Abstract
As a consequence of multiple functions of p53, its activation in response to cytotoxic stress may have proapoptotic or protective effects depending on the nature of lesions. We have previously shown that mutational inactivation of p53 results in sensitization to paclitaxel. In this study, we used cyclic pifithrin-α, a transcriptional inhibitor of p53, to further investigate the relevance of p53 function in the response of tumor cells to microtubule inhibitors. Using drug concentrations causing only antiproliferative effects, the combination of antimicrotubule agents with subtoxic pifithrin-α doses resulted in increase of sensitivity of two wild type p53 cell lines, associated with a substantial M phase cell accumulation and marked sensitization to apoptosis. Pifithrin-α had no sensitizing effect in p53 defective cells or a marginal effect in normal human fibroblasts. The apoptotic response to the combination was concomitant with p21 down-regulation, Polo-like kinase 1 up-regulation, p34cdc2 kinase dephosphorylation, and cdc25C phosphatase phosphorylation, supporting mitotic arrest. Sensitization to paclitaxel-induced apoptosis was also achieved by p53-siRNA transfection in wild type p53 H460 cells. Pifithrin-α did not enhance the apoptotic response after p53 down-regulation. The results support a protective role of the transcriptional activity of p53 in response to mitotic spindle damage. The inhibition of transcriptional activity of p53 may have therapeutic implications in the treatment of p53 wild type tumors with antimitotic agents.
Introduction
p53 function is a transcription factor, and it induces the expression of genes involved in cell cycle control, DNA repair, and apoptosis [1–4]. The p53 tumor suppressor plays a central role in the response to diverse stress stimuli [1–5]. Cell fate after activation of p53 depends on the biologic context and on the nature of response to the cytotoxic stress. P21WAF1, a p53 target protein, has been implicated as a major determinant of cell fate under stress conditions (e.g., DNA damage), because it participates in both G1 and G2 checkpoints. p53 can be activated also in response to mitotic spindle damage [6–11]. Checkpoint mechanisms play a critical role in controlling cell cycle progression and DNA repair under stress conditions, such as exposure to cytotoxic agents. Therefore, cell cycle checkpoint function is considered to be an important determinant of cellular sensitivity to various antitumor agents.
The mutation of p53 is a frequent event in most tumors (∼50%) resulting in the inactivation of its transactivating function. p53 mutations may affect the apoptotic response to some p53-activating agents such as DNA-damaging agents (e.g., platinum compounds and alkylating agents) [12,13]. However, the influence of p53 status is likely dependent on the nature of cytotoxic stress, because wild type p53 also has a protective function through the activation of p53-dependent checkpoints [14]. Indeed, we have found that ovarian carcinoma cells selected for resistance to cisplatin and characterized by mutational inactivation of p53 are hypersensitive to paclitaxel [15]. In ovarian carcinoma cells with mutant p53, paclitaxel treatment caused apoptosis after progressing to a G1-like status and DNA endoreduplication [16].
The present study is an additional attempt to analyze the impact of p53 function on cellular response to antimicrotubule agents with the use of cyclic pifithrin-α (PFT), a chemical inhibitor of p53 [17,18]. The results indicate that treatment with the PFT analog in combination with various antimicrotubule agents enforced cell accumulation in mitosis, resulting in a dramatic increase of apoptosis without evidence of progression to a G1-like status. The available results support that the transcriptional activity of p53 has a protective role in response to mitotic spindle damage.
Materials and Methods
Cell Culture and Drugs
The human ovarian carcinoma cell line IGROV-1 and the cisplatin-resistant subline IGROV-1/Pt1, selected by exposure to increasing drug concentration [12], the human lung adenocarcinoma cell line H460, the human prostate cell line PC3, and the human osteosarcoma cell line SAOS were grown in RPMI 1640 (Lonza, Vierviers, Belgium) containing 10% fetal bovine serum (Life Technologies, Inc., Gaithersburg, MD) in 5% CO2 at 37°C. Human normal embryonic lung fibroblasts were kindly provided by E. Prosperi (University of Pavia, Italy) and grown in Eagle's minimum essential medium (Lonza) supplemented with 10% fetal bovine serum (Life Technologies). Paclitaxel (Indena, Milan, Italy), thiocolchicine, and PFT (Calbiochem EMD Chemicals, Inc., La Jolla, CA) were dissolved in dimethyl sulfoxide (DMSO); vinorelbine (Navelbine, Pierre Fabre, Castres, France) was dissolved in H2O before use. In all experiments, cells were exposed to PFT 2 hours before treatment with antimicrotubule agents. The highest final concentration of DMSO in culture medium was 0.5%.
Antiproliferative Activity
Cells were seeded in duplicate into six-well plates. After 24 hours, cells were exposed to the cyclic form of PFT and to antimicrotubule agents or to both simultaneously for 72 hours. After treatment, adherent cells were trypsinized and counted by a cell counter (Coulter Electronics, Luton, UK) [19]. Analysis of drug interaction was done by a modified method of Drewinko et al. [20]: Drewinko index >1 indicates greater than additive effects (synergism; the higher the value, the greater the degree of synergy), Drewinko index =1 indicates additivity, and Drewinko index <1 indicates antagonism. In all experiments, control cells were treated with the solvent at the same concentration used in drug-exposed cells. When indicated, the method described by Chou and Talalay [21] was also applied.
