Abstract
Introduction
During the past three decades, improvements in the treatment of childhood leukemia have resulted in high cure rates, particularly for acute lymphoblastic leukemia (ALL). Unfortunately, successful therapy has come with a price, as significant morbidity can result from neurological affects which harm the brain and spinal cord. The expectation and hope is that chemotherapy, as a primary means of CNS therapy, will result in acceptable disease control with less CNS morbidity than has been observed with combinations of chemotherapy and radiotherapy over the past several decades.
Methods and results
In this review we discuss the poignant, historical aspects of CNS leukemia therapy, outline current methods of systemic and CNS leukemia therapy, and present imaging findings we have encountered in childhood leukemia patients with a variety of acute neurological conditions. A major objective of our research is to understand the neuroimaging correlates of acute and chronic effects of cancer and therapy. Specific features related to CNS leukemia and associated short-term toxicities, both disease- and therapy-related, are emphasized in this review with the specific neuroimaging findings. Specific CNS findings are similarly important when treating acute myelogenous leukemia (AML), and details of leukemic involvement and toxicities are also presented in this entity.
Conclusion
Despite contemporary treatment approaches which favor the use of chemotherapy (including intrathecal therapy) over radiotherapy in the treatment of CNS leukemia, children still occasionally experience morbid neurotoxicity. Standard neuroimaging is sufficient to identify a variety of neurotoxic sequelae in children, and often suggest specific etiologies. Specific neuroimaging findings frequently indicate a need to alter antileukemia therapy. It is important to appreciate that intrathecal and high doses of systemic chemotherapy are not innocuous and are associated with acute, specific, recognizable, and often serious neurological consequences.
Keywords: Childhood CNS leukemia, Neurotoxicity, Intrathecal therapy, CNS infection, Secondary malignancies, Methotrexate
Introduction
The efficacy of treatment for childhood leukemia has greatly improved over the last 30 years. In the early 1960s, cure rates for acute lymphoblastic leukemia (ALL) were between 5% and 10%. Today, cure rates of children with ALL approach 90% [1], and for children with acute myelogenous leukemia (AML), 40-50% [2, 3]. In ALL, chemotherapeutic regimens target both systemic disease and so-called “sanctuary sites” that require specific therapies, most importantly the central nervous system (CNS). Modern diagnostic techniques improve our ability to categorize risk and estimate potential for relapse in children with leukemia, enabling clinicians to optimize and tailor therapy including that directed to the CNS. Evolution of successful CNS-directed therapy has played a major role contributing to cure in children with ALL. Additionally, through the careful study of leukemia and cancer survivors using sophisticated neuroimaging techniques, we have further stimulated risk-adapted therapeutic approaches that aim toward maximizing disease control while minimizing deleterious effects on the CNS. In a companion article, appearing in this issue of Neuroradiology, many of the advanced neuroimaging techniques used at our institution to evaluate the brain of children with leukemia are discussed. In this respect, despite the proven effectiveness of irradiation in treating subclinical and overt CNS disease, most current ALL protocols seek to minimize or eliminate the use of cranial or craniospinal irradiation in favor of systemic and intrathecal (IT) chemotherapy regimens which offer the potential for less acute and chronic neurotoxicity.
Many of the chronic neurological sequelae affecting children with leukemia treated with combination irradiation and chemotherapy are well documented in the literature and are mentioned only briefly in this report. Neuroendocrine and neurocognitive dysfunction are examples that negatively impact the quality of life of leukemia survivors. One purpose of this review is to focus on relatively small numbers of childhood leukemia patients and survivors who experience acute neurotoxicity around the time of diagnosis and during therapy. In patients who received cranial irradiation, secondary cancers within the irradiated field including brain tumor, parotid gland or thyroid carcinoma and skin cancer can develop many years after completion of therapy [4]. It is also important to appreciate that IT and high doses of systemic chemotherapy are not innocuous and can be associated with relatively acute, specific, recognizable, often serious neurological consequences. The true extent of the long-term adverse effects from systemic and IT chemotherapy on the CNS is largely unknown.
Neurological morbidity may result acutely from disease at diagnosis, from relapse of disease, or from agents used to eradicate disease as part of therapy. At diagnosis, for example, cranial nerve palsies may signal the presence of overt CNS leukemia, and focal aggregates of leukemia cells (chloromas) may grow from the bone marrow, through cortical bone, causing symptoms from mass effects on neural structures (more commonly in AML). Intracranial hemorrhage with focal neurological deficits or altered mental status may complicate presentations with very high white blood counts (hyperleukocytosis with thrombosis and leukostasis resulting in poor CNS perfusion). Rarely, hemorrhage can occur in the spinal canal (often in the setting of thrombocytopenia) when lumbar puncture is performed in the initial assessment of CNS status. Dural venous sinus thrombosis (DVST) may result from aberrations of hemostasis due to coagulopathy at diagnosis, but in our experience, DVST occurs more commonly during therapy, as a complication of chemotherapy (e.g., asparaginase and corticosteroids). Seizures may herald intracranial pathology such as posterior reversible encephalopathy syndrome (PRES) in children who become hypertensive most often during the induction phase of chemotherapy. In rare instances, IT methotrexate (MTX) is associated with acute neurotoxicity manifesting with “stroke-like” clinical symptoms, as discussed later in this review. Immunosuppressive effects of disease and therapy predispose to systemic and CNS infections.
This review includes a general discussion of childhood leukemia, followed by a brief summary of landmark achievements in serial, prospective pediatric ALL trials now resulting in high cure rates. Specific features related to CNS leukemia and the short-term toxicities, both disease- and therapy-related, are outlined along with the specific neuroimaging findings. CNS findings in AML are also presented. Information gained from neuroimaging is often instrumental in identifying etiologies of adverse neurological conditions affecting children with leukemia and in providing information to oncologists allowing them to modify therapy to reduce morbidity. Neuroimaging findings related to acute and chronic sequelae may highlight therapeutic directions to reduce morbidity.
Clinical presentation of childhood leukemia
Leukemias account for 30% of all childhood malignancies and are the most common cancers diagnosed in children. ALL is diagnosed in approximately 3,000 children and AML in 500 children annually in the United States. Chronic myeloid leukemia (CML) is diagnosed in fewer than 100 children each year and chronic lymphocytic leukemia (CLL), while common in adults, is rarely diagnosed in children. Today, nearly all children with ALL are treated with systemic and IT therapy (for CNS disease control), while AML and CML are more recalcitrant to chemotherapy and frequently require bone marrow transplantation (BMT) to offer the greatest chance for cure.
ALL accounts for 80% of all leukemias in children and adolescents; the median age at diagnosis is 4 years, and most children are between 4 and 6 years of age at diagnosis. Boys are more commonly affected than girls. Common presenting symptoms are fever, bleeding, and bone pain. Examination often shows petechiae or ecchymoses, lymph node enlargement, and hepatosplenomegaly. Extramedullary involvement of the CNS, testes, and kidneys may be found. Immature lymphoblasts are typically identified in the peripheral blood; WBC count may be low or high and, in 15-20% of children, higher than 50×109/l. Bone marrow examination is performed to establish the diagnosis (and to later determine the subtype of leukemia) and lumbar puncture is performed at the time of initial diagnosis to determine if leukemia cells are present in the CSF (CNS leukemia).
