Abstract
The phytoestrogens genistein, daidzein and the daidzein metabolite equol have been shown previously to possess oestrogen agonist activity. However, following consumption of soya diets, they are found in the body not only as aglycones but also as metabolites conjugated at their 4′- and 7-hydroxyl groups with sulphate. This paper describes the effects of monosulphation on the oestrogen agonist properties of these three phytoestrogens in MCF-7 human breast cancer cells in terms of their relative ability to compete with [3H]oestradiol for binding to oestrogen receptor (ER), to induce a stably transfected oestrogen-responsive reporter gene (ERE-CAT) and to stimulate cell growth. In no case did sulphation abolish activity. The 4′-sulphation of genistein reduced oestrogen agonist activity to a small extent in whole-cell assays but increased the relative binding affinity to ER. The 7-sulphation of genistein, and also of equol, reduced oestrogen agonist activity substantially in all assays. By contrast, the position of monosulphation of daidzein acted in an opposing manner on oestrogen agonist activity. Sulphation at the 4′-position of daidzein resulted in a modest reduction in oestrogen agonist activity but sulphation of daidzein at the 7-position resulted in an increase in oestrogen agonist activity. Molecular modelling and docking studies suggested that the inverse effects of sulphation could be explained by the binding of daidzein into the ligand-binding domain of the ER in the opposite orientation compared with genistein and equol. This is the first report of sulphation enhancing activity of an isoflavone and inverse effects of sulphation between individual phytoestrogens.
Introduction
Many compounds produced naturally by plants (termed phytoestrogens) have been shown in vivo to modulate the actions of endogenous oestrogens giving rise to endocrine disruption and in vitro to be able to mimic oestrogen action with resulting agonist and/or antagonist actions (Woods 2003). Although several mechanisms are now recognised, a major basis for their action occurs through their ability to bind to the oestrogen receptors (ERα and ERβ) and thus to influence the subsequent ligand-activated functions of these transcription factors on patterns of gene expression (Woods 2003). Of the many phytoestrogens, the isoflavones genistein and daidzein can be consumed in relatively large amounts in soya bean-rich diets such as those found in Eastern populations where there are relatively low levels of hormone-dependent cancers and menopausal symptoms, and interest has increased therefore in their use as dietary supplements with measurable health benefits (Adlercreutz & Mazur 1997, Woods 2003).
Genistein and daidzein have been shown previously to possess oestrogen agonist activity in human breast cancer cells in vitro (Wang & Kurzer 1997, Hsieh et al. 1998, Makela et al. 1999, Dampier et al. 2001, Maggiolini et al. 2001, Chen et al. 2003, Woods 2003, Matsumura et al. 2005, Hwang et al. 2006). The isoflavan equol is a metabolite produced by the reduction of daidzein by gut micro-organisms, albeit to varying extents in different individuals (Woods 2003), and equol has also been shown to have oestrogenic activity in human breast cancer cells (Welshons et al. 1987, Matsumura et al. 2005). Although genistein and daidzein can be detected in the plasma and urine of humans who consume soya foods, the majority of these isoflavones circulate and are excreted in urine as mono- and disulphates, mono- and diglucuronides and sulphoglucuronides through conjugation at their 4′- and 7-hydroxyl groups (Adlercreutz et al. 1995, Shelnutt et al. 2000, 2002, Thomas et al. 2001, Zhang et al. 2003). Glucuronides are the most abundant conjugates (Zhang et al. 2003), but sulphates of genistein and daidzein have been shown to make up a significant proportion of the total isoflavone concentration in plasma, in one study of 21 and 20% respectively (Zhang et al. 2003) and in a second study of 8 and 23% respectively (Shelnutt et al. 2002), and in urine of 24 and 23% respectively (Zhang et al. 2003). In urine, site-specific studies have measured monosulphates as 13% of total daidzein with disulphates making up <0·1% (Clarke et al. 2002).
At target tissues, overall activity of these phytoestrogens will depend not only on the enzymatic hydrolysis of the conjugates back to the aglycones by tissue sulphatases and β-glucuronidases (Shelnutt et al. 2002) but also on any intrinsic oestrogenic activity of the conjugates themselves. Human breast cancer cells are known to possess both sulphotransferase and sulphatase activities, which additionally can be influenced by the phytoestrogens themselves (Kirk et al. 2001, Harris et al. 2004). However, little is known of the intrinsic oestrogenic activity of the conjugates. Genistein glucuronides have been reported to retain weak ER-binding capability (Zhang et al. 1999) but the effect of sulphation remains uncertain (Kinjo et al. 2004, Totta et al. 2005). We report here on the varied ability of the monosulphates of genistein, daidzein and equol to bind to cytosolic ER of MCF-7 human breast cancer cells and to act on the whole MCF-7 cells to regulate oestrogen-responsive gene expression and to increase oestrogen-dependent cell growth. The inverse effect of sulphation on oestrogen agonist activity of genistein and equol compared with daidzein led us to carry out molecular modelling and docking using the program suite GRID/GLUE (Molecular Discovery Ltd, Ponte San Giovanni, Italy) to investigate the possible binding modes in which these ligands can interact with the ligand-binding domain (LBD) of human ERα and ERβ.
Materials and Methods
Chemicals
Genistein (98% purity) was purchased from Sigma. Daidzein (98% purity), dl-equol (97–98% purity) and genistein-7-sulphate were purchased from Plantech (Reading, Berkshire, England). 17β-Oestradiol was purchased from Steraloids (Croydon, England). Daidzein-7-sulphate (95% purity) and daidzein-4′-sulphate (96% purity) were synthesised as described previously (Fairley et al. 2003). Genistein-4′-sulphate and equol-7-sulphate were synthesised by analogous methods.
All compounds were made as stock solutions in ethanol or dimethyl sulphoxide (DMSO) and diluted into culture medium. Stock solutions of genistein, genistein-4′-sulphate, equol and equol-7-sulphate were prepared at 10−2 M in ethanol. Stock solutions of daidzein and sulphates were prepared at 10−3 M in ethanol. Stock solutions of genistein-7-sulphate were prepared at 10−2 M in DMSO.
For genistein and equol, cell culture experiments using 10−5 M concentrations were performed by diluting the stock 10−2 M solutions at 1 in 1000 (v/v) in culture medium, and controls were performed with equivalent concentrations of ethanol or DMSO. For concentrations of 10−6 M and below, the stock solutions were serially diluted such that the dilution into culture medium was always 1 in 10 000 (v/v), and controls were performed with equivalent concentrations of ethanol or DMSO.
For daidzein and sulphates, the cell culture experiments using 10−6 M concentrations were performed by diluting the stock 10−3 M solutions at 1 in 1000 (v/v) in culture medium, and controls were performed with equivalent concentrations of ethanol. For concentrations of 10−7 M and below, the stock solutions were serially diluted such that the dilution into culture medium was always 1 in 10 000 (v/v), and controls were performed with equivalent concentrations of ethanol.
Culture of stock cells
MCF-7 McGrath human breast cancer cells were kindly provided by C K Osborne at passage number 390 (Osborne et al. 1987). This cell line is dependent on oestrogen for growth as described previously (Darbre & Daly 1989). Stock MCF-7 cells were grown as monolayer cultures in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 5% (v/v) fetal calf serum (FCS; Invitrogen), 10 μg/ml insulin (Sigma) and 10−8 M 17β-oestradiol in a humidified atmosphere of 10% carbon dioxide in air at 37 °C. The cell stocks were subcultured at weekly intervals by suspension with 0·06% trypsin/0·02% EDTA (pH 7·3).
