Abstract
Objective: Our objective was to determine whether subclinical thyrotoxicosis alters health status, mood, and/or cognitive function.
Design: This was a double-blinded, randomized, cross-over study of usual dose l-T4 (euthyroid arm) vs. higher dose l-T4 (subclinical thyrotoxicosis arm) in hypothyroid subjects.
Patients: A total of 33 hypothyroid subjects receiving l-T4 were included in the study.
Measurements: Subjects underwent measurements of health status, mood, and cognition: Short Form 36 (SF-36); Profile of Mood States (POMS); and tests of declarative memory (Paragraph Recall, Complex Figure), working memory (N-Back, Subject Ordered Pointing, and Digit Span Backwards), and motor learning (Pursuit Rotor). These were repeated after 12 wk on each of the study arms.
Results: Mean TSH levels decreased from 2.15 to 0.17 mU/liter on the subclinical thyrotoxicosis arm (P < 0.0001), with normal mean free T4 and free T3 levels. The SF-36 physical component summary and general health subscale were slightly worse during the subclinical thyrotoxicosis arm, whereas the mental health subscale was marginally improved. The POMS confusion, depression, and tension subscales were improved during the subclinical thyrotoxicosis arm. Motor learning was better during the subclinical thyrotoxicosis arm, whereas declarative and working memory measures did not change. This improvement was related to changes in the SF-36 physical component summary and POMS tension subscales and free T3 levels.
Conclusions: We found slightly impaired physical health status but improvements in measures of mental health and mood in l-T4 treated hypothyroid subjects when subclinical thyrotoxicosis was induced in a blinded, randomized fashion. Motor learning was also improved. These findings suggest that thyroid hormone directly affects brain areas responsible for affect and motor function.
L-thyroxine-treated hypothyroid patients deliberately rendered for subclinical thyrotoxicosis have minor decrements in general and physical health status but improvements in mood and motor learning, suggesting that thyroid hormone directly affects brain areas responsible for affect and motor function.
Subclinical thyroid dysfunction [abnormal serum TSH with normal free T4 (fT4) and free T3 (fT3) levels] may have central nervous system effects. This has been best studied in subclinical hypothyroidism, with some (but not all) reports showing adverse effects on mood and cognition, and improvements after l-T4 treatment (reviewed in Refs. 1 and 2). Subclinical hyperthyroidism has been less well studied in this regard, with reports showing increased levels of anxiety or depression and decrements in cognitive domains (3,4,5,6,7,8). However, this is not uniform (5,9,10,11), and there is little information on treatment effects.
We recently reported results of a randomized, double-blind, cross-over study in which l-T4 treated subjects received their usual dose of l-T4, or a slightly lower dose (12). Induction of subclinical hypothyroidism led to decrements in specific domains of health status, mood, and working memory. In the current study, we applied a similar experimental design to induce subclinical thyrotoxicosis. We chose to focus on memory, based on our previous data and studies suggesting that memory is preferentially affected in subjects with thyroid dysfunction (2,12,13), and because animal studies support a major role for l-T4 in brain areas that subserve memory (14,15,16,17,18). We studied three distinct forms of memory linked to specific brain systems: declarative memory (medial temporal lobe), working memory (prefrontal cortex), and motor learning (cerebellum and basal ganglia) (19). We included validated measures of health status and mood because these may be altered in subjects with subclinical thyrotoxicosis (3,4,5,6,7,8,11,20,21), and may secondarily affect cognition. We hypothesized the following:
Subjects with induced subclinical thyrotoxicosis have decrements in health status and mood compared with the euthyroid state.
Subjects with induced subclinical thyrotoxicosis have specific decrements in memory, which may be related to changes in health status or mood.
In subjects with induced subclinical thyrotoxicosis, changes in health status, mood, and/or cognition are correlated with changes in TSH, fT4, and/or fT3 levels.
Subjects and Methods
Experimental subjects
A total of 33 subjects (31 women, two men), ages 24–45 yr, with adult-onset primary hypothyroidism was recruited. There were 31 subjects that had autoimmune hypothyroidism, and two had received iodine-131 therapy for Graves’ disease. Duration of hypothyroidism averaged 7 yr (range 6 months to 25 yr). All subjects had elevated TSH levels before l-T4 treatment or while on lower l-T4 doses. Subjects were receiving l-T4 as the sole treatment in stable doses for at least 3 months, with normal TSH levels. Subjects did not have any acute or chronic illnesses and were not receiving medications that affect thyroid hormone levels, mood, or cognition. Stable doses of oral contraceptive or estrogen therapy were allowed during the study. Testing was done during the first 10 d after onset of menstrual bleeding, or in the first 10 d of an oral contraceptive cycle.
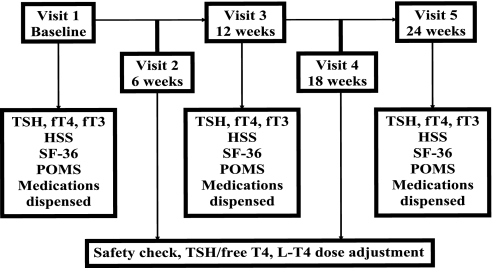
Experimental design (Fig. 1)
Figure 1.
Study flow diagram.
The protocol was approved by the Oregon Health & Science University Institutional Review Board, and subjects gave written informed consent.
