Abstract
Objective
The present study investigated whether lateralized ERP components triggered during covert manual response preparation (ADAN, LDAP) reflect effector selection, the selection of movement direction, or both.
Methods
Event-related brain potentials were recorded during a response precueing paradigm where visual cues provided either partial (Experiment 1) or full (Experiment 2) information about the response hand and the direction for a subsequent reaching movement.
Results
ADAN and LDAP components were elicited even when only partial response information was available, demonstrating that they do not require the presence of a fully specified motor program. The ADAN was elicited in a similar fashion regardless of whether effector or movement direction information was provided, suggesting that the underlying mechanisms are equally sensitive to both types of response-related information. In contrast, the LDAP was larger in response to cues providing effector information, but was also reliably present when movement direction was available.
Conclusions
ADAN and LDAP components reflect preparatory activity within anterior and posterior parts of the parieto-premotor sensorimotor network where different parameters for manual reaching movements are programmed independently.
Significance
These results support the claim of the premotor theory of attention that shared sensorimotor control mechanisms are involved in attention and motor programming.
Keywords: Motor preparation, Electroencephalography, Event-related brain potentials, Premotor cortex, Parietal cortex, Attention
1. Introduction
During the programming of motor responses, an abstract action goal is translated into structured motor programs that eventually specify a set of muscle commands (Keele, 1968, 1981). To activate a motor program, the dimensions on which a movement can vary need to be specified (Rosenbaum, 1983). Even the preparation of a simple manual pointing movement requires the specification of different parameters such as effector, movement direction, amplitude, duration, and velocity. To investigate the selection of such movement parameters, the S1–S2 movement precueing paradigm has been widely used (Rosenbaum, 1980, 1983). A response precue (S1) presented prior to an imperative stimulus (S2) in a choice reaction time (RT) task conveys full, partial, or no information about the parameters of the response. Any additional information required for response selection is provided by S2. RTs are faster when the precue provides advance information about the movement, and size of this effect depends on the amount of response-related information given by the cue (e.g., Miller, 1982; Rosenbaum, 1983).
Such response precueing benefits are usually attributed to the preparatory activation of motor cortex (e.g., Miller, 1982; Rosenbaum, 1980), although non-motor accounts (e.g., Goodman and Kelso, 1980; Reeve and Proctor, 1984), and explanations in terms of attentional mechanisms (e.g., Adam and Pratt, 2004; Bock and Eversheim, 2000) have also been proposed. A motor interpretation is supported by psychophysiological studies that have measured movement-related potentials such as the lateralized readiness potential (LRP) during response precueing (e.g., De Jong et al., 1988; Leuthold et al., 1996; Jentzsch et al., 2004). The LRP reflects an enhanced negativity over the motor cortical areas contralateral to the side of an activated response and is considered as an electrophysiological indicator of unimanual response activation in motor cortex (Eimer, 1995; Eimer and Coles, 2003; Gratton et al., 1990; Gehring et al., 1992). In studies on cued hand movement preparation (Jentzsch and Leuthold, 2002; Leuthold and Jentzsch, 2001, 2002; Leuthold et al., 1996; Sangals et al., 2002; Ulrich et al., 1998), precues provided partial, full or none information about the hand to use (right or left finger) and the movement direction (finger flexion or extension). While partial information about the hand elicited an LRP during the response preparation interval even when movement direction was still unknown, no LRP was found with partial information about the movement direction (finger flexion or extension) without hand information. LRP amplitudes were larger when precues provided full response information as compared to precues specifying hand, but not movement direction.
In addition to the LRP, which directly reflects motor activation processes, several recent ERP studies using response precueing procedures have uncovered other lateralized components that are triggered earlier during the movement preparation interval (Eimer et al., 2005, 2006; Praamstra et al., 2005; Mathews et al., 2006; Praamstra, 2006; Wauschkuhn et al., 1997; Van der Lubbe et al., 2000). When a cue specified which hand to use in an upcoming simple finger lift movement (Eimer et al., 2005, 2006; Eimer and Van Velzen, 2006), an initial negativity at anterior recording sites contralateral to the side of the cued response was found between 350 and 600 ms after precue onset and was followed by a contralateral posterior positivity between 600 and 900 ms (see also Verleger et al., 2000, for similar results). Similar lateralized components are not only triggered during manual response preparation, but can also be observed during covert saccade preparation (Eimer et al., 2006; Wauschkuhn et al., 1997; Van der Lubbe et al., 2000, 2006; but see also Van der Stigchel et al., 2006, for partially contrasting results).
Interestingly, these lateralized components found during covert manual response preparation were very similar to lateralized components found in other studies during instructed shift of spatial attention. In these studies in which a shift of attention was indicated by central symbolic cues, ERPs waveforms triggered in response to cues directing attention to the left side were compared to ERPs elicited during rightward attentional shifts (c.f., Harter et al., 1989; Yamaguchi et al., 1994; Nobre et al., 2000; Hopf and Mangun, 2000; Eimer et al., 2003a). An anterior directing attention negativity (ADAN), reflecting an enhanced negativity at anterior electrodes contralateral to the cued side of an attentional shift, was followed by a late directing attention positivity (LDAP), reflecting an enhanced posterior contralateral positivity. The fact that similar ERP components can be observed during covert shifts of attention and during the preparation of eye movements or simple manual responses has been interpreted as evidence for the premotor theory of attention (e.g., Rizzolatti et al., 1987, 1994), which claims that response programming and spatial attention are mediated by shared sensorimotor control structures.
There is as yet no consensus with respect to the neural generators underlying the ADAN and LDAP components. Praamstra et al. (2005) suggested that the ADAN is generated in dorsal premotor cortex (PMd), and that this component reflects activation of a frontoparietal attentional control network. Alternatively, the ADAN has been interpreted as indicator of saccade preparation or saccade inhibition in the frontal eye fields (FEF; Van der Lubbe et al., 2000, 2006). The LDAP is assumed to be generated in the occipitotemporal cortex (Mathews et al., 2006; Praamstra et al., 2005), although it has also been tentatively localized in the ventral intraparietal sulcus (Van der Lubbe et al., 2006). The observation that this component is eliminated during shifts of attention in congenitally blind participants (Van Velzen et al., 2006) suggests that the LDAP might reflect spatial selection processes that are predominantly based on visually mediated coordinates of external space.
The aim of the present experiment was to obtain new insights into the nature of the mechanisms that are reflected by lateralized ERP components observed during covert response preparation by investigating whether such components are also triggered during the programming of manual reaching movements. Most previous studies of saccade or manual response preparation have investigated very simple movements, such as lifting the index finger of one hand, where effector and target locations were spatially and temporally coincident. However, motor preparation usually involves more complex movements, where different movement parameters need to be specified independently. During reaching movements, the effector involved and the movement target are initially located in different regions of space. Here, response programming not just requires the localization and the selection of the response hand, but also the localization of the target with respect to the body and the hand, and the selection of movement direction and amplitude. In addition, simple finger lift responses and reaching movement also differ on the temporal dimension (discrete versus continuous; see Proctor and Wang, 1997). Thus, a complex process of sensorimotor coordinate transformations is required in order to represent the effector and the target for a reaching movement within the same frame of reference and to select the appropriate motor program. Evidence from single-cell recording studies suggests that such transformations are carried out within a network of parietal and frontal premotor areas. Different specialized frontal motor areas are interconnected with equally specific parietal areas, forming modules of largely segregated anatomical circuits that are each dedicated for a specific sensorimotor transformation (e.g., Colby and Duhamel, 1996; Gross and Graziano, 1995; Rizzolatti et al., 1994). The parietal reach region (PRR) forms part of the network specialized for planning target-directed limb movements. Neurons in this region are sensitive to movement direction (for review, see Snyder et al., 2002; Andersen and Buneo, 2002), while others appear to be coding effector-specific information (Calton et al., 2002). Further clinically relevant evidence for a specific role of parietal cortex in motor control that might be closely linked to attentional functions comes from recent TMS studies (Rushworth et al., 2001a,b), and from neuropsychological patient studies demonstrating a specific role of the parietal cortex for the planning of action (Castiello and Paine, 2002) and on-line motor control (Pisella et al., 2000).
In the only previous ERP study to date which investigated ERP lateralizations during the preparation of more complex movements (Berndt et al., 2002), participants had to point to targets presented contralateral, ipsilateral or central relative to the response hand. A frontal negativity contralateral to the response hand varied in latency and amplitude with pointing direction, and an increased negativity ipsilateral to the pointing target emerged at posterior electrodes. These anterior and posterior components were interpreted as reflecting the encoding of spatial information in arm-centered coordinates in premotor cortex, and the processing of visuo-spatial stimulus properties relevant for response selection in posterior parietal cortex, respectively. Unfortunately, the fact that response hand remained constant throughout blocks of trials in Berndt et al. study made it impossible to dissociate effector selection from movement direction or target selection.
In the present study, we investigated ERP lateralizations triggered by effector and movement direction selection during the preparation of reaching movements, in a task where these two movement parameters were specified independently by response precues. On each trial, participants had to execute one of four possible reaching movements with their right or left-hand towards a target located on the right or left hemifield (see Fig. 1). A centrally presented visual cue contained either partial (Experiment 1) or full (Experiment 2) information about the next reaching movement. In Experiment 1, cues either specified which hand to use without movement direction information (effector information condition), or movement direction (left versus right) without specifying response hand (direction information condition). The subsequent imperative stimulus provided the additional information needed to perform the movement. In Experiment 2, the cue specified both effector and movement direction, and thus provided full response information. Here, the imperative stimulus was a Go/Nogo stimulus instructing participants to execute or withheld the movement indicated by the cue.
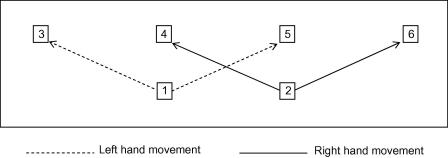
Fig. 1.
Response device used in both experiments. Numbers refer to the six keys locations (start keys: 1–2; target keys: 3–4). The left hand (start key 1) moved left to target key 3 or right to target key 5, while the right hand (start key 2) moved left to target key 4 or right to target key 6.
In previous studies investigating lateralized ERP components during unimanual response preparation (Eimer et al., 2005, 2006; Eimer and Van Velzen, 2006; Verleger et al., 2000), ADAN and LDAP components were found during the preparation of very simple movements such as finger lift responses. The first critical question addressed in the present study was whether similar lateralized components would also be present during the preparation of more complex spatially and temporally extended reaching movements. Most importantly, the current design allowed us to investigate whether such components primarily reflect effector selection, the selection of movement direction, or a combination of both, by directly comparing ERP lateralizations triggered by response precues that specified either response hand or movement direction.
