Abstract
Context: Reproductive hormones are incompletely characterized during the menopause transition (MT).
Hypothesis: Increased anovulation and decreased progesterone accompany progress through the MT.
Design: The Daily Hormone Study (DHS) of the Study of Women’s Health Across the Nation (SWAN) included 848 women aged 43–53 yr at baseline who collected daily urine for one cycle or up to 50 d annually for 3 yr.
Main Outcome Measures: LH, FSH, estrone conjugates, and pregnanediol glucuronide levels were assessed. Cycles were classified by presumed luteal (ovulatory) status and bleeding. Hormones were related to time in study, age, menopausal status, and selected variables.
Results: Ovulatory-appearing cycles declined from 80.9% at baseline to 64.7% by the third assessment (H3). Cycles presumed anovulatory and not ending with bleeding by 50 d (anovulatory/nonbleeding) increased from 8.4 to 24% by H3 and were associated with progress to early perimenopause [odds ratio (OR) = 2.66; confidence interval (CI) = 1.17–6.04] or late perimenopause (OR = 56.21; CI = 18.79–168.12; P < 0.0001), African-American ethnicity (OR = 1.91; CI = 1.06–3.43), and less than high school education (OR = 3.51; CI = 1.62–7.62). Anovulatory cycles ending with bleeding remained at about 10% from baseline to H3; compared with ovulatory cycles, they were associated with obesity (OR = 4.68; CI = 1.33–16.52) and more than high school education (OR = 2.12; CI = 1.22–3.69; P = 0.02). Serum estradiol in both the highest and lowest categories was associated with anovulatory/nonbleeding collections. Pregnanediol glucuronide decreased 6.6% for each year on study. Insulin sensitivity measures did not relate strongly to menstrual cycle hormones.
Conclusions: Anovulation without bleeding represents progression of the MT. A small but detectable decrease in luteal progesterone excretion occurs as women progress through the MT.
A longitudinal study of women aged 44-56 years of age shows that anovulation without bleeding for greater than 50 days as well as a decrease in luteal progesterone excretion occurs during progression through the menopausal transition.
Yearly (1) or biannual (2) changes in circulating reproductive hormones (3,4) have shown decreased estradiol and increased FSH (5) as women traverse the menopause. Because the FSH rise is observed before an appreciable decline in estradiol, it has been hypothesized (6) and subsequently demonstrated (7) that the earliest hormonal change as women enter the menopausal transition (MT) is reduced inhibin B. Most previous studies have lacked the ability to focus on the entire menstrual cycle. Limiting sampling to the follicular phase of the menstrual cycle, partial cycle sampling, and random sampling, although practical, does not provide a complete assessment of the dynamics of the reproductive axis.
Knowledge of the normal patterns of hormone secretion and excretion across the menstrual cycle of women during the MT may help refine the probability of fertility as menstrual cyclicity changes with reproductive aging. Changes in cycle patterns may assist in predicting the timing of the final menstrual period (FMP). Finally, the ability to identify reproductive hormonal disorders concurrent with reproductive aging would be greatly assisted by defining the ranges and limits of normal menstrual function.
We hypothesized that luteal function would decrease with increasing progress through the MT and sought to evaluate which other features previously linked to reproductive hormones in the Study of Women’s Health Across the Nation (SWAN), such as body size (8), metabolic syndrome (9), and other sociodemographic variables such as ethnicity (10,11), were associated with this decline in luteal function. The SWAN Daily Hormone Study (DHS) included 848 women in the first collection period who provided daily, first-morning voided urine samples from menses to menses or up to 50 d (whichever came first). We now report longitudinal results from the first 3 yr of DHS collection. Although only a small proportion of women had attained their FMP by the time of the third DHS collection, we further sought to identify cycle or hormone changes associated with proximity to the FMP.
Subjects and Methods
The design of SWAN, a multiethnic cohort study of middle-aged women from seven U.S. communities (12), and details of the specimen collection protocols have been published (13). Briefly, the DHS is a SWAN substudy in which a subset of women (n = 848 at baseline, or H1 collection) collect first morning voided urine samples daily for one complete menstrual cycle or 50 d (whichever comes first) once a year. FSH, LH, estrone conjugate (E1c), and pregnanediol glucuronide (Pdg) were measured in the daily urine samples, using validated methodologies that have been described in detail and prospectively established to allow for objective assessments of menstrual cycles in perimenopausal women (8,13). Objective, validated algorithms were applied to detect hormonal patterns consistent with folliculogenesis, ovulation, and corpus luteum function.
Participant population and protocol
A volunteer subset of women from all SWAN clinical sites enrolled in the DHS after recruitment of the main cohort, as previously described (8,13,14,15,16). This study was approved by all participating sites’ Institutional Review Boards, and written informed consent was obtained from each participant.
Evaluation of cycles
LH, FSH, E1c, and Pdg levels were measured using previously described and validated chemiluminescent assays (8). Data were normalized for creatinine (Cr) concentration. To detect luteal function, we employed and further validated an objective algorithm developed by Kassam et al. (17,18) for use in older reproductive-aged women (8,13). Cycles with no evidence of luteal activity were presumed anovulatory and were further subdivided into those in which the collection ended due to the onset of a bleeding episode or those in which the collection was automatically terminated at 50 d without bleeding. For the combined analysis, data from cycles from the first (H1), second (H2), and third (H3) DHS collections were used.
Metabolic markers and metabolic syndrome definition
Serum insulin was measured using a previously described RIA (9). Metabolic syndrome was characterized by three or more of the following: 1) abdominal obesity (waist circumference > 80 cm for Chinese and Japanese, >88 cm for Caucasians, African-Americans, and Hispanics); 2) hypertriglyceridemia (fasting triglycerides > 150 mg/dl); 3) high-density lipoprotein cholesterol less than 50 mg/dl; 4) high blood pressure (systolic blood pressure > 130 mm Hg, diastolic blood pressure > 85 mm Hg, or on anti-hypertensive medication); and 5) impaired fasting glucose or diabetes (fasting glucose > 110 mg/dl or on insulin therapy). These definitions are adapted from two existing sets of criteria, one from the National Cholesterol Education Program (16) and the other from the World Health Organization (21), and have been used in previous SWAN analyses (9). β-Cell function and insulin resistance were derived from fasting insulin and glucose, using homeostasis model assessment (22).
