Abstract
Analyzing the growth of fibrosarcoma lines derived from IL-1α-, IL-1β- , or IL-1αβ-knockout (-/-) mice in the immunocompetent host revealed that tumor-derived IL-1α and IL-1β exert strong and opposing effects on immune response induction, which prohibited the evaluation of a potential impact on tumorigenicity. Therefore, in vivo growth of IL-1-deficient tumor lines was evaluated in nu/nu mice and was compared with in vitro growth characteristics. All IL-1-deficient fibrosarcoma lines grow in immunocompromised mice. However, IL-1α-/-β-competent (comp) lines grow more aggressively, efficiently induce angiogenesis, and recruit inflammatory cells. Despite stronger tumorigenicity of IL-1βcomp lines, IL-1α strengthens anchorage-independent growth, but both IL-1α and IL-1β support drug resistance. Corresponding to the aggressive growth, IL-1βcomp cells display increased matrix adhesion, motility, and cable formation on matrigel, likely supported by elevated αv/β3 and matrix metalloroteinase expression. Recruitment of myeloid cells requires IL-1β but is regulated by IL-1α, because inflammatory chemokine and cytokine expression is stronger in IL-1α-/-βcomp than in IL-1wt lines. This regulatory effect of tumor-derived IL-1α is restricted to the tumor environment and does not affect systemic inflammatory response induction by tumor-derived IL-1β. Both sarcoma cell-derived IL-1α and IL-1β promote tumor growth. However, IL-1α exerts regulatory activity on the tumor cell-matrix cross-talk, and only IL-1β initiates systemic inflammation.
Introduction
The IL-1 family consists of the agonistic proteins IL-1α and IL-1β and the IL-1 receptor antagonist (IL-1Ra) [1,2], which by binding to IL-1 receptors without transmitting an activation signal acts as a physiological inhibitor [3]. IL-1α and IL-1β are synthesized as 31-kDa precursors and are processed by proteases to their mature 17-kDa forms. IL-1β-converting enzyme cleaves the inactive IL-1β precursor; ProIL-1α is processed by calpain [4,5].
Many cell types produce and secrete IL-1α, IL-1β, and IL-1Ra on activation with environmental stimuli [6,7]. Mononuclear cells secrete the highest levels, mainly of IL-1β [1,7]. Secreted IL-1α and IL-1β bind to the same receptors and exert similar biologic activities. However, IL-1α and IL-1β differ inasmuch as IL-1β is solely active as a secreted product; IL-1α is mostly active as an intracellular precursor and in its membrane-associated form. The active membrane form of IL-1α is derived from myristoylation of proIL-1α and is anchored to the membrane through a mannose-like receptor [1,2,7–9].
IL-1 is a pleiotrophic cytokine that primarily affects inflammatory responses, immune reactivity, and hematopoiesis [1,2,7,9,10]. Its potency stems from inducing cytokine, chemokine, proinflammatory molecule secretion, and adhesion molecule expression in diverse cells, thereby amplifying and sustaining the response. Both the localization of the IL-1 molecules in the producing cell and the microenvironment dictate their biologic functions [8]. Membrane-associated IL-1α is supposed to be immunostimulatory. Cytosolic proIL-1α may control gene expression, proliferation, and differentiation [5, 11–15]. Low-level secreted IL-1β induces limited inflammatory responses followed by T cell activation. High doses of IL-1β are accompanied by broad inflammation with tissue damage [1,2,7,16].
IL-1 is abundant at tumor sites, being secreted by the malignant cells and/or cells in the tumor microenvironment in response to local inflammatory signals. It can promote invasiveness and metastasis formation or induce an antitumor immune response and inhibit tumor growth [6,7,17]. Over-expression of the precursor of IL-1α by fibrosarcoma cells can initiate a strong immune response [11–13,15,18]. In contrast, IL-1β- transfected tumor cells are more invasive than wild type cells [19,20]. Increased invasiveness correlates with enhanced angiogenesis and activation of immunosuppression [18–23], which may be a consequence of tumor-derived IL-1β supporting extramedullary myelopoiesis [18,23,24].
To explore whether these effects are exclusively or at least predominantly due to tumor-derived IL-1, fibrosarcoma were induced in IL-1α-, IL-1β-, or IL-1αβ-deficient mice [19,25]. Because IL-1 expression and secretion was unimpaired in the syngeneic host, this system allowed to selectively elaborate the contribution of tumor-derived IL-1 on tumor growth. The analysis of in vivo growth of these IL-1α-, IL-1β- , or IL-1αβ-deficient lines in the immunocompetent, IL-1-competent host confirmed that tumor-derived IL-1 induces an utmost strong T cell response (IL-1α) or immunosuppression (IL-1β) [26]. Thus, it became important to explore whether tumor-derived IL-1 affects exclusively the host's immune system or also the tumor cell itself. A detailed in vitro analysis of the tumor lines and their growth in nu/nu mice revealed that IL-1βcomp tumors are more aggressive, which is a consequence of tumor-derived IL-1β locally and systemically initiating an inflammatory milieu. Tumor-derived IL-1α exerts no systemic effects, but promotes transcription of genes, which support tumor cell survival and the cross-talk of IL-1β with the tumor environment.
Material and Methods
Mice and Tumors
nu/nu mice (WIGA, Sulzfeld, Germany), kept under specific pathogen-free conditions, fed sterilized food, and water ad libitum, were used for experiments at the age of 8 to 10 weeks. IL-1RI-/- mice were purchased from Jackson Laboratories (Bar Harbor, ME). Fibrosarcoma were induced by 3-methylcholanthrene treatment of wt, IL-1α-/-, IL-1β-/-, IL-1αβ-/-, and IL-1RI-/- C57BL6 mice [19]. The IL-1-deficient mice were generously provided to R.N. A. by Prof. Yoichiro Iwakura, University of Tokyo, Tokyo, Japan [25]. 3-Methylcholanthrene (Sigma Israel, Rehovot, Israel) was dissolved in olive oil (200 µg/mouse) and was subcutaneously (s.c.) injected into the right thigh [27]. Local fibrosarcomas developed within 3 to 5 months. When tumors reached a diameter of 10 mm, mice were killed and the tumor tissue was aseptically removed. Part of the tissue was processed for the establishment of cell lines by enzymatic digestion in trypsin. The following fibrosarcoma lines were used: IL-1wt lines 2, 19, 21 (wt2, wt19, wt21), IL-1α-/- lines 3, 15, 16 (α-/-3, α-/-15, α-/-16), IL-1β-/- lines 3, 4, 17 (β-/- 3, β-/- 4, β-/- 17), IL-1αβ-/- lines 6, 11, 13 (αβ-/-6, αβ-/-11, αβ-/-13), and IL-1RI-/- lines PV and R1. Each of these 14 tumor lines was derived from a different 3-methylcholanthrene-treated C57BL6 mouse. The lines were maintained in RPMI 1640, 10% fetal calf serum.
Antibodies
Anti-CD11b, -CD54 (European Collection of Animal Cell Cultures, Salisbury, UK), -panCD44 (American Type Culture Collection, Manassas, VA), CD49d [28], -CD44v6, -CD51, -CD49c, -CD49f, -CD29, -CD61, -CD154, -CD31 (PECAM), -CD62E (E-selectin), CD105 (endoglin), -Gr-1, -CD95, -CD178 (CD95L), -CD120a (TNFRI), -CD120b (TNFRII), -CD121a (IL-1RI), -CD121b (IL-1RII), -CD87 (uPAR), -MMP2, -CCL1, -CCL2, -CCL3, -CCL5, -CCL15, -CCL17, -CCL19, -CCL20, -CXCL10, -OPN, -CCR3, -CCR4, -CCR6, -CCR7, -CCR8, native and biotinylated anti-IL-1α, -IL-1β, -IL-4, -IL-6, -IL-10, -IL-12, -IFNγ, -TNFα, -TGFβ, -ERK1,2, -pERK1,2, -pJNK, -pJun, -PTEN, -pIκBα, -pAkt, -pBAD, -Bid, -BIM, -Bcl-2, -Bcl-Xl, -BAX, -PARP, -actin, and secondary labeled [biotin, fluorescein isothiocyanate (FITC), phycoerythrin, allophycocyanin, and HRP] antibodies, were obtained commercially.
Flow Cytometry
Approximately 2.5 to 5 x 105 cells were stained according to standard protocols. For intracellular staining, cells were fixed (formaldehyde) and permeabilized (PBS, 1% Tween 20). Apoptosis was determined by Annexin V-FITC/PI staining. Fluorescence was determined using a FACStar and the CellQuest program (BD, Heidelberg, Germany).
Cytokine ELISA
Standard sandwich ELISA procedures were used to measure IL-1α, IL-1β, and IL-1Ra secretion.
