Abstract
The Notch signaling cascade is deregulated in diverse cancer types. Specific Notch function in cancer is dependent on the cellular context, the particular homologs expressed, and cross-talk with other signaling pathways. We have previously shown that components of the Notch signaling pathway are deregulated in meningiomas. However, the functional consequence of abnormal Notch signaling to meningiomas is unknown. Here, we report that exogenous expression of the Notch pathway effector, HES1, is associated with tetraploid cells in meningioma cell lines. Activated Notch1 and Notch2 receptors induced endogenous HES1 expression and were associated with tetraploidy in meningiomas. Tetraploid meningioma cells exhibited nuclear features of chromosomal instability and increased frequency of nuclear atypia, such as multipolar mitotic spindles and accumulation of cells with large nuclei. FACS-sorted tetraploid cells are viable but have higher rates of spontaneous apoptosis when compared with diploid cells. We have used spectral karyotyping to show that, in contrast to diploid cells, tetraploid cells develop a higher number of both numerical and structural chromosomal abnormalities. Our findings identify a novel function for the Notch signaling pathway in generating tetraploidy and contributing to chromosomal instability. We speculate that abnormal Notch signaling pathway is an initiating genetic mechanism for meningioma and potentially promotes tumor development.
Introduction
The Notch signaling pathway consists of Notch (Notch1–4 in mammals), a family of transmembrane receptors, that undergo proteolytic activation in response to ligand (Delta-like1,3,4 and Jagged1–2 in mammals) binding to release the intracellular domain of Notch [1]. The intracellular domain of Notch translocates to the nucleus and acts as a transcriptional activator inducing the expression of members of the HES (Hairy/Enhancer of Split) family of basic helix-loop-helix transcriptional regulators [2]. HES proteins in turn regulate the expression of downstream target genes [2].
Aberrant Notch signaling contributes to the genesis of diverse cancers. As the normal effect of Notch signaling during development differs from cell type to cell type, the tumorigenic effect of Notch is varied and depends on the tissue in which the tumor develops [3]. Notch functions as an oncogene promoting tumorigenesis in tissues where it normally functions to maintain stem cells or regulate precursor cell fates [4]. In T-cell leukemias, constitutively active Notch1 transforms cells in vitro [5] and cause mice to develop leukemia [6]. In contrast, Notch is a tumor suppressor in certain epithelial cancers where its normal function is to promote terminal differentiation [4]. Mice with Notch1-deficient epithelia develop spontaneous basal cell carcinoma-like tumors [7]. In yet other cancers, such as cervical cancers, the role of Notch is more complex. Notch signaling seems to provide a permissive environment for the development of early lesions, whereas progression to late-stage cervical lesions requires limiting Notch signaling [8,9]. More recently, Notch has been shown to function as a regulator of pathologic angiogenesis in cancer. In head and neck squamous cell carcinomas, activated Notch signaling in endothelial cells promote neovascularization [10].
We have previously shown that the Notch signaling pathway is deregulated in human meningioma tumors [11]. Over-expression of Notch pathway components occurs with equal frequency in benign and malignant meningiomas, suggesting that activation of this pathway may be important for tumor initiation [11]. Meningiomas are the most common primary brain tumor [12] and also develop in half of all patients who suffer from neurofibromatosis 2 [13]. These tumors are a considerable cause of morbidity and mortality because of their location in the central nervous system. Meningiomas often occur in difficult-to-access locations, and their continued growth impacts surrounding brain tissue, causing serious neurologic deficits [14,15]. In addition, 15% of meningiomas have malignant characteristics, and these aggressive tumors are frequently fatal [16]. Meningiomas arise in the arachnoid layer of the meninges [17,18], and the function of Notch signaling in the normal development of the meninges is unknown. It is therefore difficult to predict the nature of Notch function in meningioma tumor development.
The purpose of this study was to evaluate the functional consequences of Notch signaling to meningioma tumor growth. We show that exogenous expression of HES1 induces the formation of tetraploid cells associated with features of chromosomal instability in meningioma cells. Tetraploid meningioma cells have higher rates of spontaneous apoptosis and an increased frequency of generation of structural and numerical chromosomal abnormalities. Thus, aberrant Notch signaling functions in regulating ploidy and enhancing chromosomal instability in meningiomas.
Materials and Methods
Cell Lines
All human tissues were collected by the Neurological Surgery Tissue Bank at the University of California, San Francisco (UCSF) using protocols approved by the UCSF Committee on Human Research. Human meningioma cell lines used were SF3061, SF4068, and SF4433. The immortalization and characteristics of these cells have been described earlier [19]. Briefly, SF3061, derived from a malignant meningioma, has been immortalized by expression of telomerase, whereas SF4068 and SF4433, derived from benign meningiomas, have been immortalized by the expression of telomerase and the human papillomavirus E6/E7 genes. All three cell lines were positive for vimentin and desmoplakin, characteristic markers of meningiomas. These cell lines do not have increased Notch signaling compared to normal arachnoidal cells and were therefore chosen to exogenously express HES1. All cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and appropriate antibiotic selection markers.