Data analysis
The analytic sample at the H1–H3 visits were summarized using frequency distributions for categorical variables [ethnicity, menopausal status, categorical body mass index (BMI), smoking status (current vs. past/never), educational level, presence/absence of metabolic syndrome, and presence/absence of diabetes] and means and sd for continuous variables (age and physical activity) with log transformation when appropriate (log glucose, log insulin, log β-cell function, and log insulin resistance). Physical activity was assessed from questions adapted from the Kaiser Physical Activity Survey, based on the Baecke physical activity questionnaire regarding activity in household and caregiving, sports and exercise, and daily routine (23,24,25).
Multivariate models for presumed ovulatory vs. anovulatory collections and for whole-cycle integrated hormones included as predictors nonhormonal variables that were hypothesized to relate to cycle characteristics, i.e. ethnicity, baseline age and time since baseline, baseline and concurrent menopause status, collection length in days, baseline and concurrent BMI, smoking, education, and physical activity, as well as other hormonal (metabolic) exposures hypothesized to be related to menstrual cycle hormones. To identify factors distinguishing ovulatory from anovulatory collections, we estimated multivariate random-effects logistic regression models (26), using PROC GLIMMIX in SAS to account for within-woman correlation. Anovulatory cycles with and without (50-d collections) associated bleeding differed in their hormonal characteristics, as estimated from random-effects linear regression (27). We therefore compared each anovulatory subgroup to the ovulatory cycles in a separate logistic regression.
Log-transformed whole-cycle integrated urinary hormones were adjusted for the length of the collection and modeled using random-effects linear regression (27), using PROC MIXED in SAS to handle within-woman correlation. Model predictors included those from the logistic regressions as well as baseline ovulatory/bleeding status. From these models, we estimated adjusted mean integrated hormones for subgroups defined by concurrent ovulatory/bleeding status, baseline age and time on study, ethnicity, and baseline metabolic syndrome status.
Observations with missing covariate data were omitted from the analytic sample. Collections that did not begin with menstrual bleeding were classified into one of the three cycle categories when possible: ovulatory, anovulatory with bleeding, or anovulatory without bleeding. Separate algorithms were generated to assess whether these cycles fit the three-category classification appropriately. Results are depicted as mean ± sd.
Results
Characteristics of the sample
At baseline, reflecting study design, approximately 40% of participants were Chinese or Japanese, and about 75% were early perimenopausal (Table 1). Approximately 50% had a BMI of 25 kg/m2 or less, and only 10% were current smokers. Mean age increased by approximately a year between consecutive visits; mean BMI also increased. The percentage of perimenopausal observations rose as well, as did mean collection length, whereas the percentage of ovulatory collections declined. At H1, of 834 women providing interpretable hormone data, 26 (3.1%) were missing covariate data. Comparable numbers at H2 were 700 and 39 (5.6%), and at H3, 624 and 36 (5.8%), respectively. Attrition between H1 and H2 was 16.1%, and between H2 and H3 was 27.7%, and was related to having an anovulatory collection at H1, being early perimenopausal at H1, a longer H1 collection, or having a lower educational level. At H1, 27.0% of the participants were premenopausal (i.e. without menstrual cycle changes that defined them as having entered the MT), but by the H3 collection, only 13.3% were premenopausal (P < 0.0001 for change over time). By entry criteria, no women who contributed data at H1 had entered the late MT, but by the H3 collection, 9.5% of women had.
Table 1.
Characteristics of analytic sample at each DHS visit
| % (n) or mean (sd)
|
|||
|---|---|---|---|
| H1 (n = 834) | H2 (n = 700) | H3 (n = 624) | |
| Ethnicity | |||
| African-American | 21.0 (175) | 21.3 (149) | 20.0 (125) |
| Caucasian | 30.6 (255) | 30.0 (210) | 29.3 (183) |
| Chinese | 18.1 (151) | 18.9 (132) | 19.6 (122) |
| Hispanic | 10.0 (83) | 8.7 (61) | 8.0 (50) |
| Japanese | 20.4 (170) | 21.1 (148) | 23.1 (144) |
| Age (yr) | 47.3 (2.5) | 48.4 (2.5) | 49.3 (2.5) |
| Menopause status | |||
| Pre | 27.0 (225) | 19.3 (135) | 13.3 (83) |
| Early peri | 73.0 (609) | 73.6 (515) | 72.9 (455) |
| Late peri | 0.0 (0) | 6.6 (46) | 9.5 (59) |
| Post | 0.0 (0) | 0.6 (4) | 4.3 (27) |
| Concurrent BMI | |||
| Normal/underweight | 45.4 (379) | 43.7 (306) | 43.6 (272) |
| Overweight | 26.9 (224) | 26.4 (185) | 26.0 (162) |
| Obese | 27.7 (231) | 29.9 (209) | 30.5 (190) |
| Current smoking at baseline | |||
| No | 89.8 (749) | 89.0 (623) | 89.9 (561) |
| Yes | 10.2 (85) | 11.0 (77) | 10.1 (63) |
| Educational level | |||
| Less than High school | 8.0 (67) | 7.9 (55) | 7.1 (44) |
| High school | 18.2 (152) | 16.7 (117) | 16.5 (103) |
| Some college | 30.3 (253) | 32.1 (225) | 31.6 (197) |
| Completed college | 22.8 (190) | 22.9 (160) | 24.0 (150) |
| Postgraduate | 20.6 (172) | 20.4 (143) | 20.8 (130) |
| Routine physical activity at baseline | 2.4 (0.8) | 2.4 (0.8) | 2.4 (0.8) |
| Collection length (d) | 29.6 (8.4) | 32.0 (10.4) | 32.9 (11.0) |
| Presumed ovulatory status | |||
| Ovulatory | 80.9 (675) | 72.1 (505) | 64.7 (404) |
| Anovulatory cycle ending with bleeding | 10.7 (89) | 10.6 (74) | 11.2 (70) |
| Anovulatory/nonbleeding | 8.4 (70) | 17.3 (121) | 24.0 (150) |
Associations with change in cycle type over time
The proportion of ovulatory cycles progressively decreased over time, constituting 80.9% of cycles at H1 and declined to 64.7% of cycles by H3. Anovulatory collections that ended in an episode of bleeding within 50 d were relatively stable, about 10% of the sample, whereas the proportion of anovulatory collections that did not end with a bleeding episode by 50 d nearly tripled, increasing from 8.4% of cycles at H1 to 24% of cycles at H3.