Screening visit
Subjects were screened for general health, medication use, drug abuse, thyroid status, and mood or cognitive disorders by history, physical examination, laboratory, and electrocardiogram testing. General intelligence was measured by the Wechsler Adult Intelligence Scale-Revised (WAIS-R) Vocabulary subtest, which correlates with intelligence quotient (22). Subjects were excluded if they scored less than eight (scaled). The Symptom Checklist 90-R was administered to screen subjects for psychiatric diseases (23). Subjects who scored outside the normative range were excluded.
Baseline visit
Within 3 wk, subjects returned for a 90- to 120-min baseline visit. Subjects refrained from taking their l-T4 dose that morning. Serum TSH, fT4, and fT3 levels were obtained. The Hyperthyroid Symptom Scale (HSS) was completed (24). Subjects completed validated measures of health status and mood.
The Short Form 36 health survey (SF-36), a questionnaire about general health (22).
Higher scores on the SF-36 summary scales and subscales reflect better health status and well-being. This instrument has been used in studies involving thyroid disease (25), and decrements were found in our study of subclinical hypothyroidism (12).
The Profile of Mood States (POMS), a questionnaire about mood (22).
Higher scores on the POMS subscales reflect mood decrements, except for the vigor subscale, in which higher scores reflect improved mood.
Cognitive tests were administered by a single research assistant in a standard fashion. Based on existent literature and our previous study, we did not use a battery of general cognitive measures but, rather, validated measures targeted to specific domains likely to be affected by altered thyroid status.
Tests of declarative memory (hippocampus, medial temporal lobe)
Paragraph Recall (verbal memory)
For this subtest of the Wechsler Memory Scale-Revised, subjects were read two brief stories, and verbally recalled each of them immediately and after a 30-min interval. The outcome measure was the total number of story elements recalled at each interval (26).
Complex Figure Test (visual memory)
For the Medical College of Georgia Complex Figure Test, subjects copied a standard complex figure, and drew it from memory immediately and after a 30-min interval. The outcome measure was the total number of elements correctly reproduced (22).
Tests of working memory (prefrontal cortex)
N-back test
A series of letters was presented one at a time on a screen. Subjects responded when a letter appeared that they had seen on the previous screen (one back). The task was repeated with an increase in memory load by having the subject respond when a letter appeared three back. The outcome measure was the total number correct (27).
Subject Ordered Pointing (SOP)
Subjects were presented with stacks of cards (six, eight, 10, or 12 per set). Each card in a set showed the same array of abstract drawings, but in a different spatial arrangement. The subject was instructed to touch one drawing on each card, but not to touch the same drawing on subsequent cards in the set. Subjects erred when they touched a drawing that had been touched on a previous card. Each card set was repeated three times. The outcome measure was the total number of errors across each card set (22).
Digit Span Backwards
The examiner read number sequences of increasing length, and after each sequence the subject repeated the sequence backwards. Subjects erred when they could not successfully repeat two sequences of the same length (22).
Motor learning (basal ganglia, cerebellum)
Pursuit Rotor
Subjects held a photosensitive wand to maintain contact with a 2-cm light disk rotating on a variable speed turntable (Model 30014; Lafayette Instrument Co., Lafayette, IN). An initial block of four trials was administered at 15, 30, 45, and 60 revolutions per minute to establish the optimal speed for further trials. Two blocks of eight 20-sec trials were then administered, with a 20-sec rest after each trial and a 60-sec rest period after four trials. After a 30-min interval, the two blocks were repeated. The outcome measure was the mean total time the stylus remained on target during each trial (28).
Subjects were randomized to receive either their usual doses of l-T4 (euthyroid arm) or higher doses of l-T4, intended to lead to a TSH level of 0.01–0.4 mU/liter (subclinical thyrotoxicosis arm), while maintaining normal fT4 and T3 levels. Our target TSH level reflects the range seen in clinical practice, while avoiding overtreatment and possible symptoms. Based on average suppressive l-T4 doses in thyroid cancer patients, estimated initial l-T4 dose increases were 30%. The subjects and the physician (M.H.S.) and research assistant (P.C.) with direct subject contact were blinded, whereas the monitoring physician (K.G.S.) was unblinded, to adjust l-T4 doses and assess reported side effects. l-T4 pills were placed in gel capsules to maintain blinding.
Six-week interim visit.
Six weeks later, subjects returned for a brief visit. Compliance was assessed by pill counts. Safety was assessed by questioning for symptoms, performing electrocardiograms, serum TSH, fT4, and total T3 levels. The monitoring physician (K.G.S.) made l-T4 dose adjustments as needed to attain target TSH levels for that arm of the study, keeping fT4 and T3 levels within the normal range.
Cross-over visit.
Twelve weeks after the baseline visit, subjects returned for an extended visit. Serum TSH, fT4, and fT3 levels were measured. The HSS, SF-36, POMS, and cognitive tests were repeated in the same order as the baseline visit. Subjects were crossed over to the second arm of the study and had their new doses of l-T4 dispensed.
Eighteen-week interim visit.
This visit was identical to the 6-wk visit, with subjects now on the alternate l-T4 dose.
End of study visit.
Twelve weeks after the cross-over visit, subjects returned for a visit identical to the cross-over visit. Subjects were asked which arm of the study they thought was the higher dose arm and which arm they preferred. They were then placed back on their usual doses of l-T4.
