Abstract
Objective: Hyperandrogenia and insulin resistance are heritable family traits, likely to cluster in children of polycystic ovary syndrome (PCOS) mothers.
Design: We performed a case control study of PCOS children (n = 32) compared with children from control women (n = 38) for reproductive and metabolic abnormalities, stratifying results by three Tanner stage groupings. The children underwent history and physical examinations, a 3-h timed urine collection, a 2-h oral glucose tolerance test, and abdominal ultrasound examination (females only). Serum was obtained in older children (age > 8 yr) who consented.
Results: Urine LH levels were significantly lower in the Tanner IV–V PCOS girls compared with controls (P = 0.04). Urine testosterone levels were significantly elevated in Tanner II–III PCOS boys compared with controls (P = 0.007). There were no significant differences in dehydroepiandrosterone levels. We validated the correlation between salivary and serum levels of insulin (insulin areas under the curve) in an adult population [n =30, Pearson correlation coefficient (r) = 0.67; P < 0.0001], which also replicated in the children (2-h insulin r = 0.57; P = 0.0004). Mean area under the curve salivary insulin levels were significantly higher in the Tanner IV–V PCOS girls in the later stages of puberty when compared with controls (3625 ± 1372 vs. 1766 ± 621 min × μU/ml, 95% confidence interval 475-3242; P < 0.02).
Conclusions: Hyperinsulinism may be a familial characteristic of PCOS children (or at least girls) but does not appear until the later stages of puberty. Other reproductive abnormalities that characterize PCOS may develop later.
A case-control study comparing children of polycystic ovary syndrome (PCOS) mothers to children of healthy mothers finds that hyperinsulinism may be a familial characteristic of PCOS children, appearing in the later stages of puberty. Additionally, urine LH levels were lower in PCOS girls and urine testosterone levels were higher in PCOS boys.
Polycystic ovary syndrome (PCOS) is an ovarian disorder in reproductive-age women characterized by anovulation, hyperandrogenism, and polycystic ovaries (1). The phenotype in prepubertal or postmenarchal women and in males is unknown (2). We have shown familial clustering of hyperandrogenism in first-degree relatives of probands with PCOS, including sisters (3), brothers (4), and mothers (5). Furthermore, this reproductive phenotype of hyperandrogenism identifies women with insulin resistance and associated dyslipidemias, and these are familial traits affecting both female and male relatives (4,6,44). Specific alleles are associated with this disorder and its metabolic derangements (7), supporting a genetic component to this disorder.
Studying the children of women with PCOS offers a unique insight into the familial aspects of PCOS and also to its ontogeny. If familial clustering is characteristic of this disorder, then abnormal phenotypical characteristics of PCOS, including both reproductive and metabolic abnormalities, may be more likely in children of PCOS mothers (PCOS children) than in those from mothers without PCOS and provide some insight as to the heritability of this disorder. Studying children may expose the time sequence in which abnormal phenotypical characteristics appear (8). However, these studies are difficult to perform given the limited number of children born to mothers with PCOS because of the known subfertility of these women (9), ethical concerns about invasive and dynamic testing in children, the complex reproductive and metabolic changes associated with puberty (10), and the gender-specific differences in sexual maturation (11).
Nonetheless, the fate of children of women with PCOS remains a highly relevant clinical question both to the families and to investigators trying to understand this disorder better. We performed this study to test the hypothesis that reproductive and metabolic abnormalities are more likely present in children of women with PCOS, and are likely to appear at an early age. Furthermore, we sought to use existing methods for studying reproductive function in this vulnerable population (timed urinary collections, and add a noninvasive test of insulin measurement [salivary insulin levels during an oral glucose tolerance test (OGTT)].
Subjects and Methods
Subjects
There were 17 healthy girls and 15 healthy boys of 27 mothers with PCOS, and 21 healthy control girls and 17 healthy control boys of 30 control mothers (mean age of all children 9.3 ± 3.1 yr, range 4–14) studied in the General Clinical Research Center (GCRC) at the Hershey Medical Center in Hershey, PA, in concordance with a protocol approved by the Penn State College of Medicine institutional review board. All subjects and at least one parent gave written informed consent after the study was explained to them.
We chose a lower age limit of 4 yr based on our judgment that children older than this age would be able to understand and comply with the salivary collection procedure during the OGTTs.
Subjects for the PCOS group were recruited through our database of women who had previously participated in a PCOS research study. All mothers in this group had been studied and noted to have PCOS by the 1990 National Institutes of Health-National Institute of Child Health and Human Development criteria for PCOS (12), with a history of documented chronic anovulation (less than or equal to six menses per year) and hyperandrogenism, with the exclusion of secondary causes (3). Control children were recruited through local advertisements. We studied control children who did not have any personal or family history of diabetes, and their mothers had a history of regular menstrual cycles without hirsutism. No children were taking confounding medications known to affect sex steroids or insulin action.
We did not collect maternal age, body mass index (BMI) at pregnancy, or the use of fertility medications or other treatment to achieve pregnancy. The following self-reported major pregnancy complications were noted in our mothers: 3.3% (one of 30) of mothers with PCOS and 0% (zero of 37) of control mothers had preeclampsia; 10% (three of 30) of mothers with PCOS and 0% (zero of 37) of control mothers had gestational diabetes; and 3.3% (one of 30) of mothers with PCOS and 10.8% (four of 37) of control mothers had preterm labor.