2. Experiment 1
Experiment 1 investigated whether lateralized ERP components (ADAN and LDAP) similar to those previously observed during the preparation of simple unimanual responses and during shifts of covert attention would also be triggered during the preparation of more complex reaching movements, and whether these components predominantly reflect the selection of an effector or the selection of movement direction. In order to dissociate the relative contributions of effector and movement direction selection, the response precue (S1) presented at beginning of each trial contained only partial information about the reaching movement to prepare. In the effector information condition, the cue signalled which effector to use (left or right hand), whereas in the direction information condition, it indicated movement direction (left or right) 1100 ms after the cue onset, an imperative visual stimulus (S2) presented at fixation provided the additional information required to select a specific response (movement direction information in the effector information condition, and effector information in the direction information condition). Participants were instructed to use the information provided by the cue to prepare the next response, and to integrate it with the subsequent information given by the imperative stimulus in order to execute the correct reaching movement. Thus, only effector or movement direction selection could take place during the S1–S2 interval, as the completion of a reaching movement program had to await the arrival of S2.
ERPs elicited in the S1–S2 interval in response to precues specifying the left versus right response hand (in the effector information condition), or a leftward versus rightward response (in the direction information condition), were compared in order to identify lateralized ERP activity sensitive to the partial response information provided by the precue. If the components previously observed during cued unimanual response preparation were critically dependent on the availability of full response information required to activate a complete motor program, they should be entirely absent in Experiment 1, where only partial response information was provided. Thus, the presence of ADAN and LDAP components in this experiment would provide strong evidence that the processes reflected by these components are triggered even when manual responses can only be partially prepared. Systematic differences between ADAN and LDAP components elicited for the effector and direction information conditions would suggest that the underlying processes are differentially involved in the preparation of specific parameters of reaching movements.
Finally, we also investigated the presence of an LRP during covert manual response preparation in the S1–S2 interval. Given that the LRP is assumed to reflect the differential activation of one response hand versus the other (see Sangals et al., 2002), it should only be present in the effector information condition, but not in the direction information condition, where response hand was left unspecified until S2 was presented.
2.1. Methods
2.1.1. Participants
Eighteen healthy volunteers participated in this experiment. One participant was excluded because of a large number of eye blinks, another was excluded because of difficulties in executing the task. Thus 16 participants (9 females and 7 males; 18–42 years old; average age: 28.7 years) remained in the sample. All were right-handed and had normal or corrected-to-normal vision by self-report. Informed consent was taken prior to participation and the investigation was approved by the Ethics Committee, School of Psychology, Birkbeck College, University of London.
2.1.2. Apparatus, stimuli and procedure
Participants sat in a dimly lit sound attenuated cabin in front of a computer screen at a viewing distance of 57 cm. A custom-made response device was placed on the table just below the computer screen. The device contained six response keys that were located on a wooden panel (60 × 48 cm), tilted at an angle of approximately 30°, with its midline aligned with the screen centre (Fig. 1). The two start keys (keys 1 and 2 in Fig. 1) were located 5.5 cm to the left and right of the centre of the panel. The four target keys (keys 3–6 in Fig. 1) were horizontally aligned 5 cm above the start keys. Outer and inner target keys were located 20.5 and 5.5 cm to the left and right of the panel centre, respectively. Outer target keys (keys 3 and 6) were the goals for outward movements (left hand from start key 1 to target key 3; right hand from start key 2 to target key 6). Inner target keys (keys 4 and 5) were the goals for inward movements (left hand from key 1 to key 5; right hand from key 2 to key 4). The distance between each start and corresponding target key (movement length) was kept constant at 20 cm.
Each trial started with a 100-ms presentation of a visual response precue (S1) at fixation. This cue consisted of two adjacent triangles, presented centrally on a computer screen at a viewing distance of 55 cm (visual angle: 3.5° × 2.5°). One triangle was red, the other blue, and they always pointed in opposite directions (Fig. 2a). A central fixation cross, located in the space between the two triangles, was present throughout the experimental blocks. Left or right side was signalled by the direction of one of the triangles. For half of the participants, blue triangles were relevant, while for the other half red triangles were relevant. Relevant left-pointing or right-pointing triangles were presented with equal probability to the left or right of fixation. On each trial, the cue was followed after an interval of 1000 ms by the letter ‘L’ or ‘R’ (S2, indicating left or right side, respectively) that was presented at fixation for 100 ms, replacing the fixation cross during this period.
Fig. 2.
Different types of response precues used in Experiment 1 (smooth-edged triangles, panel a) and in Experiment 2 (triangles with smooth or jagged edges, panels a and b). Dark grey and light grey colours represent the colours blue and red.
To investigate whether covert manual response preparation results in spatially selective modulations of visual processing during the S1–S2 interval (as shown previously by Eimer et al., 2006), a single irrelevant visual probe stimulus was presented on each trial 900 ms after cue onset and 200 ms before the onset of S2. Probes consisted of a 100 ms illumination of one of two ensembles of green LEDs. Each of these ensembles was composed of six segments arranged in a circle plus one central segment (size of each LED segment: 0.4 cm; diameter of the circle: 2.4 cm). LEDs were located 1.5 cm above the start keys 1 and 2 (Fig. 1) where the left and right hand were located during response preparation at a viewing distance of approximately 39 cm. Left or right probes were presented with equal probability and in random order. Participants were instructed to completely ignore these probe stimuli throughout.1
The experiment consisted of 12 experimental blocks. Each block contained 80 trials, with 20 trials per block for each combination of information carried by the cue (left versus right) and information carried by the imperative stimulus (left versus right). Trials were presented in a pseudo-random sequence. Participants performed two different conditions of six successive blocks each. In the effector information condition, response cues indicated which hand to use, while the subsequent S2 specified the direction of the movement. In the direction information condition, response cues indicated movement direction, and S2 specified which hand to use. One training block of 80 trials was run before each experimental condition. The order in which these conditions were delivered was counterbalanced across participants.
Participants were asked to maintain a body posture such that their midline was aligned with the centre of the screen and response panel, and to place their forearms on the response panel with their hands on the start keys. They were instructed to use the information provided by the response cue to prepare the cued hand (in the effector information condition) or the cued movement direction (in the direction information condition) and to use the additional information provided by the subsequent S2 to select and execute the required movement as fast and accurately as possible. They were explicitly encouraged to maintain central eye fixation, and to wait for the imperative stimulus before moving their hand from the start key. After having pressed a target key, the hand had to be moved back to its corresponding start key that had to be pressed it in order to start the next trial. The next response cue appeared on the screen 1000 ms after the start key was pressed.
2.1.3. Recording and data analysis
EEG was recorded with Ag–AgCl electrodes and linked-earlobe reference from Fpz, F7, F3, Fz, F4, F8, FC5, FC6, T7, C3, Cz, C4, T8, CP5, CP6, P7, P3, Pz, P4, P8, and Oz (according to the 10–20 system), and from OL and OR (located halfway between O1 and P7, and O2 and P8, respectively). Horizontal EOG was recorded bipolarly from the outer canthi of both eyes. Electrode impedance was kept below 5 kΩ, and the impedances of the earlobe electrodes were kept as equal as possible. Data were recorded with a bandpass filter from 0 to 40 Hz, and a digitisation rate of 200 Hz. Trials with eyeblinks (Fpz exceeding ±80 μV), horizontal eye movements (HEOG exceeding ±30 μV), or other artefacts (a voltage exceeding ±80 μV at any electrodes) in the S1–S2 interval were excluded prior to data analysis. To detect systematic deviations of eye position indicating residual tendencies to move the eyes toward the cued location, averaged waveforms in the S1–S2 interval in response to left versus right cues were examined for each participant. None of the participants showed a residual HEOG deviation exceeding ±3 μV.
ERPs were averaged relative to a 100 ms pre-S1 baseline prior for the 1400 ms time interval following S1 onset. Statistical analyses were conducted on the basis of ERP mean amplitudes obtained within predefined measurement windows. Separate averages were computed for the effector and direction information conditions, for trials with left and right cues (specifying either response hand or movement direction in these two conditions). ERP mean amplitudes were analysed with repeated measures ANOVAs, and separate analyses were conducted for lateral anterior, central, and posterior sites. These analyses included the factors electrode site (F7/8 versus F3/4 versus FC5/6, for the anterior analysis, C3/4 versus T7/8 versus CP5/6, for the central analysis, and OL/R versus P3/4 versus P7/8, for the posterior analysis), condition (effector versus direction information), cued side (left versus right), and hemisphere (left versus right). In these analyses, the presence of ERP lateralizations sensitive to the response information provided by the cue will be indicated by significant hemisphere × cued side interactions. Additional analyses were conducted separately for the effector and direction information conditions. Whenever interactions between hemisphere, cued side and electrodes sites were found, revealing that the amplitudes of lateralized ERP components differed across individual electrode pairs, follow-up analyses were conducted for single electrode sites. All analyses were based on mean amplitudes obtained within three successive post-cue latency windows between 350 and 600 ms (where the ADAN was previously observed), between 600 and 900 ms (where the LDAP component was found), and between 900 and 1400 ms (where an LRP was expected to emerge). ERPs to irrelevant visual probe stimuli were computed relative to a 100 ms pre-stimulus baseline. ERPs obtained at lateral posterior electrodes (OL/R, P3/4, P7/8) were analysed within two post-stimulus time intervals (P1: 100–130 ms; N1: 150–200 ms), for the factors condition, visual probe location (left versus right), and hemisphere (left versus right).
Behavioural performance (response speed and accuracy) was measured for target key presses. All trials with reaction times (RTs) exceeding two standard deviations from the mean were removed.2 RTs for correct responses were analysed in a repeated measures ANOVA for the factors response information (effector versus direction information), response hand (left versus right), movement direction (left versus right), and visual probe location (left versus right).
For all analyses, Greenhouse–Geisser adjustments to the degrees of freedom were applied where appropriate.
2.2. Results
2.2.1. Behavioural performance
Trials where RTs exceeded two standard deviations from the mean (4.3% of all trials) and trials where participants failed to respond (1.4% and 1.3% of all trials in the effector and direction information conditions, respectively) were excluded from analysis. RTs did not differ between the effector and direction information conditions (755 ms versus 770 ms; F(1, 15) = 0.4). RTs were faster for movements to the right side than for movements to the left side (755 ms versus 770 ms; main effect of movement direction: F(1, 15) = 8.7, p < .01). A significant interaction between response hand and movement direction (F(1, 15) = 28.9, p < .001) was due to the fact that outward movements where response hand and movement direction were congruent, and start and target keys were one the same side of the body midline were faster than inward movements where they were incongruent and movements crossed the body midline (696 ms versus 830 ms). Furthermore, a significant interaction between condition, response hand and visual probe location was obtained (F(1, 15) = 6.4, p < .05). Separate analysis revealed a significant interaction between response hand and visual probe location in the direction information condition (F(1, 15) = 11.0, p < .005), where irrelevant visual probes appeared before the response hand was specified by S2. RTs were faster when visual probes were adjacent to this response hand (765 ms) than when they were presented on the opposite side (777 ms). No such effect was present in the effector information condition, where the response hand was known before irrelevant visual probes were presented.
