Abstract
Context: In women with polycystic ovary syndrome (PCOS), excess ovarian androgen production is driven by increased LH secretion. Studies conducted in animals suggest that the granulosa cell may influence LH-stimulated theca cell androgen production.
Objective: The objective of this study was to determine whether FSH enhances androgen production in women with PCOS compared with that of normal women.
Design: A prospective study was conducted to compare androgen production in response to FSH in two groups of women.
Setting: The study was conducted in a General Clinical Research Center in a tertiary academic medical center.
Patients: Women with PCOS, 18–35 yr (n = 20), and normal ovulatory controls, 18–35 yr (n = 10), were recruited for study.
Interventions: Serial blood samples were obtained over a 24-h period after an iv injection of recombinant human FSH (150 IU).
Main Outcome Measures: The main outcome measures were serum 17-hydroxyprogesterone (17-OHP), androstenedione (A), dehydroepiandrosterone (DHEA), testosterone (T), and inhibin B (Inh B) responses after FSH administration.
Results: Basal serum 17-OHP, A, and T levels were markedly increased in women with PCOS compared with that observed in normal women. Basal DHEA and Inh B levels were similar to those of normal controls. After FSH injection, PCOS women demonstrated enhanced production of 17-OHP, A, DHEA, and Inh B, whereas in normal women no increases were observed. T levels declined slightly in both groups.
Conclusions: These findings provide evidence that, in PCOS women, theca cell androgen production is enhanced by FSH administration and suggest a granulosa-theca cell paracrine mechanism.
In women with polycystic ovary syndrome serum, androgen levels increase following FSH stimulation. These androgen responses were associated with corresponding increases of circulating inhibin B, suggesting that excess ovarian androgen production in polycystic ovary syndrome is partially regulated by granulosa-theca cell interaction involving an intrafollicular paracrine mechanism.
Excessive ovarian androgen production is a major pathophysiological feature of polycystic ovary syndrome (PCOS). The basis for androgen overproduction has been attributed to altered theca cell responsiveness to gonadotropin stimulation in association with increased pituitary LH secretion in women with this disorder (1,2,3). In particular, hyperandrogenemic women with PCOS have exhibited exaggerated 17-hydroxyprogesterone (17-OHP) responses to GnRH agonist compared with those observed in normal women (4). These results are consistent with in vitro studies that have demonstrated substantially greater androgen production by cultured PCOS theca cells exposed to LH than amounts produced by normal theca cells similarly treated (5). However, the range of androgen responses to gonadotropin stimulation among individual PCOS women is variable and not necessarily dependent on circulating LH levels, which suggests that other factors may contribute to hyperandrogenemia in this disorder (4,6,7,8).
Exclusive of the direct LH-theca cell stimulus-response, ovarian androgen production may also be subject to paracrine regulation by factors derived from granulosa cells based on previous studies in animals. Early reports suggested an interaction between these adjacent cellular components because reduction of androgen production was observed after removal of granulosa cells from theca tissue cultures (9,10). Subsequently, it was shown that ovine theca cells coincubated with conditioned media from FSH-stimulated granulosa cell cultures produced significantly more LH-induced androgen than theca cells incubated with untreated media (11). In a later study, it was shown that LH-stimulated androgen production from cultured rat theca cells of animals pretreated with FSH was substantially greater than that produced by theca cells of animals treated with vehicle (12). These findings strongly suggested the existence of granulosa cell mediators that participate in LH-induced androgen production.
As to whether this paracrine relationship is clinically evident in women has not been examined. In particular, does FSH stimulate androgen production in normal women, and is the process altered in women with PCOS? To address this issue, basal and 24-h responses of ovarian androgens after acute FSH stimulation were examined in normal and PCOS women.
Subjects and Methods
Subjects
There were 20 women with PCOS and 10 normal women with regular menstrual cycles recruited for study. All of the PCOS subjects were either oligomenorrheic or amenorrheic, and all exhibited clinical and biochemical evidence of hyperandrogenism. Each PCOS subject exhibited ultrasound evidence of bilaterally enlarged polycystic ovaries and greater than 12 follicles per ovary. The mean ages of the PCOS and normal group, 26.4 ± 1.0 and 28.4 ± 1.9 yr, respectively, were not significantly different. The mean body mass index (BMI) in PCOS subjects, 34.8 ± 1.9 kg/m2, was significantly greater (P < 0.05) than that of normal controls, 28.9 ± 1.6 kg/m2. Late-onset congenital adrenal hyperplasia was excluded by a serum 17-OHP level of less than 3 ng/ml. Circulating TSH and prolactin levels were normal and not significantly different between groups. None of the subjects in either group had received any hormone medication for at least 3 months before study. The study had been approved by the Human Research Protection Program at the University of California, San Diego, and written informed consent was obtained from each participant before study.