Study design
The study design was a case control study. To provide comparable pubertal reference populations, children were clustered according to gender and Tanner staging into three groups: A, Tanner stage 0–I; B, Tanner stage II–III; and C, Tanner stage IV–V. Children presented after an overnight fast to the GCRC. A medical history was obtained from each child, including gestational age and birth weight of the child. Biometrical data included height, weight, waist-to-hip ratio, and blood pressure, measured in the morning in the seated position in the right arm after a 5-min rest period. The average of three measurements taken 2 min apart was the reported blood pressure used for establishing the diagnosis of hypertension. Blood pressure percentiles were calculated adjusting for age, gender, and height. The diagnosis of hypertension in adolescents was defined as a blood pressure above the 90th percentile for age, height, and gender, as defined by the National High Blood Pressure Education Program for Children and Adolescents (13).
Tanner staging of pubic hair was recorded by experienced and trained nursing personnel in the GCRC (14). Each subject was asked to collect a 3-h timed urinary sample. The urines were collected independent of menstrual bleeding and cycle day; five girls of PCOS mothers and five girls from control mothers were postmenarchal. Parents had the children void as soon as they awoke in the morning, which was discarded, and then collected all voided urine for the next 3 h. The collection period was timed so that it ended soon after the child’s arrival to the GCRC in the morning.
Children underwent a modified 2-h OGTT. Each child received an oral glucose load of 1.7 g/weight (kg). Salivary samples were collected at 0, 30, 60, 90, and 120 min to measure salivary insulin levels. Children older than the age of 8 yr who consented also had blood drawn at 0 and 120 min for glucose and insulin levels. Fasting morning blood samples also were taken in this group for measurement of lipid profiles, androgens, and gonadotropins (3). At the conclusion of the OGTT, all female subjects underwent an abdominal ultrasound to determine ovarian size and morphology. Polycystic ovary morphology, according to the criteria of Adams et al. (15), was determined during the performance of the ultrasound. Ovarian size was obtained by measuring the largest plane of the ovary in two dimensions and then turning the abdominal probe 90° to obtain a third measurement. Volume of the ovary was calculated using the formula for an ellipsoid: length × height × width × (π/6) (16).
Assays
Serum assays for total testosterone, dehydroepiandrosterone sulfate (DHEAS), and gonadotropins were performed using Diagnostic Products Corp. (Los Angeles, CA) Coat-A-Count kits (3). Plasma glucose levels were determined by the glucose oxidase technique (18). Insulin in blood and saliva was determined with a double-antibody method using reagents obtained from LINCO Research, Inc. (St. Charles, MO) (19). The assay detects free insulin, and the cross-reactivity of this assay with proinsulin is less than 0.2%. All assays had intraassay and interassay coefficients of variation (CVs) less than 10%.
The saliva sample was stored at −20 C or colder to precipitate out proteoglycans in the salivary fluid. Upon thawing, the sample was centrifuged at 3000 rpm for 10 min at room temperature to sediment the heavy proteinaceous material in the sample. The supernatant was removed and an aliquot pipetted into assay tubes for the insulin assay.
Total cholesterol was determined with a cholesterol esterase method on a Roche automated chemistry analyzer (Indianapolis, IN). High-density lipoprotein choloesterol (HDL-C) was determined with the cholesterol esterase method after selective precipitation of apolipoprotein-B containing lipoproteins with a polyanion solution. The low-density lipoprotein fraction was calculated using the Friedewald equation (20). All serum and salivary assays had method CVs less than or equal to 10% (21).
Urinary steroid hormone assays were performed using established RIA techniques that use ether extraction and celite chromatography before the RIA (14,22). The day to day reproducibility of the urinary testosterone measurements during this study had a between-run precision of 12% at a mean concentration of 45 μg/liter, whereas dehydroepiandrosterone (DHEA) averaged 11% interassay precision at a concentration of 1 μg/liter. Intraassay and interassay CVs for urinary LH and FSH values were less than 10%. The urinary gonadotropin RIA method used has been published elsewhere (23), and involves acetone precipitation of the gonadotropins from the urine sample and reconstitution in assay buffer, concentrating the sample 80-fold in the process. The low-end detection for this assay was 1.0 mIU/ml. Urinary gonadotropin levels were not run in two male children of control mothers due to reagent changes in the urine gonadotropin assay toward the end of this study.
Diagnostic criteria for the adolescent metabolic syndrome
The diagnosis of the metabolic syndrome was determined using the de Ferranti (24) criteria for adolescents, derived from an analysis of adolescents in the Third National Health and Nutrition Examination Survey: waist circumference more than the 75th percentile, hypertension defined by either systolic or diastolic blood pressure more than the 90th percentile, fasting triglycerides more than or equal to 97.3 mg/dl, fasting HDL-C less than 50.2 mg/dl, and fasting glucose more than or equal to 110 mg/dl. Subjects meeting three or more of the aforementioned criteria were diagnosed with the metabolic syndrome (25).
Statistical methods
The homeostatic index of insulin resistance (HOMA IR) was calculated as follows: HOMA IR = [fasting insulin (μU/ml) × fasting glucose (mmol/liter)]/22.5 (26). The area under the curve (AUC) for salivary insulin obtained every 30 min for a 2-h period was calculated per subject using the trapezoidal rule. The Pearson correlation coefficient was used to assess the relationship between blood and salivary insulin levels at each 30-min interval as well as for the AUC. For biometrical data comparisons, heterogeneous variance models were fit to assess the mean differences, with associated 95% confidence intervals, between PCOS and control groups in each cohort (gender and Tanner stage). The heterogeneous variance model is an extension of the ANOVA model that allows for unequal variances across the groups. No adjustments for multiple hypothesis testing were done in this small study, so interpretation of P values should be done with caution. All hypotheses tests were two-sided, and all analyses were performed using SAS software, version 9.1 (SAS Institute Inc., Cary, NC) with graphics created using S-plus, version 7.0 (Insightful Corp., Seattle, WA).