AML accounts for 15% to 20% of new leukemias and presents throughout the pediatric age range. AML is slightly more frequent in infants and adolescents and has a high incidence in young children with Down’s syndrome. Symptoms include pallor, fatigue, bleeding, and fever. Organomegaly is less common in AML; however, AML is more likely to present with chloromas which are often found in the head and neck region or near the spinal cord or brain in conjunction with acute neurological signs. Initial WBC counts in AML are typically less than 50×109/l, but hyperleukocytosis (>100×109/l) is present in about 20% of those with AML. Again, bone marrow examination is the diagnostic test used to establish the diagnosis and lumbar puncture is used to determine if there is CNS leukemia present at diagnosis.
Diagnosis and risk assessment of pediatric leukemia
Leukemias are systemic malignancies of dysregulated, clonal expansions of immature lymphoid or myeloid progenitor cells which are blocked at a particular stage of differentiation, with characteristic genotypes and pheno-types that can correlate, to various degrees, with clinical behavior. Morphology and cytochemical staining properties such as the presence of Auer rods, myeloperoxidase-positive cells, Sudan black B (myeloid pattern), and naphthyl butyrate esterase (myeloid pattern), suggest the diagnosis of AML. In our institution, ALL is subtyped by immunophenotyping with a panel of monoclonal antibodies using flow cytometry. T-cell leukemia is diagnosed by the presence of cytoplasmic CD3, and B-cell precursor by the presence of cytoplasmic CD79a. A further classification of each subtype (T- and B-cell precursor leukemias) has limited prognostic or therapeutic value.
In ALL, various criteria have been used to estimate risk and stratify therapy. At St. Jude Children’s Research Hospital (SJCRH) genetic abnormalities of leukemic cells, CNS disease status, and treatment response to remission induction are major factors used to stratify therapy. Genetic features of leukemic cells used in stratification include t(9;22) translocation (Philadelphia chromosome, Ph) with BCR-ABL fusion, t(1;19) with E2A-PBX1 fusion, t(4;11) with MLL-AF4 fusion, t(12;21) with TEL-AML1 fusion, hyperdiploidy (>50 chromosomes), and hypodiploidy (<45 chromosomes).
Examination of the CSF at diagnosis following lumbar puncture to determine if leukemia cells are present in the CNS is an important factor in assigning CNS directed therapy in children with leukemia (Fig. 1). Many children with leukemia cells in the CSF are asymptomatic, while advanced CNS disease may manifest clinically with irritability, headaches, and sometimes vomiting, seizures, or unusual weight gain. Patients with overt CNS leukemia may experience cranial nerve palsies (typically unilateral nerve VII palsy; less often nerves VI or III) [5]. Advanced CNS disease may present with papilledema and diffuse retinal disease. The international definition of overt CNS leukemia is the presence of a WBC count of 5/μl or more in the CSF with documented lymphoblasts and/or the presence of a cerebral mass or cranial nerve palsy. The current international classification system for CNS leukemia as proposed by investigators at SJCRH is presented in Table 1 [6]. Overt CNS leukemia (CNS3) is present in about 3% of children at diagnosis and as many as 15-20% of children have CNS2 status [6, 7]. With contemporary therapy, isolated CNS relapse of leukemia during therapy has been reduced to <5%. For example, the 5-year cumulative risk was only 1.2% (95% CI, 0-2.9%) in the St. Jude Total XIIIA study. Only 3 of 247 patients treated in Study XIIIB, with a median follow-up of 3 years, experienced isolated CNS relapse.
Fig. 1.
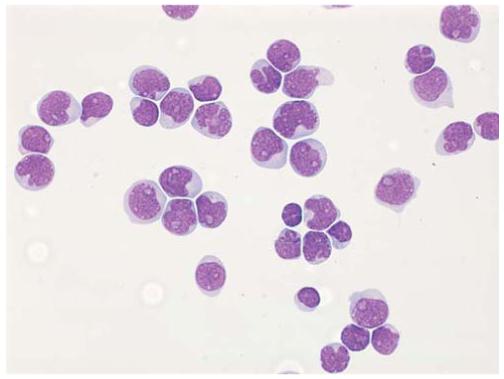
CSF involvement in acute lymphoblastic leukemia. The fluid is hypercellular and contains numerous leukemic blasts. This appearance indicates CNS3 status (Wright-Giemsa stain; original magnification ×60, oil immersion) (Courtesy of Mihaela Onciu, MD, St. Jude Children’s Research Hospital)
Table 1.
Classification of CNS status
| Classification | Lymphoblasts in CSF | WBC count (cells/μl) |
|---|---|---|
| CNS1 | None | |
| CNS2 | Present | <5 |
| CNS3 | Present | >5 |
| Traumatic lumbar puncturea with blasts | Present | Any |
Traumatic lumbar puncture with a red blood cell count of >10/μl.
Analysis of the bone marrow for minimal residual disease (MRD) after remission induction is used for risk assessment and for tailoring therapy after remission. Aspirated bone marrow tissue is assessed using flow cytometry or PCR to determine the amount of residual leukemia in the marrow. The presence of residual ALL disease in the marrow is increasingly recognized as a disease response associated with an unfavorable prognosis Similarly, the relapse risk of AML is assessed by leukemic cell genetic abnormalities, and the response to remission induction therapy.
Historical perspective of leukemia and therapy
During the early years of treatment decades ago, hematological responses were poor. As serial refinements in chemotherapy increased the length of hematological remissions, CNS relapse rates rose dramatically, creating new therapeutic challenges. Pathological findings suggested that leukemic infiltration of the dura and meninges was associated with cranial nerve palsies, that leukemia present deep in the perivascular spaces (Virchow-Robin spaces) of the brain parenchyma was associated with meningeal disease, and that leukemia identified in brain parenchyma was associated with disease in the perivascular spaces (Fig. 2) [8]. Early attempts at IT chemotherapy provided some relief from hydrocephalus, but it did not eradicate CNS leukemia. Early attempts to alter this trend by intensifying systemic chemotherapy were not any more successful in improving outcomes.
Fig. 2.
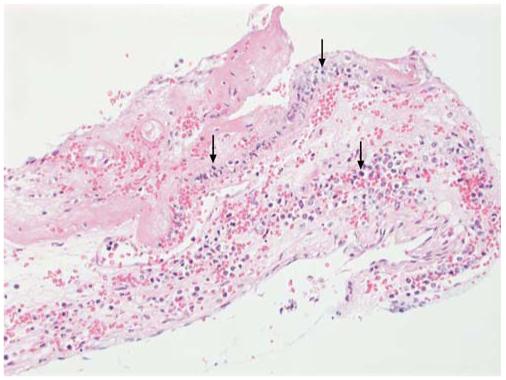
Autopsy specimen with meningeal involvement in acute myeloid leukemia. The meninges are infiltrated by leukemic blasts (arrows), with associated hemorrhage and fibrin deposition (H&E stain; original magnification ×10) (Courtesy of Mihaela Onciu, MD, St. Jude Children’s Research Hospital)
Pinkel was the first to effectively control meningeal leukemia by combining cranial irradiation (20 to 24 Gy) with combination chemotherapy and giving both early during the treatment course. This approach resulted in 5-year relapse-free survival in approximately 50% of children with ALL [9]. From the 1960s through the early 1990s, investigators at SJCRH capitalized on the growing understanding that leukemia “control” was only possible when CNS disease was adequately treated with irradiation. Investigations eventually showed that cranial irradiation to 24 Gy in conjunction with IT MTX reduced the incidence of CNS relapse from over 65% to less than 10%.