Competitive binding assay to ER of MCF-7 cytosol
MCF-7 human breast cancer cells were grown as monolayer cultures in phenol red-free RPMI 1640 medium (Invitrogen) with 5% dextran-charcoal-stripped FCS (DCFCS; Darbre et al. 1983) for a minimum of 3 days to deplete steroid hormone levels in the cells. The cells were then harvested, pelleted and homogenised in eight volumes of buffer (10 mM Tris–HCl, 1 mM EDTA, 2 mM dithiothreitol, 10% (v/v) glycerol, 0. 5 M NaCl, pH 7·4) at 4 °C. Homogenates were centrifuged at 105 000 g for 1 h at 4 °C, and the resulting supernatant was stored in aliquots at −70 °C. Competitive binding assays were performed on the cytosol using the dextran-coated charcoal method as described previously (Green & Leake 1987). Competition was assayed between the binding of [2,4,6,7-3H]oestradiol (Amersham International) at 4×10−10 M and 1- to 100 000-fold molar excess of unlabelled compounds.
Competitive binding assay to recombinant ERα
Full-length human recombinant ERα (Invitrogen) was diluted 1:2500 in buffer (10 mM Tris–HCl, 1 mM EDTA, 2 mM dithiothreitol, 10% (v/v) glycerol, 0·5 M NaCl, pH 7.4 containing 5 mg/ml yeast extract) at 4 °C. Competitive binding assays were performed in 96-well polystyrene plates for 18 h at 4 °C between the binding of [2,4,6,7-3H]oestradiol (GE Healthcare: Amersham International) at 0·8 nM and 1- to 1 000 000-fold molar excess of unlabelled phytoestrogen using 0·8 nM ER protein. Receptor–ligand complexes were separated from free ligand using the hydroxylapatite method as described previously (Green & Leake 1987).
Assay of stably transfected ERE-CAT reporter gene in MCF-7 cells
The ERE-CAT (oestrogen response element–chloramphenicol acetyl transferase) vector consisted of the ERE of the vitellogenin A2 gene from −331 to −295 bp cloned into the pBLCAT2 vector (Luckow & Schutz 1987) upstream of the thymidine kinase promoter. A clonal line of MCF-7 cells stably transfected with this vector was previously characterised and the assay was carried out exactly as published previously (Byford et al. 2002). P values were calculated using a two-tailed student t-test, two-sample assuming unequal variance, within the MS Excel 2000 software package.
Cell proliferation experiments
The cells were added to the required volume of phenol red-free RPMI 1640 medium containing 5% DCFCS at a concentration of about 0·2×105 cells/ml and plated in monolayer in 0·5 ml aliquots into 24-well plastic tissue culture dishes (Nunc, Roskilde, Denmark). After 24 h, the medium was changed to phenol red-free RPMI 1640 medium supplemented with 5% DCFCS with or without supplements of 17β-oestradiol and/or phytoestrogen. The culture medium was changed routinely every 3–4 days in all experiments. The cell counts were performed by counting released nuclei on a model ZBI Coulter Counter, as described previously (Daly & Darbre 1990). Doubling time of the cells was calculated as a function of the slope of the linear plot of log10 (cell number) against time. Doubling time was calculated as log10 2/slope. P values were calculated using a two-tailed student t-test, two-sample assuming unequal variance, within the MS Excel 2000 software package.
Molecular modelling and docking of ligand binding to the LBD of human ER
The crystal structures of 1A52, an oestradiol-ERα complex (Tanenbaum et al. 1998) and 1×7R, a genistein-ERα complex (Manas et al. 2004) were obtained from the Protein Data Bank (http://www.ebi.ac.uk). The oestrogen ligand was removed from the coordinate file of 1A52 and the resulting protein was subjected to molecular mechanics and energy minimisation using the consistent valence force field (CVFF) in InsightII (Cerius2 Modeling Environment, Release 4.8, Accelrys Inc., San Diego, CA, USA) as the starting structure for the docking studies. The structures of genistein, daidzein and equol were built using the BUILDER module in InsightII and a conformational minimisation was performed for each modelled ligand using the software Mizer (Molecular Discovery Ltd) to obtain the lowest energy conformer for the docking studies. GRID was used to calculate the molecular interaction fields (MIFs) within the active site of the protein, according to hydrophobic, hydrogen-bond donor/acceptor and electrostatic interaction capabilities and geometries (Goodford 1985, Pastor et al. 1997, Carosati et al. 2004). Eight default GRID probes (H, OH2, DRY, N1+, N1, N:=, O− and OH) were used for the MIF calculation. GLUE, a GRID-based docking program, was used to dock each ligand into the LBD of human ERα for subsequent analysis (Sciabola et al. 2005). GLUE calculates the interaction energy between an entire ligand and a protein-binding site, proposes several docking solutions, and ranks these by the values of the energy scoring function. The docking solution with the lowest and most favourable energy was chosen to compare the docking results of the different ligands in the protein-binding pocket. GLUE generates possible interactions between the ligand and the active site of the protein molecule by fitting the maps (or MIFs) generated by GRID for each probe, which mimics the functional groups on the ligands of interest, to the potential ligand-binding site. GLUE then positions the flexible hydrophobic and polar atoms of the ligands by generating several conformations (poses) over their corresponding energy maps. The most favourable modes of binding are then optimised within the active site of the protein by calculating successive torsion and translation energies driven by the protein–ligand interaction energy as computed by the GRID force field. Thus, it is expected that the resulting docked pose and its associated energy represents a realistic and potentially biologically relevant bound conformation. Indeed, to test the reliability of the docking as performed by GLUE, we first docked the crystal structure of genistein, extracted from 1×7R (an ERα – genistein co-crystallised complex), into 1A52 (with the oestrogen ligand removed). The default GRID/GLUE parameters were used for the MIF calculation and docking procedure. For binding optimisation, 1000 iterations were used. We next applied the same GRID/GLUE parameters to dock the modelled structures (using InsightII) of genistein, daidzein and equol into the active site of 1A52. The docking results were visualised using PyMOL (DeLano Scientific LLC, San Francisco, CA, USA), the hydrogen bond and van der Waals interactions were calculated using the program CONTACT available in the CCP4 suite (CCP4, 2003) and the root mean square deviation (RMSD) was calculated using the least-squares fitting procedure provided in the program ‘O’ version 9.0.7 ( Jones et al. 1990). The potential hydrogen bonds were assigned if the distance between two electronegative atoms was <3·3 Å. The van der Waals interactions were assigned where the separation between non-bonded hydrogen atoms was <4 Å.
Predicting sulphation in the ligand-binding site of the LBD of human ER
The program GRID was used to calculate the energies of interaction between a sulphoxide functional group (OS probe: oxygen sulphoxide/sulphonate) and the LBD of human ER. The box chosen for the GRID calculation was such that it was centred over the ligand-binding site of the LBD of ERα and ERβ (using 1×7R and 1QKM respectively, as the target structures). A 5 Å clearance and 0·5 Å spacing were used for the GRID calculation. The resulting MIF was visualised using GVIEW (Molecular Discovery Ltd), as provided within the GRID program (version 22a). An energy threshold of −5·0 kcal/mol was chosen for final visualisation using PyMOL.
Results
Experimental strategy
The chemical structures of the phytoestrogens and their monosulphates included in this study are given in Fig. 1. Oestrogen agonist activity was assessed for a range of concentrations for each phytoestrogen using a variety of validated assays based in MCF-7 human breast cancer cells (Byford et al. 2002, Matsumura et al. 2005): 1) ligand ability to bind to ER from MCF-7 cell lysates in a competitive binding assay, 2) ligand ability to regulate expression of a stably transfected oestrogen-responsive reporter gene (ERE-CAT) in MCF-7 cells and 3) ligand ability to regulate the proliferation of oestrogen-dependent MCF-7 cells.