2.2.2. Lateralised ERP components triggered during reaching movement preparation
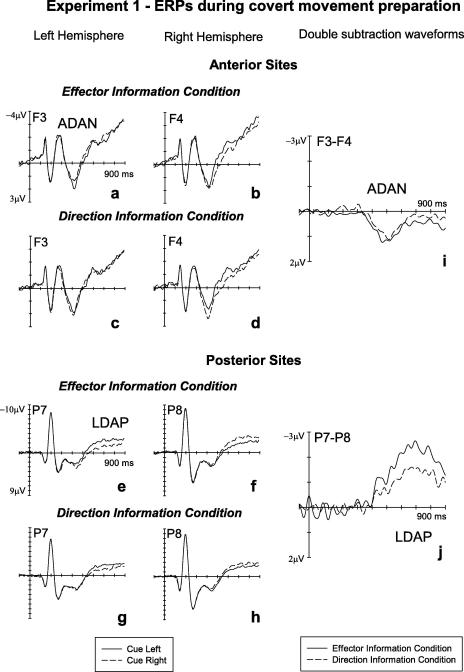
Fig. 3 shows ERPs elicited in response to right versus left cues in the 900 ms interval after cue onset at anterior sites F3/4 (panels a–d) and posterior sites P7/8 (panels e–h), separately for the effector and direction information conditions. Similar to previous ERP studies of manual response preparation, an enhanced anterior negativity (ADAN) and an enhanced posterior positivity (LDAP) contralateral to the cued side appeared to be present in both conditions. These components can also be seen in the double subtraction waveforms (Fig. 3i and j) that were generated by first subtracting ERPs in response to right cues from ERPs to left cues, and then subtracting the resulting difference waveforms obtained over the right hemisphere from difference waveforms emerging at homologous electrodes over the left hemisphere. In these double subtraction waveforms, a negativity contralateral to the cued hand or movement direction is reflected by positive amplitude values (downward-going deflections), and a contralateral positivity is indicated by negative values (upward-going deflections). While the ADAN appeared to be similar in amplitude across effector and direction information conditions (Fig. 3i), LDAP amplitudes (Fig. 3j) seemed to be substantially larger for the effector relative to the direction information condition.
Fig. 3.
Experiment 1: grand-averaged ERPs elicited in the S1–S2 interval during covert response preparation over the left and right hemisphere at anterior (F3/4, top panels) and posterior (P7/8, bottom panels) electrode pairs. Panels a, b, e, and f show ERPs in response to precues indicating a response with the left hand (solid lines) or right hand (dashed lines). Panels c, d, g, and h show ERPs in response to precues indicating a response towards the left side (solid lines) or right side (dashed lines). Negativity is plotted upwards. Panels i and j (right side) show difference waveforms reflecting lateralized ERP components sensitive to the response information provided by the cues. These were generated by subtracting ERPs in response to right cues from ERPs to left cues, and then subtracting the resulting difference waves at right electrodes from the difference waveform obtained for the corresponding left-hemisphere electrode, separately for the effector information condition (solid lines) and the direction information condition (dashed lines). Enhanced negativities contralateral to the cued side are reflected by positive values (downward deflections), and enhanced contralateral positivities are reflected by negative values (upward deflections). ADAN, anterior directing attention negativity; LDAP, late directing attention positivity.
Statistical analyses confirmed these observations. In the 350–600 ms measurement interval, a hemisphere × cued side interaction was present at lateral anterior electrodes (F(1, 15) = 24.4, p < .001), reflecting the ADAN component. At frontal electrodes the presence of a hemisphere × cued side × electrode site interaction (F(2, 30) = 4.6, p < .02) suggested that there were systematic differences in ADAN amplitude between the different electrode pairs. Follow-up analyses confirmed the presence of a reliable ADAN at all frontal electrode pairs (all F(1, 15) > 16.3, all p < .001). Importantly, no condition × hemisphere × cued side interaction was obtained for lateral anterior sites (F(1, 15) = 0.5), suggesting that this component was triggered in an analogous fashion in both cue conditions (see Fig. 3i). Analyses conducted separately for both cue conditions confirmed the presence of a significant hemisphere × cued side interaction in the effector information condition (F(1, 15) = 13.8, p < .002) as well as in the direction information condition (F(1, 15) = 13.3, p < .002).
At posterior electrode pairs, a significant hemisphere × cued side interaction (F(1, 15) = 15.1, p < .001) obtained for the 350–600 ms interval reflected the early phase of the posterior contralateral positivity (LDAP). The observation that this LDAP component was more pronounced in the effector information condition (see panel j) was substantiated by a significant condition × hemisphere × cued side interaction (F(1, 15) = 5.1, p < .04). However, analyses conducted separately for both cue conditions revealed that the early LDAP was significantly present not only in the effector information condition (hemisphere × cued side interaction: F(1, 15) = 13.8, p < .002), but also in the direction information condition (F(1, 15) = 11.3, p < .004).
In the subsequent measurement window (600–900 ms post-cue) a significant hemisphere × cued side interaction was again triggered at lateral posterior sites (F(1, 15) = 31.8, p < .001) reflecting the continued presence of the LDAP component. Analogous to the previous time window, a condition × hemisphere × cued side interaction (F(1, 15) = 11.8, p < .004) reflected the fact that the LDAP amplitudes were larger in response to effector cues as compared to movement direction cues (Fig. 3j). Additional analyses again confirmed that the LDAP was significantly present not only in the effector information condition (F(1, 15) = 29.9, p < .001), but also in the direction information condition (F(1, 15) = 25.3, p < .001).3 In both analyses, hemisphere × cued side × electrode site interactions (both F(2, 30) > 13.5, both p < .001) suggested that LDAP amplitudes differed between posterior electrode pairs. Follow-up analysis conducted for both conditions confirmed that the LDAP was significantly present at all posterior electrodes (all F(1, 15) > 16.5, all p < .001 in the effector information condition, all F(1, 15) > 5.8, all p < .03 in the direction information condition).
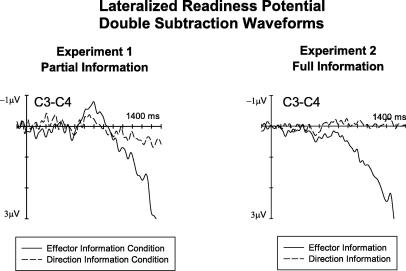
In the 900–1400 ms interval, no hemisphere × cued side interactions were found at lateral anterior, lateral central, or lateral posterior electrodes. At lateral central sites, an electrode site × condition × hemisphere × cued side interaction was obtained (F(2, 30) = 4.4, p < .03), and follow-up analyses revealed a significant condition × hemisphere × cued side interaction for electrode pair C3/4 only (F(1, 15) = 12.4, p < .003). Separate analyses conducted for both cue conditions showed that an LRP was elicited only in the effector information condition where the response precue had signalled which response hand was to be used (hemisphere × cued side interaction: F(1, 15) = 10.9, p < .005). This is further illustrated in Fig. 4 (left panel), where double subtraction waveforms obtained for electrodes C3/4 during the 1400 ms interval following S1 onset are shown for both task conditions.
Fig. 4.
Lateralized readiness potential (LRP) waveforms obtained at C3/4 by subtracting ERPs in response to right cues from ERPs to left cues, and then subtracting the resulting difference waves at C4 from the difference waveform obtained for C3. These standard procedures for deriving the LRP are described in more detail in Eimer and Coles (2003). Left panel: LRPs obtained in Experiment 1 in the effector information condition (solid line) and in the direction information condition (dashed line). Right panel: LRPs obtained in Experiment 2 in response to cues specifying a left-hand versus right-hand response, collapsed across trials where these cues indicated a leftward or rightward movement (effector information, solid line), and in response to cues signalling a leftward versus rightward movement, collapsed across trials where they specified a left-hand or right-hand response (direction information, dashed line).
2.2.3. Visual ERPs to irrelevant probe stimuli
No effect of cued response preparation on the P1 component was found. For N1 amplitudes, a main effect of visual probe location (F(1, 15) = 15.6, p < .001) was accompanied by an interaction between condition and visual probe location (F(1, 15) = 5.1, p < .04). Separate analyses conducted for both conditions showed that N1 amplitudes were enhanced when probes were presented close to the cued hand in the effector information condition (F(1, 15) = 13.2, p < .002), analogous to previous experiments where participants prepared a simple finger lift response (e.g., Eimer and Van Velzen, 2006; Eimer et al., 2006). In contrast, no such effect was present in the direction information condition. This is most likely due to the fact that response hand remained unspecified during the response preparation interval in this condition, and attention was therefore not directed to one hand versus the other.
2.3. Discussion of Experiment 1
In spite of the fact that the response cues used in Experiment 1 only provided partial information about either effector or movement direction for a subsequent reaching movement, ADAN and LDAP components were found to be reliably present during covert response preparation elicited by both types of cues. This pattern of results demonstrates conclusively that these lateralized components are not restricted to situations where full information about an upcoming movement is available, and a complete motor program can therefore be generated, but are also elicited when only one aspect of an anticipated movement can be programmed in advance. Furthermore, the fact that significant ADAN and LDAP components were triggered in response to effector cues as well as in response to cues signalling movement direction suggests that neither component is exclusively linked to the specification of one single response parameter. Instead, both seem to reflect preparatory processes that are involved in the selection of effectors as well as in the selection of movement direction. The observation that ADAN amplitudes did not differ between the effector and direction information conditions indicates that the mechanisms responsible for the ADAN may be equally sensitive to both parameters. In contrast, although the LDAP was also reliably present in both cue conditions, the LDAP amplitudes were about twice as large in response to cues providing effector information relative to cues providing information about movement direction (see Fig. 3j). This suggests that this component might predominantly reflect effector selection, although the selection of movement direction also contributes to some degree.