Acute (myelosuppression and immunosuppression) and late (neurocognitive, neuroendocrine) adverse effects of cranial irradiation led to a series of clinical trials at SJCRH and in international cooperative groups to optimize CNS-directed therapy. Today, radiotherapy is generally reserved for only those children with a poor prognosis (based on risk stratification) or relapsed disease.
Current perspectives on ALL CNS and systemic therapy
Current therapeutic techniques for ALL, which largely omit irradiation, are associated with unique neurotoxic manifestations. Given the effectiveness of systemic ALL therapy which has evolved over many years, current long-lasting remissions, and overall survival in ALL is largely dependent on successful therapy of “sanctuary sites”, most notably the CNS. Patients with standard-risk ALL can anticipate disease-free survival (DFS) rates greater than 90%, and those with high-risk ALL can anticipate 60% to 80% long-term survival.
ALL therapy includes four key phases of treatment with the intensity of each determined by risk stratification. These are remission induction, consolidation (or intensification), reinduction, and continuation. IT therapy is part of all phases of therapy. Approximately 5% to 8% of patients with very high risk ALL are treated with BMT.
Early intrathecal therapy
At SJCRH, CNS therapy is initiated at diagnosis (immediately following diagnosticlumbar puncture) with age-appropriate dosing of IT cytarabine (Ara-C). This is in keeping with the concept of early intensification of IT therapy to rapidly control CNS leukemia. This approach also reduces the risk of traumatic lumbar puncture with repeated procedures. IT cytarabine achieves therapeutic CSF levels, but has no systemic effect. IT MTX or triple IT therapy (TIT) with MTX, cytarabine, and hydrocortisone can also be used at diagnosis. However, in the presence of renal insufficiency (which occurs occasionally at diagnosis) delayed MTX clearance can occur and lead to excessive toxicities. In these patients, a blood MTX level must be obtained and leucovorin rescue should be given as appropriate.
Remission induction
The goal of induction therapy over 4 to 6 weeks is to reduce tumor burden and restore normal hematopoiesis. Remission induction is currently successful in 97% to 99% of children and includes use of prednisone, vincristine, daunorubicin, asparaginase and IT treatment, followed by cyclophosphamide plus cytarabine plus 6-mercaptopurine.
Triple IT therapy or IT MTX is administered during induction therapy and continued throughout consolidation or beyond. This risk-based, intensive IT therapy has shown success in earlier treatment protocols in treating and preventing CNS leukemia in most patients with ALL without the use of traditional radiotherapy.
Consolidation, continuation, and reinduction therapies
After induction therapy is completed successfully, consolidation (or intensification) therapy is given with high-dose systemic MTX (HD-MTX) (2.5-5 g/m2 for low risk and 5 g/m2 for standard and high risk groups). Daily oral 6-mercaptopurine (50 mg/m2) is also used. Continuation therapy is prolonged and administered for 3 years for boys and 2.5 years for girls. Reinduction treatment is an integral component of most contemporary ALL trials and is used twice at weeks 7 and 17 after bone marrow examination confirms complete remission in the St. Jude Total XV protocol.
Treatment and prognostic factors in AML
Acute myeloid leukemias are classically categorized according to their cytomorphological characteristics. The more common cell types are myeloblastic and monoblastic; less common is megakaryoblastic type. Most patients with AML have identifiable cytogenetic abnormalities from which prognostic information can be inferred.
The mainstay of therapy for AML is induction with daunorubicin (or idarubicin) in conjunction with cytarabine and thioguanine [10-13]. Postinduction therapies vary between intensified consolidation chemotherapy and allogeneic or autologous BMT. Emerging evidence suggests that autologous BMT does not improve outcome over chemotherapy, and allogeneic transplant is indicated only in patients with high-risk leukemia [12, 14]. Current treatment regimens result in a 5-year event-free survival of approximately 50% in children with AML. Those who have favorable cytogenetic features such as t (15;17), t(8;21), or inv(16) experience long-term event-free survival greater than 50% [10-12, 14-18].
The presence of MRD after remission-induction chemotherapy in pediatric patients with AML is associated with a poor outcome [19]. The incidence of CNS leukemia at diagnosis of AML is almost equivalent to that seen in ALL. It is more common in patients younger than 2 years who have a high WBC count at diagnosis [20]. As stated above, systemic relapse of AML often occurs before CNS relapse and limits survival.
Impact of leukemia on the CNS at diagnosis
Similar to patients with ALL, CNS involvement in AML is often asymptomatic. However, some patients can present with chloromas, aggregates of leukemia cells. Chloromas can occur anywhere in the body, but because these focal tumors tend to occur in the head and neck and in the spine (usually from bone marrow), symptoms are usually attributed to mass effects on critical head and neck structures including the orbits and spinal cord [21] (Fig. 3).
Fig. 3.

Four different patients with leukemia presenting with spine complications at diagnosis. a T2-weighed sagittal MR image shows a thoracic chloroma causing cord compression at T4-7(arrows) of an 11-year-old patient with Ph-positive ALL and lower extremity weakness. Emergent treatment with chemotherapy including steroids resulted in rapid resolution of symptoms. b Sagittal contrast-enhanced T1-weighted image shows tumor in the marrow of the spine in a 6-month-old male presenting with AML. The cord is compressed at T6 (arrow) which caused urinary retention. c Sagittal T1-weighted contrast-enhanced image shows extensive blood products within the epidural and subdural spaces (arrow) in a 5-year old-male with ALL who underwent lumbar puncture at diagnosis while thrombocytopenic at another institution. This improved with time allowing IT therapy to continue. d Sagittal noncontrast-enhanced T1-weighted image of a 10-year-old female who was found to have epidural lipomatosis (arrow) at diagnosis which complicated CNS therapy by impeding CSF acquisition and administration of IT therapy. A ventricular catheter was placed in the brain and successful CNS treatment was given
CT and MR imaging of the affected area usually reveal a cellular mass (dense on CT, intermediate T2 signal) which enhances [22, 23]. In both AML and ALL, prompt treatment with chemotherapy usually results in rapid lysis of the mass. Radiotherapy and/or surgical intervention are occasionally required to treat symptomatic patients. Long-term sequelae can occur, but prompt treatment of the tumor usually allows complete recovery of function. The sacral spinal canal is difficult to image and tumor in this small, confined location can produce impressive symptoms. Relatively small tumors in this location may cause a significant deficit in our experience (Fig. 4). It is important to remember that chloromas may also be a manifestation of relapse in AML and ALL (Fig. 5).
Fig. 4.