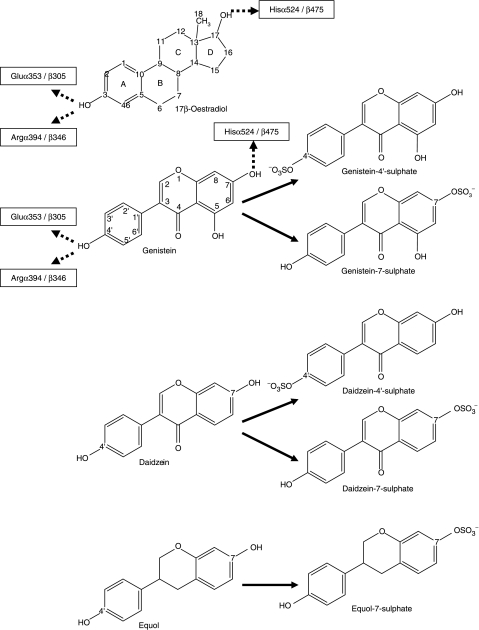
Figure 1.
Comparison of the chemical structures of 17β-oestradiol, genistein, genistein-4′-sulphate, genistein-7-sulphate, daidzein, daidzein-4′-sulphate, daidzein-7-sulphate, equol and equol-7-sulphate. Dotted arrows indicate the orientation of binding of 17β-oestradiol (Brzozowski et al. 1997) and genistein (Manas et al. 2004) into the LBD of ERα and ERβ respectively, as determined by X-ray crystallography.
Ligand binding to ER of MCF-7 cell lysates
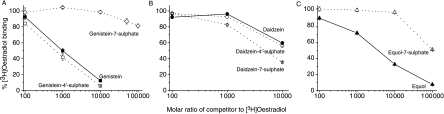
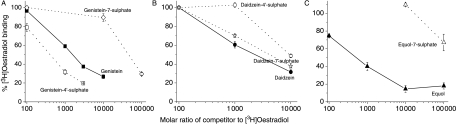
Since the first step in the action of any oestrogenic compound involves the binding of ligand to an intracellular receptor (Oettel & Schillinger 1999), experiments began by determining the effect of sulphation on binding of the phytoestrogens to ER of MCF-7 cell cytosol. In a single-point competitive binding assay, ERs from MCF-7 human breast cancer cells were incubated at 4 °C for 18 h with 4×10−10 M [2,4,6,7-3H]oestradiol and the extent of inhibition of binding was determined with increasing concentrations of unlabelled monosulphated and non-sulphated forms of genistein, daidzein and equol. Figure 2A shows that genistein-4′-sulphate was more effective at inhibiting [3H]oestradiol binding than genistein. By contrast, sulphation of genistein at the 7-position resulted in a much reduced inhibition of [3H]oestradiol binding and inhibition was only 19% even at 100 000-fold molar excess (Fig. 2A). Figure 2B shows that daidzein-7-sulphate was more effective at inhibiting [3H]oestradiol binding than daidzein. Sulphation of daidzein on the 4′-position, however, did not alter the ability of the phytoestrogen to displace [3H]oestradiol to any significant extent compared with the aglycone. By contrast, Fig. 2C shows that sulphation of equol on the 7-conjugation site reduced the ability of the ligand to displace [3H]oestradiol.
Figure 2.
Competitive binding of monosulphated and non-sulphated forms of (A) genistein, (B) daidzein and (C) equol to ER from MCF-7 human breast cancer cells. In single-point competitive binding assays, 4×10−10 M [2,4,6,7-3H]oestradiol was incubated with cytosol plus the stated molar excess of unlabelled genistein (solid square, solid line), genistein-4′-sulphate (open square, dotted line), genistein-7-sulphate (open diamond, dotted line), daidzein (solid circle, solid line), daidzein-4′-sulphate (open circle, dotted line), daidzein-7-sulphate (open star, dotted line), equol (solid triangle, solid line) and equol-7-sulphate (open triangle, dotted line). Error bars represent the mean±s.e.m. of triplicate assays.
Control experiments showed that [3H]oestradiol binding was inhibited by 96% by 10-fold molar excess and by 100% by 100-fold molar excess of diethylstilboestrol. Previous work using the same assay in our laboratory has shown that the glucocorticoid, dexamethasone, has no effect on [3H]oestradiol binding at concentrations of up to 100 000-fold molar excess (Byford et al. 2002).
Ligand ability to increase the expression of ERE-CAT reporter gene in MCF-7 cells
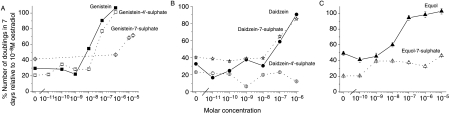
The effect of sulphation on phytoestrogen regulation of oestrogen-responsive gene expression was tested using a stably transfected oestrogen-sensitive reporter gene (ERE-CAT) in MCF-7 human breast cancer cells (Byford et al. 2002, Matsumura et al. 2005). Using a clonal line of MCF-7 cells containing a stably integrated ERE-CAT reporter gene, the cells were deprived of steroid for 7 days and then CAT gene expression was assayed after 24 hours of treatment with 17β-oestradiol or phytoestrogen. Results are shown in Fig. 3 as a % of the induction with 10−8 M 17β-oestradiol. For all the three phytoestrogens, sulphation reduced the ability of the compound to increase CAT activity but some activity was retained in every case, albeit at higher concentrations. Genistein-4′-sulphate increased CAT activity to a significantly lower extent than genistein at 10−7 M (38·6±3·0% vs 70·1±2·2%; P=0·037; Fig. 3A). Genistein-7-sulphate increased CAT activity only at concentrations of 10−5 M and above (Fig. 3A). Daidzein-7-sulphate increased CAT activity to a significantly lesser extent than daidzein at 10−7 M (7·7±3·6% vs 35·6±1·1%; P=0·097; Fig. 3B). Daidzein-4′-sulphate increased CAT activity to a significantly lower extent than daidzein at 10−7 M (2·8±2·8% vs 35·6±1·1%; P=0·037) and at 10−6 M (17·2±1·7% vs 82·3±2·7%; P=0·018) (Fig. 3B). Equol-7-sulphate increased CAT activity to a significantly lower extent than equol at 10−7 M (13·2±2·4% vs 54·0±1·7%; P=0·004) and at 10−6 M (12·8±1·8% vs 88·8±1·4%; P<0·001) (Fig. 3C). However, while 10−5 M concentrations of equol resulted in a reduced induction of CAT activity (72·5±2·1%), reduction in induction of CAT activity was not observed with 10−5 M equol-7-sulphate (24·2±2·4%) (Fig. 3C).
Figure 3.
Regulation by monosulphated and non-sulphated forms of (A) genistein, (B) daidzein and (C) equol of CAT gene expression from a stably transfected ERE-CAT gene in MCF-7 human breast cancer cells. The cells were grown in RPMI 1640 medium/5% DCFCS for 7 days, and then in the same medium for a further 24 h with genistein (solid square, solid line), genistein-4′-sulphate (open square, dotted line), genistein-7-sulphate (open diamond, dotted line), daidzein (solid circle, solid line), daidzein-4′-sulphate (open circle, dotted line), daidzein-7-sulphate (open star, dotted line), equol (solid triangle, solid line) and equol-7-sulphate (open triangle, dotted line) at the molar concentrations indicated. The results are presented graphically as the % of the induction with 10−8 M 17β-oestradiol. Bars represent the mean±s.e.m. of triplicate assays both with 17β-oestradiol and with phytoestrogen.