As expected, the LRP was present only in the effector information condition (Fig. 5, left panel) where hand-specific response information was available during response preparation, which was used to partially activate the cued effector prior to S2 onset. No hand-specific information was available during the S1–S2 interval when cues provided only movement direction information, and consequently no LRP was found in this condition. It should be noted that while the LRP was entirely absent in the movement direction condition, the ADAN component was clearly present. This dissociation is important, as it demonstrates that in spite of their similarities in terms of polarity and scalp distribution, ADAN and LRP reflect functionally distinct processes. While the LRP indicates effector-specific partial response activation processes in motor cortical areas that can only be triggered once response hand information is available, the ADAN does not critically depend on full effector information, as it is also be elicited by partial response cues that indicate movement direction while leaving response hand unspecified. In this context, it is important to note that in several previous publications from our laboratory (Eimer, 1993, 1995), lateralized ERP activity observed at electrodes C3/4 in the cue-target interval of cued covert attention tasks at latencies comparable to the ADAN latencies found in the present study was interpreted as an LRP, and was assumed to reflect the partial activation of unimanual responses. Given that the ADAN is known to be triggered during cued shifts of covert attention (e.g., Hopf and Mangun, 2000; Eimer et al., 2003a), and in light of the fact that the present experiment demonstrated a dissociation between the ADAN and the LRP, it has to be concluded that this interpretation of the lateralized effects observed in these earlier studies as LRPs is most likely incorrect. The effects observed by Eimer (1993, 1995) during the 300–500 ms interval after cue onset should be more appropriately interpreted as ADAN components.
Fig. 5.
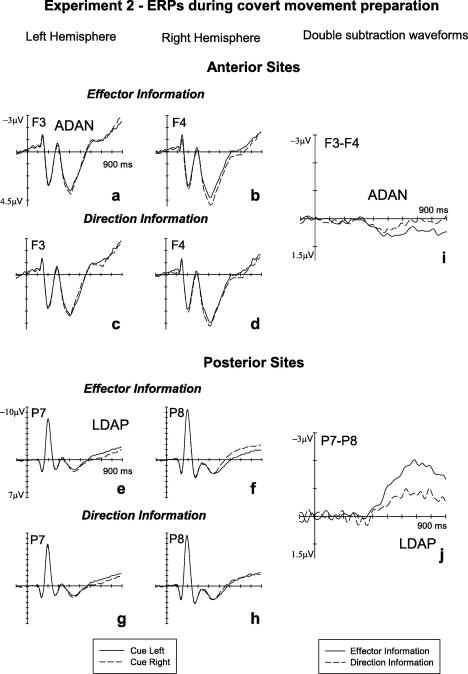
Experiment 2: grand-averaged ERPs elicited in the S1–S2 interval during covert response preparation over the left and right hemisphere at anterior (F3/4, top panels) and posterior (P7/8, bottom panels) electrode pairs. Panels a, b, e, and f show ERPs in response to precues indicating a response with the left hand (solid lines) or right hand (dashed lines), collapsed across trials where these cues indicated a leftward or rightward movement. Panels c, d, g, and h show ERPs in response to precues indicating a response towards the left side (solid lines) or right side (dashed lines), collapsed across trials where these cues indicated a left-hand or right-hand response. Negativity is plotted upwards. Panels i and j (right side) show difference waveforms reflecting lateralized ERP components sensitive to the response information provided by the cues, separately for effector information (solid lines) and direction information (dashed lines). Enhanced negativities contralateral to the cued side are reflected by positive values (downward deflections), and enhanced contralateral positivities are reflected by negative values (upward deflections).
Before the tentative conclusion can be accepted that ADAN and LDAP components reflect the selection of both effector and of movement direction, albeit to different degrees, alternative accounts of the ERP results observed in Experiment 1 need to be considered. For example, one could argue that both ADAN and LDAP primarily affect the selection of movement goals in external space, and that their presence in both the effector and direction information conditions is a result of this fact. A previous study has indeed suggested that the LDAP component is linked to the selection of target locations in external space, as it was found to be attenuated when to-be-attended positions were located close to the midline relative to blocks where they were located more peripherally (Eimer et al., 2004). Given the design of Experiment 1, and the way that response hands were mapped onto response target keys (see Fig. 1), left and right cues might have induced an attentional bias towards the left versus right side, regardless of whether they signalled the effector or movement direction. For example, when an effector cue specified the left hand for an upcoming movement, the two possible target locations for this movement were on the far left side (position 3 in Fig. 1) and on the near right side (position 5), thereby possibly resulting in an overall spatial attentional bias towards the left. Likewise, when a direction cue signalled a leftward upcoming movement, both possible target locations were located in the left hemifield (positions 3 and 4 in Fig. 1). Thus, both effector and direction cues might have triggered a tendency to shift spatial attention towards the location of response target keys on the left versus right side, and this fact alone may have been responsible for the presence of ADAN and LDAP components in both conditions.
It should be noted that this alternative explanation would predict that both ADAN and LDAP should be more pronounced in the direction information condition, where both possible response target locations were in the same hemifield. Here, a stronger spatial bias should have been elicited than in the effector information condition, where target locations were in opposite hemifields (see Fig. 1). However, this pattern of results was not observed in Experiment 1 – ADAN amplitudes were similar in size after direction and effector cues, and the LDAP was reliably larger for effector relative to direction cues. Nevertheless, the possibility that the presence of ADAN and LDAP components for both types of cues may at least in part be due to the selection of movement target locations in external space clearly needs to be ruled more conclusively. Experiment 2 was conducted for this purpose.
3. Experiment 2
Experiment 1 demonstrated that ADAN and LDAP components are triggered in the S1–S2 interval following partial response cues that provide either effector or direction information with respect to an upcoming reaching movement. While the use of partial response information cues in Experiment 1 was necessary to dissociate lateralized ERP components sensitive to effector versus movement direction information, one could argue that the results obtained with such cues may not be directly applicable to more realistic cases of response preparation, where effector and direction information are available simultaneously, effector and movement direction programming proceed in parallel, and there is no uncertainty about effector identity or movement direction. When effector and movement direction selection are allowed to operate in parallel during response preparation, one of these processes may dominate lateralized ERP components in a way that cannot be observed when only partial response information is available, as in Experiment 1.
Another feature of Experiment 1 was that while effector cues unequivocally signalled response hand, direction cues specified one of two possible target locations (see Fig. 1). Thus, these two types of cues may have differed with respect to the explicitness of spatial information provided. This fact may have been responsible for the larger LDAP in the effector information condition. If this component reflects the spatial selection of response parameters that are guided by visually mediated coordinates of external space (as suggested by the results of Van Velzen et al., 2006), it would not be all that surprising to find that it is more pronounced when response-relevant information is spatially more precise.
Experiment 2 was conducted to dissociate the relative impact of effector and direction information on ADAN and LDAP under conditions where both response parameters were simultaneously cued at the start of each trial. Precues now contained full response information about effector hand, movement direction, and goal location, so that participants knew in advance which reaching movement to perform in response to S2. Thus, these cues removed any spatial uncertainty about the upcoming movement. To avoid premature responses, S2 was either a Go stimulus (instructing participants to execute the prepared response), or, in a minority of trials, a Nogo stimulus signalling that the cued response had to be withheld. In all other respects, procedures were equivalent to Experiment 1.
Two sets of analyses were conducted. The first set included all task conditions, with cued effector and cued movement direction now independent factors. In these analyses, the existence of lateralized ERP components sensitive to the cued movement direction will be reflected by hemisphere × cued direction interactions, while lateralized effects sensitive to the cued effector will be indicated by hemisphere × cued effector interactions. The critical question was whether the pattern of results obtained in Experiment 1 (ADAN of similar size for effector and direction cues; reliable LDAP for both types of cues, but larger LDAP amplitudes in the effector information condition) would be replicated in Experiment 2 under conditions of full response information, and no spatial uncertainty.
Another aim of Experiment 2 was to further test the possibility that the presence of ADAN and LDAP components in Experiment 1 can be explained by a common purely space-based mechanism that is related to the selection of the movement goal location (see above). To provide such a test, a second analysis was carried out that included only trials where cues signalled a movement towards one of the two inner target keys (a left-hand response towards the right target key 5, or a right-hand response towards the left key 4; see Fig. 1). On these trials, the initial position of the effector and the movement goal location were equidistant from the midline, and, most importantly, always located in opposite hemifields. If the ADAN and LDAP components observed in Experiment 1 were primarily due to the spatial selection of movement goal locations on the left versus right side, these components should now be triggered contralaterally to the cued movement goal location. However, a different pattern of results should be obtained for these trials if ADAN and LDAP reflected the independent selection of effector and movement direction, as suggested by the results of Experiment 1. If effector and movement direction selection contributed equally to the ADAN, no overall significant ADAN should be observed for trials where the left hand is cued to perform a rightward movement, and the right hand is cued to perform a leftward movement, as effector-specific and direction-specific contributions will cancel each other out. For example, during the covert preparation of a rightward movement with the left hand, effector selection will produce an enhanced anterior negativity over the contralateral (right) hemisphere relative to the ipsilateral (left) hemisphere. At the same time, movement direction selection will give rise to an enhanced negativity over the left hemisphere, which is contralateral to the cued movement direction. If these ADAN components are independently elicited during effector and movement direction selection, respectively, and are of similar magnitude, as suggested by Experiment 1, the fact that they are of opposite polarity over both hemispheres implies that they will cancel each other out, resulting in the absence of any measurable ADAN effects. Following the same logic, the assumption that the LDAP is predominantly (but not exclusively) determined by effector selection leads to the prediction that an LDAP should be elicited contralaterally to the cued effector hand on these trials. However, LDAP amplitude should be considerably reduced due to the fact that the left hand was cued to perform a rightward movement, and vice versa. This should result in a smaller LDAP of opposite polarity, which would attenuate, but not completely, eliminate the LDAP that is triggered during effector selection.
3.1. Methods
3.1.1. Participants
Sixteen healthy participants (8 females and 8 males; 21–42 years old; average age: 26 years) took part in this experiment. 15 participants were right-handed, one was left-handed and all had normal or corrected-to-normal vision by self-report. Informed consent was taken prior to participation and the investigation was approved by the Ethics Committee, School of Psychology, Birkbeck College, University of London.
3.1.2. Apparatus, stimuli and procedure
These were identical to Experiment 1, with the following exceptions. Visual response cues were modified in order to simultaneously convey effector and movement direction information. One type of information was given by the direction of the relevant cue arrow (left or right), with relevant arrow colour counterbalanced across participants, as in Experiment 1. The other type of information was provided by the edges of both arrows (smooth-edged or jagged-edged, see Fig. 2b). For half of the participants, smooth edges indicated ‘right’ and jagged edges ‘left’, while opposite rules were used for the other half of participants. Triangles with smooth or jagged edges were presented with equal probability. Irrelevant visual probe stimuli were no longer presented adjacent to the left or right response hand, as in Experiment 1, but with equal probability directly 1.5 cm above one of the four target response keys. Thus, four LED ensembles (instead of just two as in Experiment 1) were used. The inner pair of LEDs was placed at a viewing distance of approximately 49 cm, while the outer pair was placed at a viewing distance of 53 cm.