Cell Cycle, Apoptosis, and CPP32 Activity
For cell cycle analysis, cells were trypsinized, fixed in 70% ethanol, stained in phosphate-buffered saline (PBS) containing 10 µg/ml propidium iodide (PI; Sigma, St. Louis, MO) and RNase A (66 U/ml; Sigma) for 18 hours, analyzed by FACScan flow cytometer (Becton Dickinson, Mountain View, CA). For apoptosis detection, adherent and floating cells were harvested and analyzed for DNA fragmentation by terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) assay (Roche, Mannheim, Germany) according to the manufacturer's recommendation. Apoptosis was assessed by flow cytometer, and the results were analyzed using the CellQuest software (Becton Dickinson). For the analysis of CPP32 activity (Oncogene Sciences, Uniondale, NY), adherent and floating cells were harvested and processed as described by the manufacturer. After washing, cells were resuspended in wash buffer, the CPP32 activity was detected by flow cytometer (FACScan), and the results were analyzed using the CellQuest software.
Western Blot Analysis
Adherent and floating cells were lysed in hot sodium dodecyl sulfate (SDS) sample buffer, and whole-cell lysates were prepared for SDS-polyacrylamide gel electrophoresis (PAGE): 0.125 M Tris-HCl (pH 6.8), 5% SDS, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml pepstatin, 12.5 µg/ml leupeptin, 100 Komberg international units of aprotinin, 1 mM sodium orthovanadate. Samples were separated by SDS-PAGE and were transferred onto nitro-cellulose filters. Blots were preblocked for 2 hours at room temperature in PBS containing 5% (w/v) dried nonfat milk. Filters were incubated overnight at 4°C with antibody anti-mitotic protein monoclonal 2 (MPM-2) and -Polo-like kinase 1 (Plk1; Upstate Biotechnology, Lake Placid, NY); anti-Raf-1 (E-10, sc-7267), -cyclin B1 (GNS1, sc-245), -cdc-25C (C-20, sc-327), -SNK/Plk2 (H-90, sc-25421), and -cyclin A (BF683, SC-239) (all from Santa Cruz Biotechnology, Santa Cruz, CA); anti-Bcl-2 and -p53 (Dako, Glostrup, Denmark); anti-p21 (Neomarker, Union City, CA); anti-PARP-1 (Oncogene Sciences); anti-cytochrome C and -Rb (BD Pharmingen, Becton Dickinson); anti-cleaved caspase 3 and anti-phospho-AKT (Ser473; Cell Signaling Technology, Beverly, MA); anti-cdc2 (Tyr15-phospho-specific; New England BioLabs, Beverly, MA); anti-actin and -β-tubulin (Sigma); anti-HSP70 mitochondrial (Abcam, Ltd., Cambridge, UK); and anti-PKBa/AKT (Transduction Laboratories, Lexington, NY).
Tubulin Polymerization Assay
Cells were seeded at a density of 1 x 106 cells in 10-cm Petri dishes. The day after, cells were exposed to paclitaxel/PFT combined treatment and, 24 hours later, were processed for the tubulin polymerization assay. Samples were prepared as described by Blagosklonny et al. [22] and Cassinelli et al. [16] and separated by SDS-PAGE (10% resolving gel and 3% stacking gel), and tubulin distribution was analyzed by immunoblot analysis using a mouse anti-β-tubulin antibody (Sigma).
Analysis of Cytochrome C Release and Subcellular Fractionation
H460 and IGROV-1 cells were exposed to paclitaxel (IC50 or IC80) alone or combined with the cyclic form of PFT. Cells were washed in PBS added with 0.1 mM ice-cold sodium orthovanadate and then incubated in permeabilization buffer (20 mM HEPESKOH, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 250 mM sucrose, 0.5 mM phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, 10 µg/ml aprotinin, and 10 µg/ml trypsin inhibitor) for 15 minutes in ice. Cells were homogenized using a glass Dounce and pestle for approximately 50 strokes. After two centrifugation cycles at 3000 rpm for 10 minutes, the supernatants were again centrifuged at 14,000 rpm for 30 minutes. Cytosolic fraction was analyzed by immunoblot analysis.
Immunofluorescence Analysis
H460 and IGROV-1 cell lines were treated with either PFT or paclitaxel alone or with their combination for 24 hours. After washing with PBS, the cells were fixed in 2% paraformaldehyde for 30 minutes and permeabilized with ice-cold methanol for 20 minutes at -20°C. After blocking with PBA (PBS containing 1% bovine serum albumin), anti-p53 antibody (DO7; Dako) was incubated for 1 hour at room temperature followed by incubation with Alexa Fluor 488 goat antimouse IgG secondary antibody (Molecular Probes, Eugene, OR) for 1 hour. Images were observed and analyzed by confocal microscopy (Microradiance 2000; Bio-Rad Laboratories, Inc., Hercules, CA) equipped with Kr/Ar (488 nm), HeNe (543 nm), and diode (638 nm) lasers. Images (525 x 525 pixels) were obtained using a x60 oil immersion lens and were analyzed using Image-Pro Plus software (Version 6.0; Media Cybernetics, Inc., Bethesda, MD). Reported images represent a single Z-section of the samples (23–51 stacks), with 0.48-µm step. The pinhole diameter was regulated according to the value suggested by the acquisition software to obtain the maximum resolution power.
Small Interfering RNA Transfection
Small interfering RNAs were synthesized by Invitrogen Corp. (Carlsbad, CA). The p53-siRNA consisted of a mixture of two siRNA duplexes targeting for a different region of the p53 mRNA (Validated stealth RNAi DuoPak). A pool of two nontargeting siRNA duplexes was used as a negative control medium GC duplex (Invitrogen). Transfection of cells with siRNA duplexes was performed using Lipofectamine 2000 Reagent (Invitrogen). H460 were transfected with control siRNA or p53-siRNA at a final concentration of 100 nM for 24 hours. After silencing, cells were replated and treated on the second day with PFT for 2 hours and, after that, with paclitaxel, as indicated. Gene silencing effects were evaluated by Western blot also on the day of the treatment.