Immunohistology
Sections (5 µm) of snap-frozen tumor were fixed (chloroform/acetone, 1:1, 4 minutes) and treated with levamisole solution to ablate tissue alkaline phosphatase activity. Nonspecific binding was blocked using an avidin-biotin blocking kit (Vector Laboratories, Burlingame, CA) and 2% normal serum from the same species as the secondary antibodies. For intracellular staining, tissues were fixed and permeabilized (4% paraformaldehyde, 0.1% Triton X-100). Tissues were incubated with the primary antibody (1 hour), the biotinylated secondary antibodies (30 minutes), and alkaline phosphatase-conjugated avidin-biotin complex solutions (5–20 minutes). Sections were counterstained with Mayer's hematoxylin. Primary antibodies were replaced by rat or rabbit IgG for negative controls.
Western Blot Analysis
Cells (5 x 105 ) were lysed in 1% riton X-100. Where indicated cells were cultured for 24 hours in the presence of recombinant IL-1α and/or IL-1β (3 (10 ng/ml) (CyoLAB/Peprotech, Rocky Hill, NJ). Lysates were resolved on 10% SDS-PAGE under reducing conditions. Proteins were transferred to nitrocellulose membranes (30 V, overnight). After blocking (5% fat-free milk powder, PBS, 0.1% Tween 20), immunoblot analysis was performed with the indicated antibodies, followed by HRP-labeled secondary antibodies. Blots were developed with the enhanced chemiluminescence detection system.
Tumor Cell Proliferation and Apoptosis
Tumor cells (1 x 104) were seeded in F-bottom 96-well plates, adding 10 µCi/ml 3H-thymidine and harvesting cultures after 16 hours. Apoptosis resistance was evaluated by the MTT assay and by Annexin V-FITC/PI staining. Cells (1 x 105), cultured overnight in F-bottom 96-well plates, were grown for 3 days in RPMI-s containing serial dilutions of cisplatin [cis-diamineplatinum(II) dichloride; Sigma, Munich, Germany], starting with 35 µg/ml.
Adhesion Assay
Cells (1 x 105) were seeded on F-bottom 96-well plates coated with collagens I and IV (10 µg/ml) or vitronectin (2 µg/ml). After 2 hours (37°C, 5% CO2), nonadherent cells were removed by washing, and adherent cells were stained with crystal violet. Absorbance was measured at 595 nm.
Soft Agar Assay
Tumor cells, suspended in 0.3% agar, were seeded on a preformed 1% agar layer. Where indicated, the agar contained 10 µg/ml anti-IL-1RI. Colonies was counted at an inverted microscope after 3 weeks.
Migration Assays
Tumor cells were seeded in 2-cm diameter, fibronectin-coated Petri dishes. Subconfluent cultures were wounded by scratching with a micropipette tip, washed, and incubated for 48 hours. Wound healing was evaluated microscopically after staining with hematoxylineosin. Alternatively, tumor cells (1 x 104) were seeded in the upper part of a Boyden chamber in 30 µl of RPMI/0.1% BSA. The lower part of the chamber, separated by an 8-µm-pore size polycarbonate membrane (Neuroprobe, Gaithersburg, MD), contained 30 µl of RPMI/10% fetal calf serum. After 4 hours, cells on the lower side of the membrane were stained with crystal violet. Absorbance was measured at 595 nm.
Zymography
Tumor cells (5 x 105) were starved for 24 hours, the conditioned medium was centrifuged (15 minutes, 15,000g), and aliquots of the supernatants were incubated with Lämmli buffer (15 minutes, 37°C) and separated in a 10% acrylamide gel containing 1 mg/ml gelatin. After washing, gels were stained with Coomassie blue.
Matrigel Assay
Tumor cells (2 x 105) were seeded on matrigel-coated 24-well plates. Cable formation was evaluated after 24 hours by light microscopy.
Tumor Growth
nu/nu mice received 5 x 105 tumor cells, s.c. Tumor growth was controlled (mean diameter) twice per week. Mice were killed at the indicated time points after tumor cell application, but latest when the s.c. tumor mass reached a mean diameter of 3 cm (survival time). Mice were bled by eye puncture, and serum was collected. Tumor, spleen, femura, and tibiae were excised. The tumor was shock frozen. The spleen was teased through fine gauze. The bones were flushed with PBS. Single spleen cell (SC) and bone marrow cell (BMC) suspensions were washed and used for flow cytometry. Animal experiments were approved by the governmental authorities for animal health care.
Statistical Analysis
Significance of differences was calculated by the Student's t-test (in vitro assays) or the Wilcoxon rank sum test (in vivo assays). P values <.05 were considered significant.
Results
Using fibrosarcoma lines derived from IL-1α-/-, IL-1β-/-, and IL-1αβ-/- mice, we recently demonstrated that tumor-derived IL-1α and IL-1β have very strong bearing on immune response induction in the immunocompetent, IL-1-competent host, such that IL-1αcomp tumor lines induce a T cell-mediated rejection response, whereas IL-1βcomp tumors induce strong immunosuppression that suffices to counterregulate immune response induction by IL-1α with the consequence that IL-1αβcomp tumors grow in the immunocompetent host [26]. These unexpectedly strong effects exclusively of tumor-derived IL-1 on the host immune system prohibited a judgment on the impact of tumor-derived IL-1 on tumorigenicity. However, an answer to this question is essential for estimating potential therapeutic efficacy of IL-1α in tumor rejection.
Tumorigenicity of IL-1α- and/or IL-1β- Deficient Tumor Cells
IL-1αcomp IL-1β-/- tumor lines do not grow in the immunocompetent host [26]. To elaborate whether this failure to grow is exclusively a consequence of induction of a potent T cell-mediated response or whether IL-1αcomp IL-1β-/- tumor lines are less tumorigenic, the experiment was repeated in nu/nu mice.
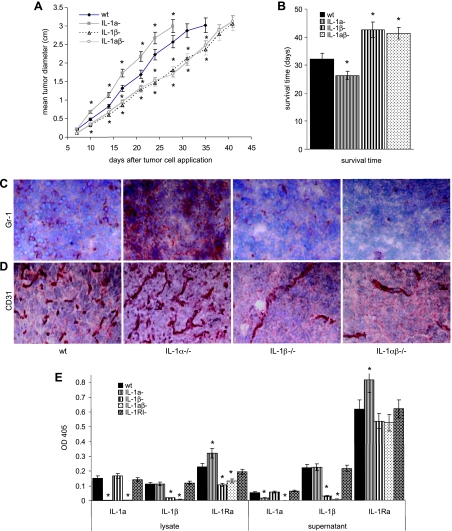
All tumor lines grew in nu/nu mice. However, the growth rate of IL-1β-/- and IL-1αβ-/- tumors was reduced, and accordingly, the mean survival time was prolonged (Figure 1, A and B). In addition, IL-1βcomp tumors grew more aggressively, infiltrated the surrounding tissue, and showed massive infiltration by myeloid cells, which were not seen in IL-1β-/- tumors (Figure 1C) but have also been seen in IL-1βcomp tumors in the immunocompetent host [26]. Importantly, too, IL-1βcomp tumors strongly supported angiogenesis, whereas only few capillaries were detected in IL-1β-/- tumors (Figure 1D).
Figure 1.
Growth of wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma lines in nu/nu mice. (A and B) nu/nu mice (10 mice/line) received a s.c. injection of 5 x 105 fibrosarcoma cells from 3 wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma lines. (A) Starting 7 days after tumor cell inoculation, tumor growth was monitored twice per week by measuring the tumor diameter. The mean tumor diameter ± SD of 30 mice/group (10 mice/line) is shown. (B) Mice were sacrificed when the mean tumor diameter reached 3 cm (survival time). The mean survival time ± SD (30 mice/group; 10 mice/line) is shown. Significant differences in the mean tumor diameter and the survival time as compared to mice receiving wt clones are indicated by an asterisk. (C and D) Wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma cells (5 x 105) were s.c. implanted into nu/nu mice. Tumors of a mean diameter of 0.5 cm were excised, shock frozen and 5 µm sections were stained with anti-Gr-1 (C) and anti-CD31 (D). Representative examples of one wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma lines are shown. (E) IL-1α, IL-1β, and IL-1Ra expression and secretion was evaluated in lysates (corresponding to 1 x 105 cells) and culture supernatants (fresh medium being added to subconfluent cultures and being collected after 24 hours) of wt, IL-1α-/-, IL-1β-/-, IL-1αβ-/-, and IL-1RI-/- fibrosarcoma lines by ELISA. Secreted IL-1α hardly could be detected in any of the tested lines. Yet, even in supernatants and lysates of IL-1wt and IL-1RI-/- cells, IL-1α and IL-1β was too low for a reliable quantification. Therefore, extinction values (mean ± SD of triplicates) are shown. Background values have been subtracted.
