Abstract
The transcription factor Gli3 (glioma-associated oncogene homolog) is essential for normal development of the mammalian forebrain. One extreme requirement for Gli3 is at the dorsomedial telencephalon, which does not form in Gli3Xt/Xt mutant mice lacking functional Gli3. In this study, we analyzed expression of Gli3 in the wild-type telencephalon and observed a highdorsal-to-lowventral gradient of Gli3 expression and predominance of the cleaved form of the Gli3 protein dorsally. This graded expression correlates with the severedorsal-to-mildventral telencephalic phenotype observed in Gli3Xt/Xt mice. We characterized the abnormal joining of the telencephalon to the diencephalon and defined the medial limit of the dorsal telencephalon in Gli3Xt/Xt mice early in corticogenesis. Based on this analysis, we concluded that some of the abnormal expression of ventral telencephalic markers previously described as being in the dorsal telencephalon is, in fact, expression in adjacent diencephalic tissue, which expresses many of the same genes that mark the ventral telencephalon. We observed occasional cells with diencephalic character in the Foxg1 (forkhead box)-expressing Gli3Xt/Xt telencephalon at embryonic day 10.5, a day after the anatomical subdivision of the forebrain vesicle. Large clusters of such cells appear in the Gli3Xt/Xt neocortical region at later ages, when the neocortex becomes highly disorganized, forming rosettes comprising mainly neural progenitors. We propose that Gli3 is indispensable for formation of an intact telencephalic–diencephalic boundary and for preventing the abnormal positioning of diencephalic cells in the dorsal telencephalon.
Keywords: Gli3, mutant, development, telencephalon, diencephalon, eminentia thalami
Introduction
Gli3 (glioma-associated oncogene homolog), a zinc finger transcription factor (Ruppert et al., 1990), is an important component of the Sonic hedgehog (Shh) signaling pathway in mammals that resembles the hedgehog (Hh) signaling pathway in Drosophila (Ingham and McMahon, 2001). In the absence of Hh signal, cubitus interruptus (Ci), the fly homolog of mammalian Gli proteins (Hui et al., 1994), is cleaved to yield a transcriptional repressor, whereas in the presence of Hh, cleavage is repressed and the full-length isoform of Ci acts as a transcriptional activator (Aza-Blanc et al., 1997; Methot and Basler, 1999). It has been shown that Shh can similarly regulate Gli3 (von Mering and Basler, 1999; Aza-Blanc et al., 2000). Insight into the function of Gli3 in vivo has been gained with the study of the extratoes (XtJ) mouse mutant, which has a 51.5 kb deletion in the Gli3 gene that includes the zinc-finger domain and is presumed to render it nonfunctional (Hui and Joyner, 1993; Maynard et al., 2002). Mice homozygous for the XtJ mutation (Gli3Xt/Xt mice) die perinatally with multiple phenotypic defects, including polydactyly and a high incidence of exencephaly, whereas non-exencephalic embryos display severe telencephalic abnormalities (Grove et al., 1998; Theil et al., 1999; Tole et al., 2000; Kuschel et al., 2003; Theil, 2005).
The telencephalic phenotype of the Gli3Xt/Xt mutant includes a reduction in the size of the dorsal telencephalon, absence of olfactory bulbs, failure of the medial wall of the dorsal telencephalon to invaginated, and absence of the choroid plexus in the lateral ventricles (Hui and Joyner, 1993; Grove et al., 1998; Theil et al., 1999; Tole et al., 2000). Recently, Gli3 has been implicated in the maintenance of a proper laminar organization of the neocortex, as well as the apical/basal cell polarity of cortical precursors (Theil, 2005).
Several studies have reported ectopic expression of ventral telencephalic markers, such as Islet1, Dlx2 (distal-less homeobox), and Mash1 (mammalian achaete-schute homolog), in the dorsal telencephalon of the Gli3Xt/Xt mutant (Tole et al., 2000; Rallu et al., 2002; Kuschel et al., 2003). However, the lack of dorsomedial telencephalon in these mice would result in an abnormal joining of the remaining dorsal telencephalon (neocortex) to the diencephalon, and previous studies might not have taken this abnormal forebrain anatomy into account when interpreting alterations in gene expression.
In this study, we performed a detailed analysis of the embryonic day 12.5 (E12.5) Gli3Xt/Xt forebrain and propose that some of the previously described ectopic dorsal expression of ventral markers in the Gli3Xt/Xt telencephalon actually reflects relatively normal gene expression in the diencephalon. We then focused on the development of the mutant neocortex. We present evidence that the telencephalic–diencephalic border in the E12.5 Gli3Xt/Xt mutants is compromised and that neocortical progenitors interspersed with diencephalic cells subsequently segregate into well organized rosettes. Finally, we analyzed Gli3Xt/Xt forebrain younger than E12.5 and traced the likely origin of the clusters of misplaced diencephalic cells in the mutant neocortex to the presence of occasional cells of diencephalic character in the mutant dorsal telencephalon at E10.5.
Materials and Methods
Animals.
Animal care was according to institutional guidelines. Gli3Xt/+ CBA mice were mated, and the morning of the vaginal plug was defined as E0.5. Embryos were genotyped by PCR, as described previously (Maynard et al., 2002), fixed in 4% paraformaldehyde, and processed into paraffin blocks.
Bromodeoxyuridine injections, immunohistochemistry, and immunofluorescence.
A 30 min pulse of bromodeoxyuridine (BrdU) (70 μg/g body weight, i.p.) was administered to pregnant dams, and E13.5 embryos were collected.
Sections were cut serially at 10 μm and reacted using standard protocols. Antigen retrieval was achieved by microwaving sections in 10 mm sodium citrate buffer. Mouse monoclonal antibodies were against BrdU (1:200; BD Biosciences, Oxford, UK), Mash1 (1:100; BD Biosciences), β-tubulin III (1:500; Sigma, Poole, UK), reelin (1:1000; Chemicon, Harrow, UK), Islet1 (1:50; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), Lim1 and Lim2 (Lim is the three gene products Lin-11/Isl-1/Mec-3) (1:200; Developmental Studies Hybridoma Bank), nestin (1:100; Developmental Studies Hybridoma Bank), and Pax6 (paired box gene) (1:400; Developmental Studies Hybridoma Bank). Rabbit polyclonals were against calretinin and calbindin (1:2000; Swant, Bellizona, Switzerland), Pax2 (1:200; Covance via Cambridge BioScience, Cambridge, UK), Foxg1 (forkhead box) (1:500; a gift from Y. Sasai, Kyoto University, Kyoto, Japan), pan-Dlx (1:100; a gift from J. Kohtz, Northwestern University, Chicago, IL), Lhx2 (Lim homeobox) and Lhx9 (1:5000; a gift from T. Jessell, Columbia University, New York, NY), and Tbr2 (T-box brain gene) (1:200; a gift from R. Hevner, University of Washington, Seattle, WA). Binding of appropriate secondary antibodies (1:200; Vector Laboratories, Peterborough, UK) was revealed with the avidin–biotin–peroxidase system (Vector Laboratories) and diaminobenzidine (Sigma). For immunofluorescence, secondary antibodies were Alexa-488-conjugated goat anti-mouse or Alexa-568-conjugated goat anti-rabbit (Invitrogen, Paisley, UK). Fluorescent images were taken using a Leica (Nussloch, Germany) TCS NT confocal microscope. At least two to three wild-type and Gli3Xt/Xt embryos were used for each of the above antibodies.
In situ hybridization.
In situ hybridizations on paraffin sections were performed as described by Nieto et al. (1996). A 611 bp fragment comprising nucleotides 560–1170 of the mouse Gli3 cDNA (a gift from T. Theil, Heinrich-Heine-University, Dusseldorf, Germany) was PCR amplified and subcloned into a pGEM-T Easy Vector (Promega, Southampton, UK). The plasmid was linearized with SpeI and transcribed with T7 RNA polymerase. A plasmid containing the Shh cDNA (a gift from J. Rubenstein, University of California, San Francisco, San Francisco, CA) was linearized with HindIII and transcribed with T3 RNA polymerase. RNA antisense probes were labeled using the digoxigenin RNA labeling kit (Roche, Welwyn Garden City, UK) according to the instructions of the manufacturer.