Procedures
Each subject was admitted to the General Clinical Research Center at the University of California, San Diego, on the day of testing. Normal subjects were studied during the midfollicular phase (d 5–8), whereas PCOS women were anovulatory and studied on a random day. Between 0800 and 1000 h, after baseline blood samples were obtained, an iv bolus of recombinant human FSH (r-hFSH) was administered at a dose of 150 IU. The r-hFSH (Gonal-F) was kindly provided by EMD Serono, Inc. (Rockland, MA). None of the PCOS subjects had experienced recent ovulation, as evidenced by a lack of menstrual bleeding for 2 months before study and serum progesterone (P4) levels of less than 1 ng/ml at the baseline sample.
An initial pilot study was conducted on seven PCOS and seven normal controls to determine the time course of androgen response. The frequency of blood sampling in the pilot study was 0, +2, +8, +16, and +24 h. For all androgens there was a gradual and progressive increase that achieved maximum values at 24 h (data not shown). Based on these results, it was determined that sampling could be performed at baseline with a repeat blood sample obtained 24 h later. Therefore, in the remaining 13 PCOS women and three normal controls, blood samples were drawn before and 24 h after the r-hFSH administration. Samples were allowed to clot, and sera were separated by centrifugation and stored at −20 C until assayed. Individual serum samples were analyzed in the same assay in duplicate.
Assays
Serum concentrations of LH and FSH were measured by RIA with intraassay and interassay coefficients of variation (CVs) of 5.4 and 8.0%, respectively, for LH, and 3.0 and 4.6%, respectively, for FSH (Diagnostic Products Corp., Los Angeles, CA). Serum concentrations of estrone (E1), estradiol (E2), androstenedione (A), testosterone (T), and dehydroepiandrosterone (DHEA) were measured by well-established RIA with an intraassay CV less than 7%. Serum P4, 17-OHP, and dehydroepiandrosterone sulfate (DHEAS) levels were measured by RIA with an intraassay CV less than 7% (Diagnostic Systems Laboratories, Inc., Webster, TX). Serum concentrations of cortisol were measured by RIA with intraassay and interassay CVs of 5.3 and 7.5%, respectively (MP Biomedical, LLC, Orangeburg, NY). Serum concentrations of inhibin B (Inh B) were measured by ELISA with interassay and intraassay CVs of 6.7 and 4.6%, respectively (Diagnostic Systems Laboratories). The highly specific two-site ELISA Kit allows for quantitative measurement of dimeric Inh B in human serum. Assay sensitivity for Inh B was 7.0 pg/ml. Serum insulin levels were measured by a double-antibody RIA with an assay sensitivity of 2 μU/ml, and intraassay and interassay CVs of 7 and 9%, respectively.
Statistical analysis
Baseline hormone values between PCOS and normal women were compared by group t tests using SPSS software (SPSS, Inc., Chicago, IL). Where applicable, significance testing was two sided at a 5% significance level. To control for the effect of the confounding variables of BMI and insulin on the difference in stimulated androgen levels between PCOS and controls, analysis of covariance was performed.
Results
Baseline studies
Baseline hormone values are shown in Table 1. In PCOS women, mean (± se) circulating levels of LH, A, T, E1, and insulin were significantly greater than those of normal controls. Basal levels of serum FSH, 17-OHP, DHEA, DHEAS, cortisol, E2, P4, and Inh B were similar in both groups.
Table 1.
Mean endocrine-metabolic values (± se) of PCOS and normal women
| PCOS (n = 20) | Normal women (n = 10) | |
|---|---|---|
| LH (mIU/ml) | 6.1 ± 0.5a | 2.7 ± 0.2 |
| FSH (mIU/ml) | 4.6 ± 0.2 | 4.8 ± 0.4 |
| T (ng/ml) | 0.6 ± 0.1a | 0.3 ± 0.1 |
| A (ng/ml) | 1.5 ± 0.1a | 0.8 ± 0.1 |
| 17-OHP (ng/ml) | 0.3 ± 0.04 | 0.2 ± 0.1 |
| DHEA (ng/ml) | 5.6 ± 0.7 | 4.3 ± 0.8 |
| DHEAS (ng/ml) | 330 ± 21 | 194 ± 13 |
| E1 (pg/ml) | 85 ± 7a | 47 ± 6 |
| E2 (pg/ml) | 58 ± 4 | 64 ± 10 |
| P4 (ng/ml) | 0.3 ± 0.03 | 0.2 ± 0.02 |
| Cortisol (mg/dl) | 19.4 ± 3.0 | 16.7 ± 3.2 |
| Inh B (pg/ml) | 126 ± 12 | 136 ± 21 |
| Insulin (μU/ml) | 33 ± 6a | 18 ± 3 |
To convert to the International System of Units, multiply by the following conversion factor: T (3.47), A (3.49), 17-OHP (3.03), DHEA (3.47), DHEAS (0.0027), E1 (3.69), E2 (3.67), P4 (3.18), cortisol (27.59), Inh B (0.00003125), and insulin (7.18).
