365-2036.2004.01992.x. [DOI] [PubMed] [Google Scholar]
- 4.Ryter S.W., Alam J., Choi A.M. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 5.Morse D., Choi A.M. Heme oxygenase-1: from bench to bedside. Am. J. Respir. Crit. Care Med. 2005;172:660–670. doi: 10.1164/rccm.200404-465SO. [DOI] [PubMed] [Google Scholar]
- 6.Poss K.D., Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poss K.D., Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koizumi S. Human heme oxygenase-1 deficiency: a lesson on serendipity in the discovery of the novel disease. Pediatr. Int. 2007;49:125–132. doi: 10.1111/j.1442-200X.2007.02353.x. [DOI] [PubMed] [Google Scholar]
- 9.Kapturczak M.H., Wasserfall C., Brusko T., Campbell-Thompson M., Ellis T.M., Atkinson M.A., Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 2004;165:1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawashima A., Oda Y., Yachie A., Koizumi S., Nakanishi I. Heme oxygenase-1 deficiency: The first autopsy case. Hum. Pathol. 2002;33:125–130. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 11.Yachie A., Niida Y., Wada T., Igarashi N., Kaneda H., Toma T., Ohta K., Kasahara Y., Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohta K., Yachie A., Fujimoto K., Kaneda H., Wada T., Toma T., Seno A., Kasahara Y., Yokoyama H., Seki H., Koizumi S. Tubular injury as a cardinal pathologic feature in human heme oxygenase-1 deficiency. Am. J. Kidney. Dis. 2000;35:863–870. doi: 10.1016/s0272-6386(00)70256-3. [DOI] [PubMed] [Google Scholar]
- 13.Farombi E.O., Surh Y.J. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J. Biochem. Mol. Biol. 2006;39:479–491. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- 14.Alam J., Cook J.L. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am. J. Respir. Cell Mol. Biol. 2007;36:166–174. doi: 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- 15.McNally S.J., Harrison E.M., Ross J.A., Garden O.J., Wigmore S.J. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int. J. Mol. Med. 2007;19:165–172. [PubMed] [Google Scholar]
- 16.Jeong G.S., Oh G.S., Pae H.O., Jeong S.O., Kim Y.C., Shin M.K., Seo B.Y., Han S.Y., Lee H.S., Jeong J.G., Koh J.S., Chung H.T. Comparative effects of curcuminoids on endothelial heme oxygenase-1 expression: ortho-methoxy groups are essential to enhance heme oxygenase activity and protection. Exp. Mol. Med. 2006;38:393–400. doi: 10.1038/emm.2006.46. [DOI] [PubMed] [Google Scholar]
- 17.Andreadi C.K., Howells L.M., Atherfold P.A., Manson M.M. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol. Pharmacol. 2006;69:1033–1040. doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]
- 18.Balogun E., Hoque M., Gong P., Killeen E., Green C.J., Foresti R., Alam J., Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogborne R.M., Rushworth S.A., O’Connell M.A. Alpha-lipoic acid-induced heme oxygenase-1 expression is mediated by nuclear factor erythroid 2-related factor 2 and p38 mitogen-activated protein kinase in human monocytic cells. Arterioscler. Thromb. Vasc. Biol. 2005;25:2100–2105. doi: 10.1161/01.ATV.0000183745.37161.6e. [DOI] [PubMed] [Google Scholar]
- 20.Choi B.M., Kim Y.M., Jeong Y.R., Pae H.O., Song C.E., Park J.E., Ahn Y.K., Chung H.T. Induction of heme oxygenase-1 is involved in anti-proliferative effects of paclitaxel on rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2004;321:132–137. doi: 10.1016/j.bbrc.2004.06.120. [DOI] [PubMed] [Google Scholar]
- 21.Anwar A.A., Li F.Y., Leake D.S., Ishii T., Mann G.E., Siow R.C. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic. Biol. Med. 2005;39:227–36. doi: 10.1016/j.freeradbiomed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Aleksunes L.M., Manautou J.E. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol. Pathol. 2007;35:459–473. doi: 10.1080/01926230701311344. [DOI] [PubMed] [Google Scholar]
- 23.Khor T.O., Huang M.T., Kwon K.H., Chan J.Y., Reddy B.S., Kong A.N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 24.Levonen A.L., Inkala M., Heikura T., Jauhiainen S., Jyrkkänen H.K., Kansanen E., Määttä K., Romppanen E., Turunen P., Rutanen J., Ylä-Herttuala S. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler. Thromb. Vasc. Biol. 2007;27:741–747. doi: 10.1161/01.ATV.0000258868.80079.4d. [DOI] [PubMed] [Google Scholar]
- 25.Crichton R.R., Wilmet S., Legssyer R., Ward R.J. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J. Inorg. Biochem. 2002;91:9–18. doi: 10.1016/s0162-0134(02)00461-0. [DOI] [PubMed] [Google Scholar]