Expression Constructs
HES1 expression construct in pReceiver was obtained from GeneCopoeia (Germantown, MD) and was transferred to the pLNCX2 retroviral vector using standard molecular biology techniques. Constructs of the activated intracellular domains of Notch1 (N1ICD) and Notch2 (N2ICD) were kind gifts from Dr. Lucio Miele (Loyola University, Chicago, IL) and Dr. Spyros Artavanis-Tsakonas (Harvard Medical School, Boston, MA), respectively. These expression constructs were transferred to the pBABE-Neo retroviral vector using standard molecular biology techniques. The HES1-promoter firefly luciferase construct was a kind gift from Dr. Lucio Miele.
Retroviral Infection and Selection of Stable Cell Populations
Retroviral supernatants were produced by transfection of the Phoenix A packaging cell line with retroviral plasmids using Lipofectamine Plus Reagent (Invitrogen, Carlsbad, CA). The 48-hour posttransfection supernatant was harvested and filtered, and polybrene (4 µg/ml) was added and used to infect meningioma cell lines. Stable cell populations were selected using 500 µg/ml of G418. In parallel, empty plasmids were used to generate vector control stable cell lines.
Western Blot Analysis
The Notch1 (bTAN20) and Notch2 (C651.6DbHN) antibodies developed by Spyros Artavanis-Tsakonas were obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA). The HES1 antibody was a kind gift from Dr. Tetsuo Sudo (Toray Scientific, Japan). The α-tubulin antibody was from Molecular Probes (Eugene, OR). Cell lysates were prepared and Western blots were performed as described earlier [11]. The molecular weights were determined with the use of prestained protein ladders.
Immunofluorescence
Indirect immunofluorescence for HES1 and α-tubulin was performed as described earlier [19]. Briefly, cells were fixed, permeabilized, blocked, and sequentially incubated with primary and secondary (4 µg/ml; Alexa 488 goat antirabbit IgG) antibodies. Cells were mounted in DAPI mounting media, examined, and photographed with a microscope (Zeiss, Thornwood, NY).
Flow Cytometry
Cells (70–80% confluent) were incubated with 1 mM bromodeoxyuridine (BrdU) for 1 hour at 37°C and processed using the fluorescein isothiocyanate (FITC) BrdU Flow Kit (BD Biosciences, San Diego, CA) following the manufacturer's instructions. Briefly, 1 x 106 trypsinized cells were fixed, permeabilized, and digested with DNAse. Cells were then stained with FITC-conjugated anti-BrdU and 7-amino-actinomycin (7-AAD). Flow cytometry was performed on a Becton Dickinson FACSCalibur machine. For each experiment, 10,000 events were counted. Data acquisition was performed with the CellQuest software (BD Biosciences), and the percentages of G1, S, and G2 phases of the diploid and tetraploid populations were calculated with the MODFIT-LT software program (Verity Software House, Topsham, ME). For the isolation of viable diploid and tetraploid cells, cells (60–70% confluency) were incubated with 5 µg/ml of Hoechst 33342 (Calbiochem, San Diego, CA) for 2 hours at 37°C, trypsinized, and sorted using a Becton Dickinson FACS Vantage machine. For evaluating the percentage of total cells that were apoptotic, the sub-G0 population (apoptotic population) was calculated using CellQuest.
Promoter Assays
Cells grown in 24-well plates were transfected in triplicate with 1 µg of various plasmid constructs using Lipofectamine (Invitrogen). The cells were cotransfected with 250 ng of pRL-CMV as a control for transfection efficiency. Forty-eight hours after transfection, the firefly and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) as recommended by the manufacturer and a Sirius Luminometer (Berthold Detection Systems, Oak Ridge, TN). Firefly luciferase activity was normalized to 5 x 106 Renilla luciferase activity units.
Spectral Karyotyping
SF3061-Vector and isolated SF3061-HES1 diploid and tetraploid cells were analyzed at passages 8 and 19 after transfection with HES1. Immortalized arachnoidal cells at similar passage numbers were used as normal controls. Cells were incubated with 10 µg/ml of colcemid for 2.5 hours, and metaphase chromosome spreads were prepared as described earlier [20]. Human spectral karyotyping (SKY) paint probes and the SKY kit were obtained from Applied Spectral Imaging (Carlsbad, CA). In situ hybridization of the SKY probes on the metaphase chromosome preparations were performed following manufacturer's instructions and as described previously [20]. Briefly, the metaphase spreads were serially digested with pepsin, denatured in formamide and hybridized to SKY probes. Chromosomespecific fluorescence spectra were developed with combinations of the rhodamine, Texas red, Cy5, Cy5.5, and FITC fluorochromes. Spectral images were captured using a SD200 SpectraCube interferometer-based spectral imaging system (Applied Spectral Imaging). Karyotypes were analyzed using the SKYView software (version 1.62). Fifteen metaphases were analyzed at each passage number for SF3061-Vector and isolated SF3061-HES1 tetraploid cells, whereas eight metaphases were analyzed for isolated SF3061-HES1 diploid cells.