Obesity was associated with an increased probability of an anovulatory cycle that ended with a bleed [odds ratio (OR) = 4.68; 95% confidence interval (CI) = 1.33–16.52); P = 0.02]. A greater than high school education was also associated with an increased probability of an anovulatory/bleeding collection (OR = 2.12; CI = 1.22–3.69; P = 0.02; Table 2).
Table 2.
Adjusted ORs from multivariate random-effects logistic regression for anovulatory cycles ending with and without a bleed vs. ovulatory cycles before adding glucose metabolism and annual hormone variables as predictors
| Predictor | OR (CI)
|
|
|---|---|---|
| Anovulatory cycles ending with a bleed vs. ovulatory cycles (1817 observations/802 women) | Anovulatory/nonbleeding collections vs. ovulatory cycles (1925 observations/831 women) | |
| Ethnicity | ||
| Caucasian | Reference | Reference |
| African-American | 1.08 (0.61–1.92) | 1.91 (1.06–3.43)a |
| Chinese | 1.66 (0.76–3.62) | 0.68 (0.32–1.46) |
| Hispanic | 2.13 (0.96–4.72) | 1.50 (0.62–3.59) |
| Japanese | 1.02 (0.48–2.19) | 0.62 (0.29–1.31) |
| Overall P value | 0.17 | 0.19 |
| Concurrent menopause status | ||
| Pre | Reference | Reference |
| Early peri | 0.96 (0.42–2.23) | 2.66 (1.17–6.04)a |
| Late peri/post | 0.78 (0.17–3.71) | 56.21 (18.79–168.12)a |
| Overall P value | 0.95 | <0.0001 |
| Concurrent BMI | ||
| Normal/underweight | Reference | Reference |
| Overweight | 1.37 (0.57–3.32) | 1.41 (0.61–3.21) |
| Obese | 4.68 (1.33–16.52)a | 2.66 (0.77–9.20) |
| Overall P value | 0.02 | 0.29 |
| Baseline smoking | ||
| No | Reference | Reference |
| Yes | 0.98 (0.54–1.78) | 1.10 (0.60–2.02) |
| P value | 0.94 | 0.76 |
| H1 age (1-yr difference) | 1.21 (1.12–1.31)a | 1.51 (1.40–1.63)a |
| P value | <0.0001 | <0.0001 |
| Time since H1 (1-yr aging) | 1.17 (0.97–1.43) | 1.74 (1.44–2.11)a |
| P value | 0.11 | <0.0001 |
| Baseline routine physical activity (1 unit difference) | 0.93 (0.73–1.19) | 1.12 (0.88–1.43) |
| P value | 0.55 | 0.36 |
| Educational level | ||
| Less than high school | 1.47 (0.67–3.20) | 3.51 (1.62–7.62)a |
| High school | Reference | Reference |
| More than high school | 2.12 (1.22–3.69)a | 1.74 (0.98–3.09) |
| Completed college | 1.10 (0.60–2.03) | 1.16 (0.61–2.19) |
| Graduate work | 1.23 (0.65–2.33) | 1.68 (0.89–3.16) |
| Overall P value | 0.02 | 0.01 |
| Collection length | ||
| <21 d | 10.59 (6.31–17.78) | |
| 22–35 d | Reference | |
| ≥36 d | 4.00 (2.64–6.05) | |
| Overall P value | <0.0001 | |
All ORs were adjusted for all other predictors in table as well as for region (East Coast/Mid-Atlantic, Midwest, West Coast), H1 baseline menopause status, and H1 categorized BMI.
P value for pairwise comparison with reference group < 0.05.
Not surprisingly, an increased OR of having an anovulatory/nonbleeding cycle was strongly associated with self-reported menstrual cycle changes at the annual interview, leading to classification as early (OR = 2.66; CI = 1.17–6.04) or late (OR = 56.21; CI = 18.79–168.12) perimenopause (P < 0.0001). No such association was observed for anovulatory cycles that ended with a bleed. Older baseline age and longer time on study were significantly positively related to both anovulatory bleeding (OR = 1.21; CI = 1.12–1.31; P < 0.0001, and OR = 1.17; CI = 0.97–1.43; P = 0.11, respectively) and anovulatory/nonbleeding collections (OR = 1.51; CI = 1.40–1.63; P < 0.0001, and OR = 1.74; CI = 1.44–2.11; P < 0.0001, respectively) compared with presumed ovulatory collections. Both short (<21 d) and long (≥36 d) collections were significantly associated with anovulatory cycles that ended with a bleed compared with ovulatory cycles (OR = 10.56; CI = 6.31–17.78, and OR = 4.00; CI = 2.64–6.05; P < 0.0001). African-American race was the only ethnicity associated with a higher probability of having an anovulatory/nonbleeding collection (OR = 1.91; CI = 1.06–3.43). Women with less than a high school education were also considerably more likely to experience an anovulatory/nonbleeding cycle at the H3 collection (OR = 3.51; CI = 1.62–7.62).
Association with changes in whole-cycle hormones
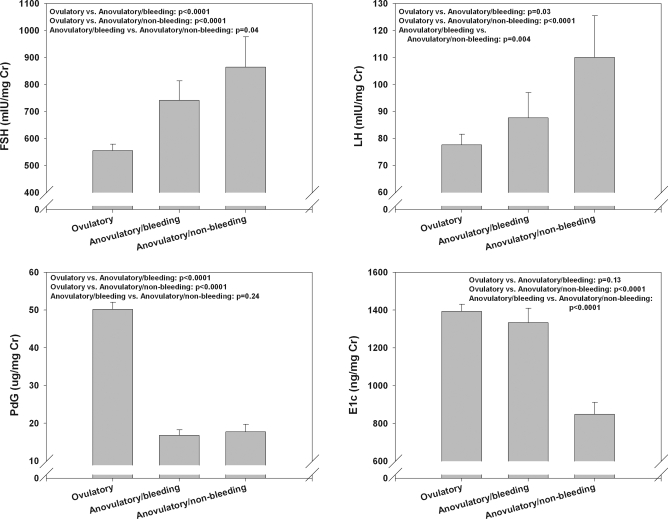
Adjusted mean integrated FSH was significantly increased in both types of anovulatory cycles (741.31 mIU/mg Cr in cycles ending with bleeding vs. 864.44 mIU/mg Cr in cycles with no bleeding) compared with ovulatory cycles (555.43 mIU/mg Cr, P < 0.0001; Fig. 1) and was higher in cycles that did not end with a bleed compared with those that did (P = 0.04; Fig. 2). Adjusted mean LH increased progressively from ovulatory (77.61 mIU/mg Cr) to anovulatory cycles with bleeding (87.59 mIU/mg Cr) to anovulatory/nonbleeding collections (110.04 mIU/mg Cr, P < 0.0001). Adjusted mean whole-cycle E1c was similar between ovulatory and anovulatory cycles ending with a bleed (1393.94 vs. 1333.46 μg/mg Cr) but was greatly decreased in anovulatory/nonbleeding collections (848.27 μg/mg Cr, P < 0.0001). Mean Pdg was, by definition, markedly lower in both types of anovulatory cycles (17.85 μg/mg Cr for cycles ending with a bleed, and 18.76 μg/mg Cr for ones that did not) compared with ovulatory cycles (51.25 μg/mg Cr).