The experiment consisted of 12 experimental blocks. Each block contained 80 trials (64 Go trials and 16 Nogo trials). Thus every block contained sixteen Go trials and four Nogo trials for each combination of cued effector (left versus right) and cued movement direction (left versus right). Trials were presented in a pseudo-random sequence. In six successive blocks, the effector was cued by the direction of the relevant arrow and movement direction was cued by edge shape. In the other six successive blocks, the effector was indicated by edge shape and movement direction by the direction of the relevant arrow. The order in which these successive blocks were delivered was counterbalanced across participants. One training block of 80 trials was run before each set of six blocks.
On each trial, the cue was followed after an interval of 1000 ms by S2, which was presented at fixation for 100 ms, replacing the fixation cross during this period. In 80% of all trials a Go stimulus (the letter ‘G’) was presented as S2, while in 20% of all trials a Nogo stimulus (the letter ‘S’) was presented instead. Participants were instructed to maintain central fixation and to prepare the movement indicated by the response cue, while leaving both hands on the start keys until S2 presentation. They were asked to move the cued hand in the cued direction to reach and press the corresponding target key as fast as possible in response to the letter ‘G’, but to refrain from responding when the letter ‘S’ was presented. As in Experiment 1, the interval between S2 on the preceding trial and response cue onset on the next trial was variable. Following a Nogo trial, this interval varied randomly between 2200 and 3000 ms relative to the onset of a Nogo stimulus. For Go trials, the cue for the next trial appeared 1000 ms after the start key was pressed by the hand which had performed the movement, as in Experiment 1.
3.1.3. Recording and data analysis
These were identical to Experiment 1, with the following exceptions. All averages were collapsed across Go and Nogo trials. In contrast to Experiment 1, where the type of response information provided by the cue was manipulated across blocks, movement direction and effector were cued independently and simultaneously at the start of each trial in Experiment 2. To investigate the relative impact of movement direction and effector information on lateralized ERP components, separate averages were computed for cues specifying the left or right hand and for cues specifying a leftward or rightward movement. In the statistical analyses of the ERP data, the factors condition and cued side that were used Experiment 1 were therefore replaced by the factors cued effector (left versus right) and cued direction (left versus right). In these analyses, the presence of ERP lateralizations sensitive to cued movement direction is reflected by cued direction × hemisphere interactions, while ERP lateralizations sensitive to the cued response hand are reflected by cued effector × hemisphere interactions.
To directly test for differences in ADAN and LDAP amplitudes computed either in terms of cued movement direction or of cued effector, these components were quantified separately as a function of the response information provided by the cues. ADAN and LDAP components sensitive to effector preparation were computed by subtracting ERP mean amplitudes obtained within the 350–600 ms and 600–900 ms post-stimulus time windows for trials with cues specifying a right-hand response from trials with left-hand cues (each collapsed across both cued movement directions). The resulting difference amplitudes obtained for right-hemisphere electrodes were then subtracted from difference amplitudes obtained for homologous sites over the left hemisphere. ADAN and LDAP components sensitive to movement direction programming were computed analogously by subtracting ERP mean amplitudes for trials where a rightward movement was cued from ERPs on trials where a leftward movement was cued (collapsed across both cued response hands) and then subtracting the resulting difference amplitudes at right anterior electrodes from difference amplitudes for left electrodes. Effector- and direction-specific ADAN and LDAP amplitudes obtained for each electrode pairs were then compared via paired t-tests.
To further investigate whether ADAN and LDAP were primarily determined by the spatial selection of movement goal locations, a second analysis was carried out for trials where cued left-hand and right-hand movements were directed towards the two inner goal locations (5 and 4 in Fig. 1). These inward movements always crossed the body midline. Separate averages were computed for cues specifying a left-hand movement towards goal key 5 on the right side, and a right-hand movement towards goal key 4 on the left, collapsed across Go and Nogo trials. In this statistical analysis, the factors cued movement (left-hand towards the right versus right-hand towards the left), hemisphere (left versus right) and electrode sites were considered. Finally, for the analyses of RTs and visual ERPs to irrelevant visual probes, the factor visual probe location now had four levels (outer left, inner left, inner right, outer right).
3.2. Results
3.2.1. Behavioural performance
Trials where RTs exceeded two standard deviations from the mean (accounting for 5.1% of all trials), with false alarms (which occurred on 1.9% of all Nogo trials), and missed responses (observed on 1.1% of all Go trials) were excluded from analysis. Similar to Experiment 1, movements in the right direction were faster than movements in the left direction (725 ms versus 742 ms; main effect of movement direction: F(1, 15) = 9.6, p < .01). A significant interaction between response hand and movement direction (F(1, 15) = 52.9, p < .001) replicated the finding of Experiment 1 that congruent outward movements were faster than incongruent inward movements (690 and 776 ms, respectively). The only effect involving visual probe location was an interaction between movement direction and visual probe location (F(3, 45) = 3.9, p < .05). RTs were faster on trials where visual probe appeared on the same side as the cued movement direction (728 ms) relative to trials where probes were presented contralaterally (737 ms).
3.2.2. Lateralised ERP components triggered during reaching movement preparation
Fig. 5 shows ERPs elicited in response to left and right response cues in the 900 ms interval after cue onset at anterior sites F3/4 (panels a–d) and posterior sites P7/8 (panels e–h), separately for cues indicating a left-hand versus right-hand movement (collapsed across trials where the cued movement direction was to the left or right), and for cues indicating a leftward versus rightward movement (collapsed across trials where the left or right hand were cued). In spite of the fact that the cues now conveyed full information with respect to both effector and movement direction, results were strikingly similar to Experiment 1, where only partial information was provided. As can be seen most easily in the double subtraction waveforms (Fig. 5i and j), ADAN amplitudes were similar for effector and direction information, while LDAP amplitudes appear twice as large when computed in terms of effector relative to movement direction information.
Statistical analysis conducted in the 350–600 ms measurement interval revealed a significant hemisphere × cued direction interaction at lateral anterior electrodes, F(1, 15) = 5.8, p < .03, reflecting the fact that the ADAN was sensitive to cued movement direction. An almost significant hemisphere × cued effector interaction (F(1, 15) = 3.9, p = .067) indicated that the ADAN was also sensitive to which hand was cued. A direct comparison of ADAN amplitudes computed with respect to cued movement direction or cued response hand for the 350–600 ms latency window (see Section 3.1) obtained no significant differences between effector- and direction-dependent ADAN amplitudes at any of the three anterior sites (all t(15) < 1.2, all p > .3), thus confirming the finding from Experiment 1 that this component is equally sensitive to effector and movement direction information.
At lateral posterior sites, a significant hemisphere × cued effector interaction (F(1, 15) = 7.1, p < .02) was present for the 350–600 ms post-cue interval, demonstrating that an early LDAP was reliably present within this interval in response to response hand information. A significant hemisphere × cued effector interaction × electrode site interaction (F(2, 30) = 7.5, p < .006) revealed that LDAP amplitudes differed across posterior electrode sites in this early time window. Follow-up analyses confirmed the presence of a reliable LDAP at P7/8 and OL/R (hemisphere × cued effector interactions: both F(1, 15) > 7.2, p < .02), but not at P3/4 (F < 1). In contrast, no significant hemisphere × cued direction interaction was present (F(1, 15) = 2.9, p = .107), thus suggesting that the early phase of the LDAP was primarily determined by effector-related information. A direct comparison of LDAP amplitudes computed in terms of effector versus movement direction information within the 350–600 ms time window revealed that the effector-dependent LDAP was significantly larger than the direction-dependent LDAP at lateral posterior electrode pair OL/R (t(15) > 3.1, p < .007), but this difference failed to reach significance at P7/8 (t(15) = 1.7, p = .09). Similar results were obtained for the LDAP in the 600–900 ms measurement interval. Here, significant hemisphere × cued effector and hemisphere × cued direction interactions were found at lateral posterior sites (F(1, 15) = 38.2, p < .001 and F(1, 15) = 12.4, p < .003, respectively), reflecting the fact that the LDAP was now present regardless of whether it was quantified in terms of movement direction or effector. However, when LDAP amplitudes computed in terms of effector versus movement direction information were directly compared, significant differences were obtained at P7/8 and at OL/R (both t(15) > 3.3, both p < .005), demonstrating that, analogous to Experiment 1, the LDAP was more strongly determined by effector information than by movement direction information (see Fig. 5j). Finally, a hemisphere × cued effector × electrode site interaction was present at lateral posterior sites in the 600–900 ms interval (F(2, 30) = 13.2, p < .001), once again suggesting a difference in LDAP amplitudes at different electrode pairs. However, follow-up analyses showed significant hemisphere × cued effector interactions al all posterior sites (all F(1, 15) > 4.7, all p < .05).
In the 900–1400 ms interval a significant electrode site × hemisphere × cued effector interaction (F(2, 30) = 38.2, p < .001) was found at lateral central electrode pairs. Follow-up analyses revealed a significant hemisphere × cued effector interaction at C3/4 (F(1, 15) = 17.5, p < .001), reflecting the emergence of the lateralized readiness potential (LRP) during the later phase of manual movement preparation (see Fig. 4, right panel).
Fig. 6 shows ERPs elicited in response to cues signalling a left-hand movement towards the right side (solid lines) and a right-hand movement towards the left side (dashed lines) in the 900 ms interval after cue onset at anterior sites F3/4 (panels a and b) and posterior sites P7/8 (panels d and e). On these trials, the starting position of the cued hand and the target location of the cued movement were always in opposite hemifields. As can be seen most easily in the double subtraction waveforms (Fig. 6c and f), the ADAN was absent, while the LDAP component appeared to be present at posterior electrode sites. Statistical analysis conducted in the 350–600 ms measurement interval revealed no significant hemisphere × cued movement interaction at lateral anterior electrodes, F(1, 15) = 0.02, p = .881, reflecting the fact that no ADAN was elicited during this time window (Fig. 6c). At lateral posterior sites, the hemisphere × cued effector interaction approached significance (F(1, 15) = 3.7, p = .07) for the 350–600 ms post-cue interval, suggesting that an early LDAP tended to be present contralaterally to the cued hand. This was substantiated in the subsequent measurement interval (600–900 ms post-cue onset), where a significant hemisphere × cued movement interaction was found at lateral posterior sites (F(1, 15) = 16.8, p < .001), reflecting the fact that the LDAP was now reliably present. Fig. 6f shows that the LDAP was elicited contralateral to the selected effector, as would be expected if this component was more strongly determined by effector information than by movement direction information.
Fig. 6.
Experiment 2: grand-averaged ERPs elicited in the S1–S2 interval during covert response preparation of inward movements over the left and right hemisphere at anterior (F3/4, top panels) and posterior (P7/8, bottom panels) electrode pairs. (a, b, d, and e) ERPs in response to precues indicating a left-hand movement towards the right side (solid lines) or a right-hand movement towards the left side (dashed lines). Negativity is plotted upwards. (c and f, right side) Difference waveforms reflecting lateralized ERP components sensitive to the response information provided by the cues during inward movement preparation. Enhanced negativities contralateral to the cued effector are reflected by positive values (downward deflections), and enhanced contralateral positivities are reflected by negative values (upward deflections).