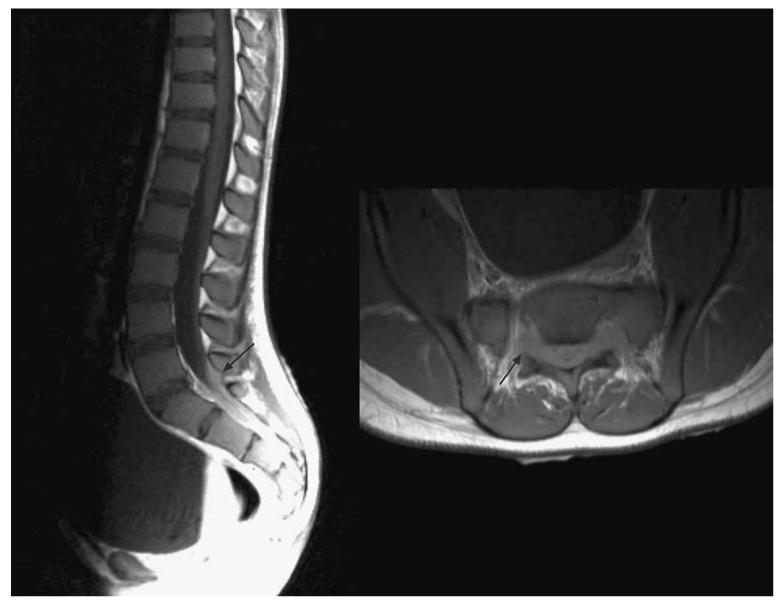
Sacral chloroma (arrows) in an 11-year-old patient with ALL resulting in symptoms of neurogenic bladder. Tumor masses in the confined space of the sacral canal can be particularly symptomatic and more difficult to image
Fig. 5.

An 8-year-old male with a sphenoid bone chloroma near the lateral right orbit which appeared as an enhancing mass on axial T1-weighted contrast-enhanced images. Six months later (right image) the patient had developed a chloroma with intracranial extension into the right cerebellopontine angle. He died shortly after this study from progressive disease including CNS relapse
Leukostasis (stagnation of leukemia blasts in the microcirculation) is more common in AML than in ALL. Leukostasis can result in death from CNS or pulmonary hemorrhage when not recognized and treated quickly [24]. A syndrome of multiple small infarcts in the cerebral hemispheres has been observed in cancer patients as a manifestation of disseminated intravascular coagulation (DIC) [25].
At SJCRH, hyperleukocytosis was identified in 178 (8% of all patients with ALL) with WBC counts >200×109/l. Four of these patients, all with WBC counts >400×109/l, had CNS hemorrhage. In this group of patients, 16 patients had neurological complications and 12 had symptoms at diagnosis [26]. Serious complications of leukostasis are uncommon in children with ALL [27]. Symptoms of leukostasis reflect the pathophysiology of the process and may range from somnolence to stupor. Leukostasis typically occurs with hyperleukocytosis, but can occur when WBC counts are lower, reflecting ill-defined factors and interactions between disease, tissue, and endothelium. Hypoxia and thrombosis may ensue; the later may result in hemorrhage in the brain parenchyma (Fig. 6). Imaging is indicated when focal neurological symptoms are suggestive of intracranial hemorrhage. Supportive therapy includes hydration, prevention of tumor lysis syndrome and DIC, and may ultimately require rapid leukocytoreduction with treatment options including induction chemotherapy, leukopheresis and exchange transfusions (in small children) (Fig. 7). AT STJCRH, leukopheresis or exchange transfusion is performed in patients with ALL with WBC counts >400×109/l and in patients with AML with WBC counts >100×109/l. However, the value of these procedures has not been proven in a randomized trial.
Fig. 6.
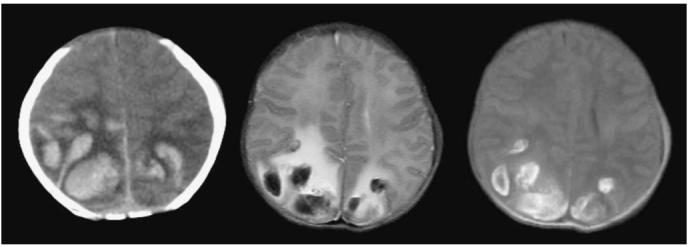
A newborn with congenital ALL presented with seizures. The WBC count was 75×109/l and the platelet count was 3×109/l. CT (left) and MR (center T2-weighted, right T1-weighted) images were obtained 72 h apart. The parietal hemorrhages were thought to be secondary to a combination of hyperleukocytosis and thrombocyto-penia. Fluid-fluid levels with a hematocrit effect are seen within the cystic hemorrhages in the T2-weighted image
Fig. 7.

An 18-year-old male with hyperleukocytosis and thrombocytopenia who complained of left central vision loss and headache and who presented with cerebellar vermis and cerebral hemorrhages detected using noncontrast-enhanced CT. Therapy included leukopheresis. On serial CT examinations, the small hemorrhages and visual acuity improved. The WBC count at diagnosis was 409×109/l and the platelet count was 34×109/l
Impact of leukemia therapy on the CNS
Second primary malignancies
The Childhood Cancer Survival Study Group, in a recently reported survey of more than 14,000 5-year survivors of childhood cancer, found that the risk of CNS neoplasms as a second malignancy is significant and most prevalent in survivors who received therapeutic or prophylactic cranial irradiation for brain tumors or leukemia [28, 29] (Fig. 8). CNS neoplasms were identified in 116 of the childhood cancer survivors. Survivors originally diagnosed with leukemia more often developed gliomas than meningiomas while the development of meningiomas was more common in patients who were first treated for CNS tumors. Only 4 of the 106 survivors (3.8%) who developed meningiomas or gliomas had not received radiation during treatment for their original cancer. The mean time from original cancer diagnosis to diagnosis for gliomas was 9 years, and that for meningiomas was 17 years. In the study of SJCRH, among 10-year event-free survivors of childhood ALL, 20% of irradiated patients developed secondary neoplasms [4]. The median time for malignant brain tumor to develop was 11 years from diagnosis and for meningioma was 21 years.
Fig. 8.