Figure 1.
Adjusted mean whole-cycle urinary reproductive hormones by cycle type. The indicated relationship and statistical significance level for each hormone are shown in the upper portion of each panel.
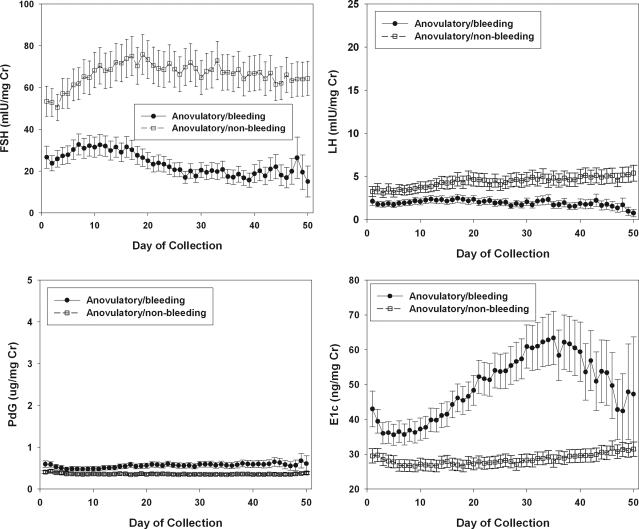
Figure 2.
Mean daily patterns of FSH, LH, Pdg, and E1c in cycles without luteal function. •, Anovulatory cycles ending with a bleed; ○, anovulatory/nonbleeding collections. Anovulatory collections that did not end with a bleed were terminated at 50 d.
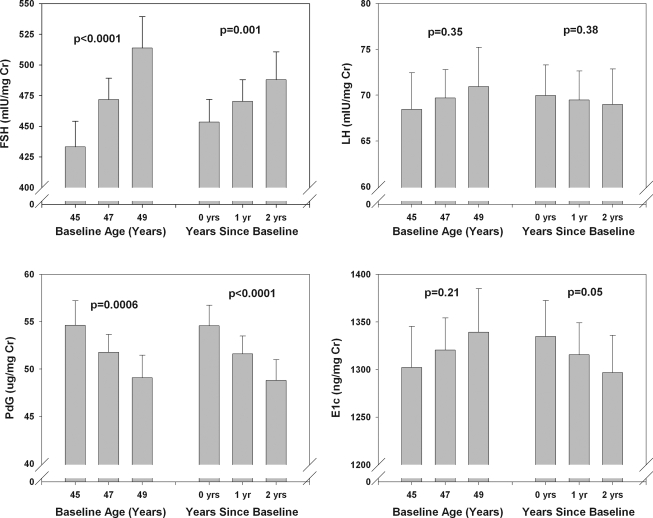
For all collections, baseline age and time in study were associated with change in whole-cycle integrated hormones (Fig. 3; data shown for ovulatory cycles only). For ovulatory cycles, FSH increased by an estimated 4.4% per year of baseline age and by 3.7% with each additional year on study. Pdg decreased significantly by 2.6% for each additional year of baseline age and by 5.4% with each additional year on study. LH was not significantly related to either baseline age (an increase of 0.9% per year, P = 0.35) or time on study (decrease of 0.7% per year, P = 0.58). E1c decreased by 1.4% per year on study (P = 0.05) but did not vary by baseline age. Relationships were similar when both types of anovulatory cycles were added to the ovulatory ones.
Figure 3.
Adjusted mean whole-cycle urinary reproductive hormones (with CI) by age and elapsed years since baseline, ovulatory cycles only. The P value for each relationship is shown above the trio of bars to which it refers.
Ethnicity and whole-cycle hormones
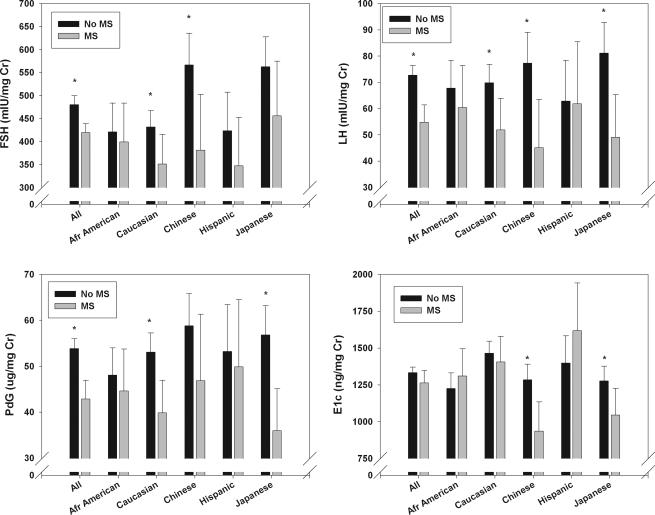
Among ovulatory cycles, integrated LH (P = 0.52) and Pdg (P = 0.97) were similar across all ethnic groups. However, FSH was significantly greater in Chinese- and Japanese-American women compared with Caucasian women (P = 0.03 and 0.009, respectively), and E1c was significantly lower (P < 0.0001) in Chinese-, Japanese-, and African-American women compared with Caucasians and Hispanic women. These relationships can be appreciated in Fig. 4.