Western blotting.
Protein was extracted from whole heads and from dissections of dorsal and ventral telencephalon of E12.5 wild-type and mutant embryos using standard methods. Tissues were homogenized with protease inhibitors and then lysed, sonicated, and boiled. Aliquots of each sample were used for protein quantification with the Pierce (Cramlington, UK) BCA protein assay, according to the instructions of the manufacturer. Equivalent amounts of protein were subjected to SDS-PAGE on a 3–8% gradient Tris-acetate gel (Invitrogen), and protein was transferred to a nitrocellulose membrane, which was incubated with rabbit polyclonal anti-Gli3 antibody (1:100; Santa Cruz Biotechnology, Heidelberg, Germany). After incubating with an HRP-conjugated anti-rabbit IgG secondary antibody (1:2000; DakoCytomation, High Wycombe, UK), signal was detected using ECL Plus detection (Amersham Biosciences, Little Chalfont, UK) according to the instructions of the manufacturer. Band intensity was measured using a densitometer and the Quantity One-4.0.3 software (Bio-Rad, Hemel Hempstead, UK).
Results
Gli3 expression in wild-type forebrain
We examined the expression of Gli3 mRNA in the mouse forebrain at midgestational stages. In situ hybridization showed that, in the telencephalon, Gli3 was expressed in the ventricular zone of the developing neocortex and dorsomedial telencephalon (Fig. 1A,B). No expression was detected in the choroid plexus (Fig. 1A,B), in agreement with previous studies (Grove et al., 1998). In the ventral telencephalon, there was a highlateral-to-lowmedial gradient of expression of Gli3 through the ventricular zone of the lateral ganglionic eminence and medial ganglionic eminence (Fig. 1B). Gli3 was also expressed in the diencephalic ventricular zone, in a high-to-low gradient from epithalamus through dorsal thalamus to ventral thalamus (Fig. 1A) and in the hypothalamus at the level of the optic chiasm (Fig. 1B). Gli3 protein exists in two forms, a long 170 kDa full-length isoform and an 80 kDa isoform formed by cleavage of the full-length product (Aza-Blanc et al., 1997; Dai et al., 1999; Wang et al., 2000). To examine the spatial distribution of these two isoforms, we performed Western blots using an antibody against the N-terminus of Gli3. The results showed bands at ∼170 and 80 kDa, in extracts from wild-type dorsal and ventral telencephalon that were absent in Gli3Xt/Xt tissue (Fig. 1C) and correspond to the previously described full-length and cleaved isoforms, respectively (Aza-Blanc et al., 1997; Dai et al., 1999; Wang et al., 2000). The antibody also detected nonspecific bands of intermediate size and unknown identity, whose intensities varied in proportion to the total amount of protein loaded in the lane and not with genotype or tissue type and which, therefore, served as a useful demonstration of the equality of protein levels loaded in each lane (Fig. 1C, arrow). Total levels of Gli3 protein were approximately twofold higher in the dorsal compared with the ventral telencephalon, reflecting the pattern of Gli3 mRNA expression (Fig. 1C). We then quantified the ratio between the full-length and cleaved forms in these tissues. Dorsally, the cleaved form was present at 2.75 ± 0.45 times (mean ± SD; n = 7) the concentration of the full-length form, whereas ventrally the ratio was lower, at 1.33 ± 0.50 (mean ± SD; n = 8) and the difference between these two ratios was significant (Student’s t test; p < 0.0005). Overall, the highest concentration of Gli3 was of the cleaved form in the dorsal telencephalon (Fig. 1C).
Figure 1.

Gli3 expression in E12.5 forebrain. A, B, In situ hybridization in coronal sections of the diencephalon shows expression of Gli3 mRNA in the epithalamus (ET) and dorsal (DT) and ventral (VT) thalamus and in the optic chiasm (op). In the telencephalon, Gli3 is expressed in the neocortex (nctx), dorsomedial telencephalon (dmT), and the lateral (LGE) and medial (MGE) ganglionic eminences and is absent from the choroid plexus (ChP), indicated by an arrowhead. Scale bars, 250 μm. C, Western blot analysis of Gli3 protein in E12.5 wild-type and Gli3Xt/Xt tissue. In dorsal (D) and ventral (V) wild-type telencephalic tissue, both the long (170 kDa) and short (80 kDa) Gli3 isoforms are present, although their relative amounts differ. Both isoforms are absent in Gli3Xt/Xt whole-head protein extracts. The arrowhead indicates a nonspecific band that is used as an internal loading control.
Identification of the limits of the dorsal telencephalon in Gli3Xt/Xt mutants
We performed a detailed rostrocaudal morphological comparison of E12.5 wild-type and Gli3Xt/Xt forebrain sections immunolabeled with Pax6, which is highly expressed in the dorsal telencephalon and diencephalon (Walther and Gruss, 1991; Stoykova and Gruss, 1994; Mastick et al., 1997), and Mash1, which is expressed in the ventral telencephalon and diencephalon (Lo et al., 1991; Guillemot and Joyner, 1993; Porteus et al., 1994). Figure 2 depicts representative sets of wild-type and mutant forebrain sections in a caudal-to-rostral order. The most caudal diencephalic parts of the mutant forebrain (epithalamus, dorsal and ventral thalamus) appear relatively normal both anatomically and in their expression of Pax6 and Mash1 (Fig. 2A,A′,B,B′,E,E′,F,F′), although the ventricular space appears slightly enlarged in mutants. At a level in which caudal telencephalic lobes are clearly visible in wild-type sections (Fig. 2B,F), only the most caudal tips of the telencephalic lobes are observed in corresponding Gli3Xt/Xt sections (Fig. 2B′,F′). This may be because of the diminished size of the Gli3Xt/Xt lobes compared with wild type and/or their altered position. Moving rostrally, the dorsomedial telencephalon and choroid plexus, seen in wild types in Figure 2, C, D, G, and H, are absent in Gli3Xt/Xt embryos, and the connection between the third and the lateral ventricle is greatly enlarged (Fig. 2C′,D′,G′,H′). The intense labeling for Pax6 in the ventral thalamus of mutant embryos (Fig. 2B′) is continuous with strong staining for Pax6 in the neocortex (Fig. 2C′), because Pax6 low-expressing tissue that would normally intervene (the dorsomedial telencephalon) (Fig. 2C) is missing in the mutant. In wild-type sections, Mash1 is expressed in the ganglionic eminences (Fig. 2G,H) and in the ventral thalamus (Fig. 2F,G) but is not observed in the neocortex (Fig. 2G,H, area of tissue between the lines). In comparable sections in the Gli3Xt/Xt mutant, Mash1 immunoreactivity is also present in the ganglionic eminences (Fig. 2G′,H′) and absent from the adjacent Pax6-positive area (Fig. 2, compare C′, G′). This suggests that the Mash1-negative region (Fig. 2G′,H′, area between the lines) is neocortical tissue, and the adjacent dorsal region of intense Mash1 staining most likely corresponds to diencephalic tissue [ventral thalamus and/or eminentia thalami (Fig. 2G′, labeled VT&EmT)].
Figure 2.

Pax6 and Mash1 immunoreactivity in the forebrain of E12.5 wild-type (Gli3+/+) (A–D, E–H) and Gli3 mutant (Gli3Xt/Xt) (A′–D′, E′–H′) embryos in comparable caudal-to-rostral coronal sections. In the diencephalon, Pax6 expression is observed in the epithalamus (ET) and dorsal (DT) and ventral (VT) thalamus, in both wild type (A, B) and mutants (A′, B′). In wild-type diencephalon, Pax6 expression is also evident in the ventricular zone of the eminentia thalami (EmT) (C). In wild-type telencephalon (Tel), Pax6 expression is observed in the neocortex (nctx) and the dorsomedial telencephalon (dmT, indicated by an arrow) and is absent from the ventral telencephalon [ganglionic eminences (GE)] (C, D). The arrowheads in C and D point to lack of Pax6 expression in the choroid plexus (ChP). In the mutant, Pax6 staining is continuous throughout the diencephalon and dorsal telencephalon, and the boundary between these two structures is not recognized (C′, D′). As in wild type (C, D), no Pax6 staining is observed in the ganglionic eminences in the mutant (C′, D′). Mash1 expression is observed in the epithalamus, ventral thalamus, hypothalamus (HT), and ganglionic eminences, in both wild type (E–H) and mutants (E′–H′). The neocortex is negative for Mash1 in both wild type (H, I) and mutant (area between the lines in G′, H′). The bracket in G′ labels a diencephalic region that corresponds to the mutant ventral thalamus and eminentia thalami. Tel, Telencephalon. Scale bars, 250 μm.