Results
Exogenous Expression of HES1 Induces Tetraploidy in Meningioma Cell Lines
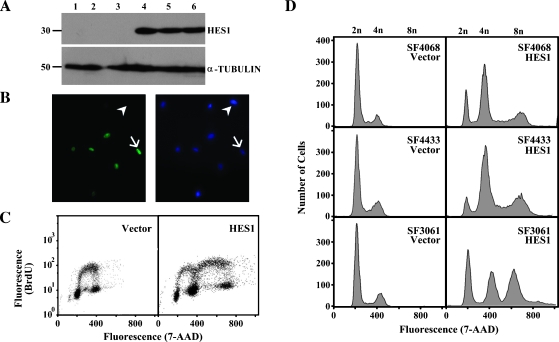
Because HES1 is a primary downstream effector of Notch signaling and is over-expressed in human meningioma tumors, we reasoned that HES1 was a good candidate to evaluate the functional consequences of Notch signaling in meningiomas. Therefore, we exogenously expressed HES1 in meningioma cell lines using retroviral mediated gene transfer. Expression and subcellular localization of HES1 in stable cell populations was verified using Western blot analysis (Figure 1A) and immunofluorescence (Figure 1B). Whereas SF4068-Vector cells were negative for HES1, SF4068-HES1 cells had strong nuclear staining in approximately 60% of the cells (Figure 1B). We evaluated the cell cycle position of individual SF4068-Vector and SF4068-HES1 cells at passage 2 (post-G418 selection) using immunofluorescence staining of incorporated BrdU and costaining for total DNA content with 7-AAD, followed by two-color flow cytometric analysis. Expression of HES1 caused 68 to 75% of the cells to double their total DNA content, i.e., they were tetraploid (Figure 1C). The relative percentage of cells in Diploid G0/1 and Diploid S in both the SF4068-Vector and SF4068-HES1 cells was not altered, suggesting that HES1 did not alter the growth rate of SF4068 cells. Similar results were obtained when HES1 was expressed in SF4433 and SF3061 meningioma cell lines (Figure 1D). In all three cases, HES1 expression was associated with the appearance of tetraploid cells.
Figure 1.
Exogenous expression of HES1 is associated with tetraploidy in meningioma cell lines. HES1 was expressed in meningioma cell lines using retroviral-mediated gene transfer. (A) Western blot analysis using a polyclonal antibody against HES1 in control (lanes 1–3) and stable (lanes 4–6) cell populations. Lane 1, SF4068-Vector; lane 2, SF4433-Vector; lane 3, SF3061-Vector; lane 4, SF4068-HES1; lane 5, SF4433-HES1; lane 6, SF3061-HES1. α-Tubulin was included as a loading control. (B) Immunofluorescence using the HES1 polyclonal antibody (left panel) was used to show nuclear localization of HES1 in SF4068-HES1 stable cell populations. Nuclei were counterstained with DAPI (right panel). Arrow indicates HES1-positive nucleus; arrowhead, HES1-negative nucleus. (C) Immunofluorescent staining for incorporated BrdU and total DNA content by 7-AAD, followed by biparametric BrdU/7-AAD flow cytometric analysis was performed in Vector and HES1 stable cell populations. The BrdU fluorescence (y-axis) for SF4068-Vector and SF4068-HES1 cells is plotted against total DNA content (x-axis). (D) The number of individual cells (y-axis) for the indicated Vector or HES1 stable cell populations is plotted against total DNA content (x-axis). The 2n, 4n, and 8n peaks are indicated above the plots.
HES1-Induced Tetraploidy Is Associated with Features Indicative of Chromosomal Instability
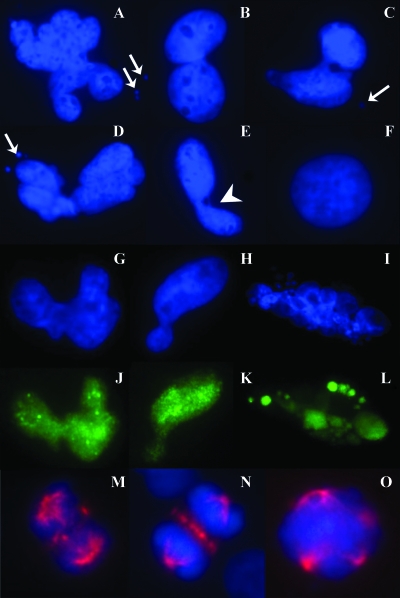
Because tetraploidization is often an intermediate step in the process to aneuploidy and enhanced aneuploidy is indicative of chromosomal instability [21], we evaluated for features of chromosomal instability in HES1 stable cells. SF4068-HES1 cells showed nuclear atypia characterized by the presence of micronuclei, nuclear bridges, and elongated and lobulated nuclei (Figure 2, A–E), none of which were observed in SF4068-Vector cells (Figure 2F). Cells with nuclear atypia were typically positive for HES1 expression (Figure 2, G–L). We performed immunofluorescence using an antibody against α-tubulin to view mitotic spindle structures. SF4068-HES1 cells had an increased frequency of aberrant mitotic spindle structures, including multipolar spindles (Figure 2M–O), none of which were observed in control cells.
Figure 2.
Tetraploidy in meningioma is associated with features of chromosomal instability and mitotic spindle anomalies. DAPI staining (blue stain) was used to show that SF4068-HES1 cells were associated with an increased frequency of nuclear atypia (A–E) characterized by the presence of micronuclei (arrows), elongated and lobulated nuclei, and nuclear bridges (arrowhead), not found in SF4068-Vector cells (F). Individual nuclei exhibiting atypia were HES1-positive (green stain; compare G–I with J–L). Staining with α-tubulin (red stain, M–O) revealed aberrant mitotic spindles, including multipolar spindles (O) in SF4068-HES1 cells.