Results
Cell Growth Inhibition
The effects of the combination of the p53 inhibitor of cyclic PFT and antimicrotubule agents were investigated in two human cancer cell lines with wild type p53 (IGROV-1 and H460), in a cisplatin-resistant subline (IGROV-1/Pt) characterized by a mutant p53 and in two p53 defective cell lines (PC3 prostate carcinoma and SAOS osteosarcoma). Cells were pretreated for 2 hours with subtoxic PFT concentrations (in the range of IC10–IC30) followed by a simultaneous 72-hour exposure to the antimitotic agent (paclitaxel, vinorelbine, or thiocolchicine; Figure 1). The IGROV-1 cells were less sensitive to the antimitotic agents than the H460 cell (IC50 values for paclitaxel 0.12 ± 0.02 and 0.03 ± 0.004 µM, respectively). In contrast, IGROV-1 cells, characterized by a higher level of p53 expression, were more sensitive to the antiproliferative effects of PFT. The pattern of sensitivity of these cell lines was apparently consistent with the level of p53 protein. Dose-response curves of various anti-microtubule agents in combination with subtoxic concentrations of PFTrevealed a supraadditive effect in both IGROV-1 and H460 cells (Figure 1, A and B). The combination of PFTwas synergic with both microtubule-stabilizing and -destabilizing drugs. The synergistic interaction was more marked in the H460 cell line. The analysis of drug interaction according to the method of Drewinko et al. [20] and to that of Chou and Talalay [21] confirmed that the antimitotic agents/PFT combination was synergic. Figure 1C shows the analysis of interaction for paclitaxel and PFT, according to the method of Chou and Talalay [21]. On the contrary, PFT did not affect the dose-response curve of paclitaxel in a cisplatin-resistant subline (IGROV-1/Pt) carrying the mutant p53, in prostate PC3 cells, and in osteosarcoma SAOS cells characterized by the loss of p53 expression (Figure 1D), suggesting that the sensitization observed in IGROV-1 or in H460 cells was related to the inhibition of the functional p53 by PFT. To better support this interpretation, we studied the effect of paclitaxel in H460 cells after transfection with p53-siRNA (Figure 1E). p53 silencing substantially reduced the accumulation of the p53 protein caused by paclitaxel treatment and resulted in a sensitization to paclitaxel comparable to the effect of PFT/paclitaxel combination in control cells. Under the same treatment conditions, PFT produced only a marginal sensitization to paclitaxel of "normal" human fibroblasts (Figure 1F).
Figure 1.
Antiproliferative effects of paclitaxel (PTX), vinorelbine (VIN), or thiocolchicine (TIO) in H460 (A); IGROV-1 (B); IGROV/Pt1, PC3, SAOS (D) and human normal fibroblasts (F) in the absence (■) or presence of 20 µM (●), 10 µM (○), and 2 µM (◯) cyclic PFT. Cells were pretreated with the indicated concentrations of PFT for 2 hours before the combined treatment for 72 hours. Pifithrin-α was used at two subtoxic concentrations (i.e., 2 µM PFT was equivalent to IC15 in IGROV-1 cells; 10 µM PFT was equivalent to IC10 in H460 and SAOS and to IC20 in IGROV-1, IGROV-1/Pt, human fibroblasts and PC3; 20 µM PFT was equivalent to IC20 in H460 and SAOS and to IC30 in IGROV-1/Pt and PC3). (E) Effects of paclitaxel (■) and its combination with PFT 10 µM (▼) in H460-negative control cells and effects of paclitaxel in p53-siRNA-transfected cells (□). The p53 expression analysis is also shown. The negative control cells are referred to cells transfected with RNAi-negative control duplex containing 48% GC. Cell transfection was performed as indicated in the Materials and Methods section. (Inset C) combination index calculated according to the Chou and Talalay analysis [21] for paclitaxel/PFT interaction.
Cell Cycle Analysis
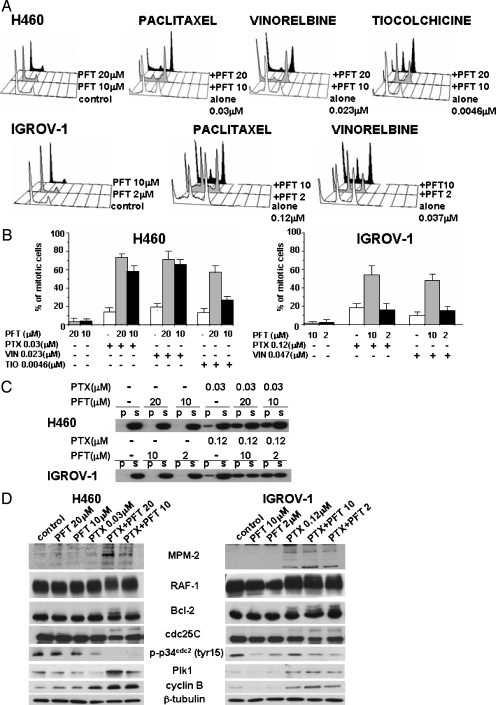
Because induction of mitotic arrest is a hallmark of microtubule inhibitors, we examined the cell cycle perturbation of H460 and IGROV-1 cells exposed to paclitaxel, vinorelbine, thiocolchicine (IC50), or to their combination with PFT. Whereas PFT itself did not induce modifications of the cell cycle distribution of H460 cells, at the concentrations used, the antimicrotubule agents caused only inhibition of cell proliferation with a moderate increase of cells in G2/M. In spite of this behavior, H460 cells exhibited a normal mitotic spindle checkpoint, because higher concentrations (IC80) caused mitotic arrest (not shown). A similar behavior has been described in other cell lines [23,24]. Pretreatment with PFT and 24 hours of simultaneous treatment with a low concentration of the antimicrotubule drug caused a cell accumulation in G2/M in H460 cells (Figure 2A). In IGROV-1 cells, paclitaxel alone caused an evident increase in the number of cells in the G2/M phase (Figure 2A). The accumulation of cells in the G2/M phase was enhanced with the PFT combined treatment in a dose-dependent manner. In particular, treatment with paclitaxel alone resulted in 50% cell accumulation in G2/M, and the combination with PFT (10 µM) enhanced the number of G2/M phase cells to 80%. The combination induced a marked increase of sub-G1 fraction in both cell lines. At drug concentrations that produced a low (<20%) extent of mitosis in wild type p53 cells, the addition of PFT resulted in a marked increase of mitotic cells as determined by the analysis with PI staining (Figure 2B). Again, the effect of combined treatment on the cell cycle was more evident in H460 cells that were also more responsive to the drug combination.