Correlation of serum and salivary insulin levels in adults and children
A total of 30 adults (23 females and seven males) who were participating in another study, which included a 2-h OGTT with a glucose load of 75 g, consented to provide salivary samples every 30 min along with routine phlebotomy samples. There were 12 normal control subjects, and 18 were women with PCOS or their first-degree relatives. None had major medical problems. All subjects had fasted overnight. The mean age of the subjects was 33.9 ± 15.1 yr (mean ± sd) with a BMI of 30.8 ± 9.3 kg/m2. Of these subjects, three had impaired glucose tolerance, and none had type 2 diabetes mellitus by the World Health Organization guidelines.
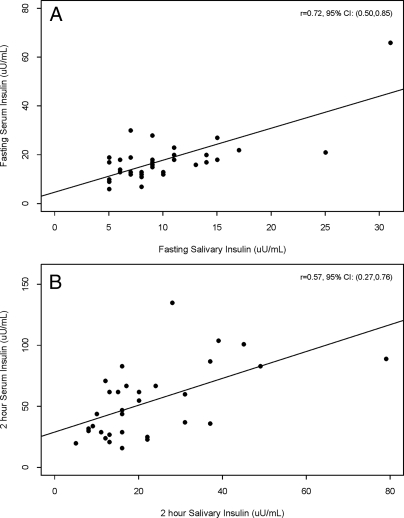
The values were adequately correlated, though the correlation dropped off with later values (≥90 min) during the test. AUC values were calculated for the serum and salivary insulin levels from the adult subjects. The results were 11,917 ± 9,719 min × μU/ml for serum and 4,107 ± 2,161 min × μU/ml for saliva. The correlation of serum and salivary AUCs in adults was found to be significant with a Pearson correlation coefficient of 0.67 (P < 0.0001). For the children, the correlation between blood and salivary levels at baseline was r = 0.72 (P < 0.0001) and at 2 h was r = 0.57 (P = 0.0004) (Fig. 1). We could not perform AUC correlations in children because we only obtained blood at 0 h(n = 32 children) and 2 h (n = 30 children).
Figure 1.
Correlation between salivary and serum insulin in children from the study. A, Fasting insulin (n = 32 children). B, One hundred twenty minute insulin (n = 30 children). The Pearson correlation coefficient (r) and associated 95% confidence interval (CI) are reported in each panel.
Results
Study subjects and biometrics
There were no differences between PCOS children and control children in terms of the length of gestation and birth weight (Table 1). The Tanner subgroups were very similar with respect to age, but girls of PCOS mothers in the Tanner II–III group were heavier than the control girls (P = 0.02) (Table 2).
Table 1.
Characteristics, urinary hormones, and salivary insulin levels in children of women with PCOS and control children
| Female
|
Male
|
|||||
|---|---|---|---|---|---|---|
| PCOS (n = 17) | Control (n = 21) | P value | PCOS (n = 15) | Control (n = 17) | P value | |
| Birth data | ||||||
| Gestational age (wk) | 40.0 (1.8) | 39.1 (1.8) | 0.14 | 40.1 (1.3) | 40.3 (0.9) | 0.73 |
| Birth weight (lb) | 7.7 (1.3) | 7.4 (1.4) | 0.57 | 8.3 (1.4) | 8.4 (1.0) | 0.88 |
| Biometric | ||||||
| Age (yr) | 9.1 (3.4) | 9.5 (3.0) | 0.74 | 9.7 (3.4) | 8.8 (3.0) | 0.40 |
| BMI (kg/m2) | 20.0 (5.3) | 18.8 (3.3) | 0.42 | 20.9 (5.1) | 19.4 (3.3) | 0.33 |
| Waist to hip ratio | 0.8 (0.1) | 0.8 (0.1) | 0.30 | 0.9 (0.1) | 0.8 (0.0) | 0.59 |
| Systolic blood pressure (mm Hg) | 109.1 (12.2) | 108.3 (10.6) | 0.84 | 110.6 (18.1) | 107.4 (9.9) | 0.54 |
| Diastolic blood pressure (mm Hg) | 66.7 (8.8) | 63.6 (6.5) | 0.24 | 65.6 (8.5) | 64.8 (7.5) | 0.77 |
| Reproductive (3-h timed urinary collections) | ||||||
| LH (mIU/h) | 190.9 (319.8) | 413.7 (882.9) | 0.34 | 137.7 (174.0) | 78.5 (59.3) | 0.23 |
| FSH (mIU/h) | 260.6 (205.4) | 358.7 (316.2) | 0.30 | 197.9 (177.8) | 161.1 (73.7) | 0.47 |
| Testosterone (ng/mg creatinine) | 21.5 (10.5) | 20.1 (13.9) | 0.75 | 30.3 (21.0) | 17.9 (17.2) | 0.09 |
| DHEA (ng/mg creatinine) | 3.3 (2.5) | 2.3 (2.5) | 0.28 | 1.9 (1.8) | 1.1 (1.1) | 0.16 |
| Metabolic (salivary insulin levels during OGTT) | ||||||
| Fasting insulin (μU/ml) | 9.1 (5.5) | 7.3 (3.3) | 0.26 | 9.5 (6.6) | 6.7 (2.0) | 0.14 |
| Insulin at 30 min (μU/ml) | 9.4 (5.2) | 8.0 (3.9) | 0.38 | 12.5 (8.4) | 8.6 (5.0) | 0.13 |
| Insulin at 60 min (μU/ml) | 24.6 (16.2) | 16.0 (6.5) | 0.06 | 20.5 (14.8) | 16.3 (11.2) | 0.38 |
| Insulin at 90 min (μU/ml) | 23.1 (16.3) | 15.4 (9.9) | 0.11 | 16.6 (14.5) | 15.6 (11.3) | 0.84 |
| Insulin at 120 min (μU/ml) | 25.0 (17.4) | 14.0 (6.9) | 0.03 | 16.7 (12.8) | 15.5 (17.2) | 0.82 |
| AUC insulin (min × μU/ml) | 2229.4 (1248.0) | 1495.0 (635.4) | 0.04 | 1881.0 (1266.7) | 1551.2 (1006.3) | 0.43 |
Values are expressed as mean (sd). Conversion factors to International System of Units: testosterone × 3.467 (nmol/liter), DHEA × 3.47 (nmol/liter), glucose × 0.0551 (mmol/liter), and insulin × 7.175 (pmol/liter).