Both Activated Notch1 and Notch2 Induce Tetraploidy in Meningiomas
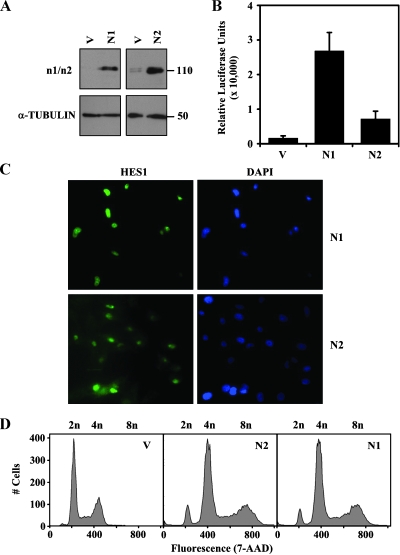
Whereas HES1 is a primary target of Notch signaling, recent studies have shown that other signaling pathways can regulate HES1 expression in a Notch-independent fashion [22]. In addition, different Notch receptor and ligand homologs can have different, nonredundant and sometimes opposing functions [23,24]. We had previously shown that meningiomas express both the Notch1 and Notch2 proteins [11]. We therefore evaluated whether Notch1 and/or Notch2 could induce tetraploidy in SF4068 cells by exogenously expressing the activated intracellular domains of Notch1 (N1ICD) and Notch2 (N2ICD; Figure 3A). Both SF4068-N1ICD and SF4068-N2ICD stable cell populations induced HES1 promoter activity (Figure 3B), and endogenous HES1 protein expression (Figure 3C) compared with SF4068-Vector cells. Both N1ICD and N2ICD expression was associated with the appearance of tetraploid cells in SF4068 (Figure 3D). Although the amplitude of induction of HES1 promoter activity by N1ICD and N2ICD was different, most cells in these stable cell populations were positive for nuclear HES1, and there was no difference in the incidence of tetraploidy. The N1ICD- and N2ICD-associated tetraploid cells exhibited features of chromosomal instability (data not shown). Similar results were obtained when N1ICD and N2ICD were exogenously expressed in SF4433 meningioma cells (data not shown). Thus, both Notch1 and Notch2 receptor homologs have similar functions in altering the ploidy of meningioma cells.
Figure 3.
Activated Notch1 and Notch2 induce endogenous HES1 expression and tetraploidy in meningioma cell lines. (A) Western blot analysis of SF4068-Vector (V), SF4068-N1ICD (N1), and SF4068-N2ICD (N2) cells using monoclonal antibodies specific for Notch1 (n1) or Notch2 (n2). α-Tubulin was included as a loading control. (B) Activity of the HES1 promoter was determined using the luciferase reporter assay. Relative luciferase units (y-axis) in SF4068-Vector (V), SF4068-N1ICD (N1), and SF4068-N2ICD (N2) cells are plotted. (C) Immunofluorescence using the HES1 polyclonal antibody (left panels) showed induction and nuclear localization of endogenous HES1 in SF4068-N1ICD (N1) and SF4068-N2ICD (N2) stable cell populations. Nuclei were counterstained with DAPI (right panels). (D) Flow cytometric analysis for total DNA content by 7-AAD was performed in SF4068-Vector (V), SF4068-N1ICD (N1), and SF4068-N2ICD (N2) cells. The number of individual cells (y-axis) is plotted against total DNA content (x-axis). The 2n, 4n, and 8n peaks are indicated above the plots.
Tetraploid Cells Are Viable and Have Slightly Higher Rates of Spontaneous Apoptosis
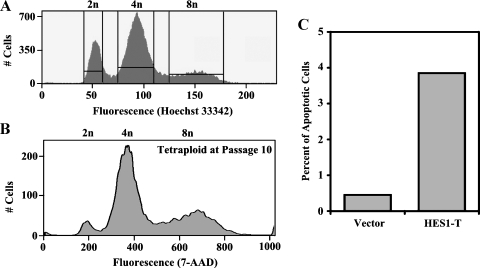
Tetraploid cells have different fates depending on the cell type and the genetic context. In cell lines with an intact tetraploidy checkpoint, they are cell cycle-arrested and eliminated, whereas in others, stable propagation of tetraploid cells is observed. In addition, tetraploid cells have altered apoptotic rates and response to apoptotic agents when compared with their diploid counterparts [25]. To evaluate whether tetraploid meningioma cells generated by expression of HES1 were viable and not undergoing massive cell death, we sorted the diploid G1 (2n) and tetraploid G2 (8n) populations and passaged them separately in culture (Figure 4A). The ploidy of the tetraploid cells was evaluated after 10 passages in culture. Greater than 90% of the cells were still tetraploid (Figure 4B). Thus, the tetraploid cells were viable and propagated stably. Next, we compared the apoptotic rates of tetraploid cells to diploid cells. Whereas only 0.5% of the SF4068-Vector diploid cells were spontaneously apoptotic, 4% of the SF4068-HES1 tetraploid cells were spontaneously apoptotic (Figure 4C). Thus, tetraploid meningioma cells had slightly elevated rates of spontaneous apoptosis.
Figure 4.
Isolated tetraploid cells are viable but have higher spontaneous apoptotic rates compared with diploid cells. (A) Viable diploid G1 (2n) cells were separated from tetraploid G2 (8n) cells using Hoechst Staining and flow cytometry and propagated in culture. (B) After 10 passages in culture, the ploidy of the tetraploid cells was measured by 7-AAD staining for total DNA content and flow cytometry. (C) The percentage of apoptotic cells (y-axis) in SF4068-Vector (Vector) and isolated tetraploid cells from SF4068-HES1 (HES1-T) are shown.