3.2.3. Visual ERPs to irrelevant probe stimuli
No significant effects of cued response preparation were found for either P1 or N1 amplitudes in response to visual probes, indicating that response preparation had no spatially selective effects on visual probe processing during the S1–S2 interval when probes were presented at response target locations.4
3.3. Discussion of Experiment 2
While only partial response information about the effector or movement direction was provided by response precues in Experiment 1, precues provided full response information in Experiment 2. In spite of this difference, and the resulting fact that effector and movement direction selection could operate in parallel in Experiment 2, the results obtained in the overall analysis across all trials almost perfectly confirmed the pattern of results found in Experiment 1.
As in Experiment 1, ADAN and LDAP components were both present. When computed separately relative to which effector was cued (collapsed across both movement directions), or relative to which movement direction was cued (collapsed across both hands), ADAN and LDAP behaved remarkably similar to what was observed in Experiment 1. ADAN amplitudes again showed equal sensitivity to effector and movement direction information (Fig. 5i). LDAP amplitudes were again twice as large when computed in terms of effector information as when quantified in terms of movement direction information (Fig. 5j). As expected, and analogous to Experiment 1, the LRP was triggered only when waveforms were computed in terms of cued effector (Fig. 4, right panel), thus again confirming that the LRP reflects effector-specific partial response activation, but is insensitive to other aspects of response programming such as the selection of movement direction.
The fact that the results of Experiment 2 were strikingly similar to the findings obtained in the first experiment provides clear evidence that these findings were not simply due to the fact that only partial response information was available during the S1–S2 interval, and just one response parameter could therefore be prepared. Even though effector selection and the specification of movement direction could occur in parallel in Experiment 2, results further supported the hypothesis that processes reflected by the ADAN component are activated to a similar degree during the programming of both of these response parameters. In addition, Experiment 2 also confirmed that the mechanisms underlying the LDAP component appear to be substantially more sensitive to effector selection than to the specification of movement direction. The fact that this was the case in spite of the fact that there was no longer any spatial uncertainty with respect to effector, movement direction, and target location in Experiment 2 demonstrates that the difference in LDAP amplitudes between effector and direction cues observed in Experiment 1 cannot be attributed to differences in the explicitness of the spatial information provided by these two types of cues.
The pattern of results obtained in the second set of analyses where only cued inward movements (leftward movements with the right hand, and rightward movements with the left hand) were considered provides clear evidence against an interpretation of ADAN and LDAP components in terms of purely space based mechanisms exclusively related to the movement goal selection such as shifts of spatial attention directed to the goal locations or the spatial localization of the target in external coordinates. According to this interpretation, these components should have been elicited contralateral to the cued movement goal location on the left or right side, but that was not what was observed. No evidence for an ADAN was obtained for these trials (Fig. 6c), which is exactly what would be expected if this component was equally sensitive to effector selection and movement direction selection. During the preparation of left-hand movements towards the right side, or right-hand movement towards the left, effector-specific and direction-specific contributions to the ADAN should cancel each other out, resulting in the absence of any systematic cue-induced lateralization. In contrast, the LDAP was reliably elicited during the preparation of inward movements, and was reflected by an enhanced positivity contralateral to the side of the cued effector, and thus ipsilateral to the side of the response target location (Fig. 6f). This is exactly the opposite of what would be expected if the LDAP reflected the spatial selection of movement target locations, but is entirely in line with the assumption that it predominantly reflects effector selection. The fact that the LDAP observed during the preparation of inward movements was considerably smaller than the LDAP observed in response to effector information when all trials were considered (Fig. 5j) is consistent with the assumption that this component is also, albeit to a smaller degree, sensitive to movement direction information. The preparation of hand movements across the body midline should result in a small direction-sensitive LDAP component of opposite polarity that attenuates the LDAP generated as a result of effector selection.
4. General discussion
In the present study, we used an S1–S2 response precueing paradigm to investigate the properties of lateralized ERP components triggered during the preparation of manual reaching movements. A visual response precue (S1) provided either partial (Experiment 1) or full (Experiment 2) information about the response hand and the direction for an upcoming reaching movement that was to be executed in response to an imperative stimulus (S2) presented 1100 ms after S1 onset. ERP waveforms recorded in the S1–S2 interval were characterized by three lateralized ERP components. An ADAN was triggered at lateral anterior electrodes, an LDAP was present at lateral posterior sites, and an LRP was elicited late during the response preparation interval at C3/4 when information about the response hand was available.
The presence of the ADAN and LDAP in the present study demonstrates that these two components are not just elicited during covert shifts of attention in the absence of any response preparation instruction (c.f., Harter et al., 1989; Yamaguchi et al., 1994; Nobre et al., 2000; Hopf and Mangun, 2000; Eimer et al., 2002, 2003b), during the preparation of simple unimanual responses (Eimer et al., 2005, 2006; Eimer and Van Velzen, 2006), and during saccade preparation (Eimer et al., 2006; Wauschkuhn et al., 1997; Van der Lubbe et al., 2000), but also during the preparation of more complex manual reaching movements. In addition, the fact that these two components were present not only when full response information was available during the S1–S2 interval (in Experiment 2), but also when response cues only provided partial information about either response hand or movement direction (in Experiment 1) demonstrates that these components do not critically depend on a fully specified motor program, but are already elicited when only one response parameter can be programmed in advance.
The main objective of the present study was to dissociate the relative contributions of advance information about the response hand or movement direction on the ADAN and LDAP components. In Experiment 1, where response cues provided information only about one of these two parameters, the ADAN was elicited in a similar fashion both when effector and when movement direction information was available during the S1–S2 interval, suggesting that the mechanisms responsible for generating this component are equally sensitive to both types of response-related information. This pattern of results was confirmed in Experiment 2, where cues provided full response information. An ADAN of similar size was observed regardless of whether ERPs were computed as a function of which hand was cued, or which movement direction was indicated by the precues, thus again indicating that the ADAN is generated during effector selection as well as during the specification of movement direction. Further evidence in favour of this interpretation comes from the fact that this component was eliminated in Experiment 2 when inward movements (left-hand movements towards the right side, or right-hand movements towards the left) were prepared, resulting in effector and movement direction selection processes that are based on incongruent spatial codes.
Given its anterior scalp distribution and its involvement in response preparation as well as covert shifts of spatial attention, the ADAN is likely to be generated in dorsolateral premotor areas (Praamstra et al., 2005), although an involvement of the frontal eye fields has also been suggested (Van der Lubbe et al., 2000, 2006). Single-cell studies on monkeys have shown that activity in premotor areas recorded during the delay period of an instructed reaching movement was initially independent of the effector used to perform this movement (Cisek et al., 2003). Furthermore, Cisek and Kalaska (2002) have demonstrated that when a monkey is presented with two mutually exclusive potential directions for a reaching movement, directional signals corresponding to both of these movements were simultaneously represented in dorsal premotor cortex. These findings suggest that premotor areas can represent movement direction and effector information independently. This information may then be integrated in order to specify a motor program (Hoshi and Tanji, 2000). The present finding that the ADAN is triggered by cues that provide either movement direction or effector information is in line with these observations, and suggests that this component might reflect activity within dorsal premotor areas that are responsible for the independent specification of different spatial parameters of an upcoming manual reaching movement.
In contrast to the ADAN, the posterior LDAP component turned out to be differentially sensitive to effector and movement direction information. In Experiment 1, where response cues specified either movement direction or response hand, the LDAP was about twice as large when effector information was available, although this component was also reliably present when only movement direction information was provided instead. This pattern of results, that was perfectly replicated in Experiment 2 when cues delivered full response information, suggests that this component is predominantly sensitive to effector selection, but also reflects processes involved in the specification of movement direction. When the spatial codes for movement direction and response hand selection were incongruent (during the preparation of inward movements in Experiment 2), an LDAP was elicited contralateral to the cued response hand, but was strongly attenuated relative to trials where these codes were congruent, again suggesting that both effector selection and movement direction selection contribute to this component, but that effector selection plays a more dominant role.
The LDAP is likely to be generated in the occipitotemporal cortex (Mathews et al., 2006; Praamstra et al., 2005), although a contribution of more dorsal parietal regions has also been suggested (Van der Lubbe et al., 2006). Single-cell recordings have shown that areas in posterior parietal cortex, and in particular the PRR, are specifically involved in the programming of manual reaching movements (Andersen and Buneo, 2002). Importantly, different PRR neurons appear to code effector information and spatial information separately. This was demonstrated by Calton et al. (2002), who trained monkeys to move their arm or their eyes to a lateral target under conditions where a response precue specified either the effector (arm or eyes) or the movement direction (by indicating its target location). One-third of PRR neurons were activated when the precue that indicated an arm movement, but left its direction unspecified. A larger and only partially overlapping set of PRR neurons was activated when the cue indicated movement direction, but not the effector. This separate coding of effector and movement direction information in PRR closely resembles the pattern of results found in the present study for the LDAP, which was triggered in response to both effector and movement direction cues. It is possible that the LDAP observed when effector information was available at least partially represents activity within PRR areas that are sensitive to effector-specific information, whereas the LDAP elicited to movement direction cues partially reflects the activity of PRR neurons that represent movement direction and target location.5 The fact that LDAP amplitudes were larger for effector as compared with movement direction information, which is not entirely analogous to the observations of Calton et al. (2002), could reflect differences between tasks involving the selection of the left versus right hand, and the selection of different effector systems (arm versus eye movements, as in Calton et al., 2002).
The hypothesis that the LDAP component observed in the present study may reflect response preparation processes in the PRR that are analogous to those identified on the basis of single-cell recordings is of course largely speculative at the present moment. Apart from the fact that the exact human homologue of the PRR identified in monkeys is still under discussion (see Connolly et al., 2003), the exact coordinates of the neural generators underlying the LDAP are still unknown. However, the results from the present study strongly suggest that the processes reflected by the LDAP are activated during the covert preparation of manual reaching movements, and that these processes are sensitive to both effector movement direction information, albeit to a different degree.
The present research also adds to the growing number of studies demonstrating that lateralized ERP components triggered during covert response preparation and components elicited during covert shifts of attention are very similar. However, although ADAN and LDAP components have now been frequently reported during covert attention tasks as well as in studies where participants prepare manual responses or eye movements, it is possible that their temporal properties might differ systematically between these two types of tasks. During covert shifts of attention, the ADAN typically precedes the LDAP component by about 200 ms (see Nobre et al., 2000; Hopf and Mangun, 2000; Eimer et al., 2002, 2003a). This temporal asymmetry has been interpreted as evidence for the hypothesis that attentional signals generated within dorsolateral frontal areas initiate and control subsequent selective attentional processes in posterior regions. In contrast, ADAN and LDAP components were triggered more or less simultaneously in the present study during the preparation of manual reaching movements. If these two components reflect the activation of dorsal prefrontal and posterior areas involved in the programming of reaching movements, as suggested above, their simultaneous presence might indicate that these areas work in parallel, rather than sequentially, during the specification of a motor program (see also Naranjo et al., 2007). This hypothesis is so far only based on informal observations of possible latency differences across studies and different tasks, and thus needs to be substantiated in experiments that are specifically designed to contrast the temporal dynamics of lateralized ERP components observed during covert attention and response preparation.