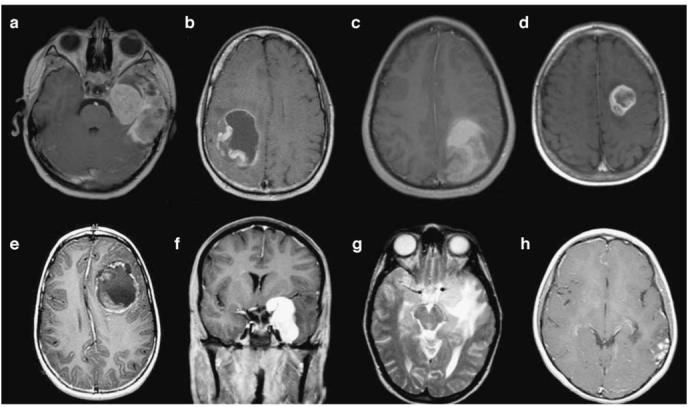
Secondary CNS malignancies in seven children with leukemia, most of whom received cranial radiation therapy for CNS leukemia prevention. a Axial contrast-enhanced T1-weighted image of a 10-year-old treated with BMT for chronic myelogenous leukemia as an infant who developed glioblastoma multiforme (no previous radiation). b Axial contrast-enhanced T1-weighted image of a 24-year-old male treated with craniospinal irradiation as part of therapy for ALL 14 years prior to development of primitive neuroectodermal tumor (PNET). c Axial noncontrast-enhanced T1-weighted image in a 16-year-old male with glioblastoma multiforme developing 13 years after craniospinal irradiation for ALL. d Axial contrast-enhanced T1-weighted image of a 13-year-old male treated with cranial irradiation as part of therapy for ALL. This anaplastic astrocytoma developed 8 years after radiotherapy . e Axial contrast-enhanced T1-weighted image of a 10-year-old male with primitive neuroectodermal tumor 6 years after cranial irradiation for ALL. f, g Coronal contrast-enhanced T1-weighted (f) and axial T2-weighted (g) images of a 20-year-old female treated with cranial irradiation for ALL 17 years earlier developed a meningioma near the left cavernous sinus. h Axial contrast-enhanced T1-weighted image of a 13-year-old female with dysembryoplastic neuroepithelial tumor (DNET) which developed 11 years after cranial irradiation therapy for ALL. This tumor later metastasized
Acute MTX-induced neurotoxicity
MTX is an antimetabolite chemotherapeutic agent which is given regularly in the treatment of ALL as both a systemic agent and as a component of IT therapy. Known acute toxic effects of MTX are myelosuppression, mucositis, nephro-toxicity, hepatotoxicity, and neurotoxicity. MTX is a folate analogue and inhibitor of dihydrofolate reductase, resulting in a block of the conversion of folate to tetrahydrofolate which inhibits cell replication. Homocysteine levels rise with MTX administration due to its effect in decreasing folate availability. MTX is capable of crossing the blood-brain barrier. Nonetheless, this drug is given intrathecally to children with ALL to improve CNS control.
Beginning in the early 1990s and continuing into the early 2000s, several reports implicated MTX as the cause of acute stroke-like syndromes [1-4, 13, 25, 30-34]. In our and others’ experience, patients typically present with focal neurological deficits such as hemiparesis, and aphasia is often present [27]. Rarely, IT therapy can result in paraplegia and neurogenic bladder (Fig. 9). This condition differs from the inflammatory polyradiculopathy induced by IT therapy by the lack of early contrast enhancement of nerve roots typical of this type of arachnoiditis [25, 35, 36].
Fig. 9.

MTX myelopathy. This 7-year-old female treated for ALL 2 years earlier, developed an isolated CNS relapse treated with cranial irradiation and five doses of IT MTX. She subsequently developed lower extremity paraparesis with urinary incontinence. Initial MR study of the spine was normal. a Sagittal T2-weighted noncontrast-enhanced image obtained after 2 weeks of persistent symptoms shows T2 hyperintensity along the dorsal aspect of the thoracic spinal cord (thick arrow). b-e Sagittal T2-weighted noncontrast-enhanced (b), sagittal T1-weighted contrast-enhanced (c), axial noncontrast-enhanced T2-weighted (d), and axial contrast-enhanced T1-weighted (e) images after 6 weeks of persistent and stable paraparesis show an increasing T2 signal abnormality dorsally in the cord with involvement of the lateral columns with faint cord enhancement (thin arrows). At the time of this report the child was slowly recovering motor function in the lower extremities and had some improvement in bladder function
While the exact mechanism by which MTX may induce these changes is unclear, some have hypothesized that it might be related to transient perfusion abnormalities and small-vessel vasculopathy, perhaps related to elevated CNS levels of homocysteine. In a small group of patients we recently reviewed with acute MTX toxicity, we were not able to detect differences in MTX pharmacokinetics between patients with or without acute encephalopathy.
MR imaging is critical in the evaluation of these patients. Typically it is diffusion-weighted (DW) imaging that secures the diagnosis. Our experience is the same as that of others: DW images tend to show bilateral areas of restricted diffusion in the cerebral white matter in areas that correspond with clinically observed neurological deficits, often without corresponding T2 prolongation, FLAIR abnormalities or enhancement on T1-weighted images (Fig. 10). One atypical patient in our cohort, was a patient with ALL who had symptoms of choreoathetosis and left caudate head abnormalities (Fig. 10a). Like others, we also have noted that follow-up imaging typically shows resolution of the diffusion abnormalities, and in a minority of patients, residual T2 or FLAIR signal changes, smaller than the abnormalities noted originally on DW images, may persist for long periods of time without detectable neurological deficits [30, 37]. The rapid reversibility of the lesions on DW images, without long-lasting clinical deficits in most patients suggests a transient restricted diffusion without irreversible cell death.
Fig. 10.

Left to right: T2-weighted, DW and apparent diffusion coefficient map (ADC map) images. Patient A. 7-year-old female with ALL developed choreoathetosis after IT MTX therapy given 12 days previously. Images show abnormal signal in the caudate head and putamen on the right, presumably correlating with the movement disorder. Patient B. 12-year-old male with ALL treated with one cycle of HD-MTX and IT MTX given 11 days before this MR scan. He was encephalopathic, dysarthric, and had uncontrollable limb movements. Arrows show the abnormalities in each patient
Our experience is similar to that reported by others. Symptoms usually resolve within a week and patients may resume MTX therapy after brief stoppages and resolution of clinical symptoms [30]. Upon reinitiating therapy careful attention is paid to providing adequate folate replacement after subsequent MTX administration and reducing the frequency of IT treatment when clinically feasible. The incidences of this complication in ALL therapy are reported to be 3-10%. We have concluded that, despite the very alarming symptoms and ominous imaging findings in this condition, there is not currently a medical therapy proven to ameliorate or hasten resolution of this condition, and it appears to be safe to resume therapy with caution after symptoms (and usually imaging changes) have resolved. Long-term neurocognitive sequelae, if any, remain unknown.
Vascular thromboses
While the pathophysiology, signs and symptoms of hyper-leukocytosis and leukostasis occurring around the time of diagnosis are discussed above, it is clear that children with AML and those with ALL are at risk of thrombosis at diagnosis and during therapy related to aberrations in hemostasis related to disease and chemotherapy. It is well established that the combination of L-asparaginase and glucocorticoids creates prothrombotic conditions in patients treated for ALL. In our experience with leukemia, vascular thromboses are overwhelmingly more common in venous structures and most frequently involve the dural venous sinuses and cortical veins.
To understand the incidences and risk factors associated with thrombosis in children with ALL and thromboses, a large meta-analysis of prospective studies included in the literature was conducted and included 1,752 children with ALL with 49 thrombotic events occurring in the CNS (only 26 were clearly identified as venous in origin, and whether the others were arterial or venous in origin was not specified) [38]. CNS thrombosis and upper extremity thromboses were most common, with a similar incidence. That review suggested that induction phase therapy and asparaginase therapy (perhaps with prolonged lower dose administrations) were overall risk factors, but no definite conclusions could be drawn about the relative influences of various asparaginase preparations, the effects of steroids, or the effects of hereditary prothrombotic factors on the incidences in this population. However, it is undisputed that concomitant administration of steroids and asparaginase results in an increased risk of thrombosis.
In our experience, children who develop DVST typically present with severe headache and usually are receiving or have recently received asparaginase together with steroids. Mental status changes, seizures, and focal neurological deficits in the setting of thrombosis usually imply a more severe pathology such as hemorrhagic venous infarction.
