Abstract
Context and Objective: Our aim was to explore the relative roles of gonadal sex steroids and inhibin B in the regulation of FSH across a spectrum of seminiferous epithelium function.
Subjects: The study included three groups: group I, healthy men (n = 31); group II, men with idiopathic hypogonadotropic hypogonadism receiving pulsatile GnRH (n = 12) selected to represent a spectrum of seminiferous tubular development, testicular size, and baseline inhibin B levels; and group III, men with functional anorchia (n = 3) receiving testosterone replacement.
Design: Subjects were studied before and after 3 d of acute sex steroid withdrawal.
Setting: The study was conducted at the Mallinckrodt General Clinical Research Center of Massachusetts General Hospital.
Interventions: Acute biochemical castration was achieved using high-dose ketoconazole (groups I and II) or withdrawal of androgen therapy (group III).
Main Outcome Measures: The relationship between FSH and inhibin B in both normal and castrate sex steroid milieu was measured.
Results: In both normal and castrate sex steroid milieus, there was a negative relationship between inhibin B and FSH, best described by a logarithmic model. Acute biochemical castration resulted in the most dramatic increases in FSH in men with the lowest baseline inhibin B levels.
Conclusions: We came to the following conclusions: 1) in the human male, inhibin B is the principal gonadal feedback regulator of FSH secretion unless seminiferous tubular function is severely compromised, and a logarithmic model best describes this relationship; and 2) sex steroid inhibition of FSH secretion is most apparent when serum inhibin B levels fall well below the normal range.
Studies of normal men and male patients with varying degrees of seminiferous tubule function defined a negative feedback relationship between inhibin B and FSH. This was best described by a logarithmic model, with the acute biochemical castration resulting in the most dramatic increases in FSH in men with the lowest inhibin B levels.
Regulation of FSH secretion in the male involves a complex interplay among the stimulatory effects of hypothalamic GnRH, autocrine/paracrine modulation by pituitary activin and follistatin, and negative feedback induced by gonadal secretion of sex steroids and inhibin B (1,2,3,4).
It appears that the sex steroid inhibition of FSH is predominantly, if not solely, attributable to the negative feedback effects of estradiol (E2) because aromatase inhibition recapitulates the rise in FSH associated with the combined ablation of both testosterone (T) and E2 (5). This conclusion is further strengthened by the observation that rises in FSH stemming from aromatase blockade occur despite a significant increase in serum T. Despite this role for sex steroids, specifically E2, in the inhibition of pituitary FSH secretion, investigation in both the human male and primate models strongly suggests that inhibin B is the principal gonadal feedback regulator of FSH when seminiferous tubular function is intact (2,6,7,8).
Circulating inhibin B levels change throughout development in the human male. Serum levels are high during infancy, decrease through mid-childhood, and increase with the initiation of puberty (9,10,11,12). During the first few decades of adult life, serum inhibin B levels are relatively stable but progressively decline with aging (12). Overall, the relevance of a feedback role for inhibin B in humans is supported by an inverse correlation between FSH and inhibin B in normal men and across a wide range of reproductive disorders (6,11,13,14). However, some investigators have shown no correlation between inhibin B and FSH in healthy men (7), and normal rather than elevated FSH levels were found in a subset of infertile men with low serum inhibin B (16).
Thus, the aim of the present study was to determine the relative roles of sex steroids and inhibin B on the feedback control of FSH secretion across the full spectrum of seminiferous epithelium function. To achieve this, we induced acute biochemical castration with high-dose ketoconazole (KC) in healthy men and men with idiopathic hypogonadotropic hypogonadism (IHH). A similar castrate milieu was induced in anorchic men by abrupt withdrawal of exogenous androgen administration. We used these manipulations in normal and functionally castrate subjects in previous studies to investigate the feedback regulation of gonadotropin secretion in men with either normal or absent seminiferous tubule function (8). To date, however, these studies have not included subjects with intermediate serum inhibin B levels. Inclusion of IHH men in the present study permits investigation of the relative roles of sex steroids and inhibin B across the full spectrum of seminiferous epithelium function.
Subjects and Methods
The study was approved by the Human Research Committee at the Massachusetts General Hospital, and all subjects provided written informed consent before the initiation of any study-related procedures.