Tetraploid Cells Acquire More Chromosomal Abnormalities Compared to Diploid Cells
We used SKY to assess the frequency of generation of numerical and structural chromosomal abnormalities in isolated SF3061-HES1 tetraploid cells compared with diploid cells. We chose SF3061 for these experiments because SF4068 and SF4433 cells have been immortalized with the E6/E7 oncogenes, which are known to enhance genomic instability [26,27]. SKY analysis confirmed that the tetraploid G2 (8n) population had double the DNA content of SF3061-Vector cells (Figure 5A). The translocations present in SF3061-Vector cells at P8 were considered baseline (Table 1 and Figure 5). These metaphases contained 9.2 translocations per metaphase and an average of 42.8 chromosomes per metaphase. In contrast, tetraploid cells at the same passage had 19.3 translocations per metaphase and an average of 89.3 chromosomes per metaphase (Table 1). Thus, both the translocations per metaphase and number of chromosomes per metaphase were slightly more than double in the tetraploid cells when compared with the corresponding diploid cells. In addition, tetraploid cells had acquired 23 translocations that were not found in the corresponding diploid metaphases. After 11 further passages in culture, tetraploid cells had acquired an average of 28.3 additional chromosomes per metaphase, whereas this number was unchanged for the diploid cells (Table 1). Moreover, tetraploid cells had acquired 137 total translocations, whereas diploid cells had only acquired 3. As an additional control, we analyzed the translocations in isolated SF3061-HES1 diploid cells at P19. Similar to the SF3061-Vector metaphases, these diploid metaphases contained 8.9 translocations per metaphase and an average of 42.3 chromosomes per metaphase. Representative SKY karyograms of diploid and tetraploid metaphase at P19 are shown in Figure 5. The tetraploid metaphase contained several new translocations that were not found in diploid metaphases such as t(2;10), t(6;4), t(10;3). In addition, this metaphase had many numerical abnormalities such as six copies of chromosome 4, 14, 16, 19, 21, and 22, six copies of the t(3;8) translocation, and three copies of the t(17;20) and the t(11;13) translocation. Thus, tetraploid cells generated by HES1 expression had a higher frequency of both numerical and structural chromosomal abnormalities when compared with diploid cells, suggesting that Notch signaling enhanced chromosomal instability in meningioma cells.
Figure 5.
Tetraploid cells develop a higher number of numerical and structural chromosomal abnormalities over time in culture when compared with diploid cells. Spectral karyotyping (SKY) was used to assess numerical and structural abnormalities in SF3061-Vector and isolated diploid and tetraploid cells from SF3061-HES1 stable cell populations. (A) Representative SKY metaphases from control nonneoplastic arachnoid (Arachnoid) cells, SF3061-Vector (Diploid), and SF3061-HES1 (Tetraploid) cells are shown. (B and C) SKY karyograms of a diploid metaphase from SF3061-Vector cells at passage 19 (B) and a tetraploid metaphase from SF3061-HES1 cells at passage 19 (C) are shown. Chromosome numbers (white) are indicated.
Table 1.
Numerical and Structural Chromosomal Abnormalities in SF3061 Meningioma Cells.
| Stable Cell Population | Passage Number | Number of Metaphases Analyzed | Total Number of Translocations | Translocations per Metaphase | Mean Number of Chromosomes per Metaphase |
| SF3061-Vector | 8 | 15 | 138 | 9.2 | 42.8 |
| SF3061-HES1 Tetraploid | 8 | 15 | 289 | 19.3 | 89.3 |
| SF3061-Vector | 19 | 15 | 141 | 9.4 | 42.9 |
| SF3061-HES1 Tetraploid | 19 | 15 | 426 | 28.4 | 117.6 |
| SF3061-HES1 Diploid | 19 | 8 | 71 | 8.9 | 42.3 |
Discussion
The contribution of aberrant Notch signaling to meningioma tumorigenesis is unknown. We show that expression of activated Notch1, Notch2, and HES1 induce tetraploidy associated with features of chromosomal instability in meningioma cell lines. In addition, we show that tetraploid cells exhibit chromosomal instability developing a higher number of numerical and structural chromosomal aberrations.
Tetraploidy occurs commonly in many cancers and is often an intermediate step in the process to aneuploidy, a hallmark of most cancers and an end feature of chromosomal instability [28]. Usually, cells with tetraploid DNA content arise as an early step in tumorigenesis and precede the formation of aneuploid cells. This has been clearly demonstrated in Barrett esophagus, a premalignant condition where patients are regularly followed by endoscopic biopsies [29,30]. A careful analysis of these patients has revealed the relatively early loss of p53, the acquisition of a significant population of tetraploid cells, and the subsequent development of aneuploidy. Also, in some tumor models, such as the elastase-simian virus 40 tumor antigen transgenic mouse model of pancreatic cancer, an increase in a tetraploid population of cells is detected early in the development of tumor [31].
Tetraploid cells are normally cell cycle-arrested and undergo apoptosis if there is an intact tetraploidy checkpoint [32]. Also, a disrupted p53 pathway has been proposed as being essential for the survival of tetraploid cells [32]. In our study, tetraploid meningioma cells associated with HES1 expression were viable albeit with a slightly elevated apoptotic rate suggesting that the meningioma cell lines used have a defective tetraploidy checkpoint. It is likely that any tetraploid cells generated by transfecting HES1 in untransformed cells that have robust cell cycle checkpoints would be eliminated.