Figure 2.
Cell cycle perturbation and analysis of mitotic arrest and of mitotic biochemical markers in cells pretreated for 2 hours with PFT followed by simultaneous 24-hour exposure to antimicrotubule agents (IC50). (A) Cell cycle distribution of H460 and IGROV-1. The representative of at least three independent experiments is shown. (B) Mitotic index in PI-stained H460 and in IGROV-1 cells determined by fluorescence microscope analysis. The results are expressed as a percentage of the total cell population and are the mean ± SD of three independent experiments. (C) Analysis of the tubulin polymerization in H460 and in IGROV-1 cells. Cell fractionation was performed as indicated in the Materials and Methods section. Soluble cytosolic (S) or polymerized (P) tubulin was detected in cell fractions by Western blot analysis after 24 hours of exposure. (D) Activation of mitosis-related factors in H460 and IGROV-1 cells. Western blot analysis of cell lysates was performed at the end of treatment (24 hours). β-Tubulin is shown as a control for protein loading.
Tubulin Polymerization
To investigate the drug effects on the microtubule system, we compared cells treated with paclitaxel (IC50) or with PFT combination for 24 hours (Figure 2C). In H460 and IGROV-1 cells, the paclitaxel/PFT combination enhanced the tubulin polymerization induced by paclitaxel. This effect is consistent with the increase of mitotic cells.
Modulation of Biochemical Markers of Mitotic Arrest
To gain further insight into the mechanism of cell arrest in the M phase, we performed additional investigation with PFT/paclitaxel combination. In cells exposed for 24 hours to the paclitaxel/PFT combination, the mitotic arrest was confirmed by the increase of mitosis-specific phosphorylated epitopes recognized by MPM-2 antibody (Figure 2D). This enhancement was less evident in IGROV-1 cells, because paclitaxel alone produced an up-regulation of phosphoproteins in keeping with the accumulation of cells in the M phase.
A hyperphosphorylation of Raf-1 and Bcl-2 is a biochemical hallmark of the cellular response to microtubule inhibitors, because these events are associated with the M phase [22,25]. In H460 cells, the PFT/paclitaxel combination treatment for 24 hours induced a parallel mobility shift of Bcl-2 and Raf-1 in SDS-PAGE, thereby supporting a drug-induced phosphorylation of the two proteins (Figure 2D). In addition, the combined treatment induced dephosphorylation of p34cdc2 kinase (at Tyr15), phosphorylation of cdc25C phosphatase, and up-regulation of cyclin B1. In IGROV-1 cells, no change in the phosphorylation of Raf-1 and Bcl-2 was detected between the treatment with paclitaxel alone or with the combination. However, after the combination treatment, cells showed a reduction of the phosphorylation of p34cdc2 kinase and an increase of phosphorylation of cdc25C. Pifithrin-α itself caused a down-regulation of the phosphorylation of p34cdc2, most evident at 10 µM. Moreover, paclitaxel combined with the higher dose of PFT induced an increase of the cyclin B1 protein levels. This event supported that PFT induced in paclitaxel-treated cells a mitotic arrest without progression to the G1-like phase.
The activation of Plk1 (a member of the Polo-like kinase family) is implicated in various processes that contribute to the activation of cyclin B/p34cdc2 complex [26]. After 24 hours of exposure, in H460 cells paclitaxel alone did not modify the Plk1 expression, whereas the PFT/paclitaxel combination caused a substantial up-regulation of Plk1. In IGROV-1 cells, paclitaxel alone caused a moderate increase of Plk1 protein, in keeping with the appearance of G2/M peak, MPM-2 reactivity, and phosphorylation of Raf-1 and Bcl-2. The PFT combination produced only a moderate increase of Plk1 expression.
Plk2, another member of the Polo-like kinase family activated during the DNA damage checkpoint in G2 phases, has been reported to be a p53 target, and the p53-dependent activation of Snk/Plk2 prevents mitotic catastrophe after spindle damage [8]. However, no modulation of Plk2 could be detected in H460 and in IGROV-1 cells in our experimental conditions (data not shown).
Apoptotis Induction and Caspase Activation
We tested the hypothesis that the sensitization effect of PFT was related to an enhanced cell susceptibility to antimicrotubule agent- induced apoptosis. The CPP32 activity assay and the TUNEL assay for the analysis of apoptotic response were performed in cells exposed to low concentrations of the antimicrotubule agents (paclitaxel, vinorelbine, or thiocolchicine), which caused only antiproliferative effects, or to their combination with subtoxic PFT concentrations for 72 hours. In both H460 and IGROV-1 cells, the FACScan analysis of CPP32-positive cells supported the increase in the activation of CPP32 after 72 hours of combined treatments (Figure 3A). Indeed, PFT induced a remarkable increase of apoptosis in paclitaxel-, vinorelbine-, or thiocolchicine-treated cells (Figure 3B). A sensitization to paclitaxel-induced apoptosis was not observed in “normal” human fibroblasts (Figure 3B), thus supporting a low susceptibility to apoptosis of normal cells.