Table 2.
Characteristics of children of women with PCOS and control children by Tanner stage
| Female
|
Male
|
|||||
|---|---|---|---|---|---|---|
| PCOS (n = 17) | Control (n = 21) | P value | PCOS (n = 15) | Control (n = 17) | P value | |
| Age (yr) | ||||||
| Tanner stage = 0, I | 6.7 (1.8) [9] | 7.8 (1.5) [13] | 0.23 | 7.1 (2.1) [8] | 7.3 (2.3) [11] | 0.88 |
| Tanner stage = II, III | 11.2 (2.7) [5] | 11.0 (3.8) [4] | 0.89 | 12.5 (1.7) [4] | 10.8 (2.1) [4] | 0.24 |
| Tanner stage = IV, V | 13.0 (1.7) [3] | 13.5 (1.0) [4] | 0.75 | 13.0 (1.0) [3] | 13.0 (0.0) [2] | 1.00 |
| BMI (kg/m2) | ||||||
| Tanner stage = 0, I | 16.7 (1.8) [9] | 17.8 (2.7) [13] | 0.48 | 18.2 (2.9) [8] | 18.5 (3.4) [11] | 0.85 |
| Tanner stage = II, III | 24.9 (6.9) [5] | 19.0 (2.8) [4] | 0.02 | 25.7 (6.4) [4] | 20.6 (3.4) [4] | 0.07 |
| Tanner stage = IV, V | 21.7 (0.7) [3] | 21.9 (4.2) [4] | 0.96 | 21.8 (3.6) [3] | 21.6 (1.2) [2] | 0.97 |
| Waist to hip ratio | ||||||
| Tanner stage = 0, I | 0.8 (0.1) [9] | 0.8 (0.1) [13] | 0.15 | 0.8 (0.0) [8] | 0.8 (0.1) [11] | 0.40 |
| Tanner stage = II, III | 0.8 (0.0) [5] | 0.9 (0.0) [4] | 0.31 | 0.9 (0.1) [4] | 0.9 (0.0) [4] | 0.28 |
| Tanner stage = IV, V | 0.8 (0.1) [3] | 0.8 (0.1) [4] | 0.25 | 0.9 (0.0) [3] | 0.8 (0.0) [2] | 0.18 |
Values are mean (sd) [number for Tanner stage].
Gonadotropins and androgens
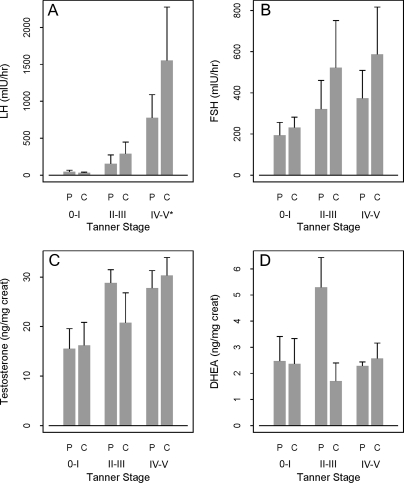
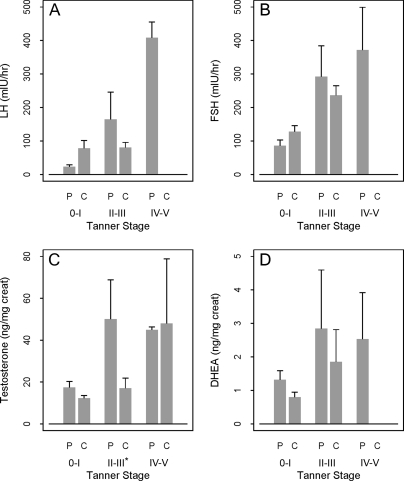
There were no significant differences in the urinary or serum FSH or LH levels between the overall male and female groups (Tables 1 and 3). However, the urine LH levels were significantly lower in the PCOS girls Tanner IV–V group compared with controls (P = 0.04) (Fig. 2). Urine testosterone levels were significantly elevated in Tanner II–III PCOS boys compared with control boys (P = 0.007) (Fig. 3). There were no significant differences in urinary DHEA or serum DHEAS between groups.
Table 3.