In vivo, we anticipate that tetraploid meningioma cells without the ability to evade apoptosis will die. Only cells with another cellular defect such as a disrupted p53 pathway would survive. This could potentially occur through a “prior genetic hit” that has provided a growth advantage to the cell. A more likely scenario is that the genetically unstable tetraploid cell itself acquires a mutation or translocation that provides a selective growth advantage allowing it to propagate.
Previous studies have shown that primary meningioma tumors contain tetraploid cells and exhibit chromosomal instability. Fluorescence in situ hybridization studies aimed at investigating chromosomal aberrations in meningiomas have frequently detected tetraploid cells [33–35]. One study, investigating chromosome 14q32 loss in 124 meningiomas, found tetraploid cells in 26% of meningiomas [33]. Using flow cytometry, a hyperdiploid phenotype was observed in 30% (44/124 tumors) of meningioma cases analyzed [36]. In a separate study, specific features of chromosomal instability have also been found at high frequency in meningiomas [37]. Early passage short-term primary cultures from 61 meningiomas were analyzed and shown to have cells with aberrant nuclear morphology including multinucleated cells, anaphase bridges, chromatin strings and nuclear blebs [37]. Chromosomal aberrations including ring chromosomes, telomere associations, and dicentric chromosomes were observed. A hyperdiploid karyotype was found in 47.5% of these tumors. The authors concluded that even slow-growing tumors such as meningiomas display chromosomal instability [37]. Our studies suggest that deregulation of the Notch signaling pathway is a potential mechanism that is responsible for this phenotype.
Notch has previously been implicated in the control of ploidy under certain cellular contexts. In the endocycle, DNA replication is uncoupled from mitosis allowing cells to dramatically increase their DNA content above diploid values. Drosophila follicle cells divide mitotically and increase in number until mid-oogenesis when they exit the mitotic cycle and enter the endocycle. The Notch signaling pathway controls this mitotic/endocycle switch. Loss of Notch or Delta results in the failure of these cells to form endocycles [38,39]. Similarly, in humans, megakaryocytes are specialized precursors of platelets that are polyploid. The Notch signaling pathway has been implicated in the generation of these polyploid megakaryocytes, although the mechanism of Notch function in this process is not understood [40,41]. Finally, in Caenorhabditis elegans, a gain of function mutation in glp1, an ortholog of Notch, prevents primordial germ cells from making the mitosis to meiosis switch (equivalent to twice the “normal” DNA content) and leads to the overgrowth of primordial germ cells and the formation of germline tumors [42]. In human germ cell tumors (seminomas and carcinoma in situ), Notch2 and Notch4 are over-expressed, and it has been proposed that deregulation of Notch causes dysfunction of the mitotic to meiotic switch leading to abnormal chromosomal segregation and the generation of aneuploid cells [43]. Thus, one of the many functions of the Notch signaling pathway in certain cell types is the regulation of ploidy. Our data show that meningiomas are one such cell type.
Tetraploidy can be induced by external signals or mutations that result in either cell fusion or an abortive cell cycle including defects in DNA replication, sister chromatid separation, mitotic spindle assembly, mitotic checkpoint regulation, or cytokinesis [21,44]. The aberrant expression of proteins regulating the G2/M phase transition such as Aurora A, cyclin B1, forkhead transcription factor M3, and mitotic spindle checkpoint proteins such as Bub and Mad have been shown to induce tetraploidy [45,46]. It is possible the Notch signaling induces tetraploidy by impacting expression of one or more of these proteins. Understanding the mechanism by which Notch signaling induces tetraploidy in meningiomas remains to be determined and will be an important topic of future work.
In conclusion, our data identify a function for Notch signaling in inducing chromosomal instability in meningiomas and potentially contributing to meningioma tumorigenesis.
Acknowledgments
The authors thank Lucio Miele and Spyros Artavanis-Tsakonas for providing expression constructs, Jingli Weier for assistance with Spectral Karyotyping analysis, Tetsuo Sudo for the HES1 antibody, and the UCSF Comprehensive Cancer Center LCA Core for technical assistance with the flow cytometric analysis. The authors also thank Katharine Striedinger and Lucia Carvalho for useful discussions and critical review of the manuscript. A. L. is a recipient of The Sontag Foundation Distinguished Scientist Award.
Footnotes
The Sontag Foundation supported this research.