To confirm that Mash1-expressing tissue adjacent to the neocortex is diencephalic and not telencephalic in origin in Gli3Xt/Xt embryos, we performed immunohistochemistry using an antibody against Foxg1. Foxg1 is expressed in the dorsal and ventral telencephalon and is absent from the dorsal and ventral diencephalon (Tao and Lai, 1992; Hanashima et al., 2002). As shown in Figure 3, A and A′, Foxg1 expression is found in the telencephalon of both wild-type and Gli3Xt/Xt embryos and is absent from the adjacent Mash1-positive tissue in the mutant (Fig. 2G′, labeled VT&EmT), confirming that this tissue is not telencephalic. These results clearly delineate the neocortical region and confirm that the Mash1-positive region dorsal to the Foxg1-positive neocortical region is diencephalic.
Figure 3.

Forebrain marker analysis in wild-type (Gli3+/+) (A–D) and Gli3 mutant (Gli3Xt/Xt) (A′–D′) E12.5 coronal sections. Foxg1 protein expression delineates telencephalic tissue in wild type (A) and mutant (A′). The area between the lines corresponds to Mash1-negative neocortex (nctx) in the mutant (see Fig. 2G′, H′). Islet1 and Dlx proteins are both found in the ventral telencephalon [ganglionic eminences (GE)] in both wild type (B, C) and mutant (B′, C′). They are also observed in the ventral thalamus (VT) in wild type and within a region in the mutant that corresponds to the ventral thalamus and eminentia thalami (EmT). Note that neither Islet1 nor Dlx proteins are expressed in neocortex of the mutant (area between the lines in B′, C′). Shh mRNA is expressed in the ZLI in wild type (D) and mutant (D′), defining the border between the dorsal thalamus (DT) and ventral thalamus. Note that the orientation of the Shh-positive ZLI is perpendicular to the main axis in the wild types but not in the mutants. Shh mRNA expression is also observed in the hypothalamus (HT) in both wild type and mutant. Scale bars, 250 μm.
To provide additional evidence for this, we examined the pattern of expression of Islet1 and Dlx in Gli3Xt/Xt E12.5 embryos (Fig. 3B′,C′). These genes are normally expressed in the ventral telencephalon and the ventral thalamus (Fig. 3B,C), and previous reports have suggested that they are ectopically expressed in the dorsal telencephalon of the Gli3Xt/Xt mutant (Tole et al., 2000; Rallu et al., 2002; Kuschel et al., 2003). As in wild types, the expression of Islet1 and Dlx is confined to (1) the ventral telencephalon and (2) the ventral thalamus and/or eminentia thalami region in Gli3Xt/Xt embryos (Fig. 3B′,C′). That this latter region corresponds to the mutant equivalent of ventral thalamus and/or eminentia thalami is further supported by the expression pattern of Shh transcript in the zona limitans intrathalamica (ZLI) (Echelard et al., 1993; Marti et al., 1995), which defines the border between the dorsal and ventral thalamus (Figdor and Stern, 1993; Rubenstein et al., 1994; Kiecker and Lumsden, 2004). Shh mRNA expression is present in both wild-type and Gli3Xt/Xt sections (Fig. 3D,D′), showing clearly that the Islet1, Dlx-positive region just below the ZLI corresponds to ventral thalamus in both wild types and mutants. Furthermore, the region that corresponds to the neocortex (Fig. 3B′,C′, area of tissue between the lines) does not express either Islet1 or Dlx. Altogether, our results show that the previously described ectopic expression of Mash1, Islet1, and Dlx in the dorsal telencephalon of Gli3Xt/Xt mutants (Tole et al., 2000; Rallu et al., 2002; Kuschel et al., 2003) is actually expression in the diencephalon (ventral thalamus and/or eminentia thalami).
We examined Pax6 and Mash1 expression patterns during later stages of Gli3Xt/Xt forebrain development by immunohistochemistry on caudal-to-rostral sets of sections from E13.5 to E15.5. At most levels, Pax6 immunoreactivity was confined mainly to the dorsal telencephalon and ventral thalamus, and Mash1 immunoreactivity was confined to the ventral telencephalon and ventral thalamus in both wild types and Gli3Xt/Xt mutants (Fig. 4A,A′,B,B′), similar to results at E12.5. Results for both Mash1 and Pax6 expression in E14.5 and E15.5 wild-type and Gli3Xt/Xt tissue were also similar (data not shown), although their interpretation at these stages became more complex because of the high degree of disorganization of the putative Gli3Xt/Xt neocortex (see below).
Figure 4.
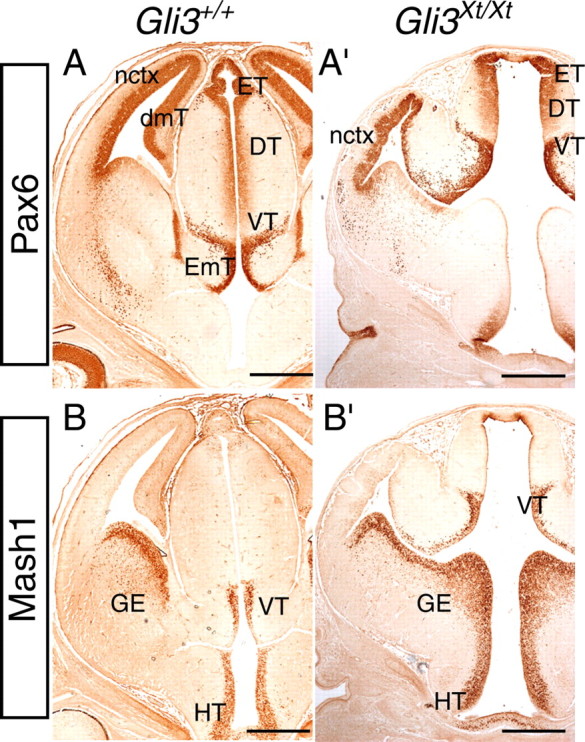
Expression patterns of Pax6 and Mash1 in E13.5 coronal wild-type (A, B) and Gli3Xt/Xt (A′, B′) sections. In wild type, Pax6 expression is observed in the neocortex (nctx), dorsomedial telencephalon (dmT), epithalamus (ET), dorsal (DT) and ventral (VT) thalamus, and the ventricular zone of the eminentia thalami (EmT) (A). In Gli3Xt/Xt mutants, expression is found in similar areas, except the dorsomedial telencephalon, which is not present (A′). Mash1 expression is observed in the ventral telencephalon [ganglionic eminences (GE)] and in the ventral thalamus and hypothalamus (HT) in wild type (B). At a comparable level, Mash1 expression is found in a similar region in Gli3Xt/Xt mutants (B′). Scale bars, 500 μm.
The Gli3Xt/Xt neocortex contains clusters of cells with characteristics of the eminentia thalami
High-power images of Foxg1 immunostaining at E12.5 revealed patchy expression of Foxg1 in the Gli3Xt/Xt neocortex (Fig. 5A′), which was not observed in the wild type (Fig. 5A). A similar patchy staining was also observed with an antibody against Lhx2 (Fig. 5B′), which is normally expressed throughout the neocortex in a highdorsal-to-lowlateral gradient (Fig. 5B) (Monuki et al., 2001).
Figure 5.