Characteristics of children who underwent serum testing
| Female
|
Male
|
|||||
|---|---|---|---|---|---|---|
| PCOS (n = 7) | Control (n = 11) | P value | PCOS (n = 10) | Control (n = 6) | P value | |
| Biometrical | ||||||
| Age (yr) | 11.6 (3.0) | 11.1 (2.5) | 0.73 | 11.1 (2.1) | 12.0 (1.3) | 0.31 |
| BMI (kg/m2) | 23.3 (6.3) | 20.5 (3.3) | 0.31 | 21.9 (4.9) | 19.9 (3.0) | 0.33 |
| Waist circumference (cm) | 72.1 (14.4) | 70.9 (7.5) | 0.84 | 80.0 (19.8) | 74.3 (3.8) | 0.39 |
| Waist to hip ratio | 0.8 (0.1) | 0.8 (0.1) | 0.64 | 0.9 (0.1) | 0.8 (0.0) | 0.28 |
| Systolic blood pressure (mm Hg) | 113.4 (14.8) | 112.0 (11.2) | 0.83 | 114.1 (20.9) | 104.8 (9.5) | 0.25 |
| Diastolic blood pressure (mm Hg) | 68.7 (10.8) | 64.7 (5.1) | 0.39 | 67.4 (10.1) | 64.0 (6.2) | 0.42 |
| Reproductive (serum) | ||||||
| LH (mIU/h) | 7.3 (12.7) | 11.9 (20.7) | 0.61 | 2.5 (1.0) | 2.0 (0.0) | 0.34 |
| FSH (mIU/h) | 5.1 (2.4) | 6.2 (2.9) | 0.43 | 3.6 (2.1) | 5.3 (3.9) | 0.47 |
| Testosterone (ng/dl) | 24.1 (10.7) | 22.6 (18.2) | 0.83 | 173.2 (229.5) | 226.2 (256.5) | 0.69 |
| DHEAS (ng/ml) | 1061.3 (425.4) | 847.0 (409.5) | 0.31 | 1138.6 (785.9) | 635.2 (327.1) | 0.10 |
| Metabolic | ||||||
| Fasting insulin (μU/ml) | 18.1 (2.3) | 15.5 (4.3) | 0.11 | 22.3 (16.9) | 15.0 (7.7) | 0.26 |
| Insulin at 120 min (μU/ml) | 61.6 (31.0) | 44.1 (20.3) | 0.22 | 55.4 (39.7) | 55.8 (25.8) | 0.98 |
| Fasting glucose (mg/dl) | 73.9 (9.7) | 79.7 (7.1) | 0.20 | 79.6 (6.4) | 77.3 (9.3) | 0.61 |
| Glucose at 120 min (mg/dl) | 77.3 (16.0) | 82.6 (9.3) | 0.46 | 90.3 (10.1) | 86.8 (14.7) | 0.63 |
| HOMA IR | 3.3 (0.6) | 3.0 (1.0) | 0.54 | 4.3 (3.0) | 2.9 (1.9) | 0.29 |
| ISI (from OGTT) | 97.2 (21.7) | 95.4 (10.3) | 0.85 | 90.3 (17.6) | 93.9 (24.1) | 0.76 |
| Total cholesterol (mg/dl) | 153.0 (15.7) | 145.3 (9.9) | 0.33 | 150.8 (27.0) | 144.4 (16.1) | 0.59 |
| HDL-C (mg/dl) | 48.8 (14.7) | 49.0 (17.1) | 0.99 | 44.3 (8.0) | 32.8 (14.6) | 0.16 |
| LDL-C (mg/dl) | 86.7 (11.7) | 83.6 (22.9) | 0.76 | 90.6 (21.0) | 90.0 (21.1) | 0.96 |
| Triglycerides (mg/dl) | 87.3 (34.7) | 63.6 (14.2) | 0.17 | 78.6 (23.6) | 108.6 (26.7) | 0.07 |
Values are expressed as mean (sd). Conversion factors to International System of Units: testostorone × 3.467 (nmol/liter), DHEAS × 0.002714 (μmol/liter), glucose × 0.0551 (mmol/liter), insulin × 7.175 (pmol/liter), cholesterol × 0.0259 9 mmol/liter, and triglycerides × 0.0113 (mmol/liter). ISI, International sensitivity index; LDL-C, low-density lipoprotein cholesterol.
Figure 2.
Urinary reproductive hormones by Tanner stage in females. A, LH. B, FSH. C, Testosterone. D, DHEA. On the horizontal axis, P denotes PCOS child, and C denotes control child. The mean and se are plotted. An asterisk (*) beside a Tanner stage on the horizontal axis denotes a P < 0.05 for comparing PCOS to control.
Figure 3.
Urinary reproductive hormones by Tanner stage in males. A, LH. B, FSH. C, Testosterone. D, DHEA. On the horizontal axis, P denotes PCOS child, and C denotes control child. The mean and se are plotted. An asterisk (*) beside a Tanner stage on the horizontal axis denotes a P < 0.05 for comparing PCOS to control.
OGTT and AUC salivary insulin results
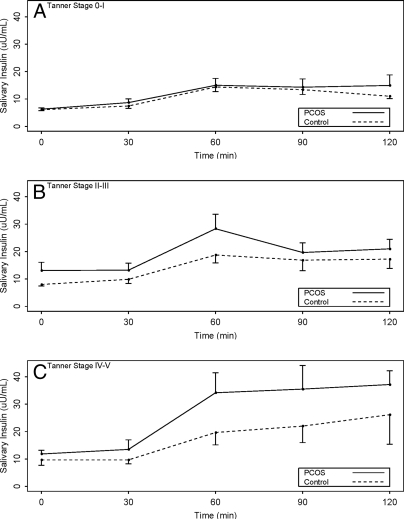
There were no significant differences in fasting insulin levels between PCOS children and control children (male or female), either with saliva or blood determinations. PCOS daughters had significantly higher 2-h salivary insulin levels compared with control daughters (P = 0.03), but this was not seen in males. However, integrated AUC salivary insulin levels were significantly higher in children of mothers with PCOS. The mean AUC salivary insulin levels were significantly higher in the daughters of PCOS mothers in the later stages of puberty (Tanner IV–V) when compared with control females in the same pubertal stage (3625 ± 1372 vs. 1766 ± 621 min × μU/ml, 95% confidence interval 475-3242; P < 0.02) (Fig. 4).
Figure 4.