Analytical methods
TSH was measured by immunochemiluminometric assay (Nichols Institute, San Juan Capistrano, CA): sensitivity 0.003 mU/liter, normal range 0.28–5.00, intraassay coefficients of variation (CVs) 9.5% at 0.03 mU/liter and 4.7% at 11.6 mU/liter, and interassay CVs 17% at 0.02 mU/liter and 4.6% at 14 mU/liter. fT4 was measured by immunochemiluminometric assay: sensitivity 0.08 ng/dl, normal range 0.7–1.8, intraassay CVs 5.7% at 0.27 ng/dl and 1% at 4.6 ng/dl, and interassay CVs 6.8% at 0.3 ng/dl and 1.6% at 3.8 ng/dl. fT3 was measured by tracer dialysis (Nichols Institute): sensitivity 25 pg/dl, normal range 210–440, intraassay CV 6%, and interassay CV 4%.
Statistical methods
Differences in outcomes between the two study arms were explored by paired t tests. Sets of quality of life, mood, and cognitive measures were then analyzed together using mixed effects models. Age, years of education, WAIS-R, and baseline measures were covariates to reduce residual variability. Generalized Estimating Equations (29) were used to estimate group differences for measures with ceiling or floor effects. Each set of tests was analyzed as a group to adjust for within-subject correlation and correlations between responses on similar tests; results are grouped together in the results tables (see Tables 2 and 3). Bonferroni adjustments were used for formal pairwise comparisons, when applicable. P values less than 0.05 were considered significant.
Table 2.
Summary statistics and P values for health status and mood measures during the two treatment arms
| Measure | Baseline (mean ± sem) | Euthyroid (mean ± sem) | Thyrotoxic (mean ± sem) | P value (paired t test) | Adjusted P values |
|---|---|---|---|---|---|
| SF-36 | |||||
| MCS | 51.72 ± 1.02 | 50.69 ± 1.46 | 52.71 ± 1.16 | 0.15 | Treatment: 0.15 |
| PCS | 51.91 ± 1.33 | 56.59 ± 0.61 | 54.75 ± 0.83 | 0.01 | Treatment: 0.01 |
| Treatment test: 0.008 | |||||
| BP | 76.00 ± 4.09 | 84.43 ± 3.01 | 81.87 ± 3.14 | 0.18 | 0.22 |
| GH | 80.84 ± 2.65 | 85.03 ± 2.23 | 81.55 ± 2.28 | 0.06 | 0.06 |
| MH | 80.38 ± 1.60 | 78.40 ± 2.28 | 84.13 ± 1.55 | 0.02 | 0.01 |
| VT | 59.84 ± 2.83 | 66.83 ± 2.57 | 69.03 ± 1.96 | 0.45 | 0.42 |
| PFa | 91.72 ± 2.82 | 96.33 ± 1.10 | 94.52 ± 1.56 | 0.50 | |
| RPa | 76.56 ± 6.54 | 93.33 ± 3.16 | 93.33 ± 3.38 | >0.99 | |
| SFa | 86.33 ± 3.86 | 93.33 ± 2.05 | 89.58 ± 3.55 | >0.99 | |
| REa | 91.67 ± 2.99 | 86.67 ± 4.40 | 88.89 ± 4.33 | 0.73 | |
| POMS | |||||
| A | 42.13 ± 0.89 | 44.50 ± 1.11 | 43.16 ± 1.21 | 0.34 | Treatment: 0.002 |
| C | 41.78 ± 1.07 | 42.44 ± 1.54 | 40.00 ± 1.03 | 0.04 | |
| D | 41.16 ± 0.73 | 42.66 ± 1.06 | 40.44 ± 0.65 | 0.06 | |
| F | 44.72 ± 1.37 | 43.38 ± 1.16 | 42.59 ± 1.03 | 0.51 | |
| T | 41.66 ± 1.06 | 42.53 ± 1.17 | 39.16 ± 1.64 | 0.05 | |
| V | 51.84 ± 1.10 | 53.16 ± 1.39 | 55.75 ± 1.28 | 0.11 | 0.11 |
P values for unadjusted paired t tests comparing the two arms of the study are shown in the fifth column. Adjusted P values in the repeated measures analysis are shown in the sixth column. These P values were adjusted for age, years of education, WAIS-R, and baseline measure for that particular variable. Significant results are highlighted in bold. For the adjusted analyses, treatment test P values were examined initially. If the treatment test P value was more than 0.1, indicating no treatment-test interaction, it is not shown in the table, and only the overall treatment effect is shown. If the treatment test P value was 0.1 or less, indicating a possible treatment-test interaction, this value is listed in the table, and subsequent analyses were done for individual measures. A, Anxiety; BP, bodily pain; C, confusion; D, depression; F, fatigue; PF, physical functioning; RE, role emotional; RP, role physical; SF, social functioning; T, tension; V, vigor; VT, vitality.
Raw scores compared using an exact McNemar’s test because many values were at ceiling.
Table 3.