Dorsal telencephalic and eminentia thalami marker analysis in wild-type (Gli3+/+) (A–G) and Gli3 mutant (Gli3Xt/Xt) (A′–G′) coronal (A–E, G and A′–E′, G′) and sagittal (F, F′) sections at E12.5. Foxg1 immunolabeling reveals the presence of patches lacking Foxg1 staining in the mutants (A′) that are never observed in corresponding wild-type sections (A). Lhx2 immunolabeling, which presents a highdorsal-to-lowlateral gradient in the wild-type neocortex (nctx) (B), reveals the presence of immunonegative patches in the mutant neocortex (B′). Calretinin expression is detected in the eminentia thalami (EmT) of both wild types (C, D) and mutants (C′, arrowhead in D′). In the mutants, calretinin-positive clusters localize in the vicinity of the Foxg1-immunonegative patches (compare A′, C′). Similarly, Lim2 is expressed in the eminentia thalami in both wild type (E) and mutant (E′) and reveals the presence of Lim2-positive clusters in the vicinity of the Foxg1-immunonegative patches in the mutant (E′). Double immunofluorescence with Foxg1 (red) and Lim2 (green) labels the neocortical region and eminentia thalami, respectively, in the wild type (F). In the mutant, Foxg1-immunonegative patches are immunopositive for Lim2 (F′). Pax2 immunostaining reveals the presence of a small population of positive cells at the most dorsolateral tip of the eminentia thalami, close to the choroid plexus (ChP) in the wild type (G). In the mutant, a similar Pax2-immunopositive population is observed in a region that is Lim2 positive and Foxg1 negative (arrow). However, additional Pax2-positive cells are observed among the Foxg1-immunonegative patches (arrowheads). C and C′ are magnifications of the boxed areas in B and B′, respectively. Sections in C, C′, D, and D′ are counterstained with cresyl violet. In F and F′, rostral is to the left. Scale bars: A, A′, B′, C′, 50 μm; B, E, E′, F′, G, G′, 100 μm; C, F, 150 μm; D, D′, 400 μm. dmT, Dorsomedial telencephalon; GE, ganglionic eminence.
We examined the nature of the Foxg1-negative cells observed in the Gli3Xt/Xt neocortical area. Results described above suggested their possible identity. They are immunonegative for Mash1, Islet1, and Dlx (Figs. 2G′,H′, 3B′,C′) (supplemental data, available at www.jneurosci.org as supplemental material), indicating that they do not share properties with neuronal progenitors or postmitotic cells from the ventral telencephalon and/or ventral thalamus. However, the tissue negative for Foxg1, Mash1, Islet1, and Dlx is positive for Pax6 (Fig. 2C′,D′) (supplemental data, available at www.jneurosci.org as supplemental material). Pax6 is expressed not only in the dorsal telencephalon and ventral thalamus but also in the ventricular zone of the eminentia thalami (Fig. 2C) (Puelles et al., 2000), which forms part of the rostral boundary between the diencephalon and the telencephalon (Rubenstein et al., 1994; Puelles and Rubenstein, 2003). We examined whether the Foxg1-immunonegative patches could have an eminentia thalami identity and used as a marker calretinin, which labels eminentia thalami postmitotic cell somata and fibers in wild-type mice (Fig. 5C,D) (supplemental data, available at www.jneurosci.org as supplemental material) (Abbott and Jacobowitz, 1999). In Gli3Xt/Xt mutants, the eminentia thalami region, as revealed by intense calretinin staining, lies above the dorsal limit of the neocortical area and displays a thinner postmitotic layer compared with the wild type (Fig. 5C′,D′) (supplemental data, available at www.jneurosci.org as supplemental material). Adjacent to this area, small, calretinin-positive cell clusters were observed in the mutant neocortex (Fig. 5C′,D′).
To provide additional evidence about the eminentia thalami nature of these clusters, we examined the expression pattern of the transcription factor Lim2, also known as Lhx5, which specifically labels the eminentia thalami and ventral thalamus in E12.5 wild types (Fig. 5E) (Sheng et al., 1997). The antibody used recognizes both Lim1 (Lhx1) and Lim2 (Lhx5) proteins, but Lim1 mRNA expression is very weak in these tissues at this age (Sheng et al., 1997). In the Gli3Xt/Xt mutant neocortex, Lim2 immunostaining presented a patchy expression (Fig. 5E′), similar to that observed with calretinin and complementary to the Foxg1-negative patches (Fig. 5, compare A′, C′, E′). To confirm this, we performed double immunofluorescence for Foxg1 and Lim2 in sagittal E12.5 wild-type and Gli3Xt/Xt sections. In wild types, Lim2 immunostaining was confined in the eminentia thalami, whereas Foxg1 specifically labeled the dorsal and ventral telencephalon (Fig. 5F). In Gli3Xt/Xt mutant, Lim2 immunostaining was found in the Foxg1-positive region in the form of patches, and the two markers did not colocalize (Fig. 5F′). Tbr1, another marker of the eminentia thalami (Puelles et al., 2000), also revealed the presence of clusters in the vicinity of the Foxg1-immunonegative patches (data not shown).
Finally, we observed that Pax2, a well described marker of the hindbrain, optic chiasm, and optic stalk (Nornes et al., 1990; Puschel et al., 1992), labels a distinct population of eminentia thalami cells, found in close proximity to the choroid plexus in wild-type sections (Fig. 5G). In the Gli3Xt/Xt mutants, Pax2 immunostaining revealed dispersed patchy-like expression (Fig. 5G′) that was similar to that revealed with the calretinin and Lim1/2 antibodies. Similar results were also obtained in a sagittal plane for all of the eminentia thalami markers examined (calretinin, Tbr1, and Pax2) (data not shown).
The above results strongly support an eminentia thalami identity for the cell clusters observed in the neocortical region of the Gli3Xt/Xt mutants. However, because calretinin, Lim2, and Tbr1 also label Cajal-Retzius cells in the marginal zone (del Rio et al., 1995; Super et al., 1998; Hevner et al., 2001, 2003; Yamazaki et al., 2004), it was possible that the calretinin-positive, Lim2-positive, and Tbr1-positive clusters in the Gli3Xt/Xt neocortex comprised this cell type. To examine this possibility, we used immunostaining with reelin, which is also found in Cajal-Retzius cells (Alcantara et al., 1998), and calbindin, which is not normally observed in this cell population (Hevner et al., 2003; Jimenez et al., 2003). We did not observe any reelin-positive cell clusters in the Gli3Xt/Xt mutant (Fig. 6A′,B′). In fact, the number of reelin-positive cells was significantly lower than in wild type (Fig. 6A,A′,B,B′), which is in agreement with recently published data (Theil, 2005). Calbindin immunostaining was detected in the ventral telencephalon in both wild type and mutant (Fig. 6C,C′), as described previously (Davila et al., 2005). However, it also labeled lightly eminentia thalami neurites in the wild type and mutant (Fig. 6D,D′) and was also detected in the vicinity of calretinin-positive clusters in the neocortex (Fig. 6C,C′,D,D′) (compare Figs. 6D′, 5C′). These results indicate that the calretinin-positive, Lim2-positive, and Tbr1-positive clusters observed among the Foxg1-negative patches are not Cajal-Retzius cells.
Figure 6.

Reelin and calbindin immunoreactivity in E12.5 coronal wild-type (A–D) and Gli3Xt/Xt (A′–D′) forebrain sections. Cajal-Retzius cells in the wild-type neocortex (nctx) are labeled with reelin (A, B). The few reelin-positive cells in the mutant neocortex (A′, B′) do not correspond to the patchy areas devoid of Foxg1 staining (see Fig. 5A′). Arrowheads (B′) indicate reelin-positive cells that are not located in the most outer layer of the Gli3Xt/Xt neocortex. Reelin also labels the eminentia thalami (EmT) in both wild type (A) and mutant (arrowhead in A′). Calbindin stains cell somata and fibers in the ganglionic eminences (GE) and fibers in EmT in both wild type (C, D) and mutant (arrowhead in C′). Adjacent to the eminentia thalami in the mutant, small clusters of calbindin-positive fibers (C′, D′) are present in the region of the neocortex. Sections in C, C′, D, and D′ are counterstained with cresyl violet. Note that D and D′ are serial sections from the same specimens as in Figure 5, A, C, A′, and C′, respectively. B, D and B′, D′ are magnifications of the boxed areas in A, C and A′, C′, respectively. Scale bars: A, A′, C, C′, 250 μm; B, B′, D, D′, 50 μm.