Subjects
Some of the subjects in this study have been previously reported as part of a study examining the feedback control of FSH secretion in the male (8). However, to represent the full spectrum of seminiferous tubule function, the study population in the present investigations was expanded to include three study cohorts.
Group I
Men with normal gonadal sex steroid levels and normal seminiferous tubule function (n = 42 enrolled; n = 31 completed). Healthy men, aged 25–45 yr with normal pubertal development, sexual function, and overall good general health, were enrolled. All subjects had a normal testicular volume 15 ml or greater by Prader orchidometer; normal semen analysis according to World Health Organization criteria (17), and normal serum levels of T, E2, inhibin B, LH, FSH, TSH, and prolactin.
Group II
This group included men with variable seminiferous tubule function (n = 14 enrolled; n = 12 completed), aged 25–45 yr, previously diagnosed with IHH (failure to go through puberty by age 18 yr, serum T < 100 ng/dl in association with inappropriately low gonadotropin levels, otherwise normal reserve testing of the anterior pituitary function, and a normal hypothalamic-pituitary region by magnetic resonance imaging). Four of these men had a history of cryptorchidism. These men had all received long-term pulsatile GnRH therapy (18,19), producing normal serum T and varying degrees of testicular development (testicular volume 13.2 ± 1 ml; range 9–20 ml). Despite the ability of pulsatile GnRH administration to restore normal levels of LH and T, this group spans a broad range of seminiferous tubule function as characterized by widely varying serum levels of inhibin B and FSH (cf. Table 1) and spermatogenesis (sperm density = 26.5 ± 39.7 × 106/ml; range: azoospermia to 111 × 106/ml) (20,21,22,23). The IHH subjects in group II continued to receive pulsatile GnRH throughout the current study.
Table 1.
Biochemical profiles of three study groups in the presence and absence of gonadal sex steroids
| Sex steroids | Normal
|
Castrate
|
||||
|---|---|---|---|---|---|---|
| Normal men (n = 31) | IHH men + GnRH (n = 12) | Agonadal men + T (n = 3) | Normal men + KC (n = 31) | IHH men + GnRH + KC (n = 12) | Agonadal men −T (n = 3) | |
| FSH (IU/liter) | 5.7 ± 0.4 (1.8–10.3) | 9.0 ± 1.8 (2.6–24.7) | 23.0 ± 5.3 (15.0–33.0) | 10.2 ± 0.7 (3.2–19.8) | 13.1 ± 2.7 (3.1–36.4) | 59.3 ± 17.6 (26.0–86.0) |
| Inhibin B (pg/ml) | 178 ± 9 (92–291) | 102 ± 14 (50–202) | <15.6 | 137 ± 9 (53–296)a | 87 ± 16 (31–246)b | <15.6 |
| LH (IU/liter) | 10.0 ± 0.6 (5.3–17.9) | 13.6 ± 0.9 (8.9–19.3) | 66.8 ± 20.1 (36.5–104.9) | 30.3 ± 1.9 (10.8–55.8)a | 19.3 ± 2.1 (10.6–31.3)b | 79.0 ± 28.5 (41.6–135) |
| T (ng/dl) | 534 ± 25 (350–941) | 552 ± 51 (345–914) | 630 ± 56 (542–735) | 37 ± 3 (15–111)a | 23 ± 2 (14–32)a | 47 ± 6 (36–57)b |
| E2 (pg/ml) | 26 ± 2 (7–52) | 26 ± 2 (19–42) | 33 ± 2 (30–38) | 7.3 ± 0.8 (5–20)a | 5.2 ± 0.2 (5–7)a | 5.1 ± 0.1 (5.0–5.2)b |
Mean ± sem (range).
P < 0.001 castrate vs. normal.
P < 0.05 castrate vs. normal.
Group III
Men with absent seminiferous tubule function (n = 3; two agonadal men aged 23 and 26 yr with castrate levels of serum T and inhibin B) were enrolled. Additionally, a 47-yr-old man with Klinefelter syndrome was also included because his clinical presentation fit the functional castrate criteria: 1 ml testes, hypogonadal serum T (59 ng/dl = 2.1 nmol/liter), and serum inhibin B level below the level of detection (<15.6 pg/ml). All three men were on transdermal T replacement therapy (Androgel, Solvay, Marietta, GA) and had normal serum T levels.