Ligand ability to stimulate proliferation of MCF-7 human breast cancer cells
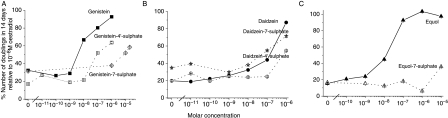
MCF-7 human breast cancer cells are dependent on oestrogen for their proliferation in monolayer culture (Darbre & Daly 1989, Byford et al. 2002, Matsumura et al. 2005). Since these growth assays were not set up as one single experiment and plating densities varied, comparison was achieved by expressing the results as the % number of doublings with the phytoestrogen compared with the number of doublings with 10−8 M 17β-oestradiol in that same assay. The cell growth assays showed that genistein, daidzein and equol could increase proliferation of MCF-7 cells after 7 (Fig. 4) or 14 days (Fig. 5), although requiring varying concentrations. Sulphation had varied effects on the ability of the phytoestrogens to increase cell proliferation.
Figure 4.
Effects of monosulphated and non-sulphated forms of (A) genistein, (B) daidzein and (C) equol on the proliferation of MCF-7 human breast cancer cells in monolayer culture. The cells were grown for 7 days in phenol red-free RPMI 1640 medium/5% DCFCS with no further addition (given at zero molar concentration) or with genistein (solid square, solid line), genistein-4′-sulphate (open square, dotted line), genistein-7-sulphate (open diamond, dotted line), daidzein (solid circle, solid line), daidzein-4′-sulphate (open circle, dotted line), daidzein-7-sulphate (open star, dotted line), equol (solid triangle, solid line) and equol-7-sulphate (open triangle, dotted line) at the molar concentrations indicated. The results are presented graphically as the % number of doublings with the phytoestrogen compared with the number of doublings with 10−8 M 17β-oestradiol in that same assay. Error bars are the standard error of all nine values from triplicate dishes with 10−8 M 17β-oestradiol and triplicate dishes with phytoestrogen.
Figure 5.
Effects of monosulphated and non-sulphated forms of (A) genistein, (B) daidzein and (C) equol on the proliferation of MCF-7 human breast cancer cells in monolayer culture. The cells were grown for 14 days in phenol red-free RPMI 1640 medium/5% DCFCS with no further addition (given at zero molar concentration) or with genistein (solid square, solid line), genistein-4′-sulphate (open square, dotted line), genistein-7-sulphate (open diamond, dotted line), daidzein (solid circle, solid line), daidzein-4′-sulphate (open circle, dotted line), daidzein-7-sulphate (open star, dotted line), equol (solid triangle, solid line) and equol-7-sulphate (open triangle, dotted line) at the molar concentrations indicated. The results are presented graphically as the % number of doublings with the phytoestrogen compared with the number of doublings with 10−8 M 17β-oestradiol in that same assay. Error bars are the standard error of all nine values from triplicate dishes with 10−8 M 17β-oestradiol and triplicate dishes with phytoestrogen.
The growth of MCF-7 cells was increased by genistein after 7 days at 10−8 M (P=0·021), 10−7 M (P=0·005) and 10−6 M (P=0·001) (Fig. 4A) and after 14 days at 10−8 M (P=0·011), 10−7 M (P=0·006) and 10−6 M (P=0·006) (Fig. 5A). By contrast, genistein-4′-sulphate did not increase growth at 10−8 M after 7 days (P=0·258) (Fig. 4A) or 14 days (P=0·209) (Fig. 5A). The growth increase at 10−7 M and 10−6 M genistein-4′-sulphate was relatively less than with genistein after both 7 days (Fig. 4A) and 14 days (Fig. 5A). Genistein-7-sulphate increased growth only at concentrations of 10−5 M and above after either 7 days (Fig. 4A) or 14 days (Fig. 5A). The growth of MCF-7 cells was increased by daidzein after 7 days at 10−7 M (P=0·052) and 10−6 M (P=0·006) (Fig. 4B) and after 14 days at 10−7 M (P=0·008) and 10−6 M (P=0·028) (Fig. 5B). The growth increase observed with daidzein-7-sulphate was very similar to that with daidzein after 7days (Fig. 4B) or 14 days (Fig. 5B). However, daidzein-4′-sulphate was only able to increase growth at 10−6 M after 14 days (P=0·012) (Fig. 5B). The growth of MCF-7 cells was increased to a small extent by equol at 10−8 M after 14 days (P=0·029), but to a greater extent at concentrations of 10−7 M and above after both 7 days (Fig. 4C) and 14 days (Fig. 5C). Equol-7-sulphate was unable to increase the growth of MCF-7 cells except very weakly after 14 days at 10−5 M concentrations (P=0·008) (Fig. 5C).
Ligand binding to recombinant ERα
Since MCF-7 cells possess sulphatase activity (Harris et al. 2004), it is possible that observed levels of activity with the sulphated compounds, which are equivalent to or below that of the aglycone could result from enzymatic desulphation within the cells. High-speed centrifugation of the MCF-7 cell lysates for receptor-binding assays would remove microsomes, but it cannot be entirely excluded that some sulphatase activity remained in the cell cytosol. Therefore, further competitive binding assays were carried out using purified recombinant human ERα (PanVera) where no sulphatase activity could have been present. Our MCF-7 cells contain mostly ERα in a ratio of ERα:ERβ of 19:1 (Shaw et al. 2006). Figure 6 shows that all the phytoestrogen sulphates could displace the binding of [3H]oestradiol to the recombinant human ERα with similar overall concentration dependence to that observed using the MCF-7 cell lysates in Fig. 2, except that daidzein displaced [3H]oestradiol at lower concentrations in this assay and daidzein-4′-sulphate was notably weaker in binding than the aglycone.
Figure 6.
Competitive binding of monosulphated and non-sulphated forms of (A) genistein, (B) daidzein and (C) equol to recombinant human ERα (Invitrogen). In single-point competitive binding assays, 0·8 nM [2,4,6,7-3H]oestradiol was incubated with 0·8 nM recombinant receptor protein plus the stated molar excess of unlabelled genistein (solid square, solid line), genistein-4′-sulphate (open square, dotted line), genistein-7-sulphate (open diamond, dotted line), daidzein (solid circle, solid line), daidzein-4′-sulphate (open circle, dotted line), daidzein-7-sulphate (open star, dotted line), equol (solid triangle, solid line) and equol-7-sulphate (open triangle, dotted line). Error bars represent the mean±s.e.m. of triplicate assays.
Assessment of relative overall oestrogenic potency
The relative oestrogenic potency of each compound was assessed by calculating for the ER-binding assays, the molar excess needed to displace 50% of the [3H]oestradiol binding to the ER (Figs 2 and 6), and for the whole-cell assays, the molar concentration needed to achieve a response equivalent to 50% of that with oestradiol (Figs 3–5). Data were assembled into Table 1 in the rank order of potency for each assay. Where 50% responses were not achieved, the maximum actual values are shown in brackets and the rank order was obtained by consideration of all the data points. Table 1 shows the sum totals of rank places in all the five assays for each compound and the compounds are listed in descending order of potency. In line with previously published work (Matsumura et al. 2005), genistein and equol showed greater oestrogenic activity than daidzein. Genistein and equol were in the top three placings in the rank order for every assay, whereas daidzein had considerably lower placings (Table 1). 7-Sulphation reduced substantially the oestrogenic activity of genistein and equol, whereas the 7-sulphate of daidzein had much higher activity than the aglycone. The 4′-sulphation increased the activity of genistein in both the ER-binding assays. Although the 4′-sulphation of genistein reduced activity in the whole-cell assays, the 4′-sulphate still retained substantial activity in marked contrast to the 7-sulphate. The 4′-sulphation of daidzein resulted in a moderate reduction in oestrogenic activity.