In summary, the present study has provided new insights into the nature of lateralized ERP components that are triggered during the programming of manual reaching movements. We have demonstrated that both LDAP and ADAN are elicited in response to precues that provide either partial or full information about an upcoming reaching response, and have dissociated the relative contributions of effector and movement direction information to these components. The ADAN and LDAP may reflect preparatory activity within the anterior and posterior parts of the parieto-premotor network (Matelli and Luppino, 2001; Luppino and Rizzolatti, 2000) that implements the complex sensory-motor transformations required to program and control visually guided reaching movements. In this context, it is interesting to note that these components have now been consistently observed not only during the covert preparation of simple and more complex manual movements, but also during saccade preparation (e.g., Eimer et al., 2006), as well as during covert shifts of visual, auditory, and tactile spatial attention (e.g., Eimer et al., 2002, 2004). This suggests that these components may reflect activity within a general-purpose neural network that is involved in the control of spatial attention shifts as well as in the programming of different types of movements. The existence of such a network is of course the basic assumption of the premotor theory of attention (Rizzolatti et al., 1994), which postulates shared sensorimotor mechanisms for the control of attentional orienting and response preparation. However, the functional significance of the brain processes that underlie the ADAN and LDAP components is still poorly understood, and attempts to dissociate the specific roles of these processes in the control of attention and motor preparation have only just begun. The present results suggest that effector and movement direction selection affect ADAN and LDAP components differently. One previous study (Van Velzen et al., 2006) has shown that the LDAP (but not the ADAN) is eliminated during attentional shifts in the congenitally blind, thus suggesting that this component might be closely linked with visually mediated spatial representations of external space. Further research is clearly needed to gain more comprehensive and systematic insights into the nature of the brain mechanisms that are responsible for these components and their specific contributions to the control of perception and action.
Acknowledgements
This research was supported by grants from the Wellcome Trust (UK) and the Medical Research Council (MRC). M.E. holds a Royal Society-Wellcome Research Merit Award. We are grateful to the Department of Social, Cognitive and Quantitative Sciences, University of Modena and Reggio Emilia, for supporting E.G. with a Ph.D. Studentship.
Footnotes
As the question whether and how covert response preparation affects visual processing is not the focus of the present paper, modulations of ERPs obtained in response to these visual probes will only be presented very briefly in Section 3.1, for completeness (see Eimer and Van Velzen, 2006; Eimer et al., 2006, for more detailed accounts of spatially specific effects of manual response preparation on early visual ERP components).
In previous behavioural studies of movement execution (e.g., Tipper et al., 1992; Pratt and Abrams, 1994; Fischer, 1997), RTs and movement times (MTs) were measured separately by instructing participants to keep start keys pressed before movement initiation, and defining RTs relative to the release of a start key, and MT as the interval between start key release and target key press. Because the continuous pressing of the start keys during the covert movement preparation interval was likely to produce sustained lateralized motor ERP components, participants were asked to leave their hands on the start keys until the onset of the imperative stimulus without pressing them. Thus, RTs for target keys reflect the joint contribution of response times to S2 and movement times.
At lateral central sites, a hemisphere × cued side interaction (F(1, 15) = 8.6, p < .01) was present during the 600–900 ms measurement interval, and was accompanied by a condition × hemisphere × cued side interaction (F(1, 15) = 6.5, p < .03). The LDAP was reliably present at lateral central sites in the effector information condition (hemisphere × cued side interaction, F(1, 15) = 12.9, p < .003) but not in the direction information condition. For the effector information condition, an electrode site × hemisphere × cued side interaction was present (F(2, 30) = 8.7, p < .003) and follow-up analyses revealed hemisphere × cued side interactions at CP5/6 (F(1, 15) = 12.8, p < .003) and T7/8 (F(1, 15) = 20.8, p < .001) but not at C3/4.
The absence of any modulations of visual processing in Experiment 2 contrast with Experiment 1, where spatially specific N1 modulations were observed in the effector information condition (see also Eimer et al., 2006, for similar findings). This difference is most likely due to the fact that visual probes were presented at one of the four response target locations in Experiment 2, while they were presented adjacent to one of the response hands in Experiment 1. It is possible that modulations of visual processing resulting from response preparation will initially be present only for visual stimulus locations close to the relevant response hand, while response-related modulations of visual probe processing at response target locations emerge only during response execution, after attention has been shifted towards the movement target location. This hypothesis needs to be investigated in future experiments.
Even though ERPs source localization studies suggest that the LDAP is primarily generated in occipitotemporal cortex (Mathews et al., 2006; Praamstra et al., 2005), it may also reflect brain activity in more parietal areas. This hypothesis is in line with the observation by Praamstra et al. (2005) of an early and transient contribution to the LDAP from parietal cortex, as well as on the fact that the posterior parietal cortex is strongly connected with extrastriate occipitotemporal visual areas (i.e., Baizer et al., 1991; Duhamel et al., 1998).
References
- Adam J.J., Pratt J. Dissociating visual attention and effector selection in spatial precueing tasks. J Exp Psychol Hum Percept Perform. 2004;30:1092–1106. doi: 10.1037/0096-1523.30.6.1092. [DOI] [PubMed] [Google Scholar]
- Andersen R.A., Buneo C.A. Intentional maps in the posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Baizer J.S., Ungerleider L.G., Desimone R. Organization of visual inputs to inferior temporal and posterior parietal cortex in monkeys. J Neurosci. 1991;11:168–190. doi: 10.1523/JNEUROSCI.11-01-00168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt I., Franz V.H., Bülthoff H.H., Wascher E. Effects of pointing direction and direction predictability on event-related lateralizations of the EEG. Hum Mov Sci. 2002;21:75–98. doi: 10.1016/s0167-9457(02)00122-7. [DOI] [PubMed] [Google Scholar]
- Bock O., Eversheim U. The mechanisms of movement preparation: a precueing study. Behav Brain Res. 2000;108:85–90. doi: 10.1016/s0166-4328(99)00134-5. [DOI] [PubMed] [Google Scholar]
- Calton J.L., Dickinson A.R., Snyder L.H. Non-spatial, motor specific activation in posterior parietal cortex. Nat Neurosci. 2002;5:580–588. doi: 10.1038/nn0602-862. [DOI] [PubMed] [Google Scholar]
- Castiello U., Paine M. Effects of left parietal injury on covert orienting of attention. J Neurol Neurosurg Psychiatry. 2002;72:73–76. doi: 10.1136/jnnp.72.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby C.L., Duhamel J.R. Spatial representations for action in parietal cortex. Cogn Brain Res. 1996;5:105–115. doi: 10.1016/s0926-6410(96)00046-8. [DOI] [PubMed] [Google Scholar]
- Cisek P., Crammond D.J., Kalaska J.F. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol. 2003;89:922–942. doi: 10.1152/jn.00607.2002. [DOI] [PubMed] [Google Scholar]
- Cisek P., Kalaska J.F. Simultaneous encoding of multiple potential reach directions in dorsal premotor cortex. J Neurophysiol. 2002;87:1149–1154. doi: 10.1152/jn.00443.2001. [DOI] [PubMed] [Google Scholar]
- Connolly J.D., Andersen R.A., Goodale M.A. FMRI evidence for a ‘parietal reach region’ in the human brain. Exp Brain Res. 2003;153:140–145. doi: 10.1007/s00221-003-1587-1. [DOI] [PubMed] [Google Scholar]
- De Jong R., Wierda M., Mulder G., Mulder L.J. Use of partial stimulus information in response processing. J Exp Psychol Hum Percept Perform. 1988;14:682–692. doi: 10.1037//0096-1523.14.4.682. [DOI] [PubMed] [Google Scholar]
- Duhamel J.R., Colby C.L., Goldberg M.E. Ventral intraparietal area of the macaque: convergent visual and somatic response properties. J Neurophysiol. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Eimer M. Spatial cueing, sensory gating and selective response preparation: an ERP study on visuo-spatial orienting. Electroencephalogr Clin Neurophysiol. 1993;88:408–420. doi: 10.1016/0168-5597(93)90017-j. [DOI] [PubMed] [Google Scholar]
- Eimer M. Stimulus-response compatibility and automatic response activation: evidence from psychophysiological studies. J Exp Psychol Hum Percept Perform. 1995;21:837–854. doi: 10.1037//0096-1523.21.4.837. [DOI] [PubMed] [Google Scholar]
- Eimer M., Coles M.G.H. The lateralized readiness potential. In: Jahanshahi M., Hallett M., editors. The bereitschafts potential: in honour of Professors Deecke and Kornhuber. Kluwer Academic/Plenum; New York: 2003. pp. 229–248. [Google Scholar]
- Eimer M., Forster B., Fieger A., Harbich S. Effects of hand posture on preparatory control processes and sensory modulations in tactile-spatial attention. Clin Neurophysiol. 2004;115:596–608. doi: 10.1016/j.clinph.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Eimer M., Forster B., Van Velzen J. Anterior and posterior attentional control systems use different spatial reference frames: ERP evidence from covert tactile-spatial orienting. Psychophysiology. 2003;40:924–933. doi: 10.1111/1469-8986.00110. [DOI] [PubMed] [Google Scholar]