Salivary insulin levels during an OGTT by Tanner stage and group. A, Tanner stage 0–I. B, Tanner stage II–III. C, Tanner stage IV–V, combined male and female data. The mean and se are plotted. There were no significant differences for any of the individual time points.
de Ferranti metabolic syndrome criteria
Overall, five of 15 PCOS children met the de Ferranti metabolic syndrome criteria, whereas one of 12 control children did. For the individual components, abnormal waist circumference, abnormal blood pressure, abnormal triglycerides, abnormal HDL-C, and abnormal fasting blood glucose, we found the following distributions for PCOS children vs. control children: 15 of 32 PCOS children and 13 of 38 control children met abnormal waist criteria; 10 of 32 PCOS children and seven of 28 control children met abnormal blood pressure criteria; five of 15 PCOS children and four of 12 control children met abnormal triglyceride criteria; and 10 of 15 PCOS children and eight of 12 control children met abnormal HDL-C criteria. No PCOS or control children met the criteria for abnormal fasting blood glucose.
Transabdominal ultrasound
Ovaries were difficult to visualize in the premenarchal girls presumably due to the absence of ovarian function and follicular development. In the Tanner 0–I group, we noted both ovaries in only two of nine females from PCOS mothers and zero of 13 from control mothers. In the Tanner II–III group, we noted ovaries in three of five subjects in the PCOS group, and one of four subjects in the control group. In the Tanner IV–V group, we noted ovaries in three of three subjects in the PCOS group and two of four subjects in the control group. There was no evidence of polycystic ovaries based on morphology in any of the subjects. The mean volume of the ovaries (when both right and left ovaries were visualized) was 25.1 cm3 in the PCOS group and 6.6 cm3 in the control group. Five girls in the PCOS group and none in the control group had a total volume from both ovaries more than 10 cm3.
Prevalence of abnormal PCOS phenotypes
We categorically defined the affected status for individual stigmata associated with the syndrome in children of women with PCOS if they had laboratory values that were higher than the mean + 1 sd for the reference Tanner stage and gender control group, though LH and insulin levels are not used in any current diagnostic criteria for adult PCOS. We would expect 16% of PCOS children to be “affected” if there were complete overlap with the control values. We found with increasing prevalence that 25% of PCOS children were affected based on elevated urinary LH levels, 26% affected based on elevated salivary insulin AUC levels, 37% affected based on elevated urinary testosterone levels, and 44% affected based on elevated urinary DHEA levels.
Discussion
Our study provides evidence for abnormalities in reproductive and metabolic parameters in PCOS children, and also insight into the ontogeny of the syndrome. Our study also provides tools (salivary and urinary measures), and a structure (case/control stratified by pubertal stage), for studying changes in reproductive and phenotypical variables associated with adult PCOS in an especially vulnerable pediatric population.
Children of mothers with PCOS, and especially those with associated insulin resistance, may be significantly impacted by the intrauterine environment (27). One group has reported an increased prevalence of small for gestational age infants to mothers with PCOS (28). This would be consistent with reports from randomized trials (9) and a metaanalysis (29) that women with PCOS are prone to complications of pregnancy such as preterm delivery and preeclampsia, which can lead to small for gestational age infants.
We found that the mean birth weights and gestational ages of the PCOS were comparable to control children and within the normal range. We did not validate a maternal report of infant gestational age and birth weight. However, studies have shown high correlations between birth weight by maternal recall and birth weight by birth certificate (30) (31). Of the maternally reported birth weights, 89% were within 1 oz of the birth certificate weight in a large study of 46, 637 Tennessee mothers (30).
We found trends between groups and differences at specific pubertal time points between PCOS children and control children, e.g. lower urinary LH levels in PCOS daughters and higher urinary testosterone levels in midpubertal PCOS boys, but we did not find a distinct reproductive phenotype among PCOS children. This may have been due to the small numbers in our subgroups, the difficulties of phenotyping children, sexually dimorphic effects, and also to the limited heritability of such traits.
We noted, counterintuitively, that urinary LH levels were lower in PCOS girls in the latter stages of puberty. In normal children, prepubertal obesity has been associated with greater circulating levels of androgen, especially free testosterone, in the early pubertal stages, and with associated decreased levels of LH (32,33). Thus, increased peripheral androgen feedback may suppress levels of LH earlier in puberty. It is tempting to speculate that this effect may persist longer throughout puberty in children of women with PCOS, perhaps due to increased intrinsic androgen production by the adrenal and gonad. In fact, PCOS with its persistent anovulation, androgen excess, disordered gonadotropin secretion, and hyperinsulinemia may be viewed as a state of arrested puberty.
Perhaps, the stepwise increasing levels of insulin during pubertal maturation may eventually exert positive feedback on LH release, leading to the adult PCOS gonadotropin phenotype (34). Admittedly, the evidence for this latter phenomenon is weak in humans, though mice with a tissue-specific brain neuron insulin receptor knockout secrete markedly lower levels of LH, implying that insulin is critical to normal (and excess) LH secretion (35).
We intentionally and preferentially recruited younger Tanner stage 0–I children into the study because we were interested in identifying a prepubertal reproductive phenotype. Our results in this earliest pubertal group suggest little difference in androgens and gonadotropin levels, and combined with the results from our salivary insulin levels, point to the emergence of abnormalities in mid to late puberty. Collecting first-morning voids rather than 3-h timed collections may have been a better screening tool for early puberty because the earliest sign of maturation of the reproductive axis is a nocturnal increase in LH pulsatility.
Other studies have tended to study older children and primary female offspring of mothers of women with PCOS with normal levels of circulating androgens compared with a control group. Anti-müllerian hormone has been increased in the daughters of mothers with PCOS (36,37). We did not measure this hormone in our study, and our ultrasound did not detect either increased follicle number, or in the overall cohort increased ovarian volume, which may be viewed as markers of increased follicular activity. However, there was a trend toward larger ovaries in PCOS children in the later pubertal group.