P < 0.05.
Serum androgen responses to r-hFSH administration
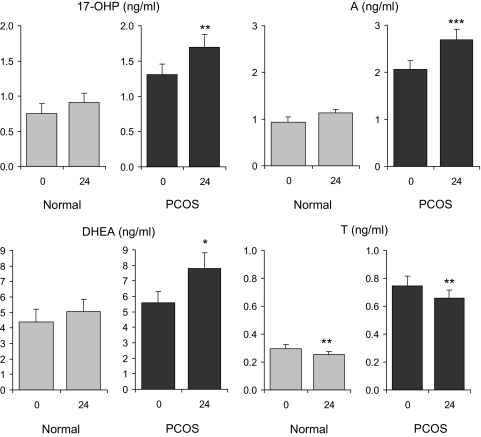
As seen in Fig. 1, administration of r-hFSH to women with PCOS resulted in significant increases of serum 17-OHP (1.3 ± 0.2 to 1.7 ± 0.2 ng/ml; P < 0.02), A (2.0 ± 0.2 to 2.7 ± 0.2 ng/ml; P < 0.001), and DHEA (5.6 ± 0.7 to 7.8 ± 1.0 ng/ml; P < 0.05). In contrast, a significant decline in circulating T (0.75 ± 0.07 to 0.66 ± 0.06 ng/ml; P < 0.02) was observed after r-hFSH injection. Among normal women, stimulated 24-h values for 17-OHP, A, and DHEA were not different from those observed at baseline. However, a significant decrement in serum T was noted, (0.30 ± 0.03 to 0.26 ± 0.02 ng/ml; P < 0.02), similar to the response pattern in PCOS women.
Figure 1.
Mean (± se) serum androgen levels before and 24 h after administration of r-hFSH (150 IU) in PCOS and normal women. A significant change from baseline is denoted by an asterisk. *, P < 0.05. **, P < 0.02. ***, P < 0.001.
Because androgen responses in PCOS women may have reflected the tendency of higher baseline values, we calculated the incremental and percent changes in both groups as seen in Table 2. In women with PCOS, the absolute and percent increases of serum 17-OHP, A, and DHEA after FSH were greater than those of normal women, however, this difference did not achieve statistical significance with the exception of an incremental change in serum A. Serum T responses to FSH were similar between groups.
Table 2.
Mean absolute and percent changes in androgen responses compared with baseline in PCOS and normal women
| Androgen | Normal women
|
PCOS
|
||
|---|---|---|---|---|
| Absolute change | Percent change | Absolute change | Percent change | |
| T (ng/ml) | −0.04 ± 0.01 | −13.95 ± 4.47 | −0.09 ± 0.03 | −11.85 ± 3.74 |
| A (ng/ml) | 0.20 ± 0.09 | 21.22 ± 11.30 | 0.63 ± 0.13a | 30.75 ± 6.61 |
| 17-OHP (ng/ml) | 0.16 ± 0.08 | 20.98 ± 20.80 | 0.39 ± 0.14 | 29.98 ± 10.34 |
| DHEA (ng/ml) | 0.70 ± 0.95 | 15.87 ± 33.35 | 2.20 ± 1.05 | 39.31 ± 16.8 |
P < 0.05.
To determine if the difference in androgen responses between the PCOS and normal women was influenced by potentially confounding variables such as BMI and insulin, an analysis of covariance was performed. The analysis revealed that maximally stimulated androgen responses were not different between groups, which suggests that BMI and insulin may have contributed to increased theca cell responsiveness in PCOS women.
Serum E2, cortisol, Inh B, and LH responses to r-hFSH administration
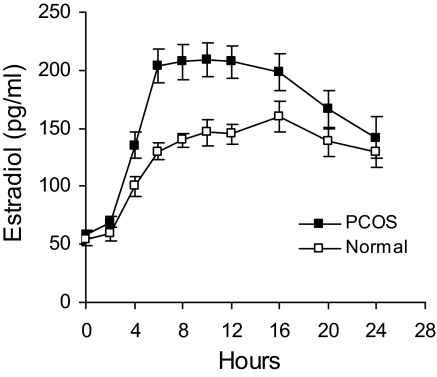
Serum E2 responses to FSH in PCOS and normal women are shown in Fig. 2. Increases in E2 were noted 2 h after FSH injection in both groups. In PCOS, maximal E2 levels occurred at 6 h and were maintained for an additional 6 h before a gradual decline of approximately 50%. In normal women the magnitude of E2 response was considerably less than that of PCOS women, and a decrease after peak levels was not observed. The data are consistent with previously published results from our laboratory (13).