Table 1.
(A) Descending rank order of the relative oestrogen agonist activities of genistein, daidzein, equol and their monosulphates for each of the five assays given in Figs 2–6. The relative oestrogenic potency is shown in terms of the molar excess for displacement of 50% of the [3H]oestradiol binding to ER and molar concentration needed to achieve 50% of the response with oestradiol. Compounds are listed in descending order of activity for each assay. Where 50% responses were not achieved, the maximal observed responses are given in brackets and rank order was based on consideration of all the data points. (B) Sum totals of rank placings in all five assays for each compound, with compounds listed in descending order of relative activity
| (A) Assay | Rank order of oestrogenic potency | |||||||
|---|---|---|---|---|---|---|---|---|
| Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 | Rank 6 | Rank 7 | Rank 8 | |
| Binding to ER/MCF-7cytosol (molar excess to achieve 50% inhibition of [3H]oestradiol binding) | G4′S 650× | Genistein 1000× | Equol 3700× | D7S 5000× | D4′S (44% at 10 000×) | Daidzein (40% at 10 000×) | Eq7S (2% at 10 000×) | G7S (19% at 100 000×) |
| Binding to recombinant ERα (molar excess to achieve 50% inhibition of [3H]oestradiol binding) | G4′S 400× | Equol 550× | Genistein 1700× | Daidzein 2500× | D7S 4000× | G7S 4500× | D4′S 10 000× | Eq7S (67% at 100 000×) |
| Induction of reporter gene (molar concentration for 50% response with 10−8 M oestradiol) | Genistein 5×10−8 M | Equol 8×10−8 M | G4′S 2×10−7 M | Daidzein 2×10−7 M | D7S 5×10−7 M | D4′S (17% at 10−6 M) | Eq7S (24% at 10−5 M) | G7S (11% at 10−5 M) |
| Cell growth after 7 days (molar concentration for 50% response with 10-8 M oestradiol) | Equol 2×10−9 M | Genistein 7×10−9 M | D7S 2×10−8 M | G4′S 3×10−8 M | Daidzein 5×10−8 M | G7S 2×10−6 M | D4′S (23% at 10−7 M) | Eq7S 10−5 M |
| Cell growth after 14 days (molar concentration for 50% response with 10−8 M oestradiol) | Genistein 4×10−9 M | Equol 10−8 M | D7S 5×10−8 M | G4′S 9×10−8 M | Daidzein 10−7 M | D4′S 7×10−7 M | G7S 8×10−6 M | Eq7S (36% at 10−5 M) |
| (B) Compound | Total sum of placings in each rank order | |||||||
|---|---|---|---|---|---|---|---|---|
| Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 | Rank 6 | Rank 7 | Rank 8 | |
| Genistein | 2 | 2 | 1 | |||||
| Equol | 1 | 3 | 1 | |||||
| Genistein-4′-sulphate (G4′S) | 2 | 1 | 2 | |||||
| Daidzein-7-sulphate (D7S) | 2 | 1 | 2 | |||||
| Daidzein | 2 | 2 | 1 | |||||
| Daidzein-4′-sulphate (D4′S) | 1 | 2 | 2 | |||||
| Genistein-7-sulphate (G7S) | 2 | 1 | 2 | |||||
| Equol-7-sulphate (Eq7S) | 2 | 3 | ||||||
Binding mode and sulphation
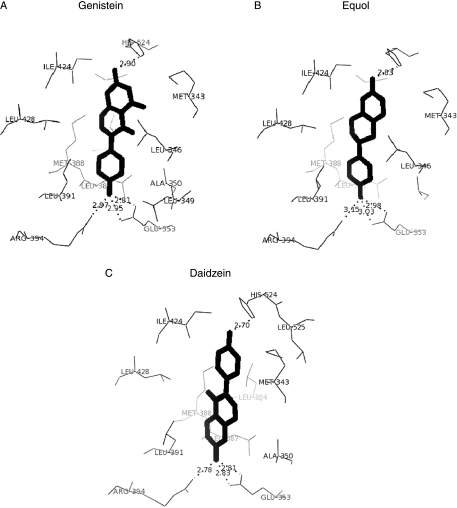
Molecular modelling and docking studies were carried out in an attempt to understand the inverse effect of sulphation on the oestrogen agonist action of daidzein compared with genistein and equol. The conformationally minimised structures of genistein, daidzein and equol were each docked into the ligand-binding site of the LBD of ERα and their docked poses were compared with that of genistein. From the crystal structure data (Manas et al. 2004), it is known that the 7-hydroxyl of genistein forms a hydrogen bond with the amino acid residue histidine 524 (His524) and the 4′-hydroxyl of genistein hydrogen bonds to glutamic acid 353 (Glu353) and arginine 394 (Arg394) (Fig. 7A). To validate the GLUE docking method, both genistein as extracted from the 1×7R crystal structure and genistein as built using InsightII and minimised using Mizer were docked into the ERα crystal structure 1A52. The modelled genistein was found to correctly dock into the ligand-binding site of 1A52, with a docked energy of −16·38 kcal/mol, in a similar bound conformation and orientation. The docking result for the modelled genistein was within 1·2 Å to that of the crystallographic genistein extracted from 1×7R with an overall RMSD value of 0·562 between the cα atoms (residues 306–544) of the two protein structures. Docking of equol into the LBD of ERα gave a docked energy value of −17·60 kcal/mol and an orientation, which resembled that of genistein, with its 7-hydroxyl within hydrogen-bonding distance of His524 and its 4′-hydroxyl group within hydrogen-bonding distance of Glu353 and Arg394 (Fig. 7B). By contrast, at its lowest and most favourable energy conformation (−14·91 kcal/mol), daidzein docked into the LBD of ERα in the opposite orientation from genistein and equol, forming hydrogen bonds between its 7-hydroxyl and Glu353/Arg394 and between its 4′-hydroxyl and His524 (Fig. 7C, Table 2).
Figure 7.
Molecular modelling and docking of (A) genistein, (B) equol and (C) daidzein into the crystal structure of human LBD of ERα (1A52). The ligands are shown in bold outline with neighbouring amino acid residues numbered according to 1A52. Hydrogen bonds (depicted by dashed lines) are shown between the ligand and binding site residues with the bond lengths given in Angstroms (Å) for the energetically favoured conformation. The docked energies for genistein, equol and daidzein were −16·38, −17·60 and −14·91 kcal/mol respectively. The number of van der Waals interactions was 42, 48 and 41 for genistein, equol and daidzein respectively.
Table 2.
Summary of the polar contacts and van der Waals interactions between the phytoestrogens genistein, daidzein and equol and the residues in the binding site of the LBD of ERα. The genistein-ERα crystal structure (1×7R) is shown for reference. Interactions of the 7-hydroxyl are shown in bold and those of the 4′-hydroxyl in normal print.
| Distance (Å) | |||||||
|---|---|---|---|---|---|---|---|
| Docked energya (kcal/mol) | Hydroxyl group | GLU353 OE1 | GLU353 OE2 | ARG394 NH2 | HIS524 ND1 | Number of van der Waals interactions | |
| Compound | |||||||
| 1×7R | 7 | 2·55 | 39 | ||||
| 4′ | 2·57 | – | 2·93 | ||||
| Genisteinb | −16·38 | 7 | 2·90 | 42 | |||
| 4′ | 2·81 | 2·95 | 2·97 | ||||
| Genistein | −15·73 | 7 | – | 2·85 | 2·92 | 47 | |
| 4′ | 2·78 | ||||||
| Equolb | −17·60 | 7 | 2·83 | 48 | |||
| 4′ | 2·98 | 3·03 | 3·15 | ||||
| Equol | −16·70 | 7 | – | 3·08 | 3·20 | 47 | |
| 4′ | 2·82 | ||||||
| Daidzeinb | −14·91 | 7 | 2·81 | 2·83 | 2·78 | 41 | |
| 4′ | 2·70 | ||||||
| Daidzein | −11·97 | 7 | 2·65 | 48 | |||
| 4′ | – | – | 2·45 | ||||
‘–’ Indicates no polar contact.