Study design
To address the relative roles of sex steroids and inhibin B in the regulation of FSH secretion, we used an acute biochemical castration model in the three study groups. Men in groups I and II received a 1-g loading dose of KC (d 0) followed by 400 mg four times a day for up to 6 d. As previously reported, this treatment reduces sex steroid levels to the castrate range within 24 h (5,24). Because KC can impair cortisol biosynthesis, glucocorticoid replacement (dexamethasone 0.5 mg orally twice a day) was given for the duration of the study. Liver function tests were monitored throughout the study, and subjects were withdrawn if transaminase levels increased greater than 3 times the upper limit of normal. Of the 56 subjects enrolled in groups I and II, 13 subjects were withdrawn due to elevated liver function tests, leaving 31 healthy normal subjects in group I and 12 IHH men in group II with complete data available for analysis. The individualized regimen of pulsatile GnRH remained the same throughout the study in the men with IHH in group II. To achieve the acute castration in group III, testosterone replacement was withdrawn in the agonadal men. In all subjects, blood samples were obtained for serum FSH, inhibin B, T, E2, and LH at baseline (d 0) and after 3 d of sex steroid ablation (d 3).
Assays
Serum LH and FSH concentrations were determined by microparticle enzyme immunoassay using the automated Abbott AxSYM system (Abbott Laboratories, Chicago, IL). The Second International Reference Preparation was used as the reference standard. The assay sensitivity for both LH and FSH was 1.6 IU/liter. The normal range for LH is 4.2–17 and 1.6–12.5 IU/liter for FSH. The intraassay coefficient of variation (CV) values for LH and FSH were less than 7% and less than 6%, respectively, with interassay CVs for both hormones of less than 7.4%. Serum T concentrations were measured using the DPC Coat-A-Count RIA kit (Diagnostic Products Corp., Los Angeles, CA), which had an intra- and interassay CV of less than 10%. The normal range for T is 280-1170 ng/dl. Inhibin B was measured using a commercially available (Serotec, Oxford, UK) double-antibody ELISA. In our use, the clinical detection limit of this assay is 15.6 pg/ml, with a CV of 4–6% within plate and 15–18% between plates and the normal range reported as 100–400 pg/ml. E2 was measured RIA using hexane ethylacetate extraction and LH-20 chromatography (Endocrine Sciences, Calabasas Hills, CA). The Endocrine Sciences E2 assay has a sensitivity of 5 pg/ml (18 pmol/liter) and based on a male serum pool has an intraassay CV of 4.9% and an interassay CV of 15%. The normal range is 8–35 pg/ml.
Statistical methods
Hormone levels were compared across cohorts using ANOVA. The relationships between FSH and inhibin B as well as the changes in FSH and inhibin B were examined by correlation analysis (Spearman rank correlation or linear correlation coefficients on the logarithmically transformed values). Subjects were also divided into quintiles according to baseline inhibin B levels. Analysis of covariance with Dunnett’s post hoc comparison was used to elucidate further the effect of inhibin B on FSH levels in the presence/absence of sex steroids by comparing mean changes in FSH after adjusting for changes in inhibin B. Values are reported as mean ± sem followed by range unless otherwise stated. P < 0.05 was considered statistically significant.