Figure 4.
Adjusted mean whole-cycle urinary reproductive hormones (with CI) by ethnicity and metabolic syndrome, ovulatory cycles only. Asterisks above each pair of bars indicate statistical significance for metabolic syndrome-related differences. *, P < 0.05.
Relationship between serum hormones and cycle types
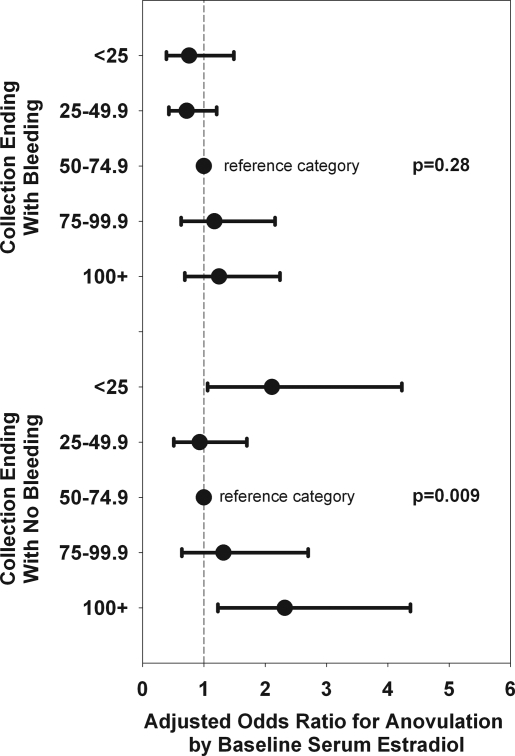
We estimated associations of annual serum hormone concentrations with cycle type, again adjusting for participant characteristics in Table 2, and for day of menstrual cycle at the annual visit’s blood draw. Serum estradiol at the SWAN baseline examination has been strongly associated with time to the final menstrual period (28). We therefore tested the association of estradiol at baseline with cycle type by dividing estradiol into quintiles, ranging from less than 25 pg/ml (6.9 nmol/ml) to 100 pg/ml or higher (27.8 nmol/ml). As seen in Fig. 5, estradiol exhibited a curvilinear association with anovulatory collections that did not end with a bleed but not with those that did end with a bleed. Both the lowest and the highest baseline estradiol categories were more likely to be anovulatory/nonbleeding collections than ovulatory cycles compared with the reference category of 50–74.9 pg/ml. Interestingly, categorized estradiol from the concurrent core visit was not significantly related to cycle type after adjustment for other factors.
Figure 5.
Adjusted OR for anovulation by baseline serum estradiol. The vertical line in the middle of the figure represents unity (OR = 1). The OR is shown as the middle of each horizontal line, with the outer limits representing the lower and upper CI.
Relationship of metabolic markers to daily hormones
We examined associations of core baseline insulin sensitivity indicators with cycle type, adjusting for participant characteristics in Table 2 and omitting women with prevalent diabetes at the core baseline measurement (Table 3). With the exception of (log-transformed) β-cell function, which was marginally associated with an increased probability of an anovulatory/nonbleeding collection vs. an ovulatory collection (OR = 1.33; CI = 0.99–1.78; P = 0.06), insulin sensitivity indicators were overall not associated with cycle type.
Table 3.
Adjusted ORs for baseline glucose metabolism indicators from multivariate random-effects logistic regression for anovulatory cycles ending with and without a bleed vs. ovulatory cycles, adjusting for all predictor variables in models in Table 2
| Baseline glucose metabolism indicatora | Anovulatory cycles ending with a bleed vs. ovulatory cycles (1666 observations/732 women)
|
Anovulatory/nonbleeding collections vs. ovulatory cycles (1758 observations/754 women)
|
||
|---|---|---|---|---|
| Adjusted OR (CI) | P value | Adjusted OR (CI) | P value | |
| Log glucose | 1.11 (0.85–1.44) | 0.44 | 0.84 (0.63–1.10) | 0.20 |
| Log insulin | 1.22 (0.90–1.65) | 0.20 | 1.18 (0.87–1.61) | 0.28 |
| Log β-cell function | 1.12 (0.84–1.49) | 0.43 | 1.33 (0.99–1.78) | 0.06 |
| Log insulin resistance | 1.23 (0.91–1.68) | 0.18 | 1.12 (0.82–1.54) | 0.47 |
| Log insulin sensitivity | 0.82 (0.61–1.11) | 0.20 | 0.85 (0.62–1.16) | 0.31 |
| Metabolic syndrome | 0.66 | 0.33 | ||
| No | Reference | Reference | ||
| Yes | 0.88 (0.51–1.55) | 0.73 (0.39–1.37) | ||
| Diabetesb | ||||
| No | Reference | 0.35 | Reference | 0.73 |
| Yes | 0.65 (0.26–1.61) | 1.16 (0.50–2.71) | ||
For all predictors except metabolic syndrome, ORs were computed comparing the 75th percentile to the 25th percentile. Each glucose metabolism indicator is included separately.
These analyses include women with prevalent diabetes mellitus at baseline.
Mean whole-cycle hormones also were compared in women with and without metabolic syndrome at the core baseline visit, after adjusting for participant characteristics in Table 2, with the exception of H1 and concurrent BMI, because BMI is highly related to waist circumference, a component of the metabolic syndrome, as well as collection length and cycle type. As can be seen in Fig. 4, in the subset of ovulatory cycles, FSH, LH, and Pdg were significantly greater in women with the metabolic syndrome, whereas E1c was lower in Chinese and higher in Hispanic women with metabolic syndrome.
Discussion
A number of investigators have attempted to address the dynamics of reproductive hormone production across the menstrual cycle of perimenopausal women. Many of these experimental designs did not use daily sampling, and durations of study from as short as one cycle to as many as 18 months have been used to attempt to provide both cross-sectional and longitudinal data. Metcalf et al. observed evidence of declining luteal function by decade of life (29) and in association with cycle irregularity in women in their 40s (30). In another study of 50 cycles of women over age 40, a variety of cycle types were categorized using weekly urine sampling (31), and a tendency for anovulatory cycles to be observed more frequently as women’s cycles departed more from normal premenopausal patterns was also observed. These studies used weekly sampling to determine Pdg excretion, an interval that might be insufficient to detect ovulatory Pdg patterns, especially if luteal phases are shorter in perimenopausal women. Others have noted reduced luteal Pdg (32) and luteal inhibin A (33,34,35) in older reproductive-aged women, using daily urinary and serum sampling, respectively. In the FREEDOM Study, daily urinary hormones were observed in a cohort of 34 women for periods of time from 6–18 months (36). In this study, reduced Pdg was associated with higher E1c excretion. On the other hand, Landgren et al. (37) examined hormones collected three times per week for 4 wk on an annual basis in a cohort of 13 women who were followed from 4–9 yr up to their FMP. Although the proportion of anovulatory cycles increased with progress to menopause, an increase in cycles with low luteal progesterone production was not reported. Another study of cycles of women in their 40s did not observe reduced luteal progesterone secretion (38).