Figure 2.
Mean (± se) serum E2 levels after administration of r-hFSH (150 IU) in PCOS and normal women.
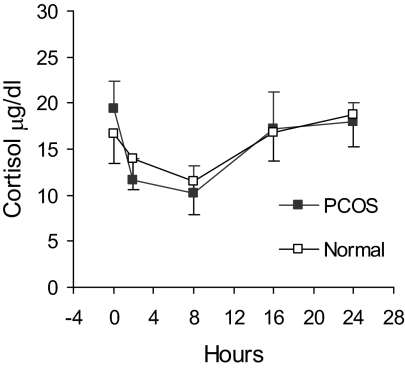
In both PCOS and normal individuals, serum cortisol responses after r-hFSH were indistinguishable because 24-h secretion patterns were essentially identical (Fig. 3). A nadir in cortisol secretion was apparent 8 h after r-hFSH, which coincided with the late afternoon and reflected normal diurnal variation.
Figure 3.
Mean (± se) serum cortisol levels after administration of r-hFSH (150 IU) in PCOS and normal women.
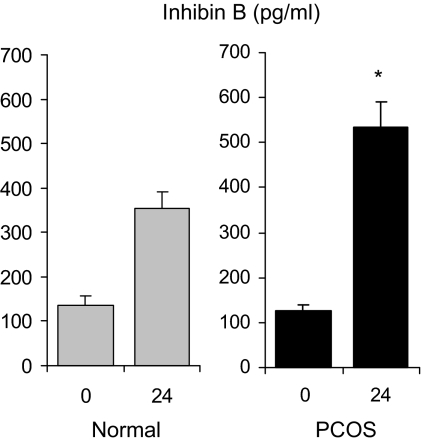
In 19 PCOS women and seven normal women, Inh B responses to FSH showed a progressive increase to achieve maximal concentrations at 16 h that were markedly greater compared with baseline values in both groups (data not shown), as reported previously (14). At 24 h, mean stimulated Inh B values in PCOS women were significantly greater (P = 0.01) than that observed in normal controls (Fig. 4).
Figure 4.
Mean (± se) serum Inh B levels before and 24 h after administration of r-hFSH (150 IU) in PCOS and normal women. *, P value for between-group comparison less than 0.001.
In seven PCOS and seven normal women, serum LH levels were assessed before, and at 2, 8, 16, and 24 h after FSH administration. Baseline LH levels were unaltered in both groups of women (data not shown).
Discussion
The results of this study have demonstrated that women with PCOS exhibit significant changes in ovarian androgen production in response to an acute injection of FSH. Notably, increases in circulating levels of 17-OHP, A, and DHEA were observed, whereas serum T levels declined after FSH. By comparison, in normal women, FSH administration failed to produce increases in serum androgen levels with the exception of T, which was reduced. Despite increased baseline androgen values in PCOS women, the absolute increments and percent changes of A, 17-OHP, and DHEA were greater than those observed for normal controls, which is consistent with a heightened responsiveness to FSH in these individuals. These findings suggest that theca cell androgen production in women with PCOS may be, in part, regulated by granulosa cells.
In women with PCOS, the relative increases in circulating androgens after FSH are consistent with previous studies that have shown that granulosa cells may contribute to theca cell androgen production. Both in vitro and in vivo studies performed in animals have indicated that FSH may amplify LH-induced ovarian androgen production (9,10,11,12). Similar studies have not been conducted on human ovarian tissues, although granulosa cell-derived proteins have been shown to enhance LH-mediated androgen production, which suggests a mechanism for FSH-stimulated androgen production. For instance, in cultured human ovarian theca cells, A responses to LH in the presence of inhibin were clearly increased compared with those observed in the absence of inhibin (15,16). In addition, inhibin, in a dose-dependent manner, was able to negate the inhibitory effect of activin (Act) on human theca cell androgen production. It has been well documented in cultured granulosa cells that inhibin production is stimulated by FSH (17,18). Accordingly, in the current study, the significant increases in ovarian androgens exhibited by PCOS women were accompanied by similar significant increments in FSH-stimulated Inh B levels compared with those of normal women.
Identification of inhibin as a paracrine mediator is underscored by the observation that addition of antiinhibin antibody to individual follicle cell cultures treated with FSH completely abrogated estrogen synthesis (19). Administration of exogenous inhibin to these cultures overcame the antibody blockade and restored estrogen production. Moreover, treatment with A in the presence of antiinhibin antibody also resulted in greater estrogen production, which suggested a consequence of increased androgen substrate rather than amplification of aromatase expression by inhibin.