Docked energy is calculated as E=ELJ+EHB+EDRY.
Indicates preferred binding mode (i.e. the pose exhibiting the lowest docked energy) as calculated by GRID/GLUE.
Crystallographic data have shown that the binding of genistein into the ligand-binding site of the LBD of ERβ occurs in a similar mode as that for ERα, with the 7-hydroxyl of genistein forming hydrogen bonds with the amino acid residue His475 and the 4′-hydroxyl of genistein hydrogen bonding to Glu305 and Arg346 (Manas et al. 2004). Docking of these three phytoestrogens into the LBD of ERβ showed that all the compounds bound with similar orientations to those shown for ERα, with daidzein preferring the opposite orientation compared with genistein and equol (data not shown).
MIFs, as calculated by GRID, have been used to indicate favourable interactions between a probe and a target molecule and have been used to inform structure-based drug design (von Itzstein et al. 1993). Using GRID to calculate the energy of interaction between a sulphoxide probe (OS) and the ligand-binding site of the LBD of ERα and ERβ indicated the most favourable site in each case (lowest energy at −7·56 and −6·43 kcal/mol for ERα and ERβ respectively) was within hydrogen-bonding contact of the two key residues involved in ligand binding, namely Glu353(ERα)/Glu305(ERβ) and Arg394(ERα)/Arg346(ERβ) (data not shown).
Discussion
The oestrogen agonist properties of the monosulphated forms of genistein, daidzein and equol have been compared with those of the aglycones in terms of their relative ability to compete with [3H]oestradiol for binding to ER, to induce a stably transfected oestrogen-responsive reporter gene (ERE-CAT) in MCF-7 cells and to stimulate growth of oestrogen-dependent MCF-7 cells. The oestrogen agonist activities of genistein and equol were found to be considerably greater than for daidzein, as previously published (Matsumura et al. 2005). All the three phytoestrogens gave full agonist responses in all MCF-7 cell-based assays but genistein and equol gave responses at consistently lower concentrations in all assays compared with daidzein (Table 1). Sulphation resulted in markedly varied alterations to the oestrogen agonist responses of these three phytoestrogens, most notably in relation to the position of sulphation and to the parent compound. In no case did sulphation completely abolish all activity, which demonstrates that monosulphation cannot be assumed to result in complete abrogation of oestrogenic activity. However, the position of the sulphation did influence in markedly different ways the oestrogen agonist responses and did so rather differently for daidzein compared with genistein and equol. The 4′-sulphation of genistein reduced oestrogen agonist activity to only a small extent in the whole-cell assays but actually increased the relative binding affinity to ER (Table 1). By contrast, 7-sulphation of genistein reduced oestrogen agonist activity very substantially in all assays such that its rank order in Table 1 dropped from top to almost bottom. The 7-sulphation of equol resulted similarly in a markedly reduced oestrogenic activity in all assays and a parallel dramatic drop in rank order in Table 1 from almost top to the bottom. By contrast, however, the position of monosulphation of daidzein acted in an opposing manner on oestrogen agonist activity. Sulphation at the 4′-position of daidzein resulted in a modest reduction in oestrogen agonist activity but in contrast to either genistein or equol, sulphation of daidzein at the 7-position resulted in an increase in oestrogen agonist activity and in a rise in ranking position in Table 1 compared with the aglycone.
Enhanced oestrogenic potency of a monosulphate relative to the aglycone can be assumed to result from an intrinsic property of the monosulphated phytoestrogen. However, reduced levels of oestrogenic activity associated with the monosulphates could have resulted not only from any intrinsic low oestrogenic properties of the monosulphated phytoestrogen itself, but also from partial desulphation of the phytoestrogen such that the low activity might have resulted from a mixture of inactive monosulphate and small amounts of active desulphated aglycone. MCF-7 cells are known to possess sulphatase activity (Harris et al. 2004) and so enzymatic desulphation could have occurred within the whole-cell assays. High-speed centrifugation of the MCF-7 cell lysates would have been expected to at least reduce the sulphatase activity present in the MCF-7 cytosolic ER-binding assay and yet oestrogenic activity was still observed for all the monosulphates tested (Table 1). However, the finding of continued weak ER-binding activity for genstein-7-sulphate, equol-7-sulphate and daidzein-4′-sulphate even when using purified recombinant human ERα, where sulphatase activity could not have been present, suggests that the weak oestrogenic activity is indeed a genuine property of the monosulphated phytoestrogen and is not an artefact of the assay following enzymatic desulphation.
One explanation for the opposing effects of 7-sulphation on daidzein compared with genistein or equol could relate to the mode in which these three phytoestrogens bind into the LBD of the ER. Crystallographic studies have shown that genistein binds into the ligand-binding site of either ERα or ERβ (Manas et al. 2004) by hydrogen bonding of its 4′-hydroxyl to Glu353(ERα)/Glu305(ERβ) and Arg394(ERα)/Arg346(ERβ) and of its 7-hydroxyl to His524(ERα)/His475(ERβ), which is analogous to that with the binding of 17β-oestradiol through its 3- and 17-hydroxyls respectively (Brzozowski et al. 1997; illustrated in Fig. 1). The molecular modelling and docking studies presented here suggest that equol can bind into the LBD of either ERα or ERβ in a similar manner to that of genistein but that the lowest energy conformation for the binding of daidzein is in the opposite orientation. Furthermore, the GRID calculation performed in this work, to find a potential sulphation site in the region of the ligand-binding site, indicates that the most energetically favourable location places a sulphoxide moiety within hydrogen-bonding distance of Glu353 and Arg394 (Glu305 and Arg346 ERβ). Together, these results would then suggest that sulphation of the hydroxyl group which hydrogen bonds to the histidine residue 524 of ERα or 475 of ERβ results in loss of oestrogen agonist activity whilst sulphation of the hydroxyl group within hydrogen-bonding distance to Glu353(ERα)/Glu305(ERβ) and Arg394(ERα)/Arg346(ERβ) can result in increased oestrogen agonist activity.
Although the 4′- and 7-hydroxyl groups of these phytoestrogens form important hydrogen bonds with amino acids in the LBD, this work demonstrates that other components of the ligand structure also play a key role. Daidzein differs from equol only by the presence of a carbonyl group and from genistein only by the lack of a hydroxyl group adjacent to the carbonyl group (Fig. 1). However, this difference in chemical structure results in marked differences to subsequent biological activity of the ligand and its metabolites. Previous work has shown that not only 4′-sulphation of genistein can decrease its ability to increase growth of oestrogen-responsive MCF-7 cells (Kinjo et al. 2004), in agreement with results here, but can also decrease its antioxidant activity (Turner et al. 2004) and its effect on platelet aggregation, inflammation, cell adhesion and chemotaxis (Rimbach et al. 2004). However, no previous work has reported any potential neither for enhanced activity of an isoflavone following sulphation nor for inverse alterations between individual phytoestrogens. Since other phytoestrogens (Woods 2003) and also xenoestrogens (Sacco & James 2005) are subject to sulphation, it will now be important to investigate the effects of sulphate conjugation on a wider range of oestrogenic ligands.