Our findings confirm a small but detectable decrease in Pdg excretion in apparently ovulatory cycles in serial collections taken 1 yr apart. Thus, our longitudinal data, taken from a larger sample than previous studies, support the notion that progressive luteal dysfunction is a characteristic of the MT.
The decrease in luteal Pdg we observed with each year of participation in the DHS is small, although statistically and perhaps biologically significant, because it may help explain the decreased cycle fecundity in women of advanced reproductive age. Reduced luteal function may be due to a primary defect of the corpus luteum; however, existing models of cycle changes in association with the menopause transition favor the hypothesis that reduced luteal function is an end result of impaired folliculogenesis (39). Reduced inhibin B in the follicular phase of the menstrual cycle was an early event in the MT in the Melbourne Women’s Health Cohort study (40). The rise in FSH that accompanies the loss of inhibin B has been associated with shortened follicular phases and abnormal follicle development compared with younger reproductive-aged women (41,42). Taken together, these observations favor the hypothesis that folliculogenesis is less effective with reproductive aging, and this leads to luteal dysfunction and anovulation. Decreasing Pdg was associated with progression of menopausal status, as defined by self-reported cycle patterns at the annual interview.
Anovulatory cycles not associated with a bleeding episode were modestly associated with subsequent progress from pre-menopause to early menopause and very strongly associated with transition from early to late perimenopausal status. In contrast, anovulatory cycles with a bleeding episode were not significantly related to progress to the early or late stages of the transition and were associated with near-normal E1c excretion. On the other hand, anovulatory/nonbleeding collections were relatively hypoestrogenic. Our data collection paradigm did not allow us to observe beyond 50 d of collection; we therefore cannot know whether or not folliculogeneis and ovulation occurred in some of the apparently anovulatory/nonbleeding collections after 50 d. Our data suggest that the progression of cycle types associated with transition to menopause begins with ovulatory changing to anovulatory/nonbleeding cycles and then to menopause. Anovulatory cycles ending with a bleed were not related to the transit time. It remains to be seen if the appearance of anovulatory/nonbleeding collections is more closely associated with time to the FMP. The relationship of anovulatory/nonbleeding cycles to both low and high early follicular-phase serum estradiol, as reported by us previously as a strong predictor of the FMP (28), argues that progress to an anovulatory/nonbleeding cycle type will prove to be predictive of a shorter time to the FMP. A similar linkage of high estrogen to a more rapid transit to menopause has been observed by others in smaller cross-sectional studies (31,36). In SWAN, which collects blood in the early follicular phase when women are cycling regularly and randomly when women become irregular, the higher estrogen levels may be due in part to the change in timing of the blood collection.
Many outcomes in SWAN have been associated with markers of the metabolic syndrome (9,43,44). In this analysis, other than a borderline association of β-cell function to ovulatory status, no direct relationship was observed between cycle types and metabolic markers. However, a small number of women who became menopausal and a larger proportion of women who did not continue to complete DHS collections beyond the first year did not contribute data to this study. The attrition of data is biased toward women with a more advanced menopausal status, a state that would be likely to be associated with worsening glucose metabolism. Therefore, a systematic underestimation of the amount of metabolic challenge incurred as women traverse the menopause may have accounted for our lack of statistically significant findings.
On the other hand, obesity per se was strongly associated with an increased likelihood of anovulation ending in bleeding vs. having an ovulatory cycle and has been previously shown to be strongly related to reduced whole-cycle (8,45,46) and early follicular-phase (10,11) hormones. A high BMI has also been shown to be associated with a higher proportion of involuntary infertility (19,47). It is likely that increasing BMI modulates reproductive hormones through the transition, perhaps by increasing the relative risk for anovulation. Obesity is a metabolic stressor that is also highly concordant with waist circumference, a key component of the definition of metabolic syndrome. Analyses of metabolic markers did not adjust for BMI for this reason. Despite this lack of adjustment, a significant relationship was still not evident. It therefore seems likely that BMI influences reproductive hormones in a manner apart from its relationship to the metabolic syndrome.
We have also observed differences in daily hormones by ethnicity in SWAN (10,11). The lower LH and E1c observed in both Chinese and Japanese women as well as the lower E1c in African-American women is not readily explainable on the basis of known characteristics of the sample. For example, if race/ethnicity interacted with BMI, accounting for these differences, one would not expect both the leaner groups (Chinese and Japanese) to be more similar to each other than to the group in SWAN with the highest BMI (African-Americans). Lower circulating estrone and estradiol have been previously reported in Japanese compared with American women and attributed to altered enzymatic metabolism (20). It is possible that these mechanisms are shared among both Asian and African-American ethnicities. However, such a relationship cannot explain the ethnic differences in the gonadotropin patterns we observed.
Educational level was related to ovulatory status in a complex way. Greater than high school education was associated with anovulatory cycles ending with a bleed, but by H3, women with less than a high school education were more likely to have anovulatory/nonbleeding collections. It is unclear how social factors such as educational level might be causally related to ovulatory status, but such factors do have a bearing on other characteristics of participants, such as BMI, which in turn appear to be strongly related to ovulatory status. Alternatively, such apparent relationships may simply be spurious and should be verified in other studies.