A direct effect of Inh B on theca cell androgen production is unlikely because a receptor for this protein has not been identified. Rather, it has been shown that Inh B binds to membrane bound binding protein, β-glycan, to form a complex that has high affinity for Act type II receptors (20). The sequestration of Act type II receptors prevents formation of the Act type II/type I receptor complex required for Act signaling and eventual inhibition of CYP17 within the theca cell. These findings provide a possible mechanism for FSH-stimulated androgen production in PCOS women. However, documentation of this interaction in human ovarian tissue and whether inhibin/Act signaling is altered in theca cells of PCOS women has not been studied.
If the enhanced production of androgen by inhibin involves blockade of Act signaling, then consideration of other factors that use type II Act receptors is warranted. In particular, some bone morphogenetic proteins (BMPs) bind to not only BMP type II receptors, but also Act type II receptors to initiate their divergent actions (21). It has been reported that BMP-4 inhibits forskolin-stimulated CYP17 mRNA expression and androgen production in a human ovarian theca-like cell culture model (22). Similarly, BMP-4, -6, and -7 suppressed CYP17 mRNA, and exhibited a dose-dependent reduction of androgen production in bovine theca cells (23). Whether the inhibitory effect of BMPs on androgen production involves BMP or Act type II receptors is unknown, however, inhibin may act to antagonize BMP signaling through binding to β-glycan (24).
In addition to inhibin, IGF-I and -II also may act to influence theca cell androgen production (25,26,27). In vitro, IGF-I has synergistically augmented LH-stimulated androgen production from cultured theca cells (15,30). This facilitative action of IGF-I has been attributed to increased LH receptor induction (31), greater expression of steroidogenic enzymes (32,33), and enhanced LH-induced cAMP production (34). However, because IGF-I mRNA and protein are not expressed in human granulosa cells, it is likely that IGF-II is the principle paracrine influence of these two proteins. In human theca cell tissue, IGF-II stimulated basal androgen release as well as enhanced LH-induced androgen production, the magnitude of which was equivalent to that of IGF-I (26). Of significant note, in the same study, these stimulatory effects of IGF-I and IGF-II on theca cell androgen production were greatly enhanced by inhibin, which suggests a predominant role for this protein among potential granulosa cell mediators.
Beyond the likelihood of an intrafollicular paracrine mechanism to explain our findings, an effect of increased mean BMI in PCOS women accompanied by hyperinsulinemia may have also contributed to greater FSH-induced androgen responses compared with those of the normal group. It has been reported that BMI is positively correlated with serum androgen levels in women with PCOS and that this relationship is enhanced in the presence of increased insulin secretion (28,29). In vitro studies have demonstrated that LH-stimulated androgen production by human theca cells is increased with the addition of insulin to the culture (25,26). Thus, the presence of obesity and hyperinsulinemia in our PCOS subjects could have, at least in part, accounted for increased androgen responsiveness to FSH.
The possibility of a direct effect of FSH on theca cells is tempered by previous observations that the FSH receptor has been demonstrated only in granulosa cells. Recent studies have shown that FSH receptor expression is absent in primordial follicles and may be identified as early as the primary stage of development (35,36). However, at this stage not all follicles were positive, and receptor expression was highly variable. Beyond the two-layered primary follicle, evidence for the FSH receptor was uniformly present. Curiously, efforts to determine whether FSH receptor mRNA is expressed in theca cells of PCO ovaries has not been reported.
There is a paucity of clinical studies designed to examine granulosa-theca cell interaction in normal or PCOS women. In a case report of a woman with an inactivating mutation in the FSH β-subunit gene resulting in undetectable serum FSH and elevated serum LH concentrations, it was observed that human chorionic gonadotropin administration stimulated only modest increases in circulating androgen levels (37). After pretreatment with FSH, repeat human chorionic gonadotropin injection 24 h later was associated with 2- to 4-fold increments in serum 17-OHP, A, T, and DHEA. These findings are, in part, consistent with those of the current study and, together, provide evidence to suggest granulosa cell amplification of theca cell androgen production. In the aforementioned case report, there was a pronounced effect of FSH on Inh B levels because the baseline concentration increased nearly 8-fold after 24 h (38). By comparison, inhibin A levels were low and failed to show any change at 24 h. We have previously reported acute 24-h patterns of Inh B release after FSH injection in normal women and women with PCOS (14). In PCOS women the magnitude of response was considerably greater than that observed in normal women, as reported here. These results support an intrafollicular mechanism that contributes to ovarian androgen excess in women with PCOS. However, further studies are necessary to delineate the precise involvement of granulosa cells in theca cell androgen production in PCOS as well as normal women.