The effects in these assays in vitro were observed at concentrations which were not incompatible with expected exposure in vivo following consumption of soya foods. The levels of genistein in adult plasma following soya consumption have been reported in the range of 1–8 μM (Woods 2003). Mean plasma concentrations of genistein and daidzein in infants fed soya-based formulae have been reported to be 684 ng/ml (equivalent to 2·5 μM) and 295 ng/ml (equivalent to 1·2 μM) respectively (Setchell et al. 1997). Assuming about 20% of each phytoestrogen would be present as a monosulphate (see Introduction), this would equate to an exposure in vivo in infants fed soya foods of 0·5 and 0·25 μM for the monosulphates of genistein and daidzein respectively. The IC50 values for genistein-4′-sulphate were below this concentration in every MCF-7 cell-based assay used here (Table 1). IC50 values for daidzein-7-sulphate were below this value for cell growth assays (Table 1), and measurable effects were found in the reporter gene assay albeit below the IC50 value (Fig. 3). In terms of breast exposure specifically, after soya consumption, nipple aspirate fluid has been reported to contain large amounts of unconjugated genistein, moderate the amounts of sulphated genistein and hardly any glucuronide compared with blood of the same patient which contained mostly genistein β-glucuronide (Peterson et al. 1998). This suggests that sulphation may be a major form of conjugation within the human breast, which is notably an oestrogen-sensitive tissue and is the target for the development of oestrogen-responsive cancers (Miller 1996). The results here demonstrate oestrogenic actions of sulphated genistein and daidzein in MCF-7 human breast cancer cells that contain predominantly ERα (Shaw et al. 2006) and therefore emphasise that sulphate conjugates must be considered as a source of biologically active ligand for breast cancer cells. Whether the level of ERβ within the breast cancer cells might influence the overall activity remains to be determined. Genistein, daidzein and equol have all been shown to more strongly bind to ERβ than to ERα (Hwang et al. 2006), and daidzein-7-sulphate has been shown to be able to transactivate reporter gene expression in ERβ-transfected HeLa cells (Totta et al. 2005). These findings highlight the complexity of the effects of sulphation on the overall biological activity of phytoestrogens but demonstrate the need to take variations in conjugation into account both between individuals and between tissues when assessing potential health benefits of soya isoflavone consumption.
Acknowledgements
This work was performed under financial support from the phytoestrogens research programme of the Food Standards Agency (N B, P D D), the Felix Trust (D P), and the Lister Institute of Preventive Medicine (KAW personal fellowship). We thank the Wellcome Trust for a Vacation Scholarship (S M). We are especially grateful to T Barlow, H Makin, J Aish and B Jeffery of the Food Standards Agency for supportive advice. We acknowledge the staff at Molecular Discovery Ltd, particularly S Sciabola and E Carosati, for providing helpful discussions and software support. We thank the School of Biological Sciences and S A Mitchell and N J Spencer at the BioCentre for providing excellent computing facilities. We thank Helen Oakley for technical support. The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Adlercreutz H, Mazur HW. Phyto-oestrogens and western diseases. Annals of Medicine. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, van der Wildt J, Kinzel J, Attalla H, Wahala K, Makela T, Hase T, Fotis T. Lignan and isoflavonoid conjugates in human urine. Journal of Steroid Biochemistry and Molecular Biology. 1995;52:97–103. doi: 10.1016/0960-0760(94)00146-d. [DOI] [PubMed] [Google Scholar]
- Brzozowski AM, Pike ACW, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- Byford JR, Shaw LE, Drew MGB, Pope GS, Sauer MJ, Darbre PD. Oestrogenic activity of parabens in MCF7 human breast cancer cells. Journal of Steroid Biochemistry and Molecular Biology. 2002;80:49–60. doi: 10.1016/s0960-0760(01)00174-1. [DOI] [PubMed] [Google Scholar]
- Carosati E, Sciabola S, Cruciani G. Hydrogen bonding interactions of covalently bonded fluorine atoms: from crystallographic data to a new angular function in the GRID force field. Journal of Medicinal Chemistry. 2004;47:5114–5125. doi: 10.1021/jm0498349. [DOI] [PubMed] [Google Scholar]
- Chen WF, Huang MH, Tzang CH, Yang M, Wong MS. Inhibitory actions of genistein in human breast cancer (MCF7) cells. Biochimica et Biophysica Acta. 2003;1638:187–196. doi: 10.1016/s0925-4439(03)00082-6. [DOI] [PubMed] [Google Scholar]
- Clarke DB, Lloyd AS, Botting NP, Oldfield MF, Needs PW, Wiseman H. Measurement of intact sulphate and glucuronide phytoestrogen conjugates in human urine using isotope dilution liquid chromatography-tandem mass spectrometry with [13C3] isoflavone internal standards. Analytical Biochemistry. 2002;309:158–172. doi: 10.1016/s0003-2697(02)00275-0. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project Number 4 1994 The CCP4 Suite: Programs for protein crystallography. Acta CrystallographyD50 760–763 [DOI] [PubMed]
- Daly RJ, Darbre PD. Cellular and molecular events in loss of estrogen sensitivity in ZR-75-1 and T-47-D human breast cancer cells. Cancer Research. 1990;50:5868–5875. [PubMed] [Google Scholar]
- Dampier K, Hudson EA, Howells LM, Manson MM, Walker RA, Gescher A. Differences between human breast cell lines in susceptibility towards growth inhibition by genistein. British Journal of Cancer. 2001;85:618–624. doi: 10.1054/bjoc.2001.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbre PD, Daly RJ. Effects of oestrogen on human breast cancer cells in culture. Proceedings of the Royal Society of Edinburgh. 1989;95B:119–132. [Google Scholar]
- Darbre P, Yates J, Curtis SA, King RJB. Effect of estradiol on human breast cancer cells in culture. Cancer Research. 1983;43:349–354. [PubMed] [Google Scholar]
- Fairley B, Botting NP, Cassidy A. The synthesis of daidzein sulfates. Tetrahedron. 2003;59:5407–5410. [Google Scholar]
- Goodford PJ. A Computational procedure for determining energetically favorable binding sites on biologically important macromolecules. Journal of Medicinal Chemistry. 1985;28:849–857. doi: 10.1021/jm00145a002. [DOI] [PubMed] [Google Scholar]
- Green B, Leake RE. Steroid Hormones: A Practical Approach. IRL Press Ltd; Oxford, UK: 1987. [Google Scholar]
- Harris RM, Wood DM, Bottomley L, Blagg S, Owen K, Hughes PJ, Waring RH, Kirk CJ. Phytoestrogens are potent inhibitors of estrogen sulfation: implications for breast cancer risk and treatment. Journal of Clinical Endocrinology and Metabolism. 2004;89:1779–1787. doi: 10.1210/jc.2003-031631. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Research. 1998;58:3833–3838. [PubMed] [Google Scholar]
- Hwang CS, Kwak HS, Lim HJ, Lee SH, Kang YS, Choe TB, Hur HG, Han KO. Isoflavone metabolites and their in vitro dual functions: they can act as estrogenic agonist or antagonist depending on the estrogen concentration. Journal of Steroid Biochemistry and Molecular Biology. 2006;101:246–253. doi: 10.1016/j.jsbmb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- von Itzstein M, Wu W-Y, Kok GB, Pegg MS, Dyason JC, Jin B, Van Phan T, Smythe ML, White HF, Oliver SW, et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- Jones TA, Bergdoll M, Kjeldgaard M. O: A macromolecular modeling environment. In: Bugg C, Ealick S, editors. Crystallographic and Modeling Methods in Molecular Design. Springer-Verlag Press; Berlin: 1990. pp. 189–195. [Google Scholar]
- Kinjo J, Tsuchihashi R, Morito K, Hirose T, Aomori T, Nagao T, Okabe H, Nohare T, Masamune Y. Interactions of phytoestrogens with estrogen receptors α and β (III). Estrogenic activities of soy isoflavone aglycones and their metabolites isolated from human urine. Biological and Pharmaceutical Bulletin. 2004;27:185–188. doi: 10.1248/bpb.27.185. [DOI] [PubMed] [Google Scholar]
- Kirk CJ, Harris RM, Wood DM, Waring RH, Hughes PJ. Do dietary phytoestrogens influence susceptibility to hormone-dependent cancer by disrupting the metabolism of endogenous oestrogens? Biochemical Society Transactions. 2001;29:209–216. doi: 10.1042/0300-5127:0290209. [DOI] [PubMed] [Google Scholar]
- Luckow B, Schutz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Research. 1987;15:5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiolini M, Bonofiglio D, Marsico S, Panno ML, Cenni B, Picard D, Ando S. Estrogen receptor α mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Molecular Pharmacology. 2001;60:595–602. [PubMed] [Google Scholar]
- Makela S, Hyder SM, Stancel GM. Environmental estrogens. In: Oettel M, Schillinger E, editors. Estrogens and Antiestrogens II, Handbook of Experimental Pharmocology. Handbook Exp Pharm. vol 135/II. Springer Verlag; Berlin: 1999. pp. 613–663. [Google Scholar]
- Manas ES, Xu ZB, Unwalla RJ, Somers WS. Understanding the selectivity of genistein for human estrogen receptor-β using X-ray crystallography and computational methods. Structure. 2004;12:2197–2207. doi: 10.1016/j.str.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Matsumura A, Ghosh A, Pope GS, Darbre PD. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. Journal of Steroid Biochemistry and Molecular Biology. 2005;94:431–443. doi: 10.1016/j.jsbmb.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Miller WR. Estrogen and Breast Cancer. Chapman and Hall; London: 1996. [Google Scholar]
- Oettel M & Schillinger E (ed) 1999 Estrogens and Antiestrogens I, Handbook of Experimental Pharmacology vol 135/I, Springer: Berlin.