Figure 3.
Induction of apoptosis and analysis of apoptosis-related factors in H460 and in IGROV-1 cells exposed to the IC50 paclitaxel (PTX), vinorelbine (VIN), thiocolchicine (TIO), cyclic PFT, or their combination. Cells were pretreated with PFT for 2 hours and then exposed to the antimicrotubule agent. (A) Caspase 3 (CPP32) activity after 72 hours of treatment determined with the colorimetric assay kit (Calbiochem) according to the manufacturer's protocol and analyzed by FACScan. The results, expressed as percentage of CPP32 activity-positive cells, are the mean ± SD of two independent experiments. (B) Apoptosis determined by TUNEL assay and FACScan analysis after 72 hours of treatment. The percentages of TUNEL-positive cells are indicated in each panel. Representative of at least three independent experiments is shown. (C) Release of cytochrome C into the cytosol. The cytosolic extracts were prepared after 24 hours of treatment with paclitaxel alone or combined with PFT. An antibody against the mitochondrial marker HSP70-mitochondrial was used as protein control to ensure the subcellular fractionation process. As a control for HSP70-mitochondrial antibody, a mitochondrial extract containing HSP70 (ctr+) is shown. (D) Cleavage of CPP32 and PARP-1. Western blot analysis with specific antibodies was performed after 24 hours of treatment. β-Tubulin is shown as a control of protein loading. The arrows indicate the cleavage products. (E) Caspase 3 activation and PARP fragmentation in response to paclitaxel (24 hours of exposure) after p53 down-regulation in p53-siRNA-transfected H460 cells.
Because proapoptotic stimuli induced by mitotic spindle damage involved mitochondrial pathway, we performed Western blot analysis of the release of cytochrome C in H460 and IGROV-1 cells at 24 hours after treatment (Figure 3C). Compared with the effect of paclitaxel alone, an increased release of cytochrome C at 24 hours was found in the combined treatment in a PFT dose-dependent manner. According to the release of cytochrome C after 24 hours of combined treatment, the apoptosis activation was further supported by Western blot analysis of caspase cleavage (Figure 3D). Barely detectable caspase activation was induced by the two drugs given alone. In contrast, in cells treated with a drug combination, a marked cleavage of caspase 3 was associated with the cleavage of the caspase substrate PARP-1, thus confirming an enhancement of the caspase-dependent apoptotic pathways. The synergistic activation of caspase, already observed at 24 hours after treatment, was more marked after 48 hours. The release of cytochrome C and caspase 3 activation were concomitant with the appearance of mitotic cells. Because under the same conditions approximately 80% of the treated cells are arrested in mitosis (Figure 2, A and B), this finding supports the interpretation that cells treated with the combination underwent apoptosis during mitosis.
In contrast to control H460 cells, the p53-siRNA-transfected cells was able to undergo apoptosis after 24 hours of exposure to a low dose of paclitaxel (0.03 µM), thus supporting the proposed protective role of p53 (Figure 3E). As expected, as a consequence of suppression of the p53 expression, this effect was not enhanced by the addition of PFT under these conditions.
Expression of p53 and p21WAF1/Cip1
The expression level of p53 and p21WAF1/Cip1 was investigated by Western blot analysis 24 hours after drug treatment. As shown in Figure 4A, paclitaxel determined a marked increase of p53 and p21 expressions in both IGROV-1 and H460 cells. Pifithrin-α had no effect on paclitaxel-induced p53 activation but had reduced the up-regulation of p21 induced by paclitaxel, likely because of the inhibition of p53 transcriptional activity. At the used subtoxic concentrations, PFT itself was unable to modify the basal expression of p21. As expected, a marginal or no induction of p21 was detected in H460 cells after p53 down-regulation with p53-siRNA (Figure 4B). Analysis by confocal microscopy revealed an increased amount of p53 in the nuclei of cells exposed to a low paclitaxel concentration (IC50; not shown). In contrast, the combination of PFT with paclitaxel resulted in a widespread distribution of p53 in the cytoplasm.
Figure 4.
Expression of p53 and p21waf1 in H460, IGROV-1 cells (A) and H460 cells transfected with RNAi-negative control duplex or with p53-siRNA (B) treated for 24 hours with paclitaxel (IC50, PTX) or cyclic PFT alone or their combination. In the combined treatment, PFT were added 2 hours before treatment with PTX. (A) Whole-cell extracts were prepared and analyzed by Western blot analysis. β-Tubulin is shown as a control of protein loading. For p21WAF1, low and high film exposures are shown.
Modulation of AKT
Because AKT is involved in the cellular response to taxanes [16,27], we examined the state of activation of this kinase in H460 and IGROV-1 cells treated with paclitaxel alone or with PFT combination for 24 hours (Figure 5). In both cell lines, AKT phosphorylation at Ser473 was marginally modulated by paclitaxel or PFT alone. On the contrary, the combination of paclitaxel with PFT resulted in a substantial decrease of AKT phosphorylation in H460 or IGROV-1 cells, associated with a down-regulation of AKT protein itself.
Figure 5.
Down-regulation of AKT and inhibition of AKT phosphorylation (Ser473) detected by Western blot analysis in H460 and IGROV-1 cells exposed to paclitaxel (IC50) and cyclic PFT in single or combined treatment. Cells were pretreated for 2 hours with PFT followed by simultaneous 24-hour exposure to the antimicrotubule agent. Filters were stripped and reprobed with the antibody recognizing AKT protein or β-tubulin to control specific or overall protein loading.