Notable among androgen responses to FSH in both normal and PCOS women was a decline in serum T levels. It is difficult to account for this subtle, but significant decrement in circulating T, particularly in women with PCOS in light of the increases noted for the other measured androgens. One consideration may be rapid activation of aromatase resulting in depletion of T substrate because marked increases of serum E2 over 24 h have been reported in PCOS women receiving a similar dose of FSH (38). However, in our PCOS subjects, a corresponding decrease in A responsiveness to FSH was not observed, thereby lessening the likelihood of aromatase activation. In PCOS women the significant increase of DHEA after FSH probably reflected theca cell androgen output as a consequence of granulosa cell stimulation because FSH receptors are unique to the granulosa cell. This notion is supported by in vitro studies in which LH-induced DHEA production from theca cell tissue culture was increased in the presence of inhibin (15). By comparison, in adrenal tissue of ovariectomized sheep, immunoreactive inhibin expression was found to decrease commensurate with increases in serum FSH, whereas inhibin protein was not detected in the adrenal glands of control animals with intact ovaries (39). Neither ACTH stimulation nor dexamethasone suppression altered the circulating levels of immunoreactive inhibin expression in ovariectomized sheep, which suggests that inhibin does not contribute to adrenal steroidogenesis. However, recently it has been reported that inhibin and Act subunit expression as well as their receptor mRNA are present in rat and human adrenocortical tissue (40). Moreover, in rat adrenocortical cells, inhibin and Act increased and decreased the CYP17 expression, respectively (41). Thus, a role for inhibin on adrenal steroidogenesis may exist, although the extent of effect is unknown. Our results showed that adrenal corticosteroid production was normal because basal and circadian cortisol secretion was comparable between groups.
The relative disparity and lack of androgen responsiveness to FSH stimulation in normal women could be attributed to increased granulosa cell sensitivity in women with PCOS. Our previous in vitro studies have documented that granulosa cells from PCOS women exhibit an 8-fold increase in sensitivity to FSH administration, which was consistent with later clinical dose-response studies that revealed greater peak responses to the highest dose of FSH in PCOS women compared with those observed in normal women (13,42). This enhanced sensitivity may relate to the reported increase of FSH binding in granulosa cells obtained from antral follicles of PCOS women compared with that of normal antral follicles (43). Thus, increased FSH-induced Inh B and androgen production in PCOS women reflects greater granulosa cell sensitivity in these women.
In summary, we have determined that serum androgen levels are significantly increased in PCOS women after FSH stimulation. In addition, increased androgen responses were accompanied by corresponding increments in circulating Inh B. These results suggest that excess ovarian androgen production in PCOS is, at least in part, regulated by granulosa-theca cell interaction involving an intrafollicular paracrine mechanism. Further investigation is necessary to characterize and delineate the precise nature of this relationship in PCOS women.
Acknowledgments
We thank Mr. Jeff Wong for his technical expertise, Andi Hartgrove for her secretarial assistance, and the nurses and staff of the University of California, San Diego, General Clinical Research Center for their dedicated care. We also thank Dr. Greg F. Erickson who reviewed the manuscript for his helpful suggestions.
Footnotes
This work was supported by the National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement (U54 HD 12303-20) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, by National Institutes of Health Grant MO1 RR00827, and by private funding from the Halopoff Family Trust.
Disclosure Statement: The authors have nothing to disclose.
First Published Online February 19, 2008
Abbreviations: A, Androstenedione; Act, activin; BMI, body mass index; BMP, bone morphogenetic protein; CV, coefficient of variation; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; E1, estrone; E2, estradiol; Inh B, inhibin B; 17-OHP, 17-hydroxyprogesterone; P4, progesterone; PCOS, polycystic ovary syndrome; r-hFSH, recombinant human FSH; T, testosterone.