- Osborne CK, Hobbs K, Trent JM. Biological differences among MCF-7 human breast cancer cell lines from different laboratories. Breast Cancer Research and Treatment. 1987;9:111–121. doi: 10.1007/BF01807363. [DOI] [PubMed] [Google Scholar]
- Pastor M, Cruciani G, Watson KA. A Strategy for the incorporation of water molecules present in a ligand binding site into a three-dimensional quantitative structure–activity relationship analysis. Journal of Medicinal Chemistry. 1997;40:4089–4102. doi: 10.1021/jm970273d. [DOI] [PubMed] [Google Scholar]
- Peterson TG, Ji GP, Kirk M, Coward L, Falany CN, Barnes S. Metabolism of the isoflavones genistein and biochanin A in human breast cancer cell lines. American Journal of Clinical Nutrition. 1998;68:1505S–1511S. doi: 10.1093/ajcn/68.6.1505S. [DOI] [PubMed] [Google Scholar]
- PyMOL(TM) Incentive Product – Copyright (C) 2006 DeLano Scientific LLC.
- Rimbach G, Weinberg PD, Pascual-Teresa S, Alonso MG, Ewins BA, Turner R, Minihane AM, Botting N, Fairley B, Matsugo S, et al. Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochimica et Biophysica Acta. 2004;1670:229–237. doi: 10.1016/j.bbagen.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Sacco JC, James MO. Sulfonation of environmental chemicals and their metabolites in the polar bear (Ursus maritimus) Drug Metabolism and Disposition. 2005;33:1341–1348. doi: 10.1124/dmd.105.004648. [DOI] [PubMed] [Google Scholar]
- Sciabola S, Carosati E, Baroni M, Mannhold R. Comparison of ligand-based and structure-based 3D-QSAR approaches: a case study on (aryl-)bridged 2-aminobenzonitriles inhibiting HIV-1 reverse transcriptase. Journal of Medicinal Chemistry. 2005;48:3756–3767. doi: 10.1021/jm049162m. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phytoestrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- Shaw LE, Sadler AJ, Pugazhendhi D, Darbre PD. Changes in oestrogen receptor-α and -β during progression to acquired resistance to tamoxifen and fulvestrant (Faslodex, ICI 182,780) in MCF7 human breast cancer cells. Journal of Steroid Biochemistry and Molecular Biology. 2006;99:19–32. doi: 10.1016/j.jsbmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Shelnutt SR, Cimino CO, Wiggins PA, Badger TM. Urinary pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein. Cancer Epidemiology, Biomarkers and Prevention. 2000;9:413–419. [PubMed] [Google Scholar]
- Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. American Journal of Clinical Nutrition. 2002;76:588–594. doi: 10.1093/ajcn/76.3.588. [DOI] [PubMed] [Google Scholar]
- Tanenbaum DM, Wang Y, Williams SP, Sigler PB. Crystallographic comparison of the estrogen and progesterone receptor's ligand binding domains. PNAS. 1998;95:5998–6003. doi: 10.1073/pnas.95.11.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BF, Zeisel SH, Busby MG, Hill JM, Mitchell RA, Scheffler NM, Brown SS, Bloeden LT, Dix KJ, Jeffcoat AR. Quantitative analysis of the principle soy isoflavones genistein, daidzein and glycitein, and theor primary metabolites in human plasma and urine using reverse-phase high-performance liquid chromatography with ultraviolet detection. Journal of Chromatography. B, Biomedical Sciences and Applications. 2001;760:191–205. doi: 10.1016/s0378-4347(01)00269-9. [DOI] [PubMed] [Google Scholar]
- Totta P, Acconcia F, Virgili F, Cassidy A, Weinberg PD, Rimbach G, Marino M. Daidzein-sulfate metabolites affect transcriptional and antiproliferative activites of estrogen receptor-β in cultured human cancer cells. Journal of Nutrition. 2005;135:2687–2693. doi: 10.1093/jn/135.11.2687. [DOI] [PubMed] [Google Scholar]
- Turner R, Baron T, Wolffram S, Minihane AM, Cassidy A, Rimbach G, Weinberg PD. Effect of circulating forms of soy isoflavones on the oxidation of low density lipoprotein. Free Radical Research. 2004;38:209–216. doi: 10.1080/10715760310001641854. [DOI] [PubMed] [Google Scholar]
- Wang C, Kurzer MS. Phytoestrogen concentration determines effects on DNA synthesis in human breast cancer cells. Nutrition and Cancer. 1997;28:236–247. doi: 10.1080/01635589709514582. [DOI] [PubMed] [Google Scholar]
- Welshons WV, Murphy CS, Koch R, Calaf G, Jordan VC. Stimulation of breast cancer cells in vitro by the environmental estrogen enterolactone and phytoestrogen equol. Breast Cancer Research and Treatment. 1987;10:169–175. doi: 10.1007/BF01810580. [DOI] [PubMed] [Google Scholar]
- Woods HF (Chairman) Phytoestrogens and Health. Crown copyright; UK: 2003. [Google Scholar]
- Zhang Y, Song TT, Cunnick JE, Murphy PA, Hendrich S. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. Journal of Nutrition. 1999;129:399–405. doi: 10.1093/jn/129.2.399. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hendrich S, Murphy PA. Glucuronides are the main isoflavone metabolites in women. Journal of Nutrition. 2003;133:399–404. doi: 10.1093/jn/133.2.399. [DOI] [PubMed] [Google Scholar]