Discussion
In this study, we have found that the inhibition of p53 transcriptional function by a specific chemical inhibitor, cyclic PFT, resulted in a marked sensitization to antimitotic agent-induced apoptosis of human tumor cells, carrying functional wild type p53. The sensitization in the combined treatment was produced by all the tested antimicrotubule agents, including both microtubule-stabilizing drugs (taxanes) and compounds that inhibit tubulin assembly (e.g., vinca alkaloids), and occurred at concentrations that caused only antiproliferative effects. Thus, subtoxic concentrations of the p53 inhibitor converted the growth-inhibitory effect of the antimicrotubule agent in a cytotoxic outcome as evidenced by a dramatic enhancement of the apoptotic response. Sensitization to paclitaxel was also found in wild type p53 cells after suppression of the p53 expression by siRNA. No or only a marginal sensitization by PFT was detected in cells characterized by defective p53 or after down-regulation of p53 by siRNA, supporting that sensitization to drug-induced apoptosis by PFT was closely related to the modulation of p53 functions, which could play a protective role. Our results are consistent with the observation that activation of p53 by MDM2 antagonists provides protection from cytotoxicity of paclitaxel [28].
The two wild type p53 cell lines were characterized by a somewhat different response to antimicrotubule agents in terms of perturbation of cell cycle progression. Indeed, the treatment with low concentrations of antimitotic agents causing only antiproliferative effects resulted in a G2/M phase accumulation that was moderate in H460 cells but substantial in IGROV-1 cells. Under these conditions, no appreciable induction of apoptosis could be detected in both cell lines, as indicated by the lack of release of cytochrome C and of the caspase activation. In contrast, the combination of the microtubule inhibitor with PFT enhanced the accumulation of cells in the M phase, with a marked increase of apoptosis in both cell lines.
Low paclitaxel concentrations may cause inhibition of cell proliferation without mitotic arrest, because under these conditions, the drug may induce both p53 and p21 causing G1 and G2 arrests [23,24]. A similar behavior was observed in H460 cells, exhibiting a partial delay in G1 and a low accumulation in late G2, and in IGROV-1 cells, exhibiting a more evident G2/M arrest. Pifithrin-α abolished in both cell lines the paclitaxel-induced up-regulation of p21 after the activation of p53. This event reflected the inhibition of the transcriptional activity of p53, because p21 is a gene target of p53. The implication of p21 in response to paclitaxel is consistent with a parallel sensitization produced by p53 down-regulation in p53-siRNA-transfected cells. Down-regulation of p21 is expected to allow the activation of the cyclin B/p34cdc2 complex [29–31], which is inactive in the G2 phase. Activation of the complex, indicated by the reduced phosphorylation of p34cdc2 at Tyr15, should allow a G2 → transition. However, a number of factors are known to be involved in regulating mitotic entry. In particular, the activation of Plk1 is expected to promote the activation of cyclin B/p34cdc2 complex, because it is implicated in the phosphorylation and activation of cdc25C, a phosphatase involved in the dephosphorylation of p34cdc2 at Tyr15 [26,32]. Moreover, Plk1 regulates the degradation of Wee1 (implicated in the G2 checkpoint) after phosphorylation [26]. Indeed, under our conditions, down-regulation of p21 in cells treated with PFT/paclitaxel combination was associated with the activation of p34cdc2/cyclin B complex, an increase of cdc25C phosphorylation, and up-regulation of Plk1, thus supporting a suppression of G2 checkpoint and a facilitation of G2/M transition [26]. A protective role of p21 against cytotoxicity of paclitaxel has been described [31,33,34], but the cellular and molecular bases of this protection are not clearly defined. Besides its involvement in multiple cell cycle checkpoints, p21 could confer resistance to antimicrotubule agents by a decrease of mitotic arrest and exit from abnormal mitosis [33,35,36]. In addition, p21 is implicated in the AKT-dependent survival pathway, because cytoplasmic accumulation of p21 in paclitaxel-treated cells could be regulated by an AKT-dependent phosphorylation [37]. In our study, the down-regulation of p21 induced by PFT in paclitaxel-treated cells (Figure 4) was associated with the release of cytochrome C and the activation of caspase 3 after 24 hours of combined treatment (Figure 3, C and D). Because p21 has been implicated in the inhibition of the caspase activation process [38], the observed down-regulation may also have a proapoptotic effect.
The observed sensitization of mitotic cells by PFT could reflect at least in part a prevalence of apoptotic signals likely activated in a p53-independent manner. In particular, although a direct involvement of p53 in the modulation of the AKT-dependent survival pathway remains to be documented in our cell systems, the available results showed an inhibition of both up-regulation and phosphorylation of AKT induced by paclitaxel. Because a protective role of AKT against taxane-induced apoptosis has been described in other cell systems [27], it is conceivable that inactivation of AKT-mediated survival pathway may favor a cell death process.
In conclusion, the present observations of the sensitization to antimicrotubule agents by a transcriptional inhibitor of p53 are consistent with multiple and sometimes opposite functions of p53, depending on the nature of cytotoxic stress. The available evidence supports that, in response to mitotic spindle damage, the transcription-mediated functions of p53 have a protective action. However, after inhibition of the transcriptional activity, p53 could retain proapoptotic functions that might be prevalent over antiapoptotic signals. The sensitization of tumor cells with wild type p53 by selective inhibition of p53 function described in this study may have obvious implications in the antitumor therapy with antimitotic agents. Indeed, it supports the potential efficacy of regimens containing low (and less toxic) doses of antimitotic agents by combination with a nontoxic modulating agent. The use of rational combinations could improve the efficacy of protracted treatment regimens with low doses of taxanes that are known to have anti-angiogenic and antitumor effects [39]. The use of p53 inhibitors has been proposed as a chemoprotective strategy to reduce the side effects caused by chemotherapy or radiotherapy or by other stress conditions implicating the activation of p53 [17]. The present study supports the potential use of PFT as a chemosensitizing agent in combination with microtubule inhibitors. In contrast to the protective effect of PFT against toxicity of DNA-damaging agents, the combination with antimicrotubule drugs could enhance toxicity against normal tissue. The potential therapeutic advantages of the combination of p53 inhibitors with antimicrotubule agents remain to be documented in in vivo models, for improvement of the “therapeutic index.” However, it should be emphasized that tumor cells seem to be more susceptible to apoptotic death than nonmalignant cells, as supported by a lack of sensitization to paclitaxel-induced apoptosis in human “normal” fibroblasts (Figure 3B).