- Eimer M., Forster B., Van Velzen J., Prabhu G. Covert manual response preparation triggers attentional shifts: ERP evidence for the premotor theory of attention. Neuropsychologia. 2005;43:957–966. doi: 10.1016/j.neuropsychologia.2004.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M., Van Velzen J. Covert manual response preparation triggers attentional modulations of visual, but not auditory processing. Clin Neurophysiol. 2006;117:1063–1074. doi: 10.1016/j.clinph.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Eimer M., Van Velzen J., Driver J. Crossmodal interactions between audition, touch and vision in endogenous spatial attention: ERP evidence on preparatory states and sensory modulations. J Cogn Neurosci. 2002;14:254–271. doi: 10.1162/089892902317236885. [DOI] [PubMed] [Google Scholar]
- Eimer M., Van Velzen J., Forster B., Driver J. Shift of attention in light and darkness: an ERP study of supramodal attentional control and crossmodal links in spatial attention. Brain Res Cogn Brain Res. 2003;15:308–323. doi: 10.1016/s0926-6410(02)00203-3. [DOI] [PubMed] [Google Scholar]
- Eimer M., Van Velzen J., Gherri E., Press C. Manual response preparation and saccade programming are linked to attention shifts: ERP evidence for covert attentional orienting and spatially specific modulations of visual processing. Brain Res. 2006;1105:7–19. doi: 10.1016/j.brainres.2005.10.060. [DOI] [PubMed] [Google Scholar]
- Fischer M.H. Attention allocation during manual movement preparation and execution. Europ J Cog Psych. 1997;9:17–51. [Google Scholar]
- Gehring W.J., Gratton G., Coles M.G.H., Donchin E. Probability effects on stimulus evaluation and response processes. J Exp Psychol Hum Percept Perform. 1992;18:198–216. doi: 10.1037/0096-1523.18.1.198. [DOI] [PubMed] [Google Scholar]
- Goodman D., Kelso J.A.S. Are movements prepared in parts? Not under compatible conditions. J Exp Psychol. 1980;109:475–495. doi: 10.1037//0096-3445.109.4.475. [DOI] [PubMed] [Google Scholar]
- Gratton G., Bosco C.M., Kramer A.F., Coles M.G., Wickens C.D., Donchin E. Event-related brain potentials as indices of information extraction and response priming. Electroencephalogr Clin Neurophysiol. 1990;75:419–432. doi: 10.1016/0013-4694(90)90087-z. [DOI] [PubMed] [Google Scholar]
- Gross C.G., Graziano M.S.A. Multiple representations of space in the brain. Neuroscientist. 1995;1:43. [Google Scholar]
- Harter M.R., Miller S.L., Price N.J., LaLonde M.E., Keyes A.L. Neural processes involved in directing attention. J Cogn Neurosci. 1989;1:223–237. doi: 10.1162/jocn.1989.1.3.223. [DOI] [PubMed] [Google Scholar]
- Hopf J.M., Mangun G.R. Shifting visual attention in space: an elctrophysiological analysis using high spatial resolution mapping. Clin Neurophysiol. 2000;111:1241–1257. doi: 10.1016/s1388-2457(00)00313-8. [DOI] [PubMed] [Google Scholar]
- Hoshi E., Tanji J. Integration of target and body-part information in the premotor cortex when planning action. Nature. 2000;408:466–470. doi: 10.1038/35044075. [DOI] [PubMed] [Google Scholar]
- Jentzsch I., Leuthold H. Advance movement preparation of eye, foot, and hand: a comparative study using movement-related brain potentials. Brain Res Cogn Brain Res. 2002;14:201–217. doi: 10.1016/s0926-6410(02)00107-6. [DOI] [PubMed] [Google Scholar]
- Jentzsch I., Leuthold H., Ridderinkhof K.R. Beneficial effects of ambiguous precues: parallel motor preparation or reduced premotoric processing time? Psychophysiology. 2004;41:231–244. doi: 10.1111/j.1469-8986.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- Keele S.W. Movement control in skilled motor performance. Psychol Bull. 1968;70:387–403. [Google Scholar]
- Keele S.W. Behavioral analysis of movement. In: Brooks V.B., editor. Handbook of physiology. American Physiological Society; Bethesda, MD: 1981. pp. 1391–1414. [Google Scholar]
- Leuthold H., Jentzsch I. Neural correlates of advance movement preparation: a dipole source analysis approach. Brain Res Cogn Brain Res. 2001;12:207–224. doi: 10.1016/s0926-6410(01)00052-0. [DOI] [PubMed] [Google Scholar]
- Leuthold H., Jentzsch I. Distinguishing neural sources of movement preparation and execution: an electrophysiological analysis. Biol Psychol. 2002;60:173–198. doi: 10.1016/s0301-0511(02)00032-7. [DOI] [PubMed] [Google Scholar]
- Leuthold H., Sommer W., Ulrich R. Partial advance information and response preparation: inferences from the lateralized readiness potential. J Exp Psychol Gen. 1996;125:307–323. doi: 10.1037//0096-3445.125.3.307. [DOI] [PubMed] [Google Scholar]
- Luppino G., Rizzolatti G. The organization of the frontal motor cortex. News Physiol Sci. 2000;15:219–224. doi: 10.1152/physiologyonline.2000.15.5.219. [DOI] [PubMed] [Google Scholar]
- Matelli M., Luppino G. Parietofrontal circuits for action and space perception in the macaque monkey. Neuroimage. 2001;14:27–32. doi: 10.1006/nimg.2001.0835. [DOI] [PubMed] [Google Scholar]
- Mathews S., Dean P.J., Sterr A. EEG dipole analysis of motor-priming foreperiod activity reveals separate sources for motor and spatial attention components. Clin Neurophysiol. 2006;117:2675–2683. doi: 10.1016/j.clinph.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Miller J. Discrete versus continuous models of human information processing: in search of partial output. J Exp Psychol Hum Percept Perform. 1982;8:273–296. doi: 10.1037//0096-1523.8.2.273. [DOI] [PubMed] [Google Scholar]
- Naranjo J.R., Brovelli A., Longo R., Budai R., Kristeva R., Battaglini P.P. EEG dynamics of the frontoparietal network during reaching preparations in humans. Neuroimage. 2007;34:1673–1682. doi: 10.1016/j.neuroimage.2006.07.049. [DOI] [PubMed] [Google Scholar]
- Nobre A.C., Sebestyen G.N., Miniussi C. The dynamics of shifting visuospatial attention revealed by event-related potentials. Neuropsychologia. 2000;38:964–974. doi: 10.1016/s0028-3932(00)00015-4. [DOI] [PubMed] [Google Scholar]
- Pisella L., Grea H., Tilikete C., Vighetto A., Desmurget M., Rode G. An ‘automatic pilot’ for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat Neurosci. 2000;3:729–736. doi: 10.1038/76694. [DOI] [PubMed] [Google Scholar]
- Praamstra P. Prior information of stimulus location: effects on ERP measures of visual selection and response selection. Brain Res. 2006;1072:153–160. doi: 10.1016/j.brainres.2005.11.098. [DOI] [PubMed] [Google Scholar]
- Praamstra P., Boutsen L., Humphreys G.W. Frontoparietal control of spatial attention and motor intention in human EEG. J Neurophysiol. 2005;94:764–774. doi: 10.1152/jn.01052.2004. [DOI] [PubMed] [Google Scholar]
- Pratt J., Abrams R.A. Action - centered inhibition: Effects of distracters on movement planning and execution. Hum Mov Sci. 1994;13:245–254. [Google Scholar]
- Proctor R.W., Wang H. Set- and element-level stimulus-response compatibility effects for different manual response sets. J Mot Behav. 1997;29:351–365. doi: 10.1080/00222899709600021. [DOI] [PubMed] [Google Scholar]
- Reeve T., Proctor R.W. On the advance preparation of discrete finger responses. J Exp Psychol Hum Percept Perform. 1984;10:541–553. doi: 10.1037//0096-1523.10.4.541. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Riggio L., Dascola I., Umiltà C. Reorienting attention across the horizontal and vertical meridians: evidence in favour of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Riggio L., Sheliga B.M. Space and selective attention. In: Umiltà C., Moscovitch M., editors. Attention and performance XV. MIT Press; Cambridge, MA: 1994. pp. 231–265. [Google Scholar]
- Rosenbaum D.A. Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen. 1980;109:444–474. doi: 10.1037//0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA. The movement precueing technique: assumptions, applications, and extensions. In: Magill RA, editor. Memory and control of action. Amsterdam: North-Holland; 1983. p. 231–74.
- Rushworth M., Krams M., Passingham R.E. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci. 2001;13:698–710. doi: 10.1162/089892901750363244. [DOI] [PubMed] [Google Scholar]
- Rushworth M., Ellison A., Walsh V. Complementary localization and lateralization of orienting and motor attention. Nat Neurosci. 2001;4:656–661. doi: 10.1038/88492. [DOI] [PubMed] [Google Scholar]
- Sangals J., Sommer W., Leuthold H. Influences of presentation mode and time pressure on the utilisation of advance information in response preparation. Acta Psychol. 2002;109:1–24. doi: 10.1016/s0001-6918(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Snyder L.H., Batista A.P., Andersen R.A. Intention-related activity in the posterior parietal cortex: a review. Vision Res. 2002;40:1433–1441. doi: 10.1016/s0042-6989(00)00052-3. [DOI] [PubMed] [Google Scholar]
- Tipper S.P., Lortie C., Baylis G.C. Selective reaching: evidence for action-centered attention. J Exp Psychol Hum Percept Perform. 1992;18:891–905. doi: 10.1037//0096-1523.18.4.891. [DOI] [PubMed] [Google Scholar]
- Ulrich R., Leuthold H., Sommer W. Motor programming of response force and movement direction. Psychophysiology. 1998;35:721–728. [PubMed] [Google Scholar]
- Van der Lubbe R.H., Wauschkuhn B., Wascher E., Niehoff T., Kompf D., Verleger R. Lateralized EEG components with direction information for the preparation of saccades versus finger movements. Exp Brain Res. 2000;132:163–178. doi: 10.1007/s002219900328. [DOI] [PubMed] [Google Scholar]
- Van der Lubbe R.H., Neggers S.F., Verleger R., Kenemans J.L. Spatiotemporal overlap between brain activation related to saccade preparation and attentional orienting. Brain Res. 2006;1072:133–152. doi: 10.1016/j.brainres.2005.11.087. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel S., Heslenfeld D.J., Theeuwes J. An ERP study of preparatory and inhibitory mechanisms in a cued saccade task. Brain Res. 2006;1105:32–45. doi: 10.1016/j.brainres.2006.02.089. [DOI] [PubMed] [Google Scholar]
- Van Velzen J., Eardley A., Forster B., Eimer M. Shifts of attention in the early blind: an ERP study of attentional control processes in the absence visual spatial information. Neuropsychologia. 2006;44:2533–2546. doi: 10.1016/j.neuropsychologia.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Verleger R., Vollmer C., Wauschkuhn B., Van der Lubbe R.H., Wascher E. Dimensional overlap between arrows as cueing stimuli and responses? Evidence from contra-ipsilateral differences in EEG potentials. Brain Res Cogn Brain Res. 2000;10:99–109. doi: 10.1016/s0926-6410(00)00032-x. [DOI] [PubMed] [Google Scholar]
- Wauschkuhn B., Wascher E., Verleger R. Lateralised cortical activity due to preparation of saccades and finger movements: a comparative study. Electroencephalogr Clin Neurophysiol. 1997;102:114–124. doi: 10.1016/s0921-884x(96)96585-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Tsuchiya H., Kobayashi S. Electroencephalographic activity associated with shifts of spatial attention. Brain. 1994;117:553–562. doi: 10.1093/brain/117.3.553. [DOI] [PubMed] [Google Scholar]