Imaging of DVST and cortical vein thrombosis can be challenging, but is very important for diagnosis. Over the past many years, MR imaging has been the modality of choice for all neuroimaging in general and for the evaluation of arterial and venous thromboses in particular. We have found MR venography and conventional MR techniques more than adequate to detect cerebral vein thromboses (Fig. 11) and its complications such as venous infarction. At the same time, we have occasionally used CT venography to aid in the diagnosis.
Fig. 11.

A 19-year-old male with a history of severe leukoencephalopathy was being followed with imaging. While asymptomatic, he was found to have cortical vein thrombosis. Imaging (left to right) using 2-D FLASH GRE T1-weighted imaging with subtraction of noncontrast-enhanced and contrast-enhanced T1-weighted sequences helps visualize an isolated cortical vein thrombosis in the patient receiving prednisone and-asparaginase. FLAIR imaging without contrast enhancement also shows the thrombosis
Because of the high quality of MR and CT imaging today, we prefer not to rely on the secondary sign of absence of venous sinus flow on MR venography to establish the diagnosis. Subtraction of axial T1-weighted images before and after contrast agent administration frequently allows us to visualize the filling defect in the venous sinuses and cortical veins in a manner similar to visualizing the empty delta sign on contrast-enhanced CT scans. We routinely use GRE T1-weighted imaging (2-D FLASH) imaging in the brain which tends to reduce ghosting artifact in the venous sinuses and tends to eliminate heterogeneous signal from the venous sinuses typically present on spin echo MR sequences. Isotropic GRE T1-weighted imaging (3-D MPRAGE) which improves resolution is useful to evaluate transverse sinuses when obtained in a sagittal plane, which is our practice. T2*-weighted GRE images can be used to check for susceptibility changes of blood products suggesting thrombosis in venous sinuses and cortical veins (Fig. 12).
Fig. 12.

DVST in a 6-year-old female with ALL while receivingl-asparaginase, IT therapy, and systemic steroids. The arrows indicate the position of a torcula clot. a, b CT images show density near the torcula (a) and the “empty delta sign” after contrast agent administration (b). c Subtraction of T1-weighted 2-D FLASH images before and after contrast agent administration produces a vascular image depicting the clot as a filling defect. d T2*-weighted GRE image shows blood products within the dural venous sinus. e, f Sagittal GRE T1-weighted MPRAGE images show the clot in the torcula (e) with resolution one week later (f) after treatment with low molecular weight heparin
CNS infections
Disease- and treatment-induced myelosuppression and immunosuppression place children with leukemia at high risk for infections that can affect the CNS. The severity and duration of neutropenia induced by chemotherapy is probably the main risk factor for infection, and fever is frequently the first indication of serious infection. In a recent review of 183 first episodes of febrile neutropenia in the setting of pediatric cancer therapy, 45 of the patients (24.6%) had ALL and 18 (9.8%) had either AML or myelodysplasia. CNS infections are relatively uncommon in our practice, probably related to aggressive antibiotic and antifungal prophylaxis and treatment. However, on rare occasions, bacterial meningitis, ventriculitis, or abscess develops within the brain (Fig. 13).
Fig. 13.

a Infant with AML who developed alpha streptococcus ventriculitis after BMT as part of her AML therapy. Axial non-contrast-enhanced FLAIR image (left) shows edema (arrow) and axial contrast-enhanced T1-weighted image (right) shows enhancement of the ependymal surfaces (arrow). b Patient who died after developing Bacillus cereus bacteremia during ALL therapy. These two sets of non-contrast-enhanced CT images, obtained 50 h apart, show progressive development of cerebral edema. The patient died shortly after the CT scan
Acute neurological complications (including infections) may affect those undergoing BMT. Conditioning regimens for allogeneic transplantation consist of myeloablative techniques which typically include high doses of chemotherapy, often cyclophosphamide and/or total body irradiation. HLA-matched sibling or unrelated-matched donor marrow is then infused into the recipient, and immuno-suppressive drugs are given thereafter to inhibit graft rejection and reduce the incidence of graft-versus-host disease.
Among patients treated with hematopoietic stem cell transplantation, CNS infections can result from a variety of opportunistic organisms including bacteria, toxoplasmosis, cytomegalovirus, herpes, and fungal, and parasitic infections. MR imaging is the modality of choice for detecting CNS infections in transplant recipients (Fig. 14).
Fig. 14.

A 16-year-old male with high-risk ALL underwent BMT and developed seizure. Many small foci of enhancement on T1-weighted contrast-enhanced images of the brain suggest infection. Later, blood cultures grew Stomatococcus mucilaginosus which was treated successfully with antibiotic therapy
Posterior reversible encephalopathy syndrome
PRES is an acute neurological condition that can arise in association with a number of medical conditions, including renal disease, connective tissue disorders, hematological and oncological disease, and eclampsia. PRES is thought to be caused by an overwhelming of autoregulatory mechanisms which help maintain normal blood pressures in the brain. Cyclosporine is a drug associated with PRES. Although PRES occurs in children with cancer, its predisposing factors and clinical and radiographic features in this population have not been well described.
Previous work from SJCRH has described PRES in 11 patients (7 girls and 4 boys) whose average age at onset of PRES was 10.4 years (range 5-16 years) [39]. Primary diagnoses included AML (n=5), ALL (n=3), non-Hodgkin lymphoma (n=2), and Ewing sarcoma (n=1). The patients appeared to be most vulnerable to PRES during induction chemotherapy. All patients had prior exposure to chemotherapy, and seven patients (63.6%) were in the induction phase of leukemia treatment at the onset of PRES. Tacrolimus was given before the onset of seizures in only three of our patients. The onset of PRES was primarily heralded by seizure, which was often preceded by headache, mental status changes, aphasia, visual changes, or a combination thereof.
The predisposing factors for PRES in the SJCRH study included hypertension (not necessarily acute) and cytotoxic or immunosuppressant drug therapy. The association of PRES with induction therapy is probably not coincidental as this is a phase of rapid tumor lysis which can impact the renal function and salt retention from prednisone treatment, resulting in hypertension. MR imaging is routinely used to evaluate these patients in our institution as it is the modality of choice to evaluate seizures and mental status changes. MR imaging findings in this patient population show typical posterior watershed region abnormalities. In some patients some anterior watershed vascular distributions were seen (Fig. 15). To date, we have not seen any patient with PRES demonstrate restricted diffusion within, adjacent to, or distant to PRES lesions, despite prompt imaging following the onset of symptoms. Given the extensive abnormalities on T2-weighted, FLAIR, and occasionally on T1-weighted contrast-enhanced images (many lesions can enhance), the pathological process at play probably was present for a period of time prior to the development of seizure and other associated symptoms. None of our patients experienced a recurrent episode with continuation of planned therapy. Long-term sequelae of PRES did occur as 6 of 11 patients had chronic radiographic or clinical sequelae. Irreversible MR imaging abnormalities were evident on images from four patients: epilepsy developed in three patients, and at the time of this report another patient continued to have an abnormal EEG without further seizures. Interestingly, epilepsy developed in only one patient with a persistent MR imaging abnormality while in two patients with normalized MR imaging, focal epilepsy developed. This finding suggests a more permanent neurological insult that is not discernible by conventional imaging [39].
Fig. 15.