Results
Relationship of FSH and inhibin B in a normal sex steroid milieu
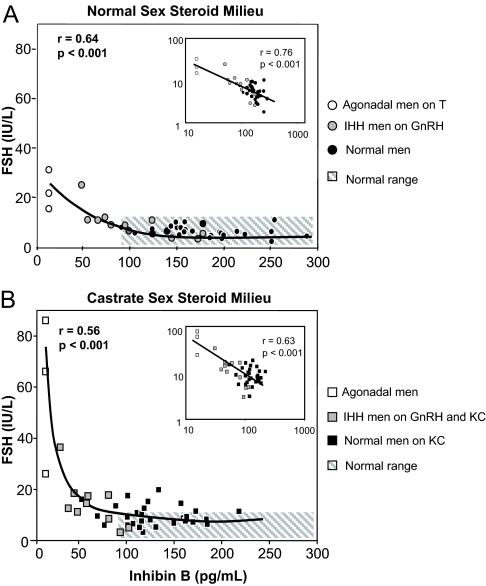
Before acute biochemical castration (baseline, d 0), men in all three groups had comparable serum T and E2 levels, well within the normal adult range (Table 1). In contrast, the serum inhibin B levels differed, with levels ranging from undetectable (<15.6 pg/ml) to the high-normal range (291 pg/ml), validating our model using three subject groups to depict the full range of seminiferous tubule function (Table 1). In the presence of normal serum sex steroids (d 0), there was a significant overall negative relationship between FSH and inhibin B (rS = −0.69, P < 0.0001, n = 46) (Fig. 1A) that was also significant when group I (rS = −0.42, n = 31, P < 0.05) and group II (rS = −0.88, n = 12, P < 0.01) were analyzed separately. The inset graph in Figure 1A depicts the log-transformed data and demonstrates a logarithmic, rather than a linear relationship between FSH and inhibin B. Serum inhibin B at day 0 did not correlate with serum T or E2 (data not shown).
Figure 1.
Serum levels of FSH vs. inhibin B in normal men (black symbols), men with IHH (gray symbols), and agonadal men (open symbols). Panel A (circles) depicts FSH and inhibin B in a normal sex steroid milieu (agonadal men on physiological T replacement and IHH men on pulsatile GnRH therapy). Panel B (squares) depicts FSH and inhibin B in a castrate sex steroid milieu (normal men and IHH men on GnRH both receiving high-dose KC; agonadal men off T replacement). The inset in each panel depicts the log-transformed data revealing a logarithmic rather than a linear relationship in both a normal and castrate sex steroid milieu. The hatched area in each panel represents the normal range for FSH and inhibin B in adult men.
Relationship of FSH and inhibin B after acute biochemical castration
The high-dose KC regimen employed in the healthy normal and IHH men (groups I and II) and withdrawal of T replacement in the agonadal men (group III) induced similar castrate levels of T and E2 across all groups by d 3 (Table 1). In the absence of sex steroids (Fig. 1B), the negative relationship between FSH and inhibin B remained significant (rS = −0.43, P < 0.01), and the log-transformed data (Fig. 1B, inset) reveal that the relationship between FSH and inhibin B after sex steroid ablation was again logarithmic. This relationship between inhibin B and FSH remained significant in group II (rS = −0.76, n = 12, P < 0.05) but not in healthy men (group I) (rS = −0.03, P < NS) in which serum FSH and E2 levels did not correlate significantly (data not shown). Notably, the mean serum inhibin B decreased significantly in subjects in both groups I and II during sex steroid ablation despite a 2-fold increase in serum FSH (Table 1).
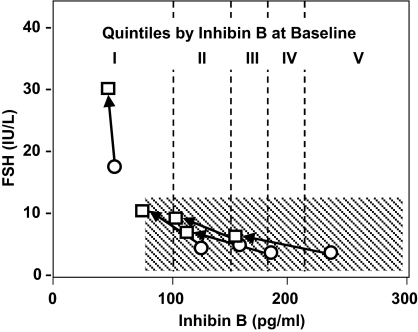
To clarify further the relative roles of serum inhibin B vs. sex steroid ablation in the control of FSH, the whole study population was pooled and then stratified into quintiles according to their baseline serum inhibin B levels (Fig. 2). The inhibin B ranges for each quintile were as follows: I = less than 100 pg/ml (n = 8), II = 100–143 pg/ml (n = 9), III = 144–165 pg/ml (n = 9), IV = 166–196 pg/ml (n = 9), and V greater than 197 pg/ml, (n = 9). Quintiles II-V, representing men with normal adult inhibin B levels, had low serum FSH levels at d 0 and experienced only modest increases in FSH after sex steroid withdrawal (d 3). This change was not significantly different across quintiles, even after adjusting for the concomitant biochemical castration-induced decrease in inhibin B. In contrast, men in quintile I exhibited the greatest increase in FSH (+11.6 ± 5.3 IU/liter) and the smallest decrease in serum inhibin B (−7.0 ± 8.8 pg/ml) in response to sex steroid ablation. The rise in FSH in quintile I was significantly different from the other quintiles (P < 0.001), even after adjusting for the change in inhibin B level.