Taken together, irrespective of IL-1 expression, 3-methylcholanthrene-induced fibrosarcoma are tumorigenic in the immunocompromised host. However, tumor-derived IL-1β appears to promote tumor growth more efficiently than tumor-derived IL-1α. Notably, the tumors express/secrete a very low level of IL-1α and IL-1β, and there is no evidence that the absence of IL-1α or IL-1β would be compensated by considerably up-regulated expression/secretion of the remaining IL-1 gene. IL-1 expression was also low in an IL-1RI-/- line. IL-1Ra expression and, particularly, secretion were slightly increased in IL-1α-/-βcomp lines and slightly reduced in IL-1β-/- lines. It was not influenced by an IL-1RI deficiency (Figure 1E). These findings suggested that low-level tumor-derived IL-1 or the host environment during carcinogenesis may have strong bearing on the expression of distinct genes. To control for these hypotheses, we evaluated several central features of tumorigenicity and tumor aggressiveness in the wt, IL-1α-/-, IL-1β-/-, and IL-1α/β-/- tumor lines. To avoid, as far as possible, in vitro selection processes, the experiments were performed with uncloned tumor lines that were cultured for less than 10 passages in vitro. Furthermore, each line was derived from a different 3-methylcholanthrene-treated mouse.
IL-1α Competence Promotes Anchorage-Independent Growth and Both Tumor-Derived IL-1α and IL-1β Support Apoptosis Resistance
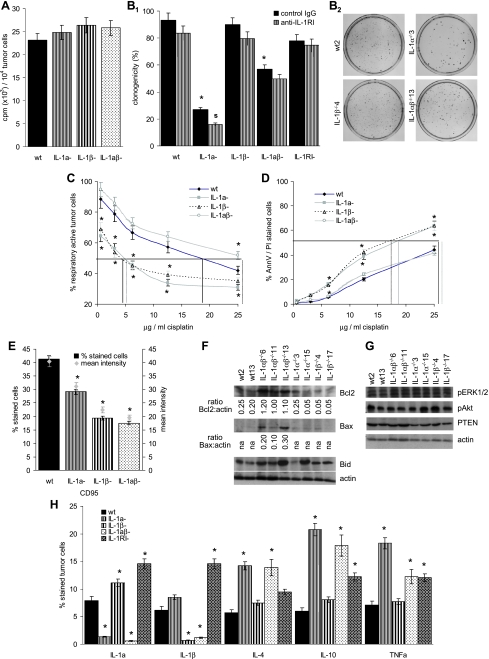
The accelerated growth of IL-1βcomp tumors could have been a consequence of accelerated cell cycle progression or pronounced apoptosis resistance. Therefore, we explored the impact of IL-1 competence on these parameters in vitro. We expected accelerated proliferation and/or high-efficacy anchorage-independent growth possibly combined with increased apoptosis resistance of IL-1βcomp lines. This has not been the case.
Neither an IL-1β nor an IL-1α deficiency influenced tumor cell proliferation (Figure 2A). In addition, IL-1α, but not IL-1β competence, supported anchorage-independent growth in soft agar, such that colony formation of IL-1α-/- lines was strongly reduced. Anchorage-independent growth of IL-1αβ-/- lines also was impaired, but less efficiently. The high cloning efficacy of IL-1αcomp lines was maintained in the presence of anti-IL-1RI. In addition, an IL-1RI-/- line also revealed high cloning efficacy. However, the low cloning efficacy of IL-1α-/-βcomp lines was further reduced in the presence of anti-IL-1RI (Figure 2B). Thus, IL-1α supports anchorage independent growth likely independent of IL-1RI expression, but IL-1β (minor contribution, if at all) might act through the IL-1RI. Apoptosis/drug resistance of the IL-1-deficient lines also did not meet our expectation. When cultured in the presence of an increasing dose of cisplatin, apoptosis resistance was strongly reduced in IL-1α-/- and IL-1β-/- cells, whereas IL-1αβ-/- cells displayed unaltered or slightly increased cisplatin resistance compared with wt cells. This has been evaluated by MTT (Figure 2C) and Annexin V/PI staining (Figure 2D) and has been confirmed by reduced 3H-thymidine uptake of cisplatin-treated IL-1α-/- and IL-1β-/-, but not IL-1αβ-/- compared with IL-1wt cells (data not shown).
Figure 2.
Tumor cell-associated IL-1, proliferation, anoikis, and apoptosis resistance. (A) Three wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma lines (2 x 104 cells) were grown for 16 hours in RPMI in the presence of 10/µCi of 3H-thymidine and incorporation was determined in a β-counter. (B1) Three wt, IL-1α-/-, IL-1β-/-, IL-1αβ-/- and 2IL-1RI--/- fibrosarcoma lines (102 cells) were suspended in 0.3% agar and seeded on a 1% agar layer. Where indicated, cultures contained 10µg/ml anti-IL-1RI. After 3 weeks of culture, colonies were counted. (B2) Representative examples for one wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma lines are shown. (C and D) Three wt, IL-1α-/-, IL-1β-/- and IL-1αβ-/- fibrosarcoma lines (1 x 105 cells) were cultured for 48 hours in the presence of 1.56 to 25 µg cisplatin. Survival was evaluated by MTT staining (C) and Annexin V-FITC/PI staining (D). The percentage of live cells (C) and apoptotic cells (D) as compared to cells grown in the absence of cisplatin (100%) is shown (mean values of triplicates). The amount of cisplatin required for a 50% reduction in cell survival is indicated. (E) CD95 expression was evaluated on three wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma lines by flow cytometry. The percentage of stained cells and the mean intensity of staining is shown. (F and G) Wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma cells were lysed. Lysates were separated by SDS-PAGE. After transfer, membranes were blotted with the indicated antibodies specific for pro- and antiapoptotic proteins and for pro- and antiapoptotic signal transducing molecules. For Bcl2 and Bax, the intensity ratio in comparison to the actin loading control is indicated. One of 3 representative experiments are shown. (H) Three wt, IL-1α-/-, IL-1β-/-, IL-1αβ-/- and 2 IL-1RI-/- fibrosarcoma lines were tested for cytokine expression by flow cytometry. The mean percentage of stained cells is shown: (A, B1, C, D, E, and H) mean ± SD of the distinct lines are presented and significant differences between wt, IL-1α-/-, IL-1β-/-, IL-1αβ-/- and IL-1RI-/- lines are indicated by an asterisk, a reduction in colony formation (B1) by anti-IL-1RI is indicated by a boldface “s.” Experiments were repeated two to three times and revealed comparable results.
Trying to explain these unexpected findings, expression of several pro- and antiapoptotic proteins was analyzed by flow cytometry and Western blot analysis. CD95 expression was reduced in IL-1α-/-, IL-1β-/-, and IL-1αβ-/- sarcoma lines (Figure 2E). Expression of CD95L and TNFRI was largely independent of tumor cell-associated IL-1. Instead, TNFRII expression was particularly low in IL-1α-/- and IL-1αβ-/- lines (Table 1). Expression of some proapoptotic proteins, such as BIM, PARP (data not shown), and Bid, varied between the individual lines and thus could not account for the uniformly decreased apoptosis resistance of IL-1α-/- and IL-1β-/- lines. However, in line with unimpaired drug resistance, both Bcl2 and proapoptotic Bax expression was significantly increased in IL-1αβ-/- lines. Decreased apoptosis resistance of IL-1β-/- lines was accompanied by reduced Bcl2 expression. In IL-1α-/- lines, a reduction in Bcl2 expression was not consistently observed (Figure 2F). These findings suggest that IL-1α and IL-1β might be distinctly involved in the regulation of both pro- and antiapoptotic signaling cascades. To support this hypothesis, Bcl2 and Bax expression was evaluated after culturing cells in the presence of recombinant IL-1α and/or IL-1β. Recombinant IL-1α and IL-1β did not influence Bax expression. This also accounted for an IL-1RI-/- line. Surprisingly, Bcl2 expression became up-regulated in the presence of exogeneous IL-1α plus IL-1β in the IL-1RI-/- line and down-regulated in an IL-1αβ-/- line. Instead, exogeneous IL-1α or IL-1β had no impact on Bcl2 expression in IL-1wt, IL-1α-/-, and IL-1β-/- cells (Table 2). Thus, one could hypothesize that possibly in the absence of IL-1α and IL-1β the Bcl2 protein may become stabilized or, at least, become less efficiently degraded. So far, we failed to identify the relevant signaling pathways. However, several pathways could be excluded. Akt phosphorylation and Pten expression were not or not consistently affected by an IL-1α or IL-1β deficiency. In addition, extracellular-regulated kinase 1/2 (ERK1/2) phosphorylation, which could have been indicative for apoptosis resistance and anchorage independence [29], was not affected by an IL-1α and/or IL-1β deficiency (Figure 2G).
Table 1.