Our study has some weaknesses that should be acknowledged. Attrition from our baseline DHS sample was not random and included more women with a variety of characteristics associated with a shorter time to their FMP. Thus, our data may be biased toward detecting less change over time than would have been detected had we been able to capture more cycles in the longitudinal sample, thereby reducing our ability to detect true changes over time. To compensate for the loss of cycles that were expected to occur as women progressed farther through their MT, we attempted to increase our ability to use data from women whose collections did not begin or end with a bleed. This adjustment appeared to introduce minimal bias when analyses were carried out with or without inclusion of these women’s cycles. Urinary hormones, although useful for interpretation of cycle patterns, do not perfectly reflect circulating sex steroids and gonadotropins. This is most true for E1c, which measures a mixture of E1cs and reflects both serum estradiol and estrone. Because our women are still in the earlier stages of the MT, the contribution of estrone to the overall pool of bioactive estrogen is relatively minor; however, the molar quantity of estrone is relatively high, and thus, urinary E1c is relatively less sensitive to fluctuations in estradiol. This aspect of the assay makes it difficult to discern estradiol-related differences in E1c between groups and over time.
In summary, we have shown that daily reproductive hormone assessments in urinary samples can provide valuable longitudinal data that further elucidate the physiology of the MT. Both declining luteal progesterone excretion in ovulatory cycles and the appearance of long, anovulatory cycles (>50 d) that do not result in bleeding appear to herald progress through the MT. Both low and high early follicular-phase estradiol also appears to herald progress through the transition. Anovulation with bleeding intervals of fewer than 50 d does not appear to be associated with progress through the transition but may be associated with obesity. As SWAN accrues more women who have undergone their FMP, the ability to form causal inferences should be strengthened.
Acknowledgments
The following clinical centers participated in the study: University of Michigan, Ann Arbor, MI [MaryFran Sowers, Principal Investigator (PI)]; Massachusetts General Hospital, Boston, MA (Robert Neer, PI 1994–1999; Joel Finkelstein, PI 1999 to present); Rush University, Rush University Medical Center, Chicago, IL (Lynda Powell, PI); University of California, Davis/Kaiser, Davis, CA (Ellen Gold, PI); University of California, Los Angeles, Los Angeles, CA (Gail Greendale, PI); University of Medicine and Dentistry–New Jersey Medical School, Newark, NJ (Gerson Weiss, PI 1994–2004; Nanette Santoro, PI 2004 to present); and the University of Pittsburgh, Pittsburgh, PA (Karen Matthews, PI).
National Institutes of Health (NIH) Program Office: National Institute on Aging, Bethesda, MD (Marcia Ory 1994–2001; Sherry Sherman 1994 to present); National Institute of Nursing Research, Bethesda, MD (Program Officers).
Central Laboratory: University of Michigan, Ann Arbor, MI (Daniel McConnell, Central Ligand Assay Satellite Services). Coordinating Center: New England Research Institutes, Watertown, MA (Sonja McKinlay, PI 1995–2001); University of Pittsburgh, Pittsburgh, PA (Kim Sutton-Tyrrell, PI 2001 to present).
Steering Committee consisted of Chris Gallagher and Susan Johnson, Chairs.
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
SWAN has grant support from the NIH, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women’s Health (Grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495).
Disclosure Statement: N.S., S.L.C., W.L.L., J.L.L., K.A.M., D.M., J.F.R., E.B.G., G.A.G., S.G.K., L.P., M.F.S., and G.W. have nothing to disclose.
First Published Online February 19, 2008
Abbreviations: BMI, Body mass index; CI, 95% confidence interval; Cr, creatinine; DHS, Daily Hormone Study; E1c, estrone conjugate; FMP, final menstrual period; H1, baseline, or first collection; MT, menopause transition; OR, odds ratio; Pdg, pregnanediol glucuronide; SWAN, Study of Women’s Health Across the Nation.
References
- Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L 1999 Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 84:4025–4030 [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Gracia CR, Kapoor S, Lin H, Liu L, Nelson DB 2005 Follicular phase hormone levels and menstrual bleeding status in the approach to menopause. Fertil Steril 83:383–392 [DOI] [PubMed] [Google Scholar]
- Ferrell RJ, O’Connor KA, Rodriguez G, Gorrindo T, Holman DJ, Brindle E, Miller RC, Schechter DE, Korshalla L, Simon JA, Mansfield PK, Wood JW, Weinstein M 2005 Monitoring reproductive aging in a 5-year prospective study: aggregate and individual changes in steroid hormones and menstrual cycle lengths with age. Menopause 12:567–577 [DOI] [PubMed] [Google Scholar]
- Metcalf MG, Livesey JH 1985 Gonadotrophin excretion in fertile women: effect of age and the onset of the menopausal transition. J Endocrinol 105:357–362 [DOI] [PubMed] [Google Scholar]
- Sherman BM, Korenman SG 1975 Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest 55:699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BM, West JH, Korenman SG 1976 The menopausal transition: analysis of LH, FSH, estradiol and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab 42:629–636 [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Robertson DM, Dennerstein L 2002 Hormonal changes in the menopause transition. Recent Prog Horm Res 57:257–275 [DOI] [PubMed] [Google Scholar]
- Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, Finkelstein JS, Greendale GA, Kelsey J, Korenman S, Luborsky JL, Matthews K, Midgeley R, Powell L, Sabatine J, Schocken M, Sowers MF, Weiss G 2004 Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition. J Clin Endocrinol Metab 89:2622–2634 [DOI] [PubMed] [Google Scholar]
- Santoro N, Torrens J, Crawford S, Allsworth JE, Finkelstein JS, Gold EB, Korenman S, Lasley WL, Luborsky JL, McConnell D, Sowers MF, Weiss G 2005 Correlates of circulating androgens in mid-life women: the study of women’s health across the nation. J Clin Endocrinol Metab 90:4836–4845 [DOI] [PubMed] [Google Scholar]
- Randolph Jr JF, Sowers MF, Gold EB, Mohr BA, Luborsky J, Santoro N, McConnell DS, Finkelstein JS, Korenman SG, Matthews KA, Sternfeld B, Lasley BL 2003 Reproductive hormones in the early menopause transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab 88:1516–1522 [DOI] [PubMed] [Google Scholar]