Summary statistics and P values for cognitive measures during the two treatment arms of the study
| Baseline (mean ± sem) | Euthyroid (mean ± sem) | Thyrotoxic (mean ± sem) | P value (paired t test) | Adjusted P values | |
|---|---|---|---|---|---|
| Declarative memory | |||||
| Paragraph Recall | Treatment: 0.25 | ||||
| Immediate | 14.06 ± 0.63 | 15.75 ± 0.61 | 16.22 ± 0.49 | 0.48 | |
| 30-min delay | 13.13 ± 0.61 | 14.56 ± 0.62 | 15.06 ± 0.48 | 0.38 | |
| Complex Figure | Treatment: 0.29 | ||||
| Copy | 34.25 ± 0.31 | 34.78 ± 0.22 | 34.84 ± 0.19 | 0.83 | |
| 3 min | 28.31 ± 0.72 | 29.78 ± 0.82 | 31.23 ± 0.66 | 0.10 | |
| 30 min | 28.11 ± 0.81 | 29.20 ± 0.83 | 30.45 ± 0.72 | 0.19 | |
| Working memory | |||||
| N-Back number correct | Treatment: 0.67 | ||||
| 1 back | 14.59 ± 0.28 | 15.00 ± 0.14 | 15.06 ± 0.16 | 0.71 | |
| 3 back | 9.25 ± 0.40 | 10.31 ± 0.35 | 10.41 ± 0.43 | 0.83 | |
| SOP errors | Treatment: 0.28 | ||||
| 6 | 0.40 ± 0.08 | 0.32 ± 0.06 | 0.32 ± 0.07 | >0.99 | |
| 8 | 0.86 ± 0.11 | 0.72 ± 0.11 | 0.62 ± 0.12 | 0.35 | |
| 10 | 1.32 ± 0.12 | 0.94 ± 0.12 | 0.77 ± 0.11 | 0.11 | |
| 12 | 1.59 ± 0.18 | 1.23 ± 0.16 | 1.30 ± 0.15 | 0.68 | |
| Digit Span Backwards | 7.56 ± 0.37 | 8.22 ± 0.42 | 8.53 ± 0.44 | 0.33 | Treatment: 0.33 |
| Motor learning | |||||
| Pursuit Rotor Trial | Treatment: <0.001 | ||||
| 1 | 23.96 ± 1.16 | 32.85 ± 2.31 | 35.19 ± 2.16 | 0.14 | |
| 2 | 26.69 ± 1.48 | 33.05 ± 2.01 | 36.34 ± 2.07 | 0.04 | |
| 3 | 28.24 ± 1.84 | 32.39 ± 2.23 | 38.08 ± 2.39 | <0.001 | |
| 4 | 28.67 ± 1.51 | 34.44 ± 2.08 | 39.62 ± 2.31 | 0.03 |
Individual cognitive tests are grouped by the three memory subdomains (first column). P values for unadjusted paired t tests comparing the two treatment arms are shown in the fifth column. Adjusted P values in the repeated measures analysis are shown in the sixth column. These P values were adjusted for age, years of education, WAIS-R, and baseline measure for that particular variable. Significant results are highlighted in bold. For the adjusted analyses, treatment test P values were examined initially. There were no treatment-test interactions, and, thus, only the overall treatment effect is shown.
To investigate associations between changes in TSH, fT4, or fT3 and outcomes, three mixed effects models were developed for each outcome, with one thyroid hormone as a covariate. In a final model for each outcome, the association between a single thyroid hormone measure was adjusted for the two other thyroid hormone measures. The number of models was large, so we were interested in statistically significant findings across similar measures and similar hormones rather than in individual P values.
To assess whether differences in cognitive measures could be explained by differences in health status or mood, the model with significant cognitive differences (Pursuit Rotor) was adjusted for changes in SF-36 and/or POMS measures. Separate models were fitted to assess effects of each individual change in SF-36 and/or POMS score that showed significant euthyroid-thyrotoxicosis differences. In a final analysis, we simultaneously adjusted for significant thyroid hormone and health status or mood associations to assess independent effects on the cognitive measures.
Results
Clinical parameters and thyroid function tests (Table 1)
Table 1.
Clinical parameters and thyroid function tests at baseline and the end of each arm of the study (euthyroid and subclinical thyrotoxicosis)
| Measure | Baseline (mean ± sem) | Euthyroid (mean ± sem) | Thyrotoxic (mean ± sem) | P value (paired t test) |
|---|---|---|---|---|
| TSH (mU/liter) | 2.58 ± 0.27 | 2.15 ± 0.31 | 0.17 ± 0.06 | <0.001 |
| fT4 (ng/dl) | 1.33 ± 0.04 | 1.40 ± 0.04 | 1.70 ± 0.06 | <0.001 |
| fT3 (pg/dl) | 262.7 ± 6.3 | 259.3 ± 6.2 | 320.2 ± 11.4 | <0.001 |
| Weight (kg) | 75.86 ± 3.83 | 74.45 ± 3.79 | 73.78 ± 3.67 | 0.38 |
| BMI (kg/m2) | 27.1 ± 1.2 | 26.7 ± 1.2 | 26.6 ± 1.2 | 0.38 |
| Pulse (beats/min) | 70.8 ± 1.8 | 70.6 ± 2.2 | 73.5 ± 2.3 | 0.29 |
| Systolic blood pressure (mm Hg) | 121.0 ± 1.9 | 120.0 ± 2.7 | 118.7 ± 2.5 | 0.52 |
| Diastolic blood pressure (mm Hg) | 72.2 ± 1.8 | 70.2 ± 2.1 | 66.7 ± 2.0 | 0.03 |
| HSS | 1.74 ± 0.30 | 1.94 ± 0.40 | 2.06 ± 0.39 | 0.73 |
| l-T4 dose (μg/kg·d) | 1.62 ± 0.09 | 1.66 ± 0.09 | 2.45 ± 0.09 | <0.001 |
To convert fT4 levels to International System of Units, multiply by 12.87. To convert fT3 levels to International System of Units, multiply by 0.01536. BMI, Body mass index.