Flow Cytometry Analysis of Apoptosis Marker Expression in wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- Fibrosarcoma Lines.
| Marker | Tumor Lines (P) | |||
| wt | α-/- | β-/- | αβ-/- | |
| CD95 | ||||
| % stained cells | 41.2 ± 4.3 | 29.2 ± 1.7 (.011) | 19.4 ± 1.8 (.001) | 17.5 ± 1.1 (<.0001) |
| Mean intensity of expression* | 42.0 ± 1.0 | 32.3 ± 2.5 (.004) | 23.0 ± 2.6 (<.001) | 19.3 ± 1.5 (<.0001) |
| CD95L | ||||
| % stained cells | 4.3 ± 0.6 | 3.5 ± 0. (ns) | 2.3 ± 0.2 (.006) | 4.6 ± 0.7 (ns) |
| Mean intensity of expression* | 11.7 ± 0.6 | 13.0 ± 2.0 (ns) | 11.0 ± 0.1 (ns) | 12.3 ± 0.6 (ns) |
| CD120a, TNFRI | ||||
| % stained cells | 15.0 ± 0.9 | 14.4 ± 1.3 (ns) | 13.9 ± 1.0 (ns) | 16.6 ± 1.7 (ns) |
| Mean intensity of expression* | 21.3 ± 1.2 | 20.3 ± 1.5 (ns) | 19.3 ± 1.5 (ns) | 23.3 ± 2.1 (ns) |
| CD120b, TNFRII | ||||
| % stained cells | 19.6 ± 2.1 | 12.0 ± 2.2 (.012) | 15.4 ± 2.7 (ns) | 12.0 ± 1.1 (.005) |
| Mean intensity of expression* | 28.7 ± 4.5 | 16.3 ± 2.3 (.014) | 21.0 ± 5.3 (ns) | 15.7 ± 0.6 (.008) |
Negative controls were set to a mean intensity of 10.
Table 2.
Regulation of Bcl2 and Bax By Recombinant IL-1 in IL-1αβ-/- and IL-1RI-/- Lines.
| Marker | Recombinant IL-1 | Ratio Marker/Actin* | ||||
| wt2 | IL-1α-/-3 | IL-1β-/-3 | IL-1αβ-/-11 | IL-1RI-/-1 | ||
| Bcl2 | None | 0.25 | 0.25 | 0.10 | 1.00 | 0.50 |
| IL-1α | 0.35 | 0.35 | 0.15 | 0.75 | 0.60 | |
| IL-1β | 0.25 | 0.35 | 0.10 | 0.50 | 0.60 | |
| IL-1α + IL-1β | 0.30 | 0.35 | 0.10 | 0.45 | 1.2 | |
| Bax | None | 0.40 | 0.35 | 0.35 | 0.75 | 1.00 |
| IL-1α | 0.55 | 0.30 | 0.40 | 0.80 | 1.00 | |
| IL-1β | 0.45 | 0.30 | 0.35 | 0.60 | 1.10 | |
| IL-1α + IL-1β | 0.40 | 0.30 | 0.30 | 0.50 | 1.15 | |
Cells were cultured for 24 hours in the presence of 10 ng/ml recombinant IL-1.
Strong differences in the marker/actin ratio are indicated in bold.
As a possible alternative explanation for the distinct effect of tumor-derived IL-1α and IL-1β on tumor cell survival, we hypothesized that IL-1β, possibly together with TNFα, might sustain an inflammatory milieu that supports the activation of an apoptotic signaling cascade. To control the latter, inflammatory cytokine expression was evaluated in the IL-1comp and IL-1-/- tumor lines. Unexpectedly, IL-10 and TNFα expression was increased in IL-1α-/- βcomp, but also, although less pronounced, in IL-1αβ-/- lines. This suggests that within the tumor cell inflammatory cytokines expression may be actively controlled and down-regulated by IL-1α. In line with this interpretation, inflammatory cytokine expression was comparably low in IL-1αcomp, IL-1RI-/- lines (Figure 2H). It should be mentioned that IL-1RI expression was unaltered in IL-1-/- cells and IL-1RII expression was low and not significantly influenced by tumor-associated IL-1 (data not shown).
Although these experiments confirm opposing effects of IL-1α and IL-1β on pro- and antiapoptotic gene expression by the IL-1-producing tumor cell and an involvement of IL-1α in proinflammatory cytokine expression, we have no unequivocal answer on the impact of tumor-derived IL-1α and IL-1β on the tumor cell's apoptosis resistance. In addition, only IL-1α promotes anchorage-independent growth, possibly through sustained TNFRII expression [30]. Both apoptosis resistance, distinctly regulated by tumor-derived IL-1α and IL-1β and anchorage-independent growth promotion by IL-1α, do not explain accelerated tumor growth of IL-1βcomp tumor lines in vivo.
Tumor Cell-Derived IL-1α and IL-1β: Cell Adhesion, Migration, and Angiogenesis
IL-1β-/- fibrosarcoma lines grow less invasively and are poorly vascularized. We interpreted the finding in the sense that tumor-derived IL-1β might modulate tumor cell adhesion, migration, matrix degradation, and angiogenesis. To support the assumption, adhesion molecule, matrix metalloproteinase (MMP), and chemokine expression was analyzed.
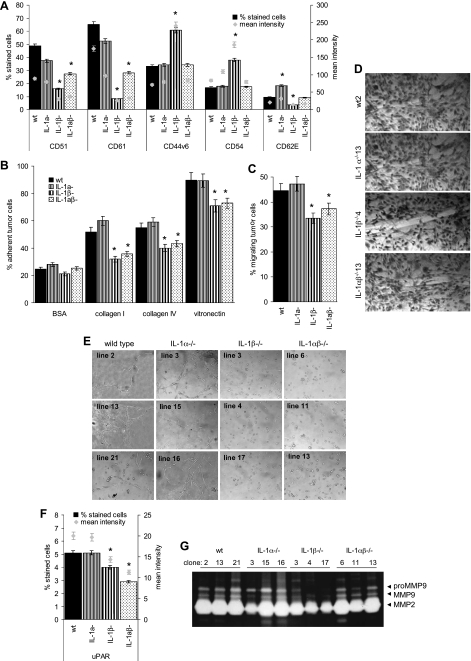
From the adhesion molecules tested, that comprised panCD44, CD44v6, the integrins CD49c (α3), CD49d (α4), CD49f (α6), CD51 (αv), CD29 (β1), CD61 (β3), CD104 (β4), CD54 (ICAM1), and CD62E (E-selectin), reduced CD51 and CD61 expression in IL-1β-/- lines was the most prominent change. CD44v6 and CD54 were up-regulated in IL-1αcompβ-/- lines. CD62E was up-regulated in IL-1α-/- βcomp and down-regulated in IL-1αcomp β-/- lines (Figure 3A). Additional changes in adhesion molecule expression were not significant (data not shown).
Figure 3.
Tumor-associated IL-1, matrix adhesion, migration, and angiogenesis induction. (A–G) Three wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma lines were tested for the following: (A) Adhesion molecule expression by flow cytometry. The percentage of stained cells and the mean intensity of staining are shown. (B) Adhesion to components of the extracellular matrix. Adhesion was measured after a 2-hour incubation by crystal violet staining. (C) Transwell migration, evaluated after a 4-hour incubation by crystal violet staining. (D) Migration into a wounded monolayer 48 hours after wounding. A representative example of one wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma line is shown. (E) Cable formation on matrigel evaluated after 24 hours. (F) uPAR expression was evaluated by flow cytometry. The percentage of stained cells and the mean intensity of staining are shown. (G) MMP2 and MMP9 expression in 3 wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma lines as revealed by zymography. (A–C and F) Mean ± SD of three distinct lines are presented, and significant differences between wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- lines are indicated by an asterisk. All experiments were repeated at least three times and revealed comparable results.
Reduced CD51/CD61 expression of IL-1β-/- and IL-1αβ-/- cells correlates with reduced adhesion to collagens I and IV and vitronectin (Figure 3B). Adhesion to hyaluronan, collagen III, fibronectin, and laminins 1 and 5 was unaltered (data not shown). IL-1β-/- and IL-1αβ-/- tumor cells also revealed reduced transwell migration and in vitro wound healing (Figure 3, C and D). In line with the reduced CD51/CD61 expression, IL-1β-/- and IL-1αβ-/- tumor cells formed small clusters but not cablelike structures on matrigel. Instead, IL-1βcomp lines formed a net of cablelike structures (Figure 3E).
Cable formation is suggested to be an angiogenesis-related morphogenic feature. Thus, cable formation by IL-1βcomp tumor lines corresponds well to the stronger in vivo angiogenesis by these tumors, and it became interesting to see whether IL-1β would have bearing on additional angiogenesis-related factors. Vascular endothelial growth factor and basic fibroblast growth factor expression was low and was not reduced in IL-1β-/- or IL-1αβ-/- lines (data not shown). Instead, in the absence of IL-1β, low uPAR expression was further reduced (Figure 3F), and MMP9 secretion was strongly diminished in IL-1αcomp β-/- and distinctly in IL-1αβ-/- lines (Figure 3G).
Taken together, IL-1βcomp tumor lines are more adhesive and motile and display angiogenesis-related features. These findings are in line with high CD51/CD61, up-regulated CD62, up-regulated uPAR expression, and increased MMP provision. Neither was there evidence for an active contribution of tumor-derived IL-1α to CD51/CD61, uPAR, and MMP expression/secretion nor has there been evidence that IL-1α may counterregulate expression of these molecules.