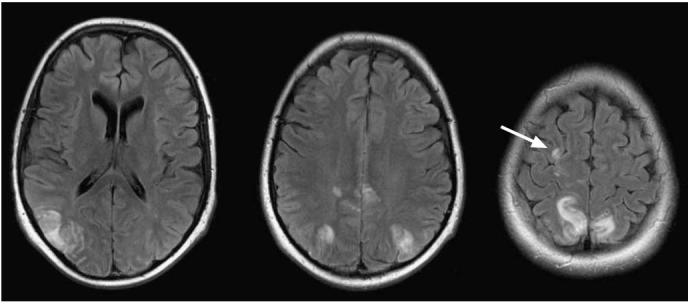
Posterior reversible encephalopathy syndrome (PRES) in a 15-year-old female patient with ALL during induction therapy. Contrast-enhanced axial FLAIR images after a generalized seizure and hypertension show areas of cortical/subcortical hyperintensity in the distribution of the posterior circulation. All imaging abnormalities resolved in 4 weeks. Watershed distribution hyperintensity is seen more anteriorly in the right hemisphere (arrow)
Bone marrow transplantation in leukemia
High-risk or relapsed AML and ALL are the most common indications for BMT in children. BMT is typically considered for children who experience early ALL relapse (i.e., within 3 years of first remission), poor initial response to induction therapy (high MRD), or genetic signs of high-risk disease (e.g., Ph-positive with BCR-ABL fusion or MLL-AF4 fusion). In BMT, patients often receive “supralethal” doses of chemotherapeutic agents (e.g., cyclophosphamide and busulfan) or combinations of total body irradiation and high-dose chemotherapy with the intent of eliminating MRD and conditioning the patient to receive donor marrow by inducing immunosuppression. The neurological toxicities and consequences of BMT can be severe and are related to the toxicity of the agents (chemotherapy and irradiation) and to the severe myelosuppression and immunosuppression. Infections account for many acute neurological problems after BMT. The neurological consequences of graft-versus-host disease is not discussed in this review.
Conclusions
The use of radiotherapy to treat overt CNS leukemia and prevent relapse is certainly associated with chronic adverse effects to the CNS. It is very important for clinicians caring for survivors of childhood leukemia (and cancer) to be cognizant of the special medical needs of these patients. Secondary malignancies usually arise years after therapy, and these patients can present with acute neurological signs and symptoms. Most acute events occurring in ALL therapy today seem to be transient. This review focuses on many of the acute neurological conditions that occur in young children and adolescents who are undergoing therapy for leukemia. Despite the limited use of radiotherapy for CNS leukemia control today, neurological conditions associated with leukemia and therapy still are problematic. For those who suffer such acute neurotoxic effects, the long-term sequelae remain unknown. Continued detailed imaging and assessments of neuroendocrine and neurocognitive function of these patients are important to determine if current efforts to reduce morbidity have been realized. Through these effects we can continue to contribute to the design of safer and more effective treatment strategies.
Acknowledgements
This work was supported in part by Cancer Center Core Grant CA (21765) and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
References
- 1.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 2.Creutzig U, Zimmermann M, Ritter J, Reinhardt D, Hermann J, Henze G, Jurgens H, Kabisch H, Reiter A, Riehm H, Gadner H, Schellong G. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005;19:2030–2042. doi: 10.1038/sj.leu.2403920. [DOI] [PubMed] [Google Scholar]
- 3.Gibson BE, Wheatley K, Hann IM, Stevens RF, Webb D, Hills RK, De Graaf SS, Harrison CJ. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–2138. doi: 10.1038/sj.leu.2403924. [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Cheng C, Leung W, Rai SN, Rivera GK, Sandlund JT, Ribeiro RC, Relling MV, Kun LE, Evans WE, Hudson MM. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 5.Kun LE. Leukemias in children. Pediatric Radiation Oncology. 2007:15–39. [Google Scholar]
- 6.Mahmoud HH, Rivera GK, Hancock ML, Krance RA, Kun LE, Behm FG, Ribeiro RC, Sandlund JT, Crist WM, Pui CH. Low leukocyte counts with blast cells in cerebrospinal fluid of children with newly diagnosed acute lymphoblastic leukemia. N Engl J Med. 1993;329:314–319. doi: 10.1056/NEJM199307293290504. [DOI] [PubMed] [Google Scholar]
- 7.Burger B, Zimmermann M, Mann G, Kuhl J, Loning L, Riehm H, Reiter A, Schrappe M. Diagnostic cerebrospinal fluid examination in children with acute lymphoblastic leukemia: significance of low leukocyte counts with blasts or traumatic lumbar puncture. J Clin Oncol. 2003;21:184–188. doi: 10.1200/JCO.2003.04.096. [DOI] [PubMed] [Google Scholar]
- 8.Price RA. Histopathology of CNS leukemia and complications of therapy. Am J Pediatr Hematol Oncol. 1979;1:21–30. [PubMed] [Google Scholar]
- 9.Pinkel D. Treatment of childhood acute lymphocytic leukemia. J Pediatr. 1970;77:1089–1091. doi: 10.1016/s0022-3476(70)80101-9. [DOI] [PubMed] [Google Scholar]
- 10.Creutzig U, Ritter J, Zimmermann M, Hermann J, Gadner H, Sawatzki DB, Niemeyer CM, Schwabe D, Selle B, Boos J, Kuhl J, Feldges A. Idarubicin improves blast cell clearance during induction therapy in children with AML: results of study AML-BFM 93. AML-BFM Study Group. Leukemia. 2001;15:348–354. doi: 10.1038/sj.leu.2402046. [DOI] [PubMed] [Google Scholar]
- 11.Krance RA, Hurwitz CA, Head DR, Raimondi SC, Behm FG, Crews KR, Srivastava DK, Mahmoud H, Roberts WM, Tong X, Blakley RL, Ribeiro RC. Experience with 2-chlorodeox-yadenosine in previously untreated children with newly diagnosed acute myeloid leukemia and myelodysplastic diseases. J Clin Oncol. 2001;19:2804–2811. doi: 10.1200/JCO.2001.19.11.2804. [DOI] [PubMed] [Google Scholar]
- 12.Woods WG, Neudorf S, Gold S, Sanders J, Buckley JD, Barnard DR, Dusenbery K, DeSwarte J, Arthur DC, Lange BJ, Kobrinsky NL. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001;97:56–62. doi: 10.1182/blood.v97.1.56. [DOI] [PubMed] [Google Scholar]
- 13.Stevens RF, Hann IM, Wheatley K, Gray RG. Marked improvements in outcome with chemotherapy alone in paediatric acute myeloid leukemia: results of the United Kingdom Medical Research Council’s 10th AML trial. MRC Childhood Leukaemia Working Party. Br J Haematol. 1998;101:130–140. doi: 10.1046/j.1365-2141.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 14.Ravindranath Y, Yeager AM, Chang MN, Steuber CP, Krischer J, Graham-Pole J, Carroll A, Inoue S, Camitta B, Weinstein HJ, Pediatric Oncology Group Autologous bone marrow transplantation versus intensive consolidation chemotherapy for acute myeloid leukemia in childhood. N Engl J Med. 1996;334:1428–1434. doi: 10.1056/NEJM199605303342203. [DOI] [PubMed] [Google Scholar]