A few dispersed reelin-positive cells in the Gli3Xt/Xt neocortex (Fig. 6B′, arrows) were found in the same region as the calretinin/calbindin-positive ectopic clusters (data not shown), suggesting that a few Cajal-Retzius cells (Fig. 6B) may contribute to these clusters of displaced eminentia thalami cells. However, it is likely that these reelin-positive cells are derived from the eminentia thalami, because reelin also labels a population of eminentia thalami cells (Fig. 6A).
Altogether, our results clearly show that the neocortex of Gli3Xt/Xt embryos contains not only neocortical cells but also cells of eminentia thalami identity, indicating that the border between the telencephalon and diencephalon is compromised in this mutant.
Eminentia thalami clusters are first observed in the Gli3Xt/Xt neocortex at E11.5
To gain insight into the developmental stage at which the eminentia thalami clusters start to form in the neocortical region of Gli3Xt/Xt embryos, we studied the expression of eminentia thalami markers in sagittal sections at E11.5 (Fig. 7). Sections through wild types (Fig. 7A–C) are at, or close to, the plane marked (I) in supplemental data A (available at www.jneurosci.org as supplemental material); sections through mutants are at the planes marked in supplemental data A′ (available at www.jneurosci.org as supplemental material) as either (II) (Fig. 7A′–C′) or (III) (Fig. 7A″–C″). E11.5 is the stage at which wild-type calretinin expression is first observed in the eminentia thalami (Abbott and Jacobowitz, 1999). In wild types, the eminentia thalami lacks Foxg1 (Fig. 7A), expresses calretinin in its differentiating cells (Fig. 7B) and Pax2 in a collection of cells close to the choroid plexus (Fig. 7C), and is located caudal to the dorsomedial telencephalon, which does not express Foxg1 (Fig. 7A) (Dou et al., 1999). In mutants, the Foxg1 nonexpressing eminentia thalami is directly caudal to the dorsal telencephalon, which is immunopositive for Foxg1 (Fig. 7A′,A″,a). As in wild types, its differentiating cells are detected by their expression of calretinin (Fig. 7B′,B″). Pax2 is expressed mainly in dorsal eminentia thalami of mutants (Fig. 7C′,C″). In Gli3Xt/Xt mutants, calretinin-positive and Pax2-positive cell clusters were observed in close proximity to the eminentia thalami and within the Foxg1-positive telencephalic region (Fig. 7B′,B″,b,C′,C″,c, arrowheads). Clusters that were positive for both calretinin and Pax2 were more numerous in the most lateral parasagittal sections of the Gli3Xt/Xt mutants (Fig. 7B″,b,C″,c), in which the Foxg1-immunonegative dorsal telencephalic patches are also more abundant (Fig. 7A″,a). These results show that, at E11.5, eminentia thalami cells are already present in the Gli3Xt/Xt neocortex in the form of clusters.
Figure 7.
Foxg1, calretinin, and Pax2 immunoreactivity in wild-type (Gli3+/+) (A–C) and Gli3 mutant (Gli3Xt/Xt) (A′–C′, A″–C″, a–c) sagittal sections at E11.5. Rostral is to the left. Foxg1 labels the dorsal (dTel) and ventral (vTel) telencephalon in both wild type (A) and mutant (A′, A″). It is absent from the dorsomedial telencephalon (dmT), eminentia thalami (EmT), and diencephalon (D) in wild types (A) and from the EmT (bracketed area in A′, A″, a) and diencephalon (A′) in mutants. The bracket in A labels the dorsomedial telencephalon and eminentia thalami regions, and the dashed line indicates the approximate border between these two regions. Foxg1-negative patches are located in the dorsal telencephalon in the mutant (a, and indicated by arrowheads in A′) and are never observed in wild types (A). Calretinin labels cell somata and fibers in the postmitotic layer of both the wild type (B) and mutant (B′, B″) EmT (indicated by brackets). In the mutant, calretinin-positive clusters (b, and arrowheads in B′ and B″) are observed in the vicinity of the Foxg1-immunonegative patches (compare B′ with A′ and b with a). Pax2 labels an eminentia thalami cell population in proximity to the choroid plexus (ChP) (indicated by an arrow) in the wild type (C). In the mutant, Pax2 labels cells in the EmT (bracketed area in C′ and C″) but is also found within the dorsal telencephalon (arrows in C′ and C″). As with the calretinin-positive clusters (B″, b), the Pax2-positive clusters are more abundant in the most lateral sections (C″, c). a–c are magnifications of the boxed areas in A″–C″, respectively. Sections B and C are midsagittal and correspond to level (I), indicated by a dashed line in supplemental data A (available at www.jneurosci.org as supplemental material). Section A is slightly more lateral to B and C. Sections A′–C′ and A″–C″ are parasagittal and correspond to levels (II) and (III) respectively, indicated by dashed lines in supplemental data A′ (available at www.jneurosci.org as supplemental material). Scale bars: A–C, A′–C′, A″–C″, 100 μm; a–c, 50 μm.
At E10.5, the choroid plexus and eminentia thalami have not differentiated yet into distinct morphological structures (Sturrock, 1979; Abbott and Jacobowitz, 1999). In addition, markers that specifically label the eminentia thalami at later developmental stages are either not expressed yet (calretinin and Tbr1) or label a broader region (Lim2) (Abbott and Jacobowitz, 1999). The only informative marker for the study of the developing eminentia thalami at E10.5 was Pax2.
Sagittal sections through the telencephalic–diencephalic boundary region of wild-type E10.5 embryos showed Pax2-positive cells in a similar relative position to that at E11.5 (Fig. 8A,a). They are within a region identified as diencephalic on the basis of morphology, intense Pax6 immunoreactivity (Fig. 8A,a) (Mastick et al., 1997), and absence of Foxg1 immunoreactivity (data not shown). These data indicate that, as at E11.5, the Pax2-expressing cells are on the diencephalic side of the diencephalic–telencephalic boundary at E10.5, in close proximity to the hippocampal primordium, which is immunonegative for Foxg1 (Dou et al., 1999).
Figure 8.

Pax2, Pax6, and Foxg1 immunoreactivity in wild-type (Gli3+/+) (A, a, B) and Gli3 mutant (Gli3Xt/Xt) (A′, a′, A″, a″, B′, B″) sagittal sections at E10.5. Pax2-immunopositive cells are located within the Pax6-positive diencephalon (D) in both wild type (A, a) and mutant (A′, a′). A few dispersed Pax2-positive cells are located in the Pax6-positive dorsal telencephalon (dTel) in the mutant (arrowheads in A′ and a′) but are never observed in the wild-type dorsal telencephalon. The big arrowhead in A′ indicates the Pax2-immunopositive optic cup. Foxg1-immunolabeling shows the limits of the dorsal telencephalon in the mutants (A″, a″). Double immunofluorescence with Pax6 (green) and Pax2 (red) reveals the presence of diencephalic cells that are either Pax2 positive and Pax6 positive (arrows) or Pax2 positive and Pax6 negative (asterisks) in both wild type (B) and mutant (B″). Some of the displaced cells found in the dorsal telencephalic region in the mutants are Pax2 positive and Pax6 negative (asterisk in B′), further supporting their eminentia thalami origin. Note that A′ and A″ are serial sections from the same specimen. a, a′, and a″ are magnifications of the boxed areas in A, A′, and A″, respectively. B′ and B″ are magnifications of the boxed areas of the inset in B′. Scale bars: A, A′, A″, 100 μm; a, a′, a″, B, B′, B″, 50 μm.
In the E10.5 mutant, Pax2-positive cells are mainly within the diencephalic region, which presents intense Pax6 immunostaining similar to that observed in the wild type (Fig. 8A′,a′). This tissue is joined directly to Foxg1/Pax6-immunopositive tissue corresponding to the Gli3Xt/Xt dorsal telencephalon (Fig. 8A″,a″), because the intervening Foxg1-immunonegative hippocampal primordium is absent in the Gli3Xt/Xt embryos, in accordance with published data (Grove et al., 1998; Theil et al., 1999; Tole et al., 2000). A few, isolated Pax2-positive cells were observed in the Foxg1/Pax6-positive dorsal telencephalon (Fig. 8A′,a′,A″,a″, arrowheads, B′, asterisk). These results show that, at E10.5, individual Pax2-immunopositive cells from the Gli3Xt/Xt diencephalon are present ectopically in the adjacent dorsal telencephalon of the mutants.