Abbreviations
- CPP32
caspase 3
- DMSO
dimethyl sulfoxide
- MPM-2
mitotic protein monoclonal-2
- PBS
phosphate-buffered saline
- PFT
pifithrin-α
- PI
propidium iodide
- Plk1
Polo-like kinase 1
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- TUNEL
terminal deoxynucleotidyl transferase-mediated nick end labeling
Footnotes
This work was partially supported by the Associazione Italiana per la Ricerca sul Cancro, Milan, by the Ministero della Salute, Rome, by the Fondazione Italo Monzino, Milan, Italy.
References
- 1.Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis—the p53 network. J Cell Sci. 2003;116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 2.Römer L, Klein C, Dehner A, Kessler H, Buchner J. p53—A natural cancer killer: structural insights and therapeutic concepts. Angew Chem Int Ed Engl. 2006;45:6440–6460. doi: 10.1002/anie.200600611. [DOI] [PubMed] [Google Scholar]
- 3.Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 4.Alimirah F, Panchanathan R, Chen J, Zhang X, Ho S-M, Choubey D. Expression of androgen receptor is negatively regulated by p53. Neoplasia. 2007;9:1152–1159. doi: 10.1593/neo.07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alimirah F, Panchanathan R, Davis FJ, Chen J, Choubey D. Restoration of p53 expression in human cancer cell lines upregulates the expression of notch: implications for cancer cell fate determination after genotoxic stress. Neoplasia. 2007;9:427–434. doi: 10.1593/neo.07211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meek DW. The role of p53 in the response to mitotic spindle damage. Pathol Biol. 2000;48:246–254. [PubMed] [Google Scholar]
- 7.Tarapore P, Fukasawa K. Loss of p53 and centrosome hyperamplification. Oncogene. 2002;21:6234–6240. doi: 10.1038/sj.onc.1205707. [DOI] [PubMed] [Google Scholar]
- 8.Burns TF, Fei P, Scata KA, Dicker DT, El-Deiry WS. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells. Mol Cell Biol. 2003;23:5556–5571. doi: 10.1128/MCB.23.16.5556-5571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castedo M, Perfettini J-L, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 10.Vogel C, Kienitz A, Hofmann I, Müller R, Bastians H. Crosstalk of the mitotic spindle assembly checkpoint with p53 to prevent polyploidy. Oncogene. 2004;23:6845–6853. doi: 10.1038/sj.onc.1207860. [DOI] [PubMed] [Google Scholar]
- 11.Blagosklonny MV. Prolonged mitosis versus tetraploid checkpoint. Cell Cycle. 2006;5:971–975. doi: 10.4161/cc.5.9.2711. [DOI] [PubMed] [Google Scholar]
- 12.Perego P, Giarola M, Righetti SC, Supino R, Caserini C, Delia D, Pierotti MA, Miyashita T, Reed JC, Zunino F. Association between cisplatin resistance and mutation of p53 gene and reduced bax expression in ovarian carcinoma cell systems. Cancer Res. 1996;56:556–562. [PubMed] [Google Scholar]
- 13.Weller M. Predicting response to cancer chemotherapy: the role of p53. Cell Tissue Res. 1998;292:435–445. doi: 10.1007/s004410051072. [DOI] [PubMed] [Google Scholar]
- 14.Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vegelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perego P, Romanelli S, Carenini N, Magnani I, Leone R, Bonetti A, Paolicchi A, Zunino F. Ovarian cancer cisplatin-resistant cell lines: multiple changes including collateral sensitivity to paclitaxel. Ann Oncol. 1998;9:423–430. doi: 10.1023/a:1008265012435. [DOI] [PubMed] [Google Scholar]
- 16.Cassinelli G, Supino R, Perego P, Polizzi D, Lanzi C, Pratesi G, Zunino F. A role for loss of p53 function in sensitivity of ovarian carcinoma cells to taxanes. Int J Cancer. 2001;92:738–747. doi: 10.1002/1097-0215(20010601)92:5<738::aid-ijc1249>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Gudkov AV, Komarova EA. Prospective therapeutic applications of p53 inhibitors. Biochem Biophys Res Commun. 2005;331:726–736. doi: 10.1016/j.bbrc.2005.03.153. [DOI] [PubMed] [Google Scholar]
- 18.Barchechat SD, Tawatao RI, Corr M, Carson DA, Cottam HB. Inhibitors of apoptosis in lymphocytes: synthesis and biological evaluation of compounds related to pifithrin-α. J Med Chem. 2005;48:6409–6422. doi: 10.1021/jm0502034. [DOI] [PubMed] [Google Scholar]
- 19.Zuco V, Zanchi C, Lanzi C, Beretta GL, Supino R, Pisano C, Barbarino M, Zanier R, Bucci F, Aulicino C, et al. Development of resistance to the atypical retinoid, ST1926, in the lung carcinoma cell line H460 is associated with reduced formation of DNA strand breaks and a defective DNA damage response. Neoplasia. 2005;7:667–677. doi: 10.1593/neo.05127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drewinko B, Loo TL, Brown JA, Gottlieb JA, Freireich EJ. Combination chemotherapy in vitro with adriamycin. Observations of additive, antagonistic, and synergistic effects when used in two-drug combination on cultured human lymphoma cells. Cancer Biochem Biophys. 1976;1:187–195. [PubMed] [Google Scholar]