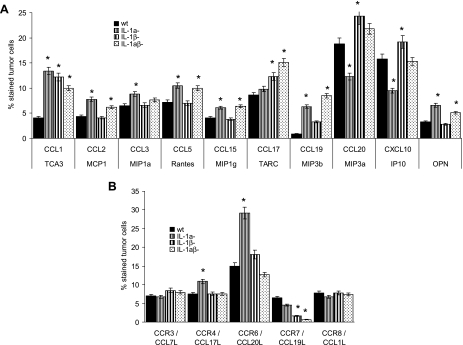
As outlined previously, this was distinct for cytokines but is also distinct for chemokine expression. A deficiency in IL-1α or IL-1β had no negative impact on chemokine secretion by the tumor cells. On the contrary, the expression of CCL2, CCL3, CCL5, CCL15, CCL19, and OPN, chemokines preferentially attracting leukocytes [31], was increased in IL-1α-/- tumor lines, indicating that IL-1α may counterregulate expression-promoting activities. A distinct effect was only seen on T cell attracting CCL20 and CXCL10 expression [32,33] that was reduced in IL-1α-/- βcomp and was high in IL-1αcomp β-/- cells (Figure 4A).
Figure 4.
Tumor cell-associated IL-1, chemokine, and chemokine receptor expression. (A and B) Three wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma lines were tested for (A) chemokine and (B) chemokine receptor expression by flow cytometry. The percentage of stained cells is shown. Mean values ± SD of 3 distinct lines are presented, and significant differences between wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- lines are indicated by an asterisk. The experiments were repeated three times and revealed comparable results.
The impact of IL-1α and IL-1β on chemokine receptor expression was less pronounced. Expression of the chemokine receptors CCR3 and CCR8 was not significantly influenced by tumor-associated IL-1α and IL-1β. Instead, CCR4 and, most pronounced, CCR6 expression was enhanced in IL-1α-/- βcomp lines, which could indicate a negative feedback of IL-1α on IL-1β- induced CCR4 and CCR6 expression, with high CCR6 expression also being described for metastatic tumor cells [34]. As could have been expected, CCR7, a chemokine receptor on inflammatory fibroblasts [35], was hardly expressed in IL-1β-/- and IL-1αβ-/- clones (Figure 4B).
Thus, mostly IL-1α-/-βcomp lines displayed up-regulated expression of leukocyte attracting chemokines and of CCR6, a chemokine receptor that has been described to support angiogenesis and metastatic spread. Because, in many instances, expression of inflammatory chemokines becomes up-regulated in the absence of IL-1α, we interpret the finding in the sense that in wt fibrosarcoma cells, IL-1α counterregulates not only inflammatory cytokine but also inflammatory chemokine induction. This implies that tumor-derived IL-1α actually could hamper the aggressiveness of IL-βcomp tumors. In line with this interpretation, IL-1α-/- βcomp tumor lines grew faster and more aggressive than IL-1wt lines. However, IL-1αβ-/- lines grew slower than IL-1wt lines. Thus, tumor-derived IL-1β may exert an additional, IL-1α-independent, systemic effect on the tumor-bearing host.
Systemic Activity of Tumor-Derived IL-1β
IL-1α and IL-1β are proinflammatory cytokines, the potency of IL-1 originating from its ability to induce cytokine and chemokine expression [1,2,7,8,16,17]. As outlined previously, cytokine and chemokine expression within the tumor cell was regulated by IL-1α, such that inflammatory cytokine and chemokine expression was strongly increased in IL-1α-/- tumor cells. Accordingly, IL-1α-/- βcomp tumors displayed increased angiogenesis and attracted myeloid cells more efficiently than IL-1wt tumors (Figure 1, C and D). However, IL-1αcomp β-/- and IL-1αβ-/- tumors were poorly vascularized and very few myeloid cells were detected. Thus, tumor-derived IL-1β obviously contributed to the cross-talk between the tumor and the surrounding.
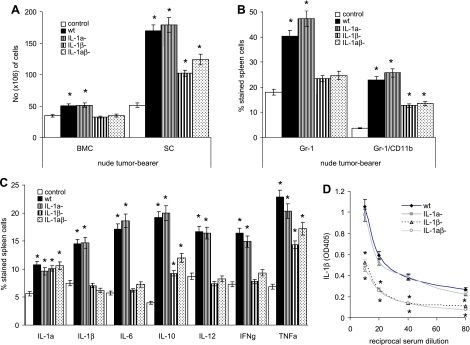
Indeed, tumor-derived IL-β strongly influenced myelopoiesis. The recovery of BMCs and SCs was significantly increased in mice bearing wt or IL-1α-/- βcomp tumors, whereas a moderate increase only of SC was seen in IL-1αcomp β-/- or IL-1αβ-/- tumor-bearing nu/nu mice (Figure 5A). The increase in SC was mostly due to myeloid cells, where up to 25% of IL-1wt or IL-1α-/- βcomp tumor-bearing mice-derived SC expressed the phenotype of myeloid-derived suppressor cells (Gr-1+ CD11b+). In the spleen of tumor-free control mice, only 4% to 6% of cells are Gr-1+CD11b+ (Figure 5B). Furthermore, in comparison to SC of tumor-free mice, the percentage of IL-6-, IL-10-, TNFα-, IL-12-, IFNγ-, and IL-1β-expressing SC was strongly increased in mice bearing IL-1βcomp tumors (Figure 5C). Furthermore, whereas IL-1β was hardly detectable in the serum of tumor-free mice (data not shown), an increased amount of IL-1β was recovered from the serum of IL-1βcomp tumor-bearing nu/nu mice (Figure 5D). None of these phenomena was seen in mice bearing IL-1αβ-/- tumors. Thus, IL-1β expression on a subcutaneously growing tumor exerts a strong systemic effect on leukopoiesis and suffices to create an inflammatory milieu. There was no evidence for a contribution of tumor-derived IL-1α.
Figure 5.
Tumor-associated IL-1β, myelopoiesis, and cytokine expression/secretion by leukocytes. Three wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma lines (5 x 105 cells) were s.c. implanted into nu/nu mice (3 mice/line). Mice were sacrificed after 4 weeks. (A) Mean ± SD of the number of BMC and SC from 5 control mice and 9 each wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma-bearing mice. (B) The percentage of Gr-1+ and of Gr-1+CD11b+ SC was evaluated by flow cytometry. Values represent the mean ± SD from six tumor-free and tumor-bearing mice per group (2 mice/group wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- fibrosarcoma lines). (C) Cytokine expression in SC, 4 weeks after tumor cell application, was evaluated by flow cytometry. SC were fixed and permeabilized in advance. Mean ± SD of SC from 3 tumor-free mice and 3 mice bearing the different wt, IL-1α-/-, IL-1β-/-, and IL-1αβ-/- lines is shown. (D) Serum was collected from 4-week-old tumor-bearing mice. The relative amount of IL-1β was determined in a sandwich ELISA. Values represent the mean ± SD of sera from 6 mice (2 mice/line). Significant differences of IL-1α-/-, IL-1β-/-, and IL-1αβ-/- compared with wt fibrosarcoma-bearing mice are indicated by an asterisk.
Discussion
IL-1α and IL-1β are pleiotropic cytokines, that despite binding to the same receptor, can exert distinct effects [1,2,7–9,16]. This relies, at least in part, on IL-1α mainly being active in the membrane-bound form and as proIL-1α in the cytoplasm [7,9,15], whereas IL-1β is secreted and acts on the microenvironment and systemically [1,2,7–9,16,17]. These distinct activities sufficed for the induction of a T cell-mediated rejection response against IL-1αcomp β-/- tumors [26]. On the contrary, IL-1βcomp tumors promote an inflammatory response that leads to the expansion of MDSC and Treg, which together prevent rejection of IL-1αβcomp tumors. Nonetheless, by the failure of IL-1αcompβ-/- tumor lines to grow in the immunocompetent host, the question arose on the feedback impact of tumor-derived IL-1α and IL-1β on the tumor cell itself. To answer this question, tumorigenicity of IL-1-/- lines was evaluated in the immunocompromised host and in vivo growth features were compared with altered gene expression in IL-1α-/-, IL-1β-/-, and IL-1αβ-/- tumor cells. Our data show that tumorigenicity of IL-1αcomp tumor lines is unimpaired. In fact, tumor-derived IL-1α contributes to essential features as anchorage-independent growth and apoptosis resistance. Nonetheless, IL-1α-/-βcomp tumors grow more aggressively, which is due to augmented inflammatory cytokine and chemokine expression in IL-1α-/- tumors and a most efficient concomitant stimulation of inflammatory cytokine expression by hematopoietic cells through tumor-derived IL-1β.
IL-1α and IL-1β Distinctly Support Tumor Growth and Tumor Cell Survival
Tumor growth in nu/nu mice provided convincing evidence for unimpaired tumorigenicity of IL-1-deficient fibrosarcoma lines. However, IL-1βcomp tumor lines grew faster and more aggressively in vivo. One possible explanation could have been that IL-1β promotes tumor cell proliferation and/or strengthens apoptosis resistance, features that actually have been reported to be associated with membrane-bound and cytoplasmic IL-1α [1,7,8,17,36].