In the diencephalon, we observe a mixed population of diencephalic cells that either express Pax2 but not Pax6 (Fig. 8B,B″, asterisks) or Pax2 and Pax6 (Fig. 8B,B″, arrows) in both wild types and mutants. In the mutant, some of the Pax2-immunopositive cells located within the dorsal telencephalon coexpress Pax2 and Pax6 (data not shown) or express Pax2 but not Pax6 (Fig. 8B′, asterisk), whereas all surrounding nonmitotic cells that do not express Pax2 express Pax6. The Pax6 expression profile of the ectopically located Pax2-positive cells in the mutant dorsal telencephalon strengthens the possibility that the Pax2-positive cells have an eminentia thalami identity.
Rosette-like structures form in the residual Gli3Xt/Xt neocortex
To study the development of the Gli3Xt/Xt neocortex and the behavior of the eminentia thalami clusters at later developmental stages, we immunostained consecutive mutant sections for calretinin and Foxg1 from E13.5 to E16.5. Foxg1-negative areas were observed in the E13.5 Gli3Xt/Xt neocortical region (Fig. 9A). At later stages of development (E14.5 and E16.5), the Gli3Xt/Xt neocortical area became highly disorganized and large Foxg1-negative patches were found in the midst of Foxg1-positive tissue (Fig. 9B,C). At E13.5 and E14.5, calretinin-positive clusters were still found among neocortical cells (Fig. 9D,E), and, in most cases, these two cell types seemed to segregate from the Foxg1-positive region (Fig. 9, compare A,B with D,E). At E16.5, no calretinin-positive clusters were detected, in agreement with the reduced calretinin expression observed in the wild-type eminentia thalami at this stage (data not shown).
Figure 9.

The development of the Gli3Xt/Xt neocortex after E12.5. As for E12.5, Foxg1-negative patches are also observed at E13.5 (A) and E14.5 (B) Gli3Xt/Xt sections and are positive for calretinin (D, E). At E16.5, Foxg1-negative patches persist (C) but are no longer labeled with calretinin. Note that A, D and B, E are serial sections from the same specimens. After E12.5, rosettes start to form in the Gli3Xt/Xt neocortex. Rosettes are positive for Foxg1 (G), indicating that this tissue is telencephalic and not diencephalic. These rosettes are immunopositive for Pax6 (H), BrdU (I), and nestin (J) and immunonegative for β-tubulin III (Tuj1) (K), demonstrating that these clusters comprise neural progenitors. Tbr2 is present in the outermost cells of the rosettes (L). Cresyl violet staining at E16.5 reveals a greater number of rosettes with a less visible lumen (F, F′). F′ is a high magnification of the boxed area in F. Scale bars: A–F, 500 μm; F′, 100 μm; G–L, 50 μm.
At E13.5, Foxg1-positive tissue in the residual neocortex had formed rosette-like structures (Fig. 9A). Although the number and size of these structures were variable, they all consistently contained a central lumen and were formed close to the ventricular zone. There was no obvious correlation between the frequency of their appearance and position within the neocortex. The fact that these rosettes were Foxg1 positive (Fig. 9A,G) and Mash1 negative (data not shown) indicated that they comprised neocortical tissue and did not have a ventral telencephalic or diencephalic character. They were immunopositive for markers of neural progenitors, such as Pax6 (Fig. 9H), BrdU (Fig. 9I), and nestin (Fig. 9J), and negative for the postmitotic neuronal marker β-tubulin III (Fig. 9K). Tbr2 staining, which is normally detected in the cortical intermediate zone and early postmitotic neurons (Englund et al., 2005), was found in the outermost cells of the rosettes (Fig. 9L). At later stages, rosettes became more numerous and were also found close to the pial surface (Fig. 9B,C,F). At E16.5, the lumen in the center of the rosettes was not as clearly distinguishable as in previous stages (Fig. 9F′). These findings indicate that the rosettes contain cells with a neocortical identity that continue to proliferate in an organized manner, near the lumen.
Discussion
The severe reduction in size of the dorsal telencephalon and the lack of dorsomedial telencephalon in the Gli3Xt/Xt mouse have been reported (Grove et al., 1998; Theil et al., 1999; Tole et al., 2000). However, previous studies neither precisely defined the extent of the remaining dorsal telencephalon nor showed how this disrupted structure is joined to its neighboring region, the diencephalon. Our study allowed us to identify the area of the Gli3Xt/Xt forebrain corresponding to neocortical tissue (summarized in supplemental data, available at www.jneurosci.org as supplemental material). By studying the expression patterns of Foxg1, which is expressed in developing telencephalon but not diencephalon (Tao and Lai, 1992; Hanashima et al., 2002), Pax6, a well characterized marker of the dorsal telencephalon and diencephalon (Walther and Gruss, 1991; Stoykova and Gruss, 1994; Mastick et al., 1997), and Mash1, which is expressed in both the ventral telencephalon and ventral thalamus (Lo et al., 1991; Guillemot and Joyner, 1993; Porteus et al., 1994), we were able to distinguish the dorsal and ventral limits of the residual Gli3Xt/Xt dorsal telencephalon. Based on this analysis, we demonstrated that the Mash1-, Dlx-, and Islet1-immunopositive regions found at the dorsal end of the Gli3Xt/Xt neocortex correspond to the ventral thalamus. This is in contrast to previous studies that have reported that these ventral telencephalic markers are ectopically expressed in the dorsal telencephalon of Gli3Xt/Xt mice (Tole et al., 2000; Rallu et al., 2002; Kuschel et al., 2003). This discrepancy can be attributed to the fact that previous studies did not define the Gli3Xt/Xt telencephalic limits relative to the diencephalon and did not consider that many ventral telencephalic markers are also expressed in the developing diencephalon.
Correspondence between the severity of the forebrain defects in Gli3Xt/Xt forebrain and the expression of Gli3
Our expression analysis of Gli3 mRNA in the developing mouse telencephalon reveals high expression dorsally and a highlateral-to-lowmedial gradient ventrally, as described previously (Grove et al., 1998), and is in accordance with the severe phenotypic defects of the Gli3Xt/Xt mutants dorsally and the absence of gross alterations ventrally. Furthermore, it agrees with our observation that levels of Gli3 protein are higher in the dorsal than in the ventral telencephalon.
Estimates of the relative amounts of the long and short isoforms of Gli3 in the developing telencephalon showed that there is significantly more of the short than the long isoform in the dorsal telencephalon, whereas there are almost equivalent levels of both isoforms ventrally. The ratio of the cleaved to the full-length isoform of Gli3 in the dorsal and ventral telencephalon are analogous to those described in the anterior and posterior limb bud, respectively (Wang et al., 2000; Litingtung et al., 2002; Chen et al., 2004). Litingtung et al. (2002) proposed that the ratio of the two Gli3 forms is crucial for digit number and identity. Similarly, regional differences in this ratio may be important for dorsoventral patterning of the telencephalon. Relatively high expression of the cleaved Gli3 repressor form in the anterior limb domain has been correlated with absence of Shh signaling (Wang et al., 2000), in a similar manner to that described in the Drosophila anterior wing bud (Methot and Basler, 1999). In the dorsal telencephalon, absence of Shh (Sussel et al., 1999; Nery et al., 2001) may account for the high levels of the processed Gli3 isoform, which might act as a repressor of the Shh signaling pathway.
Neocortical cells form rosettes intermingled with eminentia thalami cells in Gli3Xt/Xt mutants
Having defined the limits of the presumptive neocortex in the E12.5 Gli3Xt/Xt mutant, we evaluated its development at later stages. Our analyses led to the observation of two striking features. First, starting at E13.5, we noticed the formation of rosettes, which consisted of cells surrounding a lumen. These became more numerous as development proceeded. They were composed of neocortical neural progenitors, and the relative position of S-phase cells around the lumen was similar to that of S-phase cells around the ventricle in wild types. Tbr2 expression in cells surrounding the outermost surface of the rosettes resembled that observed overlying the ventricular zone in the neocortex of wild types (Englund et al., 2005). It appears that the rosettes comprise well organized neocortical progenitors that segregate from surrounding cells, many of which express markers of the eminentia thalami.