References
- 1.Kopan R. Notch: a membrane-bound transcription factor. J Cell Sci. 2002;115:1095–1097. doi: 10.1242/jcs.115.6.1095. [DOI] [PubMed] [Google Scholar]
- 2.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 3.Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 4.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 5.Capobianco AJ, Zagouras P, Blaumueller CM, Artavanis-Tsakonas S, Bishop JM. Neoplastic transformation by truncated alleles of human NOTCH1/TAN1 and NOTCH2. Mol Cell Biol. 1997;17:6265–6273. doi: 10.1128/mcb.17.11.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pear WS, Aster JC, Scott ML, Hasserjian RP, Soffer B, Sklar J, Baltimore D. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med. 1996;183:2283–2291. doi: 10.1084/jem.183.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 8.Zagouras P, Stifani S, Blaumueller CM, Carcangiu ML, Artavanis-Tsakonas S. Alterations in Notch signaling in neoplastic lesions of the human cervix. Proc Natl Acad Sci USA. 1995;92:6414–6418. doi: 10.1073/pnas.92.14.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talora C, Sgroi DC, Crum CP, Dotto GP. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 2002;16:2252–2263. doi: 10.1101/gad.988902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng Q, Li S, Chepeha DB, Giordano TJ, Li J, Zhang H, Polverini PJ, Nor J, Kitajewski J, Wang CY. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell. 2005;8:13–23. doi: 10.1016/j.ccr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Cuevas IC, Slocum AL, Jun P, Costello JF, Bollen AW, Riggins GJ, McDermott MW, Lal A. Meningioma transcript profiles reveal deregulated notch signaling pathway. Cancer Res. 2005;65:5070–5075. doi: 10.1158/0008-5472.CAN-05-0240. [DOI] [PubMed] [Google Scholar]
- 12.CBTRUS, author. Statistical Report: Primary Brain Tumors in the United States, 1998–2002. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2005. [Google Scholar]
- 13.Baser ME, R Evans DG, Gutmann DH. Neurofibromatosis 2. Curr Opin Neurol. 2003;16:27–33. doi: 10.1097/01.wco.0000053583.70044.ab. [DOI] [PubMed] [Google Scholar]
- 14.Ringel F, Cedzich C, Schramm J. Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery. 2007;60:214–221. doi: 10.1227/01.NEU.0000255415.47937.1A. [discussion 221–212] [DOI] [PubMed] [Google Scholar]
- 15.Bassiouni H, Asgari S, Stolke D. Olfactory groove meningiomas: functional outcome in a series treated microsurgically. Acta Neurochir (Wien) 2007;149:109–121. doi: 10.1007/s00701-006-1075-z. [discussion 121] [DOI] [PubMed] [Google Scholar]
- 16.Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85:2046–2056. doi: 10.1002/(sici)1097-0142(19990501)85:9<2046::aid-cncr23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Kepes JJ. Presidential address: the histopathology of meningiomas. A reflection of origins and expected behavior? J Neuropathol Exp Neurol. 1986;45:95–107. [PubMed] [Google Scholar]
- 18.O'Rahilly R, Muller F. The meninges in human development. J Neuropathol Exp Neurol. 1986;45:588–608. [PubMed] [Google Scholar]
- 19.Baia GS, Slocum AL, Hyer JD, Misra A, Sehati N, Vandenberg SR, Feuerstein BG, Deen DF, McDermott MW, Lal A. A genetic strategy to overcome the senescence of primary meningioma cell cultures. J Neurooncol. 2006;78:113–121. doi: 10.1007/s11060-005-9076-y. [DOI] [PubMed] [Google Scholar]
- 20.Fung J, Weier HU, Goldberg JD, Pedersen RA. Multilocus genetic analysis of single interphase cells by spectral imaging. Hum Genet. 2000;107:615–622. doi: 10.1007/s004390000416. [DOI] [PubMed] [Google Scholar]
- 21.Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- 22.Curry CL, Reed LL, Nickoloff BJ, Miele L, Foreman KE. Notchindependent regulation of Hes-1 expression by c-Jun N-terminal kinase signaling in human endothelial cells. Lab Invest. 2006;86:842–852. doi: 10.1038/labinvest.3700442. [DOI] [PubMed] [Google Scholar]
- 23.Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, Brat DJ, Perry A, Eberhart CG. Notch1 and Notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 24.Tohda S, Kogoshi H, Murakami N, Sakano S, Nara N. Diverse effects of the Notch ligands Jagged1 and Delta1 on the growth and differentiation of primary acute myeloblastic leukemia cells. Exp Hematol. 2005;33:558–563. doi: 10.1016/j.exphem.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Castedo M, Coquelle A, Vivet S, Vitale I, Kauffmann A, Dessen P, Pequignot MO, Casares N, Valent A, Mouhamad S, et al. Apoptosis regulation in tetraploid cancer cells. EMBO J. 2006;25:2584–2595. doi: 10.1038/sj.emboj.7601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coursen JD, Bennett WP, Gollahon L, Shay JW, Harris CC. Genomic instability and telomerase activity in human bronchial epithelial cells during immortalization by human papillomavirus-16 E6 and E7 genes. Exp Cell Res. 1997;235:245–253. doi: 10.1006/excr.1997.3670. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi M, Takeuchi K, Kohara A, Satoh M, Shioda S, Ozawa Y, Ohtani A, Morita K, Hirano T, Terai M, et al. Chromosomal instability in human mesenchymal stem cells immortalized with human papilloma virus E6, E7, and hTERT genes. In Vitro Cell Dev Biol Anim. 2007;43:129–138. doi: 10.1007/s11626-007-9021-9. [DOI] [PubMed] [Google Scholar]