The salivary insulin levels from the glucose tolerance test are the most intriguing results from this study. Advancing puberty, PCOS family history, and female gender combine to exacerbate hyperinsulinemia in comparison to control children. Hyperinsulinemia may be the first and most common abnormality in PCOS children, and appears to be associated with relative metabolic dysfunction by our limited assessment of the metabolic syndrome in these children. The children in both groups, though overweight, did not display frank diabetes or impaired glucose tolerance. Hyperinsulinemia is often triggered by insulin resistance, and it is possible that with a larger sample size or more rigorous tests of insulin sensitivity (e.g. frequently sampled iv glucose tolerance test or clamp), we may have detected insulin resistance in younger children.
Our results are supported by another study of peri-pubertal daughters of women with PCOS who displayed elevated 2-h insulin levels during an OGTT (37). Reproductive abnormalities may be induced by hyperinsulinemia but require either a longer induction period (38) or further reproductive maturation to manifest this disorder. A stronger study design to determine the ontogeny of reproductive and metabolic abnormalities would be a longitudinal study design with repeated measures of the same individual (14), but such studies are expensive and labor intensive. Therefore, we cannot conclude from our cross-sectional study whether hyperinsulinism precedes hyperandrogenism in children from mothers with PCOS, though we suspect it does.
We provide here noninvasive tools for studying reproductive and metabolic function in children. Our salivary assay correlated well with serum measures, especially with fasting levels, and appears to lose association during the later time points of the OGTT, but the 2-h and the integrated levels still display acceptable correlation value. This time lag between salivary and serum measurements has previously been reported (39,40), but our data suggest it may be less in children than adults. However, larger studies and further validation of these measures are needed.
Urinary measures of gonadotropins and androgens have been well validated to diagnose pubertal disorders in children (41,42) and have probably been underused in studies of reproductive maturation (14) or senescence (43). Timed urine collections allow for the integrative assessment of gonadotropin and steroid secretion, and minimize the pulsatility observed with spot checks (though still subject to changes in the menstrual cycle in older girls). Both saliva and urine are easy to collect and involve minimal physical or psychological trauma to a child. They are also acceptable to parents. They also may be incorporated into dynamic testing (for both research and diagnostic purposes), as we demonstrate with glucose challenge testing in our study.
The strengths of our study include the case control design that allows for matching of a variety of confounding historical and biometrical parameters between groups of children, and groups by pubertal maturation. Unfortunately, this design also limits the size within each Tanner stage and gender combination, and our results support both a larger and a longitudinal study to understand better the ontogeny of reproductive and metabolic abnormalities in children of mothers of women with PCOS. These children, based on our study, are likely at increased risk for the sequelae of PCOS compared with the normal population.
Acknowledgments
We thank the study coordinator, Barb Scheetz, for her dedication, the nursing personnel at the General Clinical Research Center at Penn State College of Medicine for its consistent care, Chris Hamilton for his timely and accurate assays provided in the Core Endocrine Laboratory, and Christy Bentley for her statistical programming and figures in the Department of Public Health Sciences. We also thank the mothers and children who volunteered and contributed to this study.
Footnotes
This work was supported by PHS K24 HD01476, U54 HD034449, General Clinical Research Center Grant MO1 RR 10732, and construction Grant C06 RR016499 to Pennsylvania State University.
Disclosure Information: R.S.L. served as a consultant to Glaxo Smith Kline and Ferring, and has been paid lecture fees by Serono, meeting support from Abbott, and grant support from Pfizer. The other authors have nothing to declare.
First Published Online February 12, 2008
For editorial see page 1576
Abbreviations: AUC, Area under the curve; BMI, body mass index; CV, coefficient of variation; DHEA, dehydroepiandrosterone; DHEAS, DHEA sulfate; GCRC, General Clinical Research Center; HDL-C, high-density lipoprotein cholesterol; HOMA IR, homeostatic index of insulin resistance; OGTT, oral glucose tolerance test; PCOS, polycystic ovary syndrome.
References
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004 Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25 [DOI] [PubMed] [Google Scholar]
- Legro RS, Strauss JF 2002 Molecular progress in infertility: polycystic ovary syndrome. Fertil Steril 78:569–576 [DOI] [PubMed] [Google Scholar]
- Legro RS, Driscoll D, Strauss JF, Fox J, Dunaif A 1998 Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA 95:14956–14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A 2002 Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab 87:2134–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam S, Legro RS, Essah PA, Apridonidze T, Dunaif A 2006 Evidence for metabolic and reproductive phenotypes in mothers of women with polycystic ovary syndrome. Proc Natl Acad Sci USA 103:7030–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam S, Legro RS, Bentley-Lewis R, Dunaif A 2005 Dyslipidemia and metabolic syndrome in the sisters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:4797–4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DR, Dombroski B, Urbanek M, Ankener W, Ewens KG, Wood JR, Legro RS, Strauss 3rd JF, Dunaif A, Spielman RS 2006 Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab 91:4112–4117 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL 2007 Identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab 92:787–796 [DOI] [PubMed] [Google Scholar]
- Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP, Coutifaris C, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Giudice LC, Leppert PC, Myers ER, Cooperative Multicenter Reproductive Medicine Network 2007 Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 356:551–566 [DOI] [PubMed] [Google Scholar]