Figure 2.
Changes in FSH and inhibin B when gonadal sex steroids are suppressed or withdrawn. Subjects from all three groups have been combined and then divided into quintiles according to their baseline serum inhibin B concentrations. The data are plotted at the mean FSH and inhibin B for each quintile in a normal sex steroid milieu at baseline (circles) and after a castrate sex steroid milieu has been established (squares). The arrows connect the paired data; the hatched area represents the normal range for FSH and inhibin B in adult men.
Discussion
With the development of sensitive and specific immunoassays for the inhibins in the last decade, great strides have been made in our understanding of the role of inhibin B in the endocrine regulation of pituitary FSH in the human male. Nonetheless, the complex interplay between T, estrogen, and inhibin B in the modulation of FSH secretion is difficult to discern when gonadal function is intact. The current study is the first to explore the relative roles of inhibin B and sex steroids in the negative feedback regulation of FSH in men across a full spectrum of seminiferous epithelium function during controlled ablation and restoration of gonadal sex steroids.
We previously demonstrated that KC treatment induces suppression of both serum T and E2 in normal men with concurrent increased serum FSH (8). Furthermore, the use of aromatase blockade in normal men led to increased serum T, suppression of E2, and a similar increase in FSH levels (4). As such, the sex steroid component of FSH-negative feedback appears to be mediated predominately, if not solely, via estrogen. Although estrogen plays an important role in the feedback regulation of FSH, previous studies in humans and primates using intact gonads or unilateral or bilateral gonadectomy have consistently concluded that inhibin B plays a major role in the restraint of GnRH-stimulated pituitary FSH secretion (26,27,28).
Previous reports from our group underscore the heterogeneity in the severity of human GnRH deficiency (22,23,29). IHH patients with histories of partial pubertal development and larger testicular volume before gonadotropin and/or GnRH replacement appear to represent a subset whose fertility outcomes are better than IHH men with a more severe phenotype of absent puberty (23). In the current study, inclusion of patients along this phenotypic spectrum of IHH, with healthy normal men on one side and anorchid men on the other, allowed us to dissect the relative roles of inhibin B and gonadal sex steroids in the feedback regulation of FSH across the full spectrum of seminiferous epithelial function.
Baseline determinations of FSH in these three groups revealed a negative correlation between FSH and inhibin B that remained evident in a castrate sex steroid milieu. Furthermore, when the relationship between FSH and inhibin B was examined within each group, the negative correlation remained significant. In addition, the negative correlation between FSH and inhibin B in both normal and castrate sex steroid milieus was best described by a logarithmic model. This model is characteristic of other endocrine feedback systems as exemplified by the relationship between thyroxine and TSH (30,31).
Changes in serum FSH levels after KC induced sex steroid ablation were particularly revealing when the subjects were stratified according to baseline serum inhibin B levels. The rise in FSH was significant yet modest in subjects whose baseline levels of inhibin B fell within the adult male range (≥ 100 pg/ml). Despite the fact that serum FSH levels doubled after inducing a castrate sex steroid milieu, serum FSH levels predominantly remained within the normal range. As such, the restraint imposed by inhibin B on FSH secretion appears to be of paramount significance across a wide range of serum inhibin B levels. Put another way, within the normal range of seminiferous tubule function (normal serum inhibin B), the impact of sex steroids on the feedback regulation of FSH secretion is small relative to inhibin B. Conversely, in the setting of impaired seminiferous tubule function (low serum inhibin B), the key role of sex steroid-negative feedback on FSH is unmasked by our model of acute castration. It is worth noting that the IHH men in group II were maintained on a fixed regimen of pulsatile GnRH during acute sex steroid ablation. Further studies will be required to determine whether the rise in FSH is more dramatic when the frequency of GnRH administration is adjusted to recapitulate the free-running GnRH frequency in the castrate (8). The fact that the rises in FSH during sex steroid withdrawal were comparable in the normal men (whose endogenous GnRH frequency is not fixed) and the IHH men (whose GnRH frequency was clamped) suggests that changes in the stimulatory inputs from the hypothalamus play less of a role in modulating FSH then negative feedback from the gonads.