- 28.Olaharski AJ, Sotelo R, Solorza-Luna G, Gonsebatt ME, Guzman P, Mohar A, Eastmond DA. Tetraploidy and chromosomal instability are early. events during cervical carcinogenesis. 2006;Carcinogenesis 27:337–343. doi: 10.1093/carcin/bgi218. [DOI] [PubMed] [Google Scholar]
- 29.Galipeau PC, Cowan DS, Sanchez CA, Barrett MT, Emond MJ, Lvine DS, Rabinovitch PS, Reid BJ. 17p (p53) allelic losses, 4N (G2/tetraploid) populations, and progression to aneuploidy in Barrett's esophagus. Proc Natl Acad Sci USA. 1996;93:7081–7084. doi: 10.1073/pnas.93.14.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrett MT, Pritchard D, Palanca-Wessels C, Anderson J, Reid BJ, Rabinovitch PS. Molecular phenotype of spontaneously arising 4N (G2-tetraploid) intermediates of neoplastic progression in Barrett's esophagus. Cancer Res. 2003;63:4211–4217. [PubMed] [Google Scholar]
- 31.Levine DS, Sanchez CA, Rabinovitch PS, Reid BJ. Formation of the tetraploid intermediate is associated with the development of cells with more than four centrioles in the elastase-simian virus 40 tumor antigen transgenic mouse model of pancreatic cancer. Proc Natl Acad Sci USA. 1991;88:6427–6431. doi: 10.1073/pnas.88.15.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margolis RL, Lohez OD, Andreassen PR. G1 tetraploidy checkpoint and the suppression of tumorigenesis. J Cell Biochem. 2003;88:673–683. doi: 10.1002/jcb.10411. [DOI] [PubMed] [Google Scholar]
- 33.Tabernero MD, Espinosa AB, Maillo A, Sayagues JM, Alguero Mdel C, Lumbreras E, Diaz P, Goncalves JM, Onzain I, Merino M, et al. Characterization of chromosome 14 abnormalities by interphase in situ hybridization and comparative genomic hybridization in 124 meningiomas: correlation with clinical, histopathologic, and prognostic features. Am J Clin Pathol. 2005;123:744–751. [PubMed] [Google Scholar]
- 34.Maillo A, Diaz P, Sayagues JM, Blanco A, Tabernero MD, Ciudad J, Lopez A, Goncalves JM, Orfao A. Gains of chromosome 22 by fluorescence in situ hybridization in the context of an hyperdiploid karyotype are associated with aggressive clinical features in meningioma patients. Cancer. 2001;92:377–385. doi: 10.1002/1097-0142(20010715)92:2<377::aid-cncr1333>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 35.Sayagues JM, Tabernero MD, Maillo A, Diaz P, Rasillo A, Bortoluci A, Gomez-Moreta J, Santos-Briz A, Morales F, Orfao A. Incidence of numerical chromosome aberrations in meningioma tumors as revealed by fluorescence in situ hybridization using 10 chromosome-specific probes. Cytometry. 2002;50:153–159. doi: 10.1002/cyto.10075. [DOI] [PubMed] [Google Scholar]
- 36.Sayagues JM, Tabernero MD, Maillo A, Espinosa A, Rasillo A, Diaz P, Ciudad J, Lopez A, Merino M, Goncalves JM, et al. Intratumoral patterns of clonal evolution in meningiomas as defined by multicolor interphase fluorescence in situ hybridization (FISH): is there a relationship between histopathologically benign and atypical/anaplastic lesions? J Mol Diagn. 2004;6:316–325. doi: 10.1016/S1525-1578(10)60527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Tilborg AA, Al Allak B, Velthuizen SC, de Vries A, Kros JM, Avezaat CJ, de Klein A, Beverloo HB, Zwarthoff EC. Chromosomal instability in meningiomas. J Neuropathol Exp Neurol. 2005;64:312–322. doi: 10.1093/jnen/64.4.312. [DOI] [PubMed] [Google Scholar]
- 38.Deng WM, Althauser C, Ruohola-Baker H. Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development. 2001;128:4737–4746. doi: 10.1242/dev.128.23.4737. [DOI] [PubMed] [Google Scholar]
- 39.Shcherbata HR, Althauser C, Findley SD, Ruohola-Baker H. The mitotic-to-endocycle switch in Drosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell-cycle transitions. Development. 2004;131:3169–3181. doi: 10.1242/dev.01172. [DOI] [PubMed] [Google Scholar]
- 40.Sun L, Tan P, Yap C, Hwang W, Koh LP, Lim CK, Aw SE. In vitro biological characteristics of human cord blood-derived megakaryocytes. Ann Acad Med Singapore. 2004;33:570–575. [PubMed] [Google Scholar]
- 41.Singh N, Phillips RA, Iscove NN, Egan SE. Expression of notch receptors, notch ligands, and fringe genes in hematopoiesis. Exp Hematol. 2000;28:527–534. doi: 10.1016/s0301-472x(00)00146-6. [DOI] [PubMed] [Google Scholar]
- 42.Berry LW, Westlund B, Schedl T. Germ-line tumor formation caused by activation of glp-1, a Caenorhabditis elegans member of the Notch family of receptors. Development. 1997;124:925–936. doi: 10.1242/dev.124.4.925. [DOI] [PubMed] [Google Scholar]
- 43.Adamah DJ, Gokhale PJ, Eastwood DJ, Rajpert De-Meyts E, Goepel J, Walsh JR, Moore HD, Andrews PW. Dysfunction of the mitotic:meiotic switch as a potential cause of neoplastic conversion of primordial germ cells. Int J Androl. 2006;29:219–227. doi: 10.1111/j.1365-2605.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen HG, Ravid K. Tetraploidy/aneuploidy and stem cells in cancer promotion: the role of chromosome passenger proteins. J Cell Physiol. 2006;208:12–22. doi: 10.1002/jcp.20565. [DOI] [PubMed] [Google Scholar]
- 45.Meraldi P, Draviam VM, Sorger PK. Timing and checkpoints in the regulation of mitotic progression. Dev Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. EMBO J. 2002;21:483–492. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]