Previous work described the formation of aberrant structures in the Gli3Xt/Xt dorsal telencephalon but did not characterize the cell types involved (Theil et al., 1999). Rosette-like structures forming close to the ventricle have also been described in mice with loss-of-function mutations in the genes for the membrane-associated protein Lgl1 (lethal giant larvae homolog 1) (Klezovitch et al., 2004) and the myosin II-B heavy chain (Tullio et al., 2001). Their formation has been attributed to alterations in the adhesive properties of the neuroepithelial cells and in their apical–basal cell polarity, defects that have been described recently in Gli3Xt/Xt neocortical tissue (Theil, 2005) and may be the primary cause for the rosette formation in this mutant.
The second important observation in the E12.5 Gli3Xt/Xt dorsal telencephalon was the presence of patches of Foxg1-negative cells that expressed markers of the neighboring eminentia thalami among the Foxg1-positive neocortical cells. Calretinin, which labels postmitotic cells and fibers found in the eminentia thalami (Abbott and Jacobowitz, 1999), was expressed by cells in these patches that were located near the marginal zone. Additional evidence for the origin of these clusters was their expression of Lim2 and Tbr1, known markers of eminentia thalami (Sheng et al., 1997; Puelles et al., 2000), and of Pax2, a newly described marker of an eminentia thalami population found close to the choroid plexus in wild types.
In mammals, the eminentia thalami is a transitional developmental structure (Keyser, 1972), is proposed to act as an organizer for the diencephalon, and appears at approximately E11 (Abbott and Jacobowitz, 1999). It is found in the rostralmost diencephalic area (prosomere 3) (Puelles and Rubenstein, 2003) and forms part of the rostral boundary between the diencephalon and the telencephalon (Trujillo et al., 2005). We found that eminentia thalamic clusters first appear among the dorsal Gli3Xt/Xt telencephalon at E11.5. At E10.5, when eminentia thalami cells have not yet started to differentiate and the medial walls of the dorsal telencephalon have not invaginated, we observed a few dispersed Pax2-positive cells within the Foxg1/Pax6-positive dorsal telencephalic area, close to the presumptive mutant diencephalic–telencephalic boundary. These Pax2-positive cells are most likely the precursors of the Pax2-positive clusters observed 1 d later. The most straightforward hypothesis to explain the aberrant presence of cells of eminentia thalami identity in neocortical tissue in Gli3Xt/Xt mutants is that the medial wall of the dorsal telencephalon normally prevents eminentia thalami cells reaching the neocortex. If the dorsomedial telencephalon is lost, as in Gli3Xt/Xt mutants, the lifting of this restriction on the movement of eminentia thalami cells might allow them to mix with neocortical cells. The cortical hem, a Bmp/Wnt (bone morphogenetic protein/wingless-type MMTV integration site family)-rich tissue found in the dorsomedial telencephalon, is suggested to act as a signaling center (Grove et al., 1998; Grove and Tole, 1999) and is missing in Gli3Xt/Xt mutants (Grove et al., 1998; Theil et al., 1999; Tole et al., 2000). This tissue might be the source of molecular signals that prevent mislocation of diencephalic cells in the telencephalon. It is interesting that cells characteristic of eminentia thalami mislocated in the dorsal telencephalon at E10.5 are not respecified to the fate of the majority of their neighbors, suggesting a strong commitment to an eminentia thalami fate. The fact that some of these Pax2-mislocated cells do not express Pax6 provides additional evidence as to their eminentia thalami origin, because Pax6 is expressed by all dorsal telencephalic precursors at this developmental stage (Walther and Gruss, 1991).
Other factors might contribute to the abnormalities described here. For example, a well studied diencephalic boundary, the ZLI, which separates the dorsal and ventral thalamus (Figdor and Stern, 1993; Rubenstein et al., 1994; Kiecker and Lumsden, 2004), is a source of Shh (Hashimoto-Torii et al., 2003; Kiecker and Lumsden, 2004) and is considered a secondary organizer of the diencephalon (Echevarria et al., 2003; Vieira et al., 2005). Although Shh expression in the presumptive ZLI is maintained in the Gli3Xt/Xt mutants, the characteristic wild-type shape of the ZLI, perpendicular to the main axis of the neural tube (Kiecker and Lumsden, 2004) (Fig. 3D), is altered. This change in ZLI orientation might affect the signaling properties that the ZLI exerts on the developing diencephalon and might directly or indirectly influence the formation of an intact diencephalic–telencephalic boundary.
In summary, the present study challenges the concept of widespread ventralization in the dorsal telencephalon of Gli3Xt/Xt mutants and shows that the absence of functional Gli3 results in abnormal localization of cells of diencephalic identity in the mutant neocortical region. Our results highlight the importance of considering the relative position of the diencephalon to the telencephalon when analyzing the telencephalic defects of this mutant.
Footnotes
This work was funded by the National Institute of Health [a National Eye Institute individual National Research Service Award postdoctoral fellowship (P.A.Z.)], research grants from the Biotechnology and Biological Sciences Research Council and the Medical Research Council, and The Wellcome Trust (J.O.M., D.J.P.). We thank C. Morrison and L. Ronaldson for excellent technical assistance, D. McNeil for valuable help with the mouse colony management, Linda Wilson for help with the confocal microscopy, R. Hevner for the Tbr2 antibody, T. Jessell for the Lhx 2 and Lhx9 antibody, J. Kohtz for the pan-Dlx antibody, Y. Sasai for the Foxg1 antibody, T. Theil for the Gli3 cDNA, and J. Rubenstein for the Shh probe. The Islet1 antibody was generated by T. Jessell, the Lim1 and Lim2 antibody by T. Jessell and S. Brenner-Morton, the nestin antibody by S. Hockfield, and the Pax6 antibody by A. Kawakami. They were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa (Department of Biological Sciences, Iowa City, IA).