As we reported in normal men, serum inhibin B decreases during the acute biochemical castration induced by high-dose KC administration (8). This decrease was noted in the current study in both normal and IHH men. This modest but statistically significant decline in serum inhibin B levels occurs despite a 2-fold rise in FSH (that in other settings would have been expected to generate an increase in inhibin B secretion). The explanation of this modest decline in inhibin B is likely the suppression of endogenous sex steroids production by the testis by KC therapy. The impact of high, intragonadal sex steroid concentrations on inhibin B production may be either direct or indirect; the latter may include influences on spermatogenesis and the complex cross talk that is evident between Leydig and Sertoli cells, Sertoli and germ cells, and/or tubular elements and the peritubular myoid cells (15,25,32,33,34,35,36,37,38). Alternatively, KC itself may be having direct effects on these systems that are not mediated via changes in sex steroid production. Whereas raising interesting questions regarding the complex feedback-feed-forward interplay between FSH and inhibin B, elucidation of these mechanisms goes beyond the aims of the current study. Because high-dose KC induces such an effective blockade of gonadal sex steroid synthesis, it is difficult to conceive of a model that permits restoration of high intragonadal sex steroid concentrations in the face of ongoing KC administration.
Taken together, the data generated in the current series of studies serve to further establish inhibin as the principal gonadal feedback regulator of FSH across a wide range of seminiferous epithelial function in the human male. The inhibin B-induced clamp on FSH secretion remains largely effective even when inhibin B concentrations fall to levels at or just below the lower limit of the adult male range (∼100 pg/ml). With the inhibin clamp in place, wide fluctuations in gonadal sex steroids (including their acute ablation by high-dose KC) have little impact on FSH levels. However, in men with anorchia or severely compromised seminiferous epithelial function in whom the inhibin clamp on FSH has been removed, sex steroids (specifically estradiol) take on the principal role in restraining FSH via negative feedback.
Acknowledgments
We gratefully acknowledge the nurses of the General Clinical Research Center for their excellent clinical care and the technicians of the Reproductive Endocrine Sciences Center RIA Core for their superb technical contributions to this study.
Footnotes
This work was supported by National Institutes of Health Grants R01 HD15788, U54 HD 028138, and M01-RR-01066 of the National Center for Research Resources, General Clinical Research Centers Program.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 12, 2008
Abbreviations: CV, Coefficient of variation; E2, estradiol; IHH, idiopathic hypogonadotropic hypogonadism; KC, ketoconazole; T, testosterone.
References
- Ying SY 1988 Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev 9:267–293 [DOI] [PubMed] [Google Scholar]
- Plant TM, Marshall GR 2001 The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev 22:764–786 [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Blount AL, Leal AM, Donaldson CJ, Fischer WH, Vale WW 2004 Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin. Mol Cell Endocrinol 225:29–36 [DOI] [PubMed] [Google Scholar]
- Hayes FJ, Hall JE, Boepple PA, Crowley Jr WF 1998 Clinical review 96: differential control of gonadotropin secretion in the human: endocrine role of inhibin. J Clin Endocrinol Metab 83:1835–1841 [DOI] [PubMed] [Google Scholar]
- Hayes FJ, DeCruz S, Seminara SB, Boepple PA, Crowley Jr WF 2001 Differential regulation of gonadotropin secretion by testosterone in the human male: absence of a negative feedback effect of testosterone on follicle-stimulating hormone secretion. J Clin Endocrinol Metab 86:53–58 [DOI] [PubMed] [Google Scholar]