A total of 47 subjects volunteered for the study, and 33 qualified after the screening visit. Interim l-T4 dose adjustment was necessary in four subjects during the euthyroid arm (two increased dose, two decreased dose) and in 27 during the subclinical thyrotoxic arm (20 increased dose further, seven decreased to intermediate dose). Per study design, mean TSH levels decreased to 0.17 mU/liter, whereas mean fT4 and fT3 levels increased at the end of the subclinical thyrotoxicosis arm. A total of 22 subjects had TSH levels less than 0.1 mU/liter, whereas 11 had TSH levels more than 0.1 mU/liter. Four subjects had slightly high fT4 levels, one with a slightly high fT3 level. Thus, we were successful in inducing subclinical thyrotoxicosis, with TSH levels in our target range, and almost all serum fT4 and fT3 levels in the normal range.
All subjects completed the study. There were no differences in weight, pulse, systolic blood pressure, or HSS at the end of the two arms of the study; diastolic blood pressure was slightly lower at the end of the subclinical thyrotoxicosis arm (P = 0.03).
Subjects did not reliably predict which arm was the subclinical thyrotoxicosis arm: 15 guessed correctly, 10 guessed incorrectly, and eight had no opinion [P = not significant (NS) by binomial calculation]. There were 15 that preferred the actual higher dose, seven preferred the actual lower euthyroid dose, and 11 had no preference (P = NS). Sixteen preferred the dose they perceived was the higher dose, five preferred the dose they perceived was the euthyroid dose, and 12 had no preference (P = NS).
Health status and mood (SF-36, POMS) (Table 2)
Physical health status. The SF-36 physical component summary (PCS) scale score was slightly worse at the end of the subclinical thyrotoxicosis arm (P = 0.01), and the general health subscale was marginally worse (P = 0.06).
Mental health (MH) status.
The mental component summary (MCS) scale score was slightly but not significantly better (P = 0.15) at the end of the subclinical thyrotoxicosis arm, and the MH subscale was improved (P = 0.01). The confusion, depression, and tension subscales of the POMS were improved during the subclinical thyrotoxicosis arm (P = 0.04–0.06). There were no significant effects on other SF-36 or POMS subscales.
Changes in the SF-36 MCS and general health subscale scores were directly related to changes in fT3 levels between the two study arms (P = 0.01). Changes in the SF-36 MCS were also directly related to changes in fT4 levels (P = 0.02). There were no other associations between changes in the SF-36 or POMS scales and changes in TSH, fT4, or fT3 levels (data not shown).
Cognitive tests (Table 3 and Fig. 2)
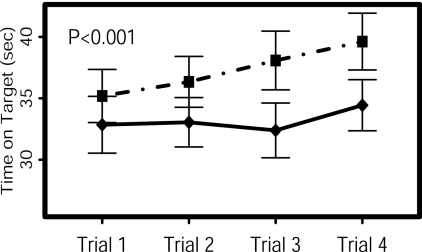
Figure 2.
Pursuit Rotor results at the end of the euthyroid arm (solid line) and subclinical thyrotoxicosis arm (dashed line). The x-axis shows each of the four trials, whereas the y-axis shows time on target for each trial (sec). Results were better during the subclinical thyrotoxicosis arm of the study (P < 0.001 by mixed effects model).
Declarative memory.
There were no differences between the two study arms in Paragraph Recall (verbal memory) or performance on the Complex Figure Test (visual memory).
Working memory.
There were no differences between the two study arms in N-Back number correct, SOP, or Digit Span Backwards.
Motor learning.
The Pursuit Rotor results were significantly better at the end of the subclinical thyrotoxicosis arm, compared with the euthyroid arm (P < 0.001 by adjusted t tests) (Fig. 2). By unadjusted t tests, there was no difference between the two arms for the initial Pursuit Rotor trial, whereas the subsequent trials were all better at the end of the subclinical thyrotoxicosis arm (P < 0.001 to 0.04).
The differences in Pursuit Rotor were marginally related to changes in fT3 levels between the two study arms (P = 0.06). The Pursuit Rotor differences were not related to changes in fT4 or TSH levels (data not shown).
The differences in Pursuit Rotor between the two study arms were also related to changes in the SF-36 PCS scale score (P = 0.003) and the POMS tension subscale (P = 0.008), but not to the other POMS subscales. These relationships were independent of changes in fT3 levels.
There were slight differences between baseline and euthyroid arm levels for some of the health status, mood, and cognitive tests, likely reflecting effects due to close monitoring during the study, and learning effects of repeated testing. We accounted for these differences by randomized order of the two arms, and by using baseline levels as a covariate in our mixed models.