- Tanner JM 1989 Foetus into man: physical growth from conception to maturity. 2nd ed. Ware [Herts]: Castlemead Publications [Google Scholar]
- Travers SH, Jeffers BW, Bloch CA, Hill JO, Eckel RH 1995 Gender and Tanner stage differences in body composition and insulin sensitivity in early pubertal children. J Clin Endocrinol Metab 80:172–178 [DOI] [PubMed] [Google Scholar]
- Zawadski JK, Dunaif A 1992 Diagnostic criteria for polycystic ovary syndrome; towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, eds. Polycystic ovary syndrome. Boston: Blackwell Scientific; 377–384 [Google Scholar]
- 1996 Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics 98:649–658 [PubMed] [Google Scholar]
- Legro RS, Lin HM, Demers LM, Lloyd T 2000 Rapid maturation of the reproductive axis during perimenarche independent of body composition. J Clin Endocrinol Metab 85:1021–1025 [DOI] [PubMed] [Google Scholar]
- Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, Morris DV, Price J, Jacobs HS 1985 Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet 2:1375–1379 [DOI] [PubMed] [Google Scholar]
- Pache TD, Hop WC, Wladimiroff JW, Schipper J, Fauser BC 1991 Transvaginal sonography and abnormal ovarian appearance in menstrual cycle disturbances. Ultrasound Med Biol 17:589–593 [DOI] [PubMed] [Google Scholar]
- Tremblay RR, Dube JY 1974 Plasma concentrations of free and non-TeBG bound testosterone in women on oral contraceptives. Contraception 10:599–605 [DOI] [PubMed] [Google Scholar]
- Dunaif A, Finegood DT 1996 β-Cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab 81:942–947 [DOI] [PubMed] [Google Scholar]
- Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A 2002 Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab 87:2128–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS 1972 Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502 [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dunaif A 2001 Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med 111:607–613 [DOI] [PubMed] [Google Scholar]
- Lloyd T, Schaeffer JM, Walker MA, Demers LM 1991 Urinary hormonal concentrations and spinal bone densities of premenopausal vegetarian and nonvegetarian women. Am J Clin Nutr [Erratum (1992) 56:954] 54:1005–1010 [DOI] [PubMed] [Google Scholar]
- Santner SJ, Santen RJ, Kulin HE, Demers LM 1981 A model for validation of radioimmunoassay kit reagents: measurement of follitropin and lutropin in blood and urine. Clin Chem 27:1892–1895 [PubMed] [Google Scholar]
- de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N 2004 Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation 110:2494–2497 [DOI] [PubMed] [Google Scholar]
- Coviello AD, Legro RS, Dunaif A 2005 Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab 91:492–497 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Cresswell JL, Barker DJ, Osmond C, Egger P, Phillips DI, Fraser RB 1997 Fetal growth, length of gestation, and polycystic ovaries in adult life. Lancet 350:1131–1135 [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburú B, Gazitúa R, Recabarren S, Cassorla F 2005 Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod 20:2122–2126 [DOI] [PubMed] [Google Scholar]
- Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS 2006 A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 12:73–683 [DOI] [PubMed] [Google Scholar]
- Gayle HD, Yip R, Frank MJ, Nieburg P, Binkin NJ 1988 Validation of maternally reported birth weights among 46,637 Tennessee WIC program participants. Public Health Rep 103:143–147 [PMC free article] [PubMed] [Google Scholar]
- Catov JM, Newman AB, Kelsey SF, Roberts JM, Sutton-Tyrrell KC, Garcia M, Ayonayon HN, Tylavsky F, Ness RB 2006 Accuracy and reliability of maternal recall of infant birth weight among older women. Ann Epidemiol 16:429–431 [DOI] [PubMed] [Google Scholar]
- McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC 2006 The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 91:1714–1722 [DOI] [PubMed] [Google Scholar]
- McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC 2007 Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 92:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F 1976 Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest 57:1320–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR 2000 Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Codner E, Maliqueo M, Echiburú B, Hitschfeld C, Crisosto N, Pérez-Bravo F, Recabarren SE, Cassorla F 2006 Increased anti-Müllerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 91:3105–3109 [DOI] [PubMed] [Google Scholar]
- Crisosto N, Codner E, Maliqueo M, Echiburú B, Sánchez F, Cassorla F, Sir-Petermann T 2007 Anti-Müllerian hormone levels in peripubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 92:2739–2743 [DOI] [PubMed] [Google Scholar]
- Mehta RV, Patel KS, Coffler MS, Dahan MH, Yoo RY, Archer JS, Malcom PJ, Chang RJ 2005 Luteinizing hormone secretion is not influenced by insulin infusion in women with polycystic ovary syndrome despite improved insulin sensitivity during pioglitazone treatment. J Clin Endocrinol Metab 90:2136–2141 [DOI] [PubMed] [Google Scholar]
- Marchetti P, Benzi L, Masoni A, Cecchetti P, Giannarelli R, Di Cianni G, Ciccarone AM, Navalesi R 1986 Salivary insulin concentrations in type 2 (non-insulin-dependent) diabetic patients and obese non-diabetic subjects: relationship to changes in plasma insulin levels after an oral glucose load. Diabetologia 29:695–698 [DOI] [PubMed] [Google Scholar]
- Pasic J, Pickup JC 1988 Salivary insulin in normal and type I diabetic subjects. Diabetes Care 11:489–494 [DOI] [PubMed] [Google Scholar]
- Kulin HE, Bell PM, Santen RJ, Ferber AJ 1975 Integration of pulsatile gonadotropin secretion by timed urinary measurements: an accurate and sensitive 3-hour test. J Clin Endocrinol Metab 40:783–789 [DOI] [PubMed] [Google Scholar]
- Witchel SF, Baens-Bailon RG, Lee PA 1996 Treatment of central precocious puberty: comparison of urinary gonadotropin excretion and gonadotropin-releasing hormone (GnRH) stimulation tests in monitoring GnRH analog therapy. J Clin Endocrinol Metab 81:1353–1356 [DOI] [PubMed] [Google Scholar]
- Santoro N, Lasley B, McConnell D, Allsworth JE, Finkelstein JS, Gold EB, Korenman S, Lasley WL, Luborsky JL, McConnell D, Sowers MF, Weiss G 2004 Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women’s Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab 89:2622–2631 [DOI] [PubMed] [Google Scholar]
- Sam S, Sung YA, Legro RS, Dunait A2008 Evidence for pancreatic B-cell dysfunction in brothers of women with polycystic ovary syndrome. Metabolism 57:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]