Proliferative activity did not significantly differ between IL-1comp and IL-1-/- lines, but anchorage-independent growth was strongly reduced in IL-1α-/- and moderately in IL-1αβ-/- lines. CD44v6 expression is up-regulated in IL-1αcomp β-/- tumor lines. Because CD44v6 engagement promotes activation of the matrix-associated protein kinase pathway [37,38], we speculated that high CD44v6 expression in IL-1αcomp β-/- lines may support anchorage-independent growth through ERK phosphorylation [39] but ERK phosphorylation was unaltered. However, TNFRII expression was reduced in IL-1α-/- lines. Thus, reduced anchorage-independent growth of IL-1α-/- lines may rely on reduced TNFRII expression and, as a consequence, reduced induction of gene expression for cell survival [30,40–42]. Irrespective of this remaining open question, our data provide convincing evidence that anchorage independence is not induced by IL-1α binding to the IL-1RI, because anti-IL-1RI did not influence the cloning efficacy of IL-1αcomp lines. In addition, IL-1RI-/- lines displayed a high cloning efficacy. Nonetheless, a reduction in the lower cloning efficacy of IL-1β-/- lines by anti-IL-1RI indicates that the minor contribution of IL-1β may be IL-1RI-dependent.
In line with the impaired clonogenicity, drug resistance, too, was mitigated not only in IL-1α-/- but also in IL-β-/- lines. Unexpectedly, drug resistance was unaltered in IL-1αβ-/- lines. So far, we cannot explain these findings. CD95 expression was reduced in IL-1α-/-, IL-1β-/-, and IL-1αβ-/- lines. CD95L and TNFRI expression was not affected by IL-1 competence. Analyzing mitochondrial pro- and anti-apoptotic gene expression revealed, corresponding to the unimpaired apoptosis resistance of IL-1αβ-/- lines, high Bcl2, and proapoptotic Bax expression. Thus, an IL-1α plus IL-1β deficiency may support pro- and antiapoptotic protein expression. However, neither in IL-1α-/- βcomp nor in IL-1 αcomp β-/- that Bcl2 or Bax expression was elevated. But Bcl2 expression was reduced in IL- 1β-/- lines. It was variably expressed in IL-1α-/ lines. The reduction in Bcl2 expression would be in line with the reduced apoptosis resistance of IL-1β-/- lines. Thus, we evaluated whether Bcl2 may become stabilized in the IL-1β-/- line by exogeneous IL-1α and/or IL-1β and whether exogeneous IL-1α and/or IL-1β may exert a distinct effect on IL-1αβ-/- lines. Exogeneous IL-1α and/or IL-1β did not support Bcl2 recovery in the IL-1β-/- line. However, a reduction in Bcl2 was noted in IL-1αβ-/- cells by exogeneous IL-1β. Conversely, in IL-1αβcomp IL-1RI-/- cells, addition of IL-1β resulted in an increased Bcl2 recovery. The finding indicates that in these fibrosarcoma lines, Bcl2 is not regulated through IL-1RI signaling but may be promoted at the level of pro-IL-1β, caspase 1, and the inflammosome [43]. Exclusion of the ERK pathway has already been mentioned, and Akt phosphorylation [44] and apoptosis-promoting PTEN [45] were variably regulated in IL-1α-/-, IL-1β-/-, and IL-1αβ-/- lines, which excluded the differing apoptosis susceptibility/resistance of these lines to be regulated by the classic PI3K/Akt pathway. Finally, TNFα expression was high in IL-1α-/- βcomp lines but was lower in IL-1αβ-/- lines, which suggests that IL-1α actively suppress and IL-1β promote TNFα expression. Thus, the high TNFα level in the IL-1α-/- βcomp lines may account for the decrease of apoptosis resistance in these lines, whereas lower TNFα levels in IL-1αβ-/- lines could support unaltered apoptosis resistance [46].
Taken together, our data provide clear evidence that apoptosis resistance of fibrosarcomas is differently regulated by IL-1α and IL-1β. However, further studies with IL-1 over-expressing tumor lines and with tumor lines from double knockout mice for IL-1, TNFα, TNFR, and/or NFκB are required to define the signaling components of receptor or mitochondrial apoptosis pathways that account for distinct apoptosis susceptibility of IL-1α-/-, IL-1 β-/-, and IL-1αβ-/- tumor lines. Irrespective of this open question, the in vitro analyses on anchorage-independent growth and apoptosis resistance did not provide a convincing explanation for the more aggressive growth of IL-1βcomp fibrosarcoma lines.
Tumor-Derived IL-1 and the Cross-talk with the Tumor Stroma
The cross-talk between the tumor and the surrounding stroma strongly influences tumor growth and progression [47]. IL-1βcomp tumors growing faster, being more efficiently infiltrated by myeloid cells, and supporting angiogenesis, tumor-derived IL-1β obviously contributes to creating a tumor growth-favoring milieu. In line with the in vivo growth features, IL-1βcomp tumor lines are more adhesive, display stronger migratory activity, and most strikingly, have the capacity to grow in cablelike structures on matrigel, which are considered as morphogenic features related to angiogenesis induction. In fact, IL-1βcomp tumor lines show higher CD51/CD61 and slightly increased uPAR expression and more pronounced MMP9 secretion. The stronger adhesiveness of IL-1βcomp cells to collagens and vitronectin also may have contributed to angiogenesis induction [48]. Increased CD62E expression, which can be supported by IL-1β [49], may further contribute to the migratory activity of IL-1βcomp lines. Whether higher CD54 and low CD62E expression in IL-1β-/- lines actively hampers migratory activity [49,50], remains to be explored. Finally, CXCL10 expression has been described to inhibit angiogenesis [51]. Notably and in line with increased angiogenesis induction by IL-1βcomp tumor lines, CXCL10 expression was reduced in IL-1α-/- βcomp tumor lines. On the contrary, it was increased in IL-1αcomp β-/- lines, and it may have actively hampered angiogenesis induction in these lines. Taken together, adhesion molecule and matrix degrading enzyme expression in IL-1βcomp lines are well in line with promoting the cross-talk between the tumor and its environment [7,8,17]. Instead, up-regulated CD54 expression in IL-1αcomp β-/- lines could be counteracting.
Of particular importance for creating a tumor growth-promoting environment are inflammatory cytokines and chemokines, whose expressions were increased in IL-1α-/- tumor lines. We interpret the finding in the sense that in fibrosarcoma lines, possibly distinct to hematopoietic cells, IL-1α can actively down-regulate inflammatory cytokine and chemokine expression, which is well in line with cytosolic proIL-1α-influencing gene expression [5,15], but does not necessarily exclude transcriptional activation by IL-1β. Tumor-associated IL-1α down-regulation accounted for proinflammatory cytokines and chemokines involved in the recruitment of myeloid cells [31]. Instead, expression of the T cell-recruiting cytokines CCL20 and CXCL10 [32,33] was reduced in IL-1α-/- cells.
Tumor-derived IL-1-dependent differences in chemokine receptor expression were only observed for CCR7 and CCR6. Low CCR7 expression in IL-1β-/- lines is in line with CCR7 expression mainly in inflammatory fibroblasts [35]. Conversely, up-regulated CCR7 expression on IL-1α-/-βcomp tumor lines can well promote response to its ligands, which are, besides others, expressed on lymphatic endothelial cells [52]. Aberrant tumor expression on certain tumor types has been liked to prosurvival and invasive pathways [52]. CCR6 expression has also been observed in several tumors [34,53] and IL-1β may contribute to its induction [54]. Whether up-regulated CCR6 expression, which has been observed particularly in metastatic tumors [34], in IL-1α-/- βcomp lines contributes to their increased tumorigenicity remains to be explored.
Our data by no means exclude a contribution of host cells to the tumor-stroma interaction. However, significant differences being observed by a selective deficit of IL-1 in the tumor cells argue for a considerable contribution of tumor-derived IL-1 in initiating and sustaining its microenvironment. Thus, cytokine/chemokine expression of IL-1 βcomp tumor lines obviously supports a high-efficacy angiogenesis and the recruitment of myeloid cells into the tumor tissue. Notably, tumor-derived IL-1α may locally control overshooting inflammatory reactions by tumor-derived IL-1β, which could impair tumor cell survival. However, the cross-talk-supporting activities of IL-1β are dominating, because IL-1αβ-/- tumor lines do not attract myeloid cells and do not support angiogenesis.
Tumor-Associated IL-1β and Systemic Inflammation
It recently has come into focus that tumor cells exert systemic effects that influence the premetastatic organ and the hematopoietic system. Although cytokines and chemokines are a powerful means to exert systemic effects [55], it was most surprising and unexpected that differences in low-level IL-1 expression in fibrosarcoma cells suffice for an induction of a strong systemic response.