We repeated our analysis after exclusion of four subjects with slightly elevated fT4 levels at the end of the subclinical thyrotoxicosis arm. The results were similar, although the significance level decreased slightly for some of the results due to the smaller sample size. Statistical significance was lost for the SF-36 general health subscale and the POMS depression subscale.
Discussion
We investigated effects of subclinical thyrotoxicosis on health status, mood, and cognition by controlling l-T4 doses in subjects with treated hypothyroidism. This model circumvents limitations in studying endogenous subclinical hyperthyroidism: subject recruitment and heterogeneity, variable natural history, and subjects’ awareness of their thyroid status. It also circumvents limitations in studying patients receiving suppressive l-T4 doses for nodular thyroid disease or thyroid cancer, who may have health status alterations due to the underlying disease. In one previous report of a similar model, Walsh et al. (10) also safely and effectively induced subclinical thyrotoxicosis in a blinded, controlled fashion.
Our subjects did not experience changes in weight, pulse, or systolic blood pressure during the subclinical thyrotoxicosis arm; the slight decrease in diastolic blood pressure is consistent with known effects of thyroid hormone on systemic vascular resistance (30). There were no changes in the HSS, and our subjects did not accurately guess which arm included the higher l-T4 dose. This confirms adequate blinding during the study.
Our first hypothesis was that subjects would have decrements in health status and/or mood during the subclinical thyrotoxicosis arm. We did find slight decrements in the SF-36 PCS score and general health subscale. In contrast, there were consistent improvements in MH measures, including the SF-36 MCS score and MH subscale, and POMS confusion, depression, and tension subscales. This suggests specific positive effects of mild thyrotoxicosis on MH and mood, despite decrements in physical health indices. These effects are likely to be clinically relevant because the magnitude of the differences in the SF-36 scales are similar to changes reported with weight loss among overweight adults (31), rosiglitazone treatment of polycystic ovarian syndrome (32), and gabapentin treatment of diabetic neuropathy (33).
Previous studies reported variable effects of subclinical thyrotoxicosis on health status and mood, with most finding decrements compared with euthyroid subjects (3,4,5,6,7,8,20,21), or no differences (9,10,34). Only a few studies reported improved mood or well-being in subclinical thyrotoxicosis subjects (11,35,36). Our study had the advantage of subjects being blinded to their treatment status. Previous findings may have been influenced by patients’ knowledge of their thyroid status. However, our findings are discordant with a recent large cross-sectional study in elderly subjects, which found no mood alterations in subclinical thyroid disease (9). Our results are also discrepant with the one other randomized, blinded study in hypothyroid subjects (10). Subjects in that study were older (mean age 53 yr), l-T4 dose alterations were less, and no specific measure of mood such as the POMS was used, which may explain the differences.
Our second hypothesis was that subjects would have decrements in memory during the subclinical thyrotoxicosis arm. Instead, we found no changes in declarative or working memory, in agreement with the recent large cross-sectional study (9). Using a similar study design as ours, Walsh et al. (10) also reported no changes in working memory. However, we did find improvements in motor learning, a form of implicit or nondeclarative memory. This was mostly independent of motor speed, and changes in health status and mood. The magnitude of the difference in Pursuit Rotor performance was similar to that seen with acute alcohol ingestion in healthy young men (37) and likely has clinical relevance.
Previous studies investigated motor speed and psychomotor function in hyperthyroidism, reporting either decrements or no differences compared with euthyroid controls (6,8,33). However, to our knowledge there are no studies of motor learning in overt or subclinical hyperthyroidism. Our findings suggest that motor learning is an important target for future studies of mild thyroid dysfunction.
Our final hypothesis was that changes in outcomes would be correlated with changes in thyroid hormone levels. This hypothesis was not consistently supported. One explanation is that serum thyroid hormone and TSH levels do not adequately reflect thyroid hormone action in the brain. Thyroid hormone levels are tightly regulated within the brain in a tissue-specific fashion that is not completely dependent on serum levels, and there may also be other mediators of thyroid hormone action in those tissues (38).
It is interesting to compare our current results with our previous study of subclinical hypothyroidism (12). Using an identical design and outcome measures, we found that subclinical hypothyroidism was associated with decrements in the SF-36 general health and POMS fatigue subscales. Together, these studies suggest that there is an optimal “window” of thyroid function for overall health (SF-36 PCS and general health subscale) but that MH and mood may continue to improve (SF-36 MCS and POMS) as thyroid function progresses from subclinical hypothyroidism to subclinical thyrotoxicosis. Subclinical hypothyroidism was associated with decrements in working memory, but not motor learning. In contrast, subclinical thyrotoxicosis was associated with improved motor learning, but no changes in working memory. This suggests that there may be different “optimal” thyroid hormone levels for the brain areas that control these functions.
There are five limitations to this study: sample size, variability in thyroid hormone levels, the study’s duration, the cross-over design, and the age range. Our study was a priori powered on the cognitive measures. We accounted for multiple comparisons by statistical adjustment, and we found positive results for a number of health status and cognitive measures. We do not feel that variability in thyroid hormone levels is a major limitation because analysis excluding subjects with elevated fT4/fT3 levels did not alter our results. We limited the time of subclinical thyrotoxicosis to 12 wk, and we recognize that most subjects required interim l-T4 dose adjustment during this time. It is unclear whether a longer time period would magnify or attenuate our findings. The cross-over design could lead to problems with learning effects during repeated testing, and carryover effects. To minimize this we used alternative test forms and randomized the treatment order. Finally, we studied younger subjects to avoid risks of inducing subclinical thyrotoxicosis, and we recognize our results may not be generalizable to older subjects.