- Anawalt BD, Bebb RA, Matsumoto AM, Groome NP, Illingworth PJ, McNeilly AS, Bremner WJ 1996 Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab 81:3341–3345 [DOI] [PubMed] [Google Scholar]
- Illingworth PJ, Groome NP, Byrd W, Rainey WE, McNeilly AS, Mather JP, Bremner WJ 1996 Inhibin-B: a likely candidate for the physiologically important form of inhibin in men. J Clin Endocrinol Metab 81:1321–1325 [DOI] [PubMed] [Google Scholar]
- Hayes FJ, Pitteloud N, DeCruz S, Crowley Jr WF, Boepple PA 2001 Importance of inhibin B in the regulation of FSH secretion in the human male. J Clin Endocrinol Metab 86:5541–5546 [DOI] [PubMed] [Google Scholar]
- Andersson AM, Muller J, Skakkebaek NE 1998 Different roles of prepubertal and postpubertal germ cells and Sertoli cells in the regulation of serum inhibin B levels. J Clin Endocrinol Metab 83:4451–4458 [DOI] [PubMed] [Google Scholar]
- Andersson AM, Juul A, Petersen JH, Muller J, Groome NP, Skakkebaek NE 1997 Serum inhibin B in healthy pubertal and adolescent boys: relation to age, stage of puberty, and follicle-stimulating hormone, luteinizing hormone, testosterone, and estradiol levels. J Clin Endocrinol Metab 82:3976–3981 [DOI] [PubMed] [Google Scholar]
- Crofton PM, Illingworth PJ, Groome NP, Stirling HF, Swanston I, Gow S, Wu FC, McNeilly A, Kelnar CJ 1997 Changes in dimeric inhibin A and B during normal early puberty in boys and girls. Clin Endocrinol (Oxf) 46:109–114 [DOI] [PubMed] [Google Scholar]
- Byrd W, Bennett MJ, Carr BR, Dong Y, Wians F, Rainey W 1998 Regulation of biologically active dimeric inhibin A and B from infancy to adulthood in the male. J Clin Endocrinol Metab 83:2849–2854 [DOI] [PubMed] [Google Scholar]
- Sikaris K, McLachlan RI, Kazlauskas R, de Kretser D, Holden CA, Handelsman DJ 2005 Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. J Clin Endocrinol Metab 90:5928–5936 [DOI] [PubMed] [Google Scholar]
- Anderson RA, Wallace EM, Groome NP, Bellis AJ, Wu FC 1997 Physiological relationships between inhibin B, follicle stimulating hormone secretion and spermatogenesis in normal men and response to gonadotrophin suppression by exogenous testosterone. Hum Reprod 12:746–751 [DOI] [PubMed] [Google Scholar]
- Pineau C, Sharpe RM, Saunders PT, Gerard N, Jegou B 1990 Regulation of Sertoli cell inhibin production and of inhibin α-subunit mRNA levels by specific germ cell types. Mol Cell Endocrinol 72:13–22 [DOI] [PubMed] [Google Scholar]
- von Eckardstein S, Simoni M, Bergmann M, Weinbauer GF, Gassner P, Schepers AG, Nieschlag E 1999 Serum inhibin B in combination with serum follicle-stimulating hormone (FSH) is a more sensitive marker than serum FSH alone for impaired spermatogenesis in men, but cannot predict the presence of sperm in testicular tissue samples. J Clin Endocrinol Metab 84:2496–2501 [DOI] [PubMed] [Google Scholar]
- World Health Organization 1992 WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 3rd ed. Cambridge, UK: Cambridge University Press [Google Scholar]
- Hoffman AR, Crowley Jr WF 1982 Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med 307:1237–1241 [DOI] [PubMed] [Google Scholar]
- Whitcomb RW, Crowley Jr WF 1990 Clinical review 4: diagnosis and treatment of isolated gonadotropin-releasing hormone deficiency in men. J Clin Endocrinol Metab 70:3–7 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Boepple PA, Nachtigall LB, Pralong FP, Khoury RH, Sluss PM, Lecain AE, Crowley Jr WF 1996 Inhibin B in males with gonadotropin-releasing hormone (GnRH) deficiency: changes in serum concentration after short-term physiologic GnRH replacement—a clinical research center study. J Clin Endocrinol Metab 81:3692–3696 [DOI] [PubMed] [Google Scholar]