References
- Yen SS 1980 The polycystic ovary syndrome. Clin Endocrinol (Oxf) 12:177–207 [DOI] [PubMed] [Google Scholar]
- Franks S 1995 Polycystic ovary syndrome. N Engl J Med 333:853–861 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA 2005 Polycystic ovary syndrome. N Engl J Med 352:1223–1236 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Barnes RB, Ehrmann DA 1994 Studies of the nature of 17-hydroxyprogesterone hyperresponsiveness to gonadotropin-releasing hormone agonist challenge in functional ovarian hyperandrogenism. J Clin Endocrinol Metab 79:1686–1692 [DOI] [PubMed] [Google Scholar]
- Gilling-Smith C, Willis DS, Beard RW, Franks S 1994 Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab 79:1158–1165 [DOI] [PubMed] [Google Scholar]
- Gilling-Smith C, Story H, Rogers V, Franks S 1997 Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol (Oxf) 47:93–99 [DOI] [PubMed] [Google Scholar]
- Ibanez L, Potau N, Zampolli M, Prat N, Gussinye M, Saenger P, Vicens-Calvet E, Carrascosa A 1994 Source localization of androgen excess in adolescent girls. J Clin Endocrinol Metab 79:1778–1784 [DOI] [PubMed] [Google Scholar]
- Chang PL, Lindheim SR, Lowre C, Ferin M, Gonzalez F, Berglund L, Carmina E, Sauer MV, Lobo RA 2000 Normal ovulatory women with polycystic ovaries have hyperandrogenic pituitary-ovarian responses to gonadotropin-releasing hormone-agonist testing. J Clin Endocrinol Metab 85:995–1000 [DOI] [PubMed] [Google Scholar]
- Fortune JE, Armstrong DT 1977 Androgen production by theca and granulosa isolated from proestrous rat follicles. Endocrinology 100:1341–1347 [DOI] [PubMed] [Google Scholar]
- Moor RM 1977 Sites of steroid production in ovine graafian follicles in culture. J Endocrinol 73:143–150 [DOI] [PubMed] [Google Scholar]
- Lischinsky A, Armstrong DT 1983 Granulosa cell stimulation of thecal androgen synthesis. Can J Physiol Pharmacol 61:472–477 [DOI] [PubMed] [Google Scholar]
- Smyth CD, Miro F, Whitelaw PF, Howles CM, Hillier SG 1993 Ovarian thecal/interstitial androgen synthesis is enhanced by a follicle-stimulating hormone-stimulated paracrine mechanism. Endocrinology 133:1532–1538 [DOI] [PubMed] [Google Scholar]
- Coffler MS, Patel KS, Dahan MH, Malcom PJ, Kawashima T, Deutsch R, Chang RJ 2003 Evidence for abnormal granulosa cell responsiveness to follicle-stimulating hormone in women with polycystic ovary syndrome. J Clin Endocrinol Metab 88:1742–1747 [DOI] [PubMed] [Google Scholar]
- Wachs DS, Coffler MS, Malcom PJ, Chang RJ 2006 Comparison of follicle-stimulating-hormone-stimulated dimeric inhibin and estradiol responses as indicators of granulosa cell function in polycystic ovary syndrome and normal women. J Clin Endocrinol Metab 91:2920–2925 [DOI] [PubMed] [Google Scholar]
- Hillier SG, Yong EL, Illingworth PJ, Baird DT, Schwall RH, Mason AJ 1991 Effect of recombinant inhibin on androgen synthesis in cultured human thecal cells. Mol Cell Endocrinol [Erratum (1991) 79:177] 75:R1–R6 [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Dahl KD, Vaughan J, Tucker E, Rivier J, Bardin CW, Vale W 1987 Heterodimers and homodimers of inhibin subunits have different paracrine action in the modulation of luteinizing hormone-stimulated androgen biosynthesis. Proc Natl Acad Sci USA 84:5082–5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier SG, Wickings EJ, Illingworth PI, Yong EL, Reichert LEJ, Baird DT, McNeilly AS 1991 Control of immunoactive inhibin production by human granulosa cells. Clin Endocrinol (Oxf) 35:71–78 [DOI] [PubMed] [Google Scholar]
- Bicsak TA, Tucker EM, Cappel S, Vaughan J, Rivier J, Vale W, Hsueh AJ 1986 Hormonal regulation of granulosa cell inhibin biosynthesis. Endocrinology 119:2711–2719 [DOI] [PubMed] [Google Scholar]
- Smyth CD, Gosden RG, McNeilly AS, Hillier SG 1994 Effect of inhibin immunoneutralization on steroidogenesis in rat ovarian follicles in vitro. J Endocrinol 140:437–443 [DOI] [PubMed] [Google Scholar]
- Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W 2000 Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature 404:411–414 [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF 2004 The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 25:72–101 [DOI] [PubMed] [Google Scholar]
- Dooley CA, Attia GR, Rainey WE, Moore DR, Carr BR 2000 Bone morphogenetic protein inhibits ovarian androgen production. J Clin Endocrinol Metab 85:3331–3337 [DOI] [PubMed] [Google Scholar]
- Glister C, Richards SL, Knight PG 2005 Bone morphogenetic proteins (BMP) -4, -6, and -7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology 146:1883–1892 [DOI] [PubMed] [Google Scholar]
- Wiater E, Vale W 2003 Inhibin is an antagonist of bone morphogenetic protein signaling. J Biol Chem 278:7934–7941 [DOI] [PubMed] [Google Scholar]
- Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjo T 1993 Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril 59:323–331 [DOI] [PubMed] [Google Scholar]
- Nahum R, Thong KJ, Hillier SG 1995 Metabolic regulation of androgen production by human thecal cells in vitro. Hum Reprod 10:75–81 [DOI] [PubMed] [Google Scholar]
- Cara JF, Rosenfield RL 1988 Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology 123:733–739 [DOI] [PubMed] [Google Scholar]
- Holte J, Bergh T, Gennarelli G, Wide L 1994 The independent effects of polycystic ovary syndrome and obesity on serum concentrations of gonadotrophins and sex steroids in premenopausal women. Clin Endocrinol (Oxf) 41:473–481 [DOI] [PubMed] [Google Scholar]
- Ciampelli M, Fulghesu AM, Cucinelli F, Pavone V, Ronsisvalle E, Guido M, Caruso A, Lanzone A 1999 Impact of insulin and body mass index on metabolic and endocrine variables in polycystic ovary syndrome. Metabolism 48:167–172 [DOI] [PubMed] [Google Scholar]
- Magoffin DA, Weitsman SR 1993 Differentiation of ovarian theca-interstitial cells in vitro: regulation of 17 α-hydroxylase messenger ribonucleic acid expression by luteinizing hormone and insulin-like growth factor-I. Endocrinology 132:1945–1951 [DOI] [PubMed] [Google Scholar]
- Adashi EY, Resnick CE, Svoboda ME, Van Wyk JJ 1985 Somatomedin-C enhances induction of luteinizing hormone receptors by follicle-stimulating hormone in cultured rat granulosa cells. Endocrinology 116:2369–2375 [DOI] [PubMed] [Google Scholar]
- Magoffin DA, Weitsman SR 1993 Insulin-like growth factor-I stimulates the expression of 3 beta-hydroxysteroid dehydrogenase messenger ribonucleic acid in ovarian theca-interstitial cells. Biol Reprod 48:1166–1173 [DOI] [PubMed] [Google Scholar]
- Magoffin DA, Weitsman SR 1993 Effect of insulin-like growth factor-I on cholesterol side-chain cleavage cytochrome P450 messenger ribonucleic acid expression in ovarian theca-interstitial cells stimulated to differentiate in vitro. Mol Cell Endocrinol 96:45–51 [DOI] [PubMed] [Google Scholar]
- Magoffin DA, Weitsman SR 1994 Insulin-like growth factor-I regulation of luteinizing hormone (LH) receptor messenger ribonucleic acid expression and LH-stimulated signal transduction in rat ovarian theca-interstitial cells. Biol Reprod 51:766–775 [DOI] [PubMed] [Google Scholar]
- Oktay K, Briggs D, Gosden RG 1997 Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab 82:3748–3751 [DOI] [PubMed] [Google Scholar]
- Rice S, Ojha K, Whitehead S, Mason H 2007 Stage-specific expression of androgen receptor, follicle-stimulating hormone receptor, and anti-Müllerian hormone type II receptor in single, isolated, human preantral follicles: relevance to polycystic ovaries. J Clin Endocrinol Metab 92:1034–1040 [DOI] [PubMed] [Google Scholar]
- Barnes RB, Rosenfield RL, Namnoum A, Layman LC 2000 Effect of follicle-stimulating hormone on ovarian androgen production in a woman with isolated follicle-stimulating hormone deficiency. N Engl J Med 343:1197–1198 [DOI] [PubMed] [Google Scholar]
- Barnes RB, Namnoum AB, Rosenfield RL, Layman LC 2002 The role of LH and FSH in ovarian androgen secretion and ovarian follicular development: clinical studies in a patient with isolated FSH deficiency and multicystic ovaries. Hum Reprod 17:88–91 [DOI] [PubMed] [Google Scholar]
- Peeters R, Vanmontfort D, Van Isterdael J, Verhoeven G, Rombauts L, Decuypere E 1997 Evidence for the presence of immunoreactive inhibin in extragonadal tissues of ovariectomized ewes. Anim Reprod Sci 48:257–268 [DOI] [PubMed] [Google Scholar]
- Hofland J, van Nederveen FH, Timmerman MA, Korpershoek E, de Herder WW, Lenders JW, Verhofstad AA, de Krijger RR, de Jong FH 2007 Expression of activin and inhibin subunits, receptors and binding proteins in human pheochromocytomas: a study based on mRNA analysis and immunohistochemistry. Clin Endocrinol (Oxf) 66:335–340 [DOI] [PubMed] [Google Scholar]
- Farnworth PG, Stanton PG, Wang Y, Escalona R, Findlay JK, Ooi GT 2006 Inhibins differentially antagonize activin and bone morphogenetic protein action in a mouse adrenocortical cell line. Endocrinology 147:3462–3471 [DOI] [PubMed] [Google Scholar]
- Erickson GF, Magoffin DA, Cragun JR, Chang RJ 1990 The effects of insulin and insulin-like growth factors-I and -II on estradiol production by granulosa cells of polycystic ovaries. J Clin Endocrinol Metab 70:894–902 [DOI] [PubMed] [Google Scholar]
- Almahbobi G, Anderiesz C, Hutchinson P, McFarlane JR, Wood C, Trounson AO 1996 Functional integrity of granulosa cells from polycystic ovaries. Clin Endocrinol (Oxf) 44:571–580 [DOI] [PubMed] [Google Scholar]