Low-level tumor-derived IL-1β strongly induces myelopoiesis in the immunocompetent [26] and the nude mouse. As described for the immunocompetent host, most of these myeloid cells are Gr-1+CD11b+ myeloid-derived suppressor cells [56]. MDSC frequently expand during tumor growth [56,57], and our finding indicates that tumor-derived IL-1β significantly contributes to MDSC expansion [23,24,26,58,59]. Importantly, too, tumor-derived IL-1β also sufficed for a systemic induction of an inflammatory cytokine milieu, which could well affect T cell-mediated immune responses [60,61]. Notably, cytokine induction in hematopoietic cells differed from the one within the tumor cell inasmuch as it exclusively depended on IL-1β, i.e., inflammatory cytokines were not induced in mice bearing IL-1αcompβ-/- or IL-1αβ-/- tumors. Thus, counter-regulation of cytokine expression by IL-1α, seen in the tumor cells, may not account for cells of the hematopoietic system. Nonetheless, as already mentioned, IL-1 expression in the described fibrosarcoma lines is very low and does not exceed the one of nonactivated fibroblasts. Thus, we hypothesize that additional, not yet defined factors secreted by IL-1β-expressing fibrosarcoma co-operate with tumor-derived IL-1β in the induction of an inflammatory milieu. In addition, IL-1β expression levels in the growing tumor may be higher than in vitro (R.N. A., unpublished observation), e.g., it is known that, besides others, hypoxia induces IL-1β transcription [62,63]. Second, although not necessarily, cleavage of pro-IL-1β by caspase-1 can be accompanied by cell death [64], increasing the local concentration of IL-1β and of pro-IL-1β that can also be cleaved by other proteases, including MMPs [65], which are expressed at an increased level in IL-1βcomp lines. After the recruitment of myeloid cells, which are a major source of IL-1β [66], Gr-1+CD11b+ cells could well become the initiators of a systemic inflammatory response that can initiate and sustain the elevated levels of IL-1β at the systemic level [67]. Gr-1+CD11b+ cells have also been described to promote IL-10, TNFα, and TGFβ cytokine secretion [56,57,68], with the expression of these inflammatory cytokines being increased in the spleen of IL-1βcomp tumor-bearing mice.
Taken together, IL-1α and IL-1β have long been suggested to exert similar functional activity and have been considered as potential therapeutic drugs [1,6,12,13]. We here demonstrate that tumor-derived IL-1α is advantageous for the tumor cell itself but hardly exerts systemic effects in the nu/nu mouse. On the contrary, even the small amount of tumor-derived IL-1β suffices to promote tumor cell adhesion and migration and further supports tumor growth by angiogenesis induction. These features and the strong expansion of MDSC and the up-regulated expression of inflammatory cytokines only in mice bearing IL-1βcomp tumors suggests that, opposite to expectation, an efficient interference with tumor-derived IL-1β may well be of therapeutic benefit. Indeed, increased IL-1β levels have repeatedly been reported to be associated with poor prognosis in cancer patients [69]. Recently, most elegant studies on the inflammosome system and pharmacological inhibition of IKKβ in septic shock and multiorgan dysfunction also strengthen the critical role of IL-1β in systemic inflammation [43,70,71].
Acknowledgments
We thank J. McAlear for her help with editing.
Abbreviations
- BMC
bone marrow cells
- IL-1α-/-/IL-1β-/-/IL-1αβ-/- fibrosarcoma
tumors derived from mice with a targeted deletion of the corresponding genes
- IL-1comp
IL-1 competent
- IL-1Ra
IL-1 receptor antagonist
- i.v.
intravenously
- MDSC
myeloid-derived suppressor cells
- MMP
matrix metalloproteinase
- RPMI-s
RPMI 1640, supplemented with 10% fetal calf serum, antibiotics, l-glutamine
- SC
spleen cells
- s.c.
subcutaneously
- wt
wild type
Footnotes
This work was supported by the Israel Ministry of Science in cooperation with the German Cancer Research Center, Heidelberg, Germany (M.Z., R.N.A.), the Tumorzentrum Heidelberg/Mannheim (M.Z.), the United States-Israel Binational foundation (R.N.A.), the Israel Science Foundation founded by the Israel Academy of Sciences and Humanities (R.N.A.), the Israel Ministry of Health Chief Scientist's Office (R.N.A., E.V.), Association for International Cancer Research (R.N.A.), the German-Israeli DIP collaborative program (R.N.A.), the Israel Cancer Association (E.V.), the Concern Foundation (E.V.), and the Israel Cancer Association (E.V.). R.N.A. is an incumbent of the Irving Isaac Sklar Chair in Endocrinology and Cancer.
References
- 1.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apte RN, Voronov E. Interleukin-1—a major pleiotropic cytokine in tumor-host interactions. Semin Cancer Biol. 2002;12:277–290. doi: 10.1016/s1044-579x(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 3.Perrier S, Darakhshan F, Hajduch E. IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde? FEBS Lett. 2006;580:6289–6294. doi: 10.1016/j.febslet.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 4.Swaan PW, Knoell DL, Helsper F, Wewers MD. Sequential processing of human ProIL-1beta by caspase-1 and subsequent folding determined by a combined in vitro and in silico approach. Pharm Res. 2001;18:1083–1090. doi: 10.1023/a:1010958406364. [DOI] [PubMed] [Google Scholar]
- 5.Sultana T, Wahab-Wahlgren A, Assmus M, Parvinen M, Weber G, Soder O. Expression and regulation of the prointerleukin-1alpha processing enzymes calpain I and II in the rat testis. Int J Androl. 2003;26:37–45. doi: 10.1046/j.1365-2605.2003.00386.x. [DOI] [PubMed] [Google Scholar]
- 6.Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med. 2006;4:48. doi: 10.1186/1479-5876-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 8.Apte RN, Krelin Y, Song X, Dotan S, Recih E, Elkabets M, Carmi Y, Dvorkin T, White RM, Gayvoronsky L, et al. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour-host interactions. Eur J Cancer. 2006;42:751–759. doi: 10.1016/j.ejca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 10.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douvdevani A, Huleihel M, Zöller M, Segal S, Apte RN. Reduced tumorigenicity of fibrosarcomas which constitutively generate IL-1 alpha either spontaneously or following IL-1 alpha gene transfer. Int J Cancer. 1992;51:822–830. doi: 10.1002/ijc.2910510526. [DOI] [PubMed] [Google Scholar]
- 12.Voronov E, Weinstein Y, Benharroch D, Cagnano E, Ofir R, Dobkin M, White RM, Zöller M, Barak V, Segal S, et al. Antitumor and immunotherapeutic effects of activated invasive T lymphoma cells that display short-term interleukin 1alpha expression. Cancer Res. 1999;59:1029–1035. [PubMed] [Google Scholar]
- 13.Dvorkin T, Song X, Argov S, White RM, Zöller M, Segal S, Dinarello CA, Voronov E, Apte RN. Immune phenomena involved in the in vivo regression of fibrosarcoma cells expressing cell-associated IL-1alpha. J Leukoc Biol. 2006;80:96–106. doi: 10.1189/jlb.0905509. [DOI] [PubMed] [Google Scholar]
- 14.Zöller M, Douvdevani A, Segal S, Apte RN. Interleukin-1 production by transformed fibroblasts: II. Influence on antigen presentation and T-cell-mediated anti-tumor response. Int J Cancer. 1992;50:450–457. doi: 10.1002/ijc.2910500321. [DOI] [PubMed] [Google Scholar]
- 15.Werman A, Werman-Venkert R, White R, Lee JK, Werman B, Krelin Y, Voronov E, Dinarello CA, Apte RN. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci USA. 2004;101:2434–2439. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello CA. The paradox of pro-inflammatory cytokines in cancer. Cancer Metastasis Rev. 2006;25:307–313. doi: 10.1007/s10555-006-9000-8. [DOI] [PubMed] [Google Scholar]
- 18.Song X, Voronov E, Dvorkin T, Fima E, Cagnano E, Benharroch D, Shendler Y, Bjorkdahl O, Segal S, Dinarello CA, et al. Differential effects of IL-1 alpha and IL-1 beta on tumorigenicity patterns and invasiveness. J Immunol. 2003;171:448–6456. doi: 10.4049/jimmunol.171.12.6448. [DOI] [PubMed] [Google Scholar]
- 19.Krelin Y, Voronov E, Dotan S, Elkabets M, Reich E, Fogel M, Huszar M, Iwakura Y, Segal S, Dinarello CA, et al. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogeninduced tumors. Cancer Res. 2007;67:1062–1071. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- 20.Saijo Y, Tanaka M, Miki M, Usui K, Suzuki T, Maemondo M, Hong X, Tazawa R, Kikuchi T, Matsushima K, et al. Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J Immunol. 2002;169:469–475. doi: 10.4049/jimmunol.169.1.469. [DOI] [PubMed] [Google Scholar]
- 21.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S, Sonoda KH, Saijo Y, Nukiwa T, Strieter RM, et al. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, Voronov E, Apte RN. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J Immunol. 2005;175:8200–8208. doi: 10.4049/jimmunol.175.12.8200. [DOI] [PubMed] [Google Scholar]