- 21.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 22.Blagosklonny MV, Giannakakou P, El-Deiry WS, Kingston DG, Higgs PI, Neckers L, Fojo T. Raf-1/bcl-2 phosphorylation: a step from microtubule damage to cell death. Cancer Res. 1997;57:130–135. [PubMed] [Google Scholar]
- 23.Giannakakou P, Robey R, Fojo T, Blagosklonny MV. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel-induced cytotoxicity. Oncogene. 2001;20:3806–3813. doi: 10.1038/sj.onc.1204487. [DOI] [PubMed] [Google Scholar]
- 24.Panno ML, Giordano F, Mastroianni F, Morelli C, Brunelli E, Palma MG, Pellegrino M, Aquila S, Miglietta A, Mauro L, et al. Evidence that low doses of taxol enhance the functional transactivatory properties of p53 on p21 waf promoter in MCF-7 breast cancer cells. FEBS Letters. 2006;580:2371–2380. doi: 10.1016/j.febslet.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 25.Ling Y-H, Tornos C, Perez-Soler R. Phosphorylation of Bcl-2 is a marker of M phase events and not a determinant of apoptosis. J Biol Chem. 1998;273:18984–18991. doi: 10.1074/jbc.273.30.18984. [DOI] [PubMed] [Google Scholar]
- 26.Van Vugt MATM, Modema RH. Getting in and out of mitosis with polo-like kinase-1. Oncogene. 2005;24:2844–2859. doi: 10.1038/sj.onc.1208617. [DOI] [PubMed] [Google Scholar]
- 27.Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–9086. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- 28.Carvajal D, Tovar C, Yang H, Vu BT, Heimbrook DC, Vassilev LT. Activation of p53 by MDM2 antagonists can protect proliferating cells from mitotic inhibitors. Cancer Res. 2005;65:1918–1924. doi: 10.1158/0008-5472.CAN-04-3576. [DOI] [PubMed] [Google Scholar]
- 29.Smits VAJ, Klompmaker R, Vallenius T, Rijksen G, Makela TP, Medema RH. P21 inhibits Thr161 phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. J Biol Chem. 2000;275:30638–30643. doi: 10.1074/jbc.M005437200. [DOI] [PubMed] [Google Scholar]
- 30.Smits VAJ, Medema RH. Checking out the G2/M transition. Biochim Biophys Acta. 2001;1519:1–12. doi: 10.1016/s0167-4781(01)00204-4. [DOI] [PubMed] [Google Scholar]
- 31.Stewart ZA, Mays D, Pietenpol JA. Defective G1-S cell cycle checkpoint function sensitizes cells to microtubule inhibitor-induced apoptosis. Cancer Res. 1999;59:3831–3837. [PubMed] [Google Scholar]
- 32.Van Vugt MATM, Bras A, Medema RH. Restarting the cell cycle when the checkpoint comes to a halt. Cancer Res. 2005;65:7037–7040. doi: 10.1158/0008-5472.CAN-05-1054. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed W, Rahmani M, Dent P, Grant S. The cyclin-dependent kinase inhibitor p21 (CIP1/WAF1) blocks paclitaxel-induced G2M arrest and attenuates mitochondrial injury and apoptosis in p53-null human leukemia cells. Cell Cycle. 2004;3:1305–1311. doi: 10.4161/cc.3.10.1161. [DOI] [PubMed] [Google Scholar]
- 34.Yu D, Jing T, Liu B, Yao J, Tan M, McDonnell TJ, Hung MC. Overexpression of ErbB2 blocks taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34cdc2 kinase. Mol Cell. 1998;2:581–591. doi: 10.1016/s1097-2765(00)80157-4. [DOI] [PubMed] [Google Scholar]
- 35.Barboule N, Chadebech P, Baldin V, Vidal S, Valette A. Involvement of p21 in mitotic exit after paclitaxel treatment in MCF-7 breast adenocarcinoma cell line. Oncogene. 1997;15:2867–2875. doi: 10.1038/sj.onc.1201469. [DOI] [PubMed] [Google Scholar]
- 36.Li W, Fan J, Banerjee D, Bertino JR. Overexpression of p21waf1 decreases G2-M arrest and apoptosis induced by paclitaxel in human sarcoma cells lacking both p53 and functional Rb protein. Mol Pharmacol. 1999;55:1088–1093. doi: 10.1124/mol.55.6.1088. [DOI] [PubMed] [Google Scholar]
- 37.Heliez C, Baricault L, Barboule N, Valette A. Paclitaxel increases p21 synthesis and accumulation of its AKT-phosphorylated form in the cytoplasm of cancer cells. Oncogene. 2003;22:3260–3268. doi: 10.1038/sj.onc.1206409. [DOI] [PubMed] [Google Scholar]
- 38.Sohn D, Essmann F, Schulze-Osthoff K, Janicke RU. P21 blocks irradiation-induced apoptosis downstream of mitochondria by inhibition of cyclicdependent kinase-mediated caspase-9 activation. Cancer Res. 2006;66:11254–11262. doi: 10.1158/0008-5472.CAN-06-1569. [DOI] [PubMed] [Google Scholar]
- 39.Pasquier E, Honoré S, Braguer D. Microtubule-targeting agents in angiogenesis: where do we stand? Drug Resist Updat. 2006;9:74–86. doi: 10.1016/j.drup.2006.04.003. [DOI] [PubMed] [Google Scholar]