- 26.Morita T., Mitsialis S.A., Koike H., Liu Y., Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J. Biol. Chem. 1997;272:32804–32809. doi: 10.1074/jbc.272.52.32804. [DOI] [PubMed] [Google Scholar]
- 27.Brüne B., Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol. Pharmacol. 1987;32:497–504. [PubMed] [Google Scholar]
- 28.Günther L., Berberat P.O., Haga M., Brouard S., Smith R.N., Soares M.P., Bach F.H., Tobiasch E. Carbon monoxide protects pancreatic beta-cells from apoptosis and improves islet function/survival after transplantation. Diabetes. 2002;51:994–999. doi: 10.2337/diabetes.51.4.994. [DOI] [PubMed] [Google Scholar]
- 29.Pae H.O., Oh G.S., Choi B.M., Chae S.C., Kim Y.M., Chung K.R., Chung H.T. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J. Immunol. 2004;172:4744–4751. doi: 10.4049/jimmunol.172.8.4744. [DOI] [PubMed] [Google Scholar]
- 30.Mannaioni P.F., Vannacci A., Masini E. Carbon monoxide: the bad and the good side of the coin, from neuronal death to anti-inflammatory activity. Inflamm. Res. 2006;55:261–273. doi: 10.1007/s00011-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 31.Choi B.M., Pae H.O., Kim Y.M., Chung H.T. Nitric oxide-mediated cytoprotection of hepatocytes from glucose deprivation-induced cytotoxicity: involvement of heme oxygenase-1. Hepatology. 2003;37:810–823. doi: 10.1053/jhep.2003.50114. [DOI] [PubMed] [Google Scholar]
- 32.Song R., Mahidhara R.S., Liu F., Ning W., Otterbein L.E., Choi A.M. Carbon monoxide inhibits human airway smooth muscle cell proliferation via mitogen-activated protein kinase pathway. Am. J. Respir. Cell Mol. Biol. 2002;27:603–610. doi: 10.1165/rcmb.4851. [DOI] [PubMed] [Google Scholar]
- 33.Balla J., Vercellotti G.M., Jeney V., Yachie A., Varga Z., Eaton J.W., Balla G. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Mol. Nutr. Food Res. 2005;49:1030–1043. doi: 10.1002/mnfr.200500076. [DOI] [PubMed] [Google Scholar]
- 34.Balla J., Vercellotti G.M., Jeney V., Yachie A., Varga Z., Jacob H.S., Eaton J.W., Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid. Redox. Signal. 2007;9:2119–2137. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- 35.Ollinger R., Wang H., Yamashita K., Wegiel B., Thomas M., Margreiter R., Bach F.H. Therapeutic applications of bilirubin and biliverdin in transplantation. Antioxid. Redox. Signal. 2007;9:2175–2185. doi: 10.1089/ars.2007.1807. [DOI] [PubMed] [Google Scholar]
- 36.Ryter S.W., Morse D., Choi A.M. Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am. J. Respir. Cell Mol. Biol. 2007;36:175–182. doi: 10.1165/rcmb.2006-0333TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogborne R.M., Rushworth S.A., Charalambos C.A., O’Connell M.A. Haem oxygenase-1: a target for dietary antioxidants. Biochem. Soc. Trans. 2004;32:1003–1005. doi: 10.1042/BST0321003. [DOI] [PubMed] [Google Scholar]
- 38.Scapagnini G., Foresti R., Calabrese V., Giuffrida Stella A.M., Green C.J., Motterlini R. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol. Pharmacol. 2002;61:554–561. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K., Tanaka I., Nakanishi I., Kurematsu A., Yakumaru H., Ikota N., Ishihara H. Drastic effect of several caffeic acid derivatives on the induction of heme oxygenase-1 expression revealed by quantitative real-time RT-PCR. Biofactors. 2006;28:151–158. doi: 10.1002/biof.5520280301. [DOI] [PubMed] [Google Scholar]
- 40.Pae H.O., Jeong G.S., Jeong S.O., Kim H.S., Kim S.A., Kim Y.C., Yoo S.J., Kim H.D., Chung H.T. Roles of heme oxygenase-1 in curcumin-induced growth inhibition in rat smooth muscle cells. Exp. Mol. Med. 2007;39:267–277. doi: 10.1038/emm.2007.30. [DOI] [PubMed] [Google Scholar]
- 41.Sawle P., Foresti R., Mann B.E., Johnson T.R., Green C.J., Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br. J. Pharmacol. 2005;145:800–810. doi: 10.1038/sj.bjp.0706241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee B.S., Heo J., Kim Y.M., Shim S.M., Pae H.O., Kim Y.M., Chung H.T. Carbon monoxide mediates heme oxygenase 1 induction via Nrf2 activation in hepatoma cells. Biochem. Biophys. Res. Commun. 2006;343:965–972. doi: 10.1016/j.bbrc.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 43.Kim K.M., Pae H.O., Zheng M., Park R., Kim Y.M., Chung H.T. Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ. Res. 2007;101:919–927. doi: 10.1161/CIRCRESAHA.107.154781. [DOI] [PubMed] [Google Scholar]