- 24.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 25.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marhaba R, Nazarenko I, Knöfler D, Reich E, Voronov E, Vitacolonna M, Hildebrand D, Elter E, Apte RN, Zöller M. Opposing effects of fibrosarcoma cell-derived IL-1α and IL-1β on immune response induction. Int J Cancer. 2008 doi: 10.1002/ijc.23503. (Apr 15, Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 27.Kono K, Petersson M, Ciupitu AM, Wen T, Klein G, Kiessling R. Methylcholanthrene-induced mouse sarcomas express individually distinct major histocompatibility complex class I-associated peptides recognized by specific CD8+ T-cell lines. Cancer Res. 1995;55:5648–5655. [PubMed] [Google Scholar]
- 28.Hession C, Moy P, Tizard R, Chisholm P, Williams C, Wysk M, Burkly L, Miyake K, Kincade P, Lobb R. Cloning of murine and rat vascular cell adhesion molecule-1. Biochem Biophys Res Commun. 1992;183:163–169. doi: 10.1016/0006-291x(92)91623-x. [DOI] [PubMed] [Google Scholar]
- 29.Jørgensen K, Skrede M, Cruciani V, Mikalsen SO, Slipicevic A, Flørenes VA. Phorbol ester phorbol-12-myristate-13-acetate promotes anchorageindependent growth and survival of melanomas through MEK-independent activation of ERK1/2. Biochem Biophys Res Commun. 2005;329:266–274. doi: 10.1016/j.bbrc.2005.01.143. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal BB. Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Ann Rheum Dis. 2000;59(Suppl 1):i6–i16. doi: 10.1136/ard.59.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 32.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 33.Luster AD, Leder P. IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med. 1993;78:1057–1065. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubie C, Oliveira-Frick V, Rau B, Schilling M, Wagner M. Chemokine receptor CCR6 expression in colorectal liver metastasis. J Clin Oncol. 2006;24:5173–5174. doi: 10.1200/JCO.2006.07.9095. [DOI] [PubMed] [Google Scholar]
- 35.Pierce EM, Carpenter K, Jakubzick C, Kunkel SL, Flaherty KR, Martinez FJ, Hogaboam CM. Therapeutic targeting of CC ligand 21 or CC chemokine receptor 7 abrogates pulmonary fibrosis induced by the adoptive transfer of human pulmonary fibroblasts to immunodeficient mice. Am J Pathol. 2007;170:1152–1164. doi: 10.2353/ajpath.2007.060649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 37.Marhaba R, Bourouba M, Zöller M. CD44v6 promotes T cell proliferation by persisting activation of MAP kinases. Cell Signal. 2005;17:961–973. doi: 10.1016/j.cellsig.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Cheng C, Yaffe MB, Sharp PA. A positive feedback loop couples Ras activation and CD44 alternative splicing. Genes Dev. 2006;20:1715–1720. doi: 10.1101/gad.1430906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang LH. Molecular signaling regulating anchorage-independent growth of cancer cells. Mt Sinai J Med. 2004;71:361–367. [PubMed] [Google Scholar]
- 40.MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- 41.Dobrovolskaia MA, Kozlov SV. Inflammation and cancer: when NF-kappaB amalgamates the perilous partnership. Curr Cancer Drug Targets. 2005;5:325–344. doi: 10.2174/1568009054629645. [DOI] [PubMed] [Google Scholar]
- 42.Roy D, Sarkar S, Felty Q. Levels of IL-1 beta control stimulatory/inhibitory growth of cancer cells. Front Biosci. 2006;11:889–898. doi: 10.2741/1845. [DOI] [PubMed] [Google Scholar]
- 43.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zbuk KM, Eng C. Cancer phenomics: RETand PTEN as illustrative models. Nat Rev Cancer. 2007;7:35–45. doi: 10.1038/nrc2037. [DOI] [PubMed] [Google Scholar]
- 46.Wajant H. CD95L/FasL and TRAIL in tumour surveillance and cancer therapy. Cancer Treat Res. 2006;130:141–165. doi: 10.1007/0-387-26283-0_7. [DOI] [PubMed] [Google Scholar]
- 47.Zalatnai A. Molecular aspects of stromal-parenchymal interactions in malignant neoplasms. Curr Mol Med. 2006;6:685–693. doi: 10.2174/156652406778195053. [DOI] [PubMed] [Google Scholar]
- 48.Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–658. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 49.Okada T, Okuno H, Mitsui Y. A novel in vitro assay system for transendothelial tumor cell invasion: significance of E-selectin and alpha 3 integrin in the transendothelial invasion by HT1080 fibrosarcoma cells. Clin Exp Metastasis. 1994;12:305–314. doi: 10.1007/BF01753837. [DOI] [PubMed] [Google Scholar]
- 50.Liu K, Caldwell SA, Abrams SI. Cooperative disengagement of Fas and intercellular adhesion molecule-1 function in neoplastic cells confers enhanced colonization efficiency. Cancer Res. 2005;65:1045–1054. [PubMed] [Google Scholar]
- 51.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182:219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mburu YK, Wang J, Wood MA, Walker WH, Ferris RL. CCR7 mediates inflammation-associated tumor progression. Immunol Res. 2006;36:61–72. doi: 10.1385/IR:36:1:61. [DOI] [PubMed] [Google Scholar]
- 53.Brand S, Olszak T, Beigel F, Diebold J, Otte JM, Eichhorst ST, Göke B, Dambacher J. Cell differentiation dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt signaling resulting in proliferation and migration of colorectal cancer cells. J Cell Biochem. 2006;97:709–723. doi: 10.1002/jcb.20672. [DOI] [PubMed] [Google Scholar]
- 54.Inoue Y, Tsushima H, Ando K, Sawayama Y, Sakai M, Yamasaki R, Matsuo E, Tsutsumi C, Imaizumi Y, Iwanaga M, et al. Chemokine expression in human erythroid leukemia cell line AS-E2: macrophage inflammatory protein-3alpha/CCL20 is induced by inflammatory cytokines. Exp Hematol. 2006;34:19–26. doi: 10.1016/j.exphem.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bonemarrow-derived cells. Trends Mol Med. 2007;13:72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006;25:323–331. doi: 10.1007/s10555-006-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young MR, Aquino S, Young ME. Differential induction of hematopoiesis and immune suppressor cells in the bone marrow versus in the spleen by Lewis lung carcinoma variants. J Leukoc Biol. 1989;45:262–273. doi: 10.1002/jlb.45.3.262. [DOI] [PubMed] [Google Scholar]
- 59.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-Arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–306. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 60.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Sullivan BJ, Thomas HE, Pai S, Santamaria P, Iwakura Y, Steptoe RJ, Kay TW, Thomas R. IL-1 beta breaks tolerance through expansion of CD25+ effector T cells. J Immunol. 2006;176:7278–7287. doi: 10.4049/jimmunol.176.12.7278. [DOI] [PubMed] [Google Scholar]
- 62.Zhang W, Petrovic JM, Callaghan D, Jones A, Cui H, Howlett C, Stanimirovic D. Evidence that hypoxia-inducible factor-1 (HIF-1) mediates transcriptional activation of interleukin-1beta (IL-1beta) in astrocyte cultures. J Neuroimmunol. 2006;174:63–73. doi: 10.1016/j.jneuroim.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Frede S, Berchner-Pfannschmidt U, Fandrey J. Regulation of hypoxia-inducible factors during inflammation. Methods Enzymol. 2007;435:405–419. doi: 10.1016/S0076-6879(07)35021-0. [DOI] [PubMed] [Google Scholar]
- 64.Lamkanfi M, Kalai M, Saelens X, Declercq W, Vandenabeele P. Caspase-1 activates nuclear factor of the kappa-enhancer in B cells independently of its enzymatic activity. J Biol Chem. 2004;279:24785–24793. doi: 10.1074/jbc.M400985200. [DOI] [PubMed] [Google Scholar]
- 65.Russo R, Siviglia E, Gliozzi M, Amantea D, Paoletti A, Berliocchi L, Bagetta G, Corasaniti MT. Evidence implicating matrix metalloproteinases in the mechanism underlying accumulation of IL-1beta and neuronal apoptosis in the neocortex of HIV/gp120-exposed rats. Int Rev Neurobiol. 2007;82:407–421. doi: 10.1016/S0074-7742(07)82023-X. [DOI] [PubMed] [Google Scholar]
- 66.Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 67.Simi A, Lerouet D, Pinteaux E, Brough D. Mechanisms of regulation for interleukin-1beta in neurodegenerative disease. Neuropharmacology. 2007;52:1563–1569. doi: 10.1016/j.neuropharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 68.Young MR, Wright MA, Pandit R. Myeloid differentiation treatment to diminish the presence of immune-suppressive CD34+ cells within human head and neck squamous cell carcinomas. J Immunol. 1997;159:990–996. [PubMed] [Google Scholar]
- 69.Deans DA, Wigmore SJ, Gilmour H, Paterson-Brown S, Ross JA, Fearon KC. Elevated tumour interleukin-1beta is associated with systemic inflammation: a marker of reduced survival in gastro-oesophageal cancer. Br J Cancer. 2006;95:1568–1575. doi: 10.1038/sj.bjc.6603446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 71.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Göktuna SI, Neuenhahn M, Fierer J, Paxian S, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]