In summary, we induced subclinical thyrotoxicosis in a controlled, blinded fashion in subjects with primary hypothyroidism. We found minor decrements in general and physical health status, but improvements in mood and motor learning. Given these mixed effects, as well as the known deleterious effects of subclinical hyperthyroidism on other organ systems (39), we do not recommend that hypothyroid subjects with benign thyroid disease be treated with suppressive doses of levothyroxine.
Footnotes
This work was supported in part by R21 DK062787 (to M.H.S.) and UL1 RR024120 (Oregon Health & Science University Clinical and Translational Research Center).
Disclosure Summary: The authors have nothing to declare.
First Published Online February 19, 2008
Abbreviations: CV, Coefficient of variation; fT3, free T3; fT4, free T4; HSS, Hyperthyroid Symptom Scale; MCS, mental component summary; MH, mental health; NS, not significant; PCS, physical component summary; POMS, Profile of Mood States; SF-36, Short Form 36; SOP, Subject Ordered Pointing; WAIS-R, Wechsler Adult Intelligence Scale-Revised.
References
- Boelaert K, Franklyn JA 2005 Thyroid hormone in health and disease. J Endocrinol 187:1–15 [DOI] [PubMed] [Google Scholar]
- Davis JD, Tremont G 2007 Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol 32:49–65 [PubMed] [Google Scholar]
- Gulseren S, Gulseren L, Hekimsoy Z, Cetinay P, Ozen C, Tokatlioglu B 2006 Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch Med Res 37:133–139 [DOI] [PubMed] [Google Scholar]
- Röckel M, Teuber J, Schmidt R, Kaumeier S, Hafner H, Usadel KH 1987 [Correlation of “latent hyperthyroidism” with psychological and somatic changes]. Klin Wochenschr 65:264–273 (German) [DOI] [PubMed] [Google Scholar]
- Schlote B, Nowotny B, Schaaf L, Kleinbohl D, Schmidt R, Teuber J, Paschke R, Vardarli I, Kaumeier S, Usadel KH 1992 Subclinical hyperthyroidism: physical and mental state of patients. Eur Arch Psychiatry Clin Neurosci 241:357–364 [DOI] [PubMed] [Google Scholar]
- Bommer M, Eversmann T, Pickardt R, Leonhardt A, Naber D 1990 Psychopathological and neuropsychological symptoms in patients with subclinical and remitted hyperthyroidism. Klin Wochenschr 68:552–558 [DOI] [PubMed] [Google Scholar]
- Sait Gonen M, Kisakol G, Savas Cilli A, Dikbas O, Gungor K, Inal A, Kaya A 2004 Assessment of anxiety in subclinical thyroid disorders. Endocr J 51:311–315 [DOI] [PubMed] [Google Scholar]
- Botella-Carretero JI, Galan JM, Caballero C, Sancho J, Escobar-Morreale HF 2003 Quality of life and psychometric functionality in patients with differentiated thyroid carcinoma. Endocr Relat Cancer 10:601–610 [DOI] [PubMed] [Google Scholar]
- Roberts LM, Pattison H, Roalfe A, Franklyn J, Wilson S, Hobbs FD, Parle JV 2006 Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med 145:573–581 [DOI] [PubMed] [Google Scholar]
- Walsh JP, Ward LC, Burke V, Bhagat CI, Shiels L, Henley D, Gillett MJ, Gilbert R, Tanner M, Stuckey BG 2006 Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab 91:2624–2630 [DOI] [PubMed] [Google Scholar]
- Eustatia-Rutten CFA, Corssmit EPM, Pereira AM, Frolich M, Bax JJ, Romijn JA, Smit JWA 2006 Quality of life in longterm exogenous subclinical hyperthyroidism and the effects of restoration of euthyroidism, a randomized controlled trial. Clin Endocrinol (Oxf) 64:284–291 [DOI] [PubMed] [Google Scholar]
- Samuels MH, Schuff KG, Carlson NE, Carello P, Janowsky JS 2007 Health status, mood and cognition in experimentally-induced subclinical hypothyroidism. J Clin Endocrinol Metab 92:2545–2551 [DOI] [PubMed] [Google Scholar]
- Samuels MH, Schuff KG, Carlson NE, Carello P, Janowsky JS 2007 Health status, psychological symptoms, mood and cognition in L-thyroxine treated hypothyroid subjects. Thyroid 17:249–258 [DOI] [PubMed] [Google Scholar]
- Sinha AK, Pickard MR, Kim KD, Ahmed MT, al Yatama F, Evans IM, Elkins RP 1994 Perturbation of thyroid hormone homeostasis in the adult and brain function. Acta Med Austriaca 21:35–43 [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS 1991 The hippocampal formation: morphological changes induced by thyroid, gonadal and adrenal hormones. Psychoneuroendocrinology 16:67–84 [DOI] [PubMed] [Google Scholar]
- Madeira MD, Sousa N, Lima-Andrade M, Calheiros F, Cadete-Leite A, Paula-Barbosa MM 1992 Selective vulnerability of the hippocampal pyramidal neurons to hypothyroidism in male and female rats. J Comp Neurol 322:501–508 [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM, Yang J 1994 A time course of altered thyroid states on the noradrenergic system in rat brain by quantitative autoradiography. Neuroendocrinology 59:235–244 [DOI] [PubMed] [Google Scholar]