- 15.Boulad F, Kernan NA. Treatment of childhood acute nonlymphoblastic leukemia: a review. Cancer Invest. 1993;11:534–553. doi: 10.3109/07357909309011672. [DOI] [PubMed] [Google Scholar]
- 16.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 17.Raimondi SC, Chang MN, Ravindranath Y, Behm FG, Gresik MV, Steuber CP, Weinstein HJ, Carroll AJ. Chromosomal abnormalities in 478 children with acute myeloid leukemia: clinical characteristics and treatment outcome in a cooperative pediatric oncology group study - POG 8821. Blood. 1999;94:3707–3716. [PubMed] [Google Scholar]
- 18.Bloomfield CD, Shuma C, Regal L, Philip PP, Hossfeld DK, Hagemeijer AM, Garson OM, Peterson BA, Sakurai M, Alimena G, Berger R, Rowley JD, Ruutu T, Mitelman F, Dewald GW, Swansbury J. Long-term survival of patients with acute myeloid leukemia: a third follow-up of the Fourth International Workshop on Chromosomes in Leukemia. Cancer. 1997;80:2191–2198. [PubMed] [Google Scholar]
- 19.Sievers EL, Lange BJ, Buckley JD, Smith FO, Wells DA, Igneault-Creech CA, Shults KE, Bernstein ID, Loken MR. Prediction of relapse of pediatric acute myeloid leukemia by use of multidimensional flow cytometry. J Natl Cancer Inst. 1996;88:1483–1488. doi: 10.1093/jnci/88.20.1483. [DOI] [PubMed] [Google Scholar]
- 20.Pui CH, Dahl GV, Kalwinsky DK, Look AT, Mirro J, Dodge RK, Simone JV. Central nervous system leukemia in children with acute nonlymphoblastic leukemia. Blood. 1985;66:1062–1067. [PubMed] [Google Scholar]
- 21.Graham A, Hodgson T, Jacubowski J, Norfolk D, Smith C. MRI of perineural extramedullary granulocytic sarcoma. Neuroradiology. 2001;43:492–495. doi: 10.1007/s002340000514. [DOI] [PubMed] [Google Scholar]
- 22.Kingsley DP, Kendall BE. Cranial computed tomography in leukaemia. Neuroradiology. 1978;16:543–546. doi: 10.1007/BF00395355. [DOI] [PubMed] [Google Scholar]
- 23.Kitajima M, Korogi Y, Shigematsu Y, Liang L, Matsuoka M, Yamamoto T, Jhono M, Eto K, Takahashi M. Central nervous system lesions in adult T-cell leukemia: MRI and pathology. Neuroradiology. 2002;44:559–567. doi: 10.1007/s00234-002-0787-x. [DOI] [PubMed] [Google Scholar]
- 24.Majhail NS, Lichtin AE. Acute leukemia with a very high leukocyte count: confronting a medical emergency. Cleve Clin J Med. 2004;71:633–637. doi: 10.3949/ccjm.71.8.633. [DOI] [PubMed] [Google Scholar]
- 25.Chen CY, Zimmerman RA, Faro S, Bilaniuk LT, Chou TY, Molloy PT. Childhood leukemia: central nervous system abnormalities during and after treatment. AJNR Am J Neuroradiol. 1996;17:295–310. [PMC free article] [PubMed] [Google Scholar]
- 26.Eguiguren JM, Schell MJ, Crist WM, Kunkel K, Rivera GK. Complications and outcome in childhood acute lymphoblastic leukemia with hyperleukocytosis. Blood. 1992;79:871–875. [PubMed] [Google Scholar]
- 27.Lowe EJ, Pui CH, Hancock ML, Geiger TL, Khan RB, Sandlund JT. Early complications in children with acute lymphoblastic leukemia presenting with hyperleukocytosis. Pediatr Blood Cancer. 2005;45:10–15. doi: 10.1002/pbc.20178. [DOI] [PubMed] [Google Scholar]
- 28.Neglia JP, Robison LL, Stovall M, Liu Y, Packer RJ, Hammond S, Yasui Y, Kasper CE, Mertens AC, Donaldson SS, Meadows AT, Inskip PD. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 29.Paakko E, Talvensaari K, Pyhtinen J, Lanning M. Late cranial magnetic resonance imaging after cranial irradiation in survivors of childhood cancer. Neuroradiology. 1999;36:652–655. doi: 10.1007/BF00600433. [DOI] [PubMed] [Google Scholar]
- 30.Rollins N, Winick N, Bash R, Booth T. Acute methotrexate neurotoxicity: findings on diffusion-weighted imaging and correlation with clinical outcome. AJNR Am J Neuroradiol. 2004;25:1688–1695. [PMC free article] [PubMed] [Google Scholar]
- 31.Mahoney DH, Jr, Shuster JJ, Nitschke R, Lauer SJ, Steuber CP, Winick N, Camitta B. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy - a Pediatric Oncology Group study. J Clin Oncol. 1998;16:1712–1722. doi: 10.1200/JCO.1998.16.5.1712. [DOI] [PubMed] [Google Scholar]
- 32.Pui CH, Evans WE. Acute lymphoblastic leukemia in infants. J Clin Oncol. 1999;17:438–440. doi: 10.1200/JCO.1999.17.2.438. [DOI] [PubMed] [Google Scholar]
- 33.Winick NJ, Bowman WP, Kamen BA, Roach ES, Rollins N, Jacaruso D, Buchanan GR. Unexpected acute neurologic toxicity in the treatment of children with acute lymphoblastic leukemia. J Natl Cancer Inst. 1992;84:252–256. doi: 10.1093/jnci/84.4.252. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen H, Clausen N. The development of cerebral CT changes during treatment of acute lymphocytic leukemia in childhood. Neuroradiology. 1981;22:79–84. doi: 10.1007/BF00344778. [DOI] [PubMed] [Google Scholar]
- 35.Morgan GW, Barohn RJ, Bazan C, III, King RB, Klucznik RP. Nerve root enhancement with MRI in inflammatory demyelinating polyradiculoneuropathy. Neurology. 1993;43:618–620. doi: 10.1212/wnl.43.3_part_1.618. [DOI] [PubMed] [Google Scholar]
- 36.Georgy BA, Chong B, Chamberlain M, Hesselink JR, Cheung G. MR of the spine in Guillain-Barre syndrome. AJNR Am J Neuroradiol. 1994;15:300–301. [PMC free article] [PubMed] [Google Scholar]
- 37.Paakko E, Vainionpaa L, Phytinen J, Lanning M. Minor changes on cranial MRI during treatment in children with acute lymphoblastic leukaemia. Neuroradiology. 1996;38:264–268. doi: 10.1007/BF00596544. [DOI] [PubMed] [Google Scholar]
- 38.Caruso V, Iacoviello L, Di Castelnuovo A, Storti S, Mariani G, de Gaetano G, Donati MB. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108:2216–2222. doi: 10.1182/blood-2006-04-015511. [DOI] [PubMed] [Google Scholar]
- 39.Morris EB, Laningham FH, Sandlund JT, Khan RB. Posterior reversible encephalopathy syndrome in children with cancer. Pediatr Blood Cancer. 2007;48:152–159. doi: 10.1002/pbc.20703. [DOI] [PubMed] [Google Scholar]