References
- Abbott LC, Jacobowitz DM. Developmental expression of calretinin-immunoreactivity in the thalamic eminence of the fetal mouse. Int J Dev Neurosci. 1999;17:331–345. doi: 10.1016/s0736-5748(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Alcantara S, Ruiz M, D’Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aza-Blanc P, Ramirez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Lin HY, Ruiz i Altaba A, Kornberg TB. Expression of the vertebrate Gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development. 2000;127:4293–4301. doi: 10.1242/dev.127.19.4293. [DOI] [PubMed] [Google Scholar]
- Chen Y, Knezevic V, Ervin V, Hutson R, Ward Y, Mackem S. Direct interaction with Hoxd proteins reverses Gli3-repressor function to promote digit formation downstream of Shh. Development. 2004;131:2339–2347. doi: 10.1242/dev.01115. [DOI] [PubMed] [Google Scholar]
- Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem. 1999;274:8143–8152. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- Davila JC, Real MA, Olmos L, Legaz I, Medina L, Guirado S. Embryonic and postnatal development of GABA, calbindin, calretinin, and parvalbumin in the mouse claustral complex. J Comp Neurol. 2005;481:42–57. doi: 10.1002/cne.20347. [DOI] [PubMed] [Google Scholar]
- del Rio JA, Martinez A, Fonseca M, Auladell C, Soriano E. Glutamate-like immunoreactivity and fate of Cajal-Retzius cells in the murine cortex as identified with calretinin antibody. Cereb Cortex. 1995;5:13–21. doi: 10.1093/cercor/5.1.13. [DOI] [PubMed] [Google Scholar]
- Dou CL, Li S, Lai E. Dual role of brain factor-1 in regulating growth and patterning of the cerebral hemispheres. Cereb Cortex. 1999;9:543–550. doi: 10.1093/cercor/9.6.543. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Echevarria D, Vieira C, Gimeno L, Martinez S. Neuroepithelial secondary organizers and cell fate specification in the developing brain. Brain Res Brain Res Rev. 2003;43:179–191. doi: 10.1016/j.brainresrev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figdor MC, Stern CD. Segmental organization of embryonic diencephalon. Nature. 1993;363:630–634. doi: 10.1038/363630a0. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S. Patterning events and specification signals in the developing hippocampus. Cereb Cortex. 1999;9:551–561. doi: 10.1093/cercor/9.6.551. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Joyner AL. Dynamic expression of the murine Achaete-Scute homologue Mash-1 in the developing nervous system. Mech Dev. 1993;42:171–185. doi: 10.1016/0925-4773(93)90006-j. [DOI] [PubMed] [Google Scholar]
- Hanashima C, Shen L, Li SC, Lai E. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J Neurosci. 2002;22:6526–6536. doi: 10.1523/JNEUROSCI.22-15-06526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Torii K, Motoyama J, Hui CC, Kuroiwa A, Nakafuku M, Shimamura K. Differential activities of Sonic hedgehog mediated by Gli transcription factors define distinct neuronal subtypes in the dorsal thalamus. Mech Dev. 2003;120:1097–1111. doi: 10.1016/j.mod.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Neogi T, Englund C, Daza RA, Fink A. Cajal-Retzius cells in the mouse: transcription factors, neurotransmitters, and birthdays suggest a pallial origin. Brain Res Dev Brain Res. 2003;141:39–53. doi: 10.1016/s0165-3806(02)00641-7. [DOI] [PubMed] [Google Scholar]
- Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jimenez D, Rivera R, Lopez-Mascaraque L, De Carlos JA. Origin of the cortical layer I in rodents. Dev Neurosci. 2003;25:105–115. doi: 10.1159/000072260. [DOI] [PubMed] [Google Scholar]
- Keyser A. The development of the diencephalon of the Chinese hamster. An investigation of the validity of the criteria of subdivision of the brain. Acta Anat Suppl (Basel) 1972;59:1–178. [PubMed] [Google Scholar]
- Kiecker C, Lumsden A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat Neurosci. 2004;7:1242–1249. doi: 10.1038/nn1338. [DOI] [PubMed] [Google Scholar]
- Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschel S, Ruther U, Theil T. A disrupted balance between Bmp/Wnt and Fgf signaling underlies the ventralization of the Gli3 mutant telencephalon. Dev Biol. 2003;260:484–495. doi: 10.1016/s0012-1606(03)00252-5. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418:979–983. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- Lo LC, Johnson JE, Wuenschell CW, Saito T, Anderson DJ. Mammalian achaete-scute homolog 1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev. 1991;5:1524–1537. doi: 10.1101/gad.5.9.1524. [DOI] [PubMed] [Google Scholar]
- Marti E, Takada R, Bumcrot DA, Sasaki H, McMahon AP. Distribution of Sonic hedgehog peptides in the developing chick and mouse embryo. Development. 1995;121:2537–2547. doi: 10.1242/dev.121.8.2537. [DOI] [PubMed] [Google Scholar]
- Mastick GS, Davis NM, Andrew GL, Easter SS., Jr Pax-6 functions in boundary formation and axon guidance in the embryonic mouse forebrain. Development. 1997;124:1985–1997. doi: 10.1242/dev.124.10.1985. [DOI] [PubMed] [Google Scholar]
- Maynard TM, Jain MD, Balmer CW, LaMantia AS. High-resolution mapping of the Gli3 mutation extra-toes reveals a 51.5-kb deletion. Mamm Genome. 2002;13:58–61. doi: 10.1007/s00335-001-2115-x. [DOI] [PubMed] [Google Scholar]
- Methot N, Basler K. Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell. 1999;96:819–831. doi: 10.1016/s0092-8674(00)80592-9. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Nery S, Wichterle H, Fishell G. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development. 2001;128:527–540. doi: 10.1242/dev.128.4.527. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Patel K, Wilkinson DG. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 1996;51:219–235. doi: 10.1016/s0091-679x(08)60630-5. [DOI] [PubMed] [Google Scholar]
- Nornes HO, Dressler GR, Knapik EW, Deutsch U, Gruss P. Spatially and temporally restricted expression of Pax2 during murine neurogenesis. Development. 1990;109:797–809. doi: 10.1242/dev.109.4.797. [DOI] [PubMed] [Google Scholar]
- Porteus MH, Bulfone A, Liu JK, Puelles L, Lo LC, Rubenstein JL. DLX-2, MASH-1, and MAP-2 expression and bromodeoxyuridine incorporation define molecularly distinct cell populations in the embryonic mouse forebrain. J Neurosci. 1994;14:6370–6383. doi: 10.1523/JNEUROSCI.14-11-06370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Puschel AW, Westerfield M, Dressler GR. Comparative analysis of Pax-2 protein distributions during neurulation in mice and zebrafish. Mech Dev. 1992;38:197–208. doi: 10.1016/0925-4773(92)90053-m. [DOI] [PubMed] [Google Scholar]
- Rallu M, Machold R, Gaiano N, Corbin JG, McMahon AP, Fishell G. Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development. 2002;129:4963–4974. doi: 10.1242/dev.129.21.4963. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Martinez S, Shimamura K, Puelles L. The embryonic vertebrate forebrain: the prosomeric model. Science. 1994;266:578–580. doi: 10.1126/science.7939711. [DOI] [PubMed] [Google Scholar]
- Ruppert JM, Vogelstein B, Arheden K, Kinzler KW. GLI3 encodes a 190-kilodalton protein with multiple regions of GLI similarity. Mol Cell Biol. 1990;10:5408–5415. doi: 10.1128/mcb.10.10.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng HZ, Bertuzzi S, Chiang C, Shawlot W, Taira M, Dawid I, Westphal H. Expression of murine Lhx5 suggests a role in specifying the forebrain. Dev Dyn. 1997;208:266–277. doi: 10.1002/(SICI)1097-0177(199702)208:2<266::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Stoykova A, Gruss P. Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J Neurosci. 1994;14:1395–1412. doi: 10.1523/JNEUROSCI.14-03-01395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock RR. A morphological study of the development of the mouse choroid plexus. J Anat. 1979;129:777–793. [PMC free article] [PubMed] [Google Scholar]
- Super H, Soriano E, Uylings HB. The functions of the preplate in development and evolution of the neocortex and hippocampus. Brain Res Brain Res Rev. 1998;27:40–64. doi: 10.1016/s0165-0173(98)00005-8. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Tao W, Lai E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron. 1992;8:957–966. doi: 10.1016/0896-6273(92)90210-5. [DOI] [PubMed] [Google Scholar]
- Theil T. Gli3 is required for the specification and differentiation of preplate neurons. Dev Biol. 2005;286:559–571. doi: 10.1016/j.ydbio.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Theil T, Alvarez-Bolado G, Walter A, Ruther U. Gli3 is required for Emx gene expression during dorsal telencephalon development. Development. 1999;126:3561–3571. doi: 10.1242/dev.126.16.3561. [DOI] [PubMed] [Google Scholar]
- Tole S, Ragsdale CW, Grove EA. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toes(J) Dev Biol. 2000;217:254–265. doi: 10.1006/dbio.1999.9509. [DOI] [PubMed] [Google Scholar]
- Trujillo CM, Alonso A, Delgado AC, Damas C. The rostral and caudal boundaries of the diencephalon. Brain Res Brain Res Rev. 2005;49:202–210. doi: 10.1016/j.brainresrev.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Tullio AN, Bridgman PC, Tresser NJ, Chan CC, Conti MA, Adelstein RS, Hara Y. Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain. J Comp Neurol. 2001;433:62–74. doi: 10.1002/cne.1125. [DOI] [PubMed] [Google Scholar]
- Vieira C, Garda AL, Shimamura K, Martinez S. Thalamic development induced by Shh in the chick embryo. Dev Biol. 2005;284:351–363. doi: 10.1016/j.ydbio.2005.05.031. [DOI] [PubMed] [Google Scholar]
- von Mering C, Basler K. Distinct and regulated activities of human Gli proteins in Drosophila. Curr Biol. 1999;9:1319–1322. doi: 10.1016/s0960-9822(00)80054-8. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Sekiguchi M, Takamatsu M, Tanabe Y, Nakanishi S. Distinct ontogenic and regional expressions of newly identified Cajal-Retzius cell-specific genes during neocorticogenesis. Proc Natl Acad Sci USA. 2004;101:14509–14514. doi: 10.1073/pnas.0406295101. [DOI] [PMC free article] [PubMed] [Google Scholar]