- Hemmings SJ, Shuaib A 1998 Hypothyroidism-evoked shifts in hippocampal adrenergic receptors: implications to ischemia-induced hippocampal damage. Mol Cell Biochem 185:161–169 [DOI] [PubMed] [Google Scholar]
- Budson AE, Price BH 2005 Memory dysfunction. N Engl J Med 352:692–699 [DOI] [PubMed] [Google Scholar]
- Biondi B, Palmieri EA, Fazio S, Cosco C, Nocera M, Sacca L, Filetti S, Lombardi G, Perticone F 2000 Endogenous subclinical hyperthyroidism affects quality of life and cardiac morphology and function in young and middle-aged patients. J Clin Endocrinol Metab 85:4701–4705 [DOI] [PubMed] [Google Scholar]
- Bianchi GP, Zaccheroni V, Solaroli E, Verscini F, Cerutti R, Zoli M, Marchesini G 2004 Health-related quality of life in patients with thyroid disorders. Qual Life Res 13:45–54 [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss EA 1998 A compendium of neuropsychological tests: administration, norms, and commentary. 2nd ed. New York: Oxford University Press [Google Scholar]
- Wetzler S, Marlowe DB 1993 The diagnosis and assessment of depression, mania, and psychosis by self-report. J Pers Assess 60:1–31 [DOI] [PubMed] [Google Scholar]
- Klein I, Trzepacz PT, Roberts M, Levey GS 1988 Symptom rating scale for assessing hyperthyroidism. Arch Intern Med 148:387–390 [PubMed] [Google Scholar]
- Razvi S, McMillan CV, Weaver JU 2005 Instruments used in measuring symptoms, health status and quality of life in hypothyroidism: a systematic qualitative review. Clin Endocrinol (Oxf) 63:617–624 [DOI] [PubMed] [Google Scholar]
- Lezak MD 1995 Neuropsychological assessment. 3rd ed. New York: Oxford University Press [Google Scholar]
- Cohen JD, Forman S, Braver TS, Casey BJ, Servan-Schreiber D, Noll DC 1993 Activation of prefrontal cortex in a nonspatial working memory task with functional MRI. Hum Brain Map 1:293–304 [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Altshuler L, Theberge DC, Mintz J 1999 Declarative and procedural memory in bipolar disorder. Biol Psychiatry 46:525–531 [DOI] [PubMed] [Google Scholar]
- Shults J, Whitt MC, Kumanyika S 2004 Analysis of data with multiple sources of correlation in the framework of generalized estimating equations. Stat Med 23:3209–3226 [DOI] [PubMed] [Google Scholar]
- Danzi S, Klein I 2004 Thyroid hormone and the cardiovascular system. Minerva Endocrinol 29:139–150 [PubMed] [Google Scholar]
- Blissmer B, Riebe D, Dye G, Ruggiero L, Greene G, Caldwell M 2006 Health-related quality of life following a clinical weight loss intervention among overweight and obese adults: intervention and 24 month follow-up effects. Health Qual Life Outcomes 4:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S, Benson S, Elsenbruch S, Pleger K, Tan S, Mann K, Schedlowski M, van Halteren WB, Kimmig R, Janssen OE 2006 Metformin treatment of polycystic ovary syndrome improves health-related quality-of-life, emotional distress and sexuality. Hum Reprod 21:1925–1934 [DOI] [PubMed] [Google Scholar]
- Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, LaMoreaux L, Garofalo E 1998 Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 280:1831–1836 [DOI] [PubMed] [Google Scholar]
- Larisch R, Kley K, Nikolaus S, Sitte W, Franz M, Hautzel H, Tress W, Muller HW 2004 Depression and anxiety in different thyroid function states. Horm Metab Res 36:650–653 [DOI] [PubMed] [Google Scholar]
- Carr D, McLeod DT, Parry G, Thornes HM 1988 Fine adjustment of thyroxine replacement dosage: comparison of the thyrotrophin releasing hormone test using a sensitive thyrotrophin assay with measurement of free thyroid hormones and clinical assessment. Clin Endocrinol (Oxf) 28:325–333 [DOI] [PubMed] [Google Scholar]
- Kathmann N, Kuisle U, Bommer M, Naber D, Muller OA, Engel RR 1994 Effects of elevated triiodothyronine on cognitive performance and mood in healthy subjects. Neuropsychobiology 29:136–142 [DOI] [PubMed] [Google Scholar]
- Harrison EL, Fillmore MT 2005 Social drinkers underestimate the additive impairing effects of alcohol and visual degradation on behavioral functioning. Psychopharmacology (Berl) 177:459–464 [DOI] [PubMed] [Google Scholar]
- Bernal J 2007 Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab 3:249–259 [DOI] [PubMed] [Google Scholar]
- Biondi B, Palmieri EA, Klain M, Schlumberger M. Biletti S, Lombardi G 2005 Subclinical hyperthyroidism: clinical features and treatment options. Eur J Endocrinol 152:1–9 [DOI] [PubMed] [Google Scholar]