- Nachtigall LB, Boepple PA, Seminara SB, Khoury RH, Sluss PM, Lecain AE, Crowley Jr WF 1996 Inhibin B secretion in males with gonadotropin-releasing hormone (GnRH) deficiency before and during long-term GnRH replacement: relationship to spontaneous puberty, testicular volume, and prior treatment—a clinical research center study. J Clin Endocrinol Metab 81:3520–3525 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Hayes FJ, Boepple PA, DeCruz S, Seminara SB, MacLaughlin DT, Crowley Jr WF 2002 The role of prior pubertal development, biochemical markers of testicular maturation, and genetics in elucidating the phenotypic heterogeneity of idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 87:152–160 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, Crowley Jr WF 2002 Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 87:4128–4136 [DOI] [PubMed] [Google Scholar]
- Sonino N 1987 The use of ketoconazole as an inhibitor of steroid production. N Engl J Med 317:812–818 [DOI] [PubMed] [Google Scholar]
- Petersen C, Soder O 2006 The Sertoli cell—a hormonal target and ‘super’ nurse for germ cells that determines testicular size. Horm Res 66:153–161 [DOI] [PubMed] [Google Scholar]
- Abeyawardene SA, Plant TM 1989 Bilateral orchidectomy and concomitant testosterone replacement in the juvenile male rhesus monkey (Macaca mulatta) receiving an invariant intravenous gonadotropin-releasing hormone (GnRH) infusion results, as in the hypothalamus lesioned GnRH-driven adult male, in a selective hypersecretion of follicle-stimulating hormone. Endocrinology 125:257–259 [DOI] [PubMed] [Google Scholar]
- Majumdar SS, Mikuma N, Ishwad PC, Winters SJ, Attardi BJ, Perera AD, Plant TM 1995 Replacement with recombinant human inhibin immediately after orchidectomy in the hypophysiotropically clamped male rhesus monkey (Macaca mulatta) maintains follicle-stimulating hormone (FSH) secretion and FSH beta messenger ribonucleic acid levels at precastration values. Endocrinology 136:1969–1977 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Marshall GR, McNeilly AS, Plant TM 1999 Evidence that in a physiological setting Sertoli cell number is the major determinant of circulating concentrations of inhibin B in the adult male rhesus monkey (Macaca mulatta). J Androl 20:430–434 [PubMed] [Google Scholar]
- Waldstreicher J, Seminara SB, Jameson JL, Geyer A, Nachtigall LB, Boepple PA, Holmes LB, Crowley Jr WF 1996 The genetic and clinical heterogeneity of gonadotropin-releasing hormone deficiency in the human. J Clin Endocrinol Metab 81:4388–4395 [DOI] [PubMed] [Google Scholar]
- Spencer CA, LoPresti JS, Patel A, Guttler RB, Eigen A, Shen D, Gray D, Nicoloff JT 1990 Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab 70:453–460 [DOI] [PubMed] [Google Scholar]
- Reichlin S, Utiger RD 1967 Regulation of the pituitary-thyroid axis in man: relationship of TSH concentration to concentration of free and total thyroxine in plasma. J Clin Endocrinol Metab 27:251–255 [DOI] [PubMed] [Google Scholar]
- Leung PC, Steele GL 1992 Intracellular signaling in the gonads. Endocr Rev 13:476–498 [DOI] [PubMed] [Google Scholar]
- Saez JM 1994 Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr Rev 15:574–626 [DOI] [PubMed] [Google Scholar]
- Griswold MD 1995 Interactions between germ cells and Sertoli cells in the testis. Biol Reprod 52:211–216 [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I, Toppari J 1995 Endocrine, paracrine and autocrine regulation of testicular steroidogenesis. Adv Exp Med Biol 377:33–54 [DOI] [PubMed] [Google Scholar]
- Verhoeven G, Hoeben E, De Gendt K 2000 Peritubular cell-Sertoli cell interactions: factors involved in PmodS activity. Andrologia 32:42–45 [PubMed] [Google Scholar]
- de Kretser DM, Buzzard JJ, Okuma Y, O’Connor AE, Hayashi T, Lin SY, Morrison JR, Loveland KL, Hedger MP 2004 The role of activin, follistatin and inhibin in testicular physiology. Mol Cell Endocrinol 225:57–64 [DOI] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE 2004 Hormonal regulation of spermatogenesis. Int J Androl 27:335–342 [DOI] [PubMed] [Google Scholar]