- Randolph Jr JF, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ 2004 Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab 89:1555–1561 [DOI] [PubMed] [Google Scholar]
- Sowers MF, Crawford SL, Sternfeld B 2000 SWAN: a multicenter, multiethnic community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: biology and pathobiology. New York: Academic Press; 175–188 [Google Scholar]
- Santoro N, Crawford SL, Allsworth JE, Gold EB, Greendale GA, Korenman S, Lasley BL, McConnell D, McGaffigan P, Midgely R, Schocken M, Sowers M, Weiss G 2003 Assessing menstrual cycles using urinary hormone assays. Am J Physiol Endocrinol Metab 284:E521–E530 [DOI] [PubMed] [Google Scholar]
- Brambilla DJ, McKinlay SM, Johannes CB 1994 Defining the perimenopause for application in epidemiologic investigations. Am J Epidemiol 140:1091–1095 [DOI] [PubMed] [Google Scholar]
- Dudley EC, Hopper JL, Taffe J, Guthrie JR, Burger HG, Dennerstein L 1998 Using longitudinal data to define the perimenopause by menstrual cycle characteristics. Climacteric 1:18–25 [DOI] [PubMed] [Google Scholar]
- Expert Panel on the Detection, Evaluation and Treatment of High Blood Cholesterol in Adults 2001 Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on the Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL 1996 Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environ Health Perspect 104:408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL 1998 Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. Am J Epidemiol 147:1071–1080 [DOI] [PubMed] [Google Scholar]
- Gesink-Law DC, Maclehose RF, Longnecker MP 2007 Obesity and time to pregnancy. Hum Reprod 22:414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Ross RK, Bernstein L, Pike MC, Henderson BE 1990 Serum oestrogen levels in postmenopausal women: comparison of American whites and Japanese in Japan. Br J Cancer 62:451–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkau B, Charles MA, Drivsholm T, Borch-Johnsen K, Wareham N, Yudkin JS, Morris R, Zavaroni I, van Dam R, Feskins E, Gabriel R, Diet M, Nilsson P, Hedblad B; European Group For The Study Of Insulin Resistance (EGIR) 2002 Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab 28:364–376 [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Ainsworth BA, Quesenberry Jr CP 1999 Physical activity patterns in a diverse population of women. Prev Med 28:313–323 [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Wang H, Quesenberry Jr CP, Abrams B, Everson Rose, SA, Greendale GA, Matthews KA, Torrens JI, Soweers M 2004 Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women’s Health Across the Nation. Am J Epidemiol 160:912–922 [DOI] [PubMed] [Google Scholar]
- Baecke JAH, Burema J, Fritjers JER 1982 A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36:936–942 [DOI] [PubMed] [Google Scholar]
- Molenberghs G, Verbeke G 2005 Models for discrete longitudinal data. New York: Springer [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH 2004 Applied longitudinal analysis. New York: John Wiley [Google Scholar]
- Santoro N, Brockwell S, Johnston J, Crawford SL, Gold EB, Harlow SD, Matthews KA, Sutton-Tyrrell K 2007 Helping midlife women predict the onset of the final menses: SWAN, the Study of Women’s Health Across the Nation. Menopause 14:415–424 [DOI] [PubMed] [Google Scholar]
- Metcalf MG, Donald RA, Livesey JH 1981 Pituitary ovarian function in normal women during the menopausal transition. Clin Endocrinol (Oxf) 14:234–255 [DOI] [PubMed] [Google Scholar]
- Metcalf MG 1979 Incidence of ovulatory cycles in women approaching menopause. J Biosoc Sci 11:39–48 [DOI] [PubMed] [Google Scholar]
- Metcalf MG, Donald RA, Livesey JH 1981 Classification of menstrual cycles in pre- and perimenopausal women. J Endocrinol 91:1–10 [DOI] [PubMed] [Google Scholar]
- Santoro N, Brown JR, Adel T, Skurnick JH 1996 Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab 81:1495–1501 [DOI] [PubMed] [Google Scholar]
- Reame NE, Wyman TL, Phillips DJ, de Kretser DM, Padmanabhan V 1998 Net increase in stimulatory input resulting from a decrease in inhibin B and an increase in activin A may contribute in part to the rise in follicular phase follicle-stimulating hormone of aging cycling women. J Clin Endocrinol Metab 83:3302–3307 [DOI] [PubMed] [Google Scholar]
- Santoro N, Adel T, Skurnick JH 1999 Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril 71:658–662 [DOI] [PubMed] [Google Scholar]
- Welt CK, McNicholl DJ, Taylor AE, Hall JE 1999 Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab 84:105–111 [DOI] [PubMed] [Google Scholar]
- Miro F, Parker SW, Aspinall J, Coley J, Perry PW, Ellis JE 2004 Origins and consequences of the elongation of the human menstrual cycle during the menopausal transition: the FREEDOM Study. J Clin Endocrinol Metab 89:4910–4915 [DOI] [PubMed] [Google Scholar]
- Landgren BM, Collins A, Csemiczky G, Buger HG, Baksheev L, Robertson DM 2004 Menopause transition: annual changes in serum hormonal patterns over the menstrual cycle in women during a nine-year period prior to menopause. J Clin Endocrinol Metab 89:2763–2769 [DOI] [PubMed] [Google Scholar]
- Batista MC, Cartledge TP, Zellmer AW, Merino MJ, Axiotis C, Bremner WJ, Nieman LK 1995 Effects of aging on menstrual cycle hormones and endometrial maturation. Fertil Steril 64:492–499 [PubMed] [Google Scholar]
- Sherman BM, Korenman SG 1974 Measurement of serum LH, FSH, estradiol and progesterone in disorders of the human menstrual cycle: the inadequate luteal phase. J Clin Endocrinol Metab 39:145–149 [DOI] [PubMed] [Google Scholar]
- Burger HG, Cahir N, Robertson DM, Groome N, Dudley EC, Green A, Dennerstein L 1998 Serum inhibins A and B fall differentially as FSH rises in perimenopausal women. Clin Endocrinol (Oxf) 48:09–813 [DOI] [PubMed] [Google Scholar]
- Klein NA, Illingworth PJ, Groome NP, McNeilly AS, Battaglia DE, Soules MR 1996 Decreased inhibin B secretion is associated with the monotropic rise of FSH in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous cycles. J Clin Endocrinol Metab 81:2742–2745 [DOI] [PubMed] [Google Scholar]
- Santoro N, Isaac B, Neal-Perry G, Adel T, Weingart L, Nussbaum A, Thakur S, Jinnai H, Khosla N, Barad D 2003 Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab 88:5502–5509 [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, Pasternak RC, Lloyd-Jones D, Sowers MF, Torrens JI; SWAN Investigators 2005 Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation 111:1242–1249 [DOI] [PubMed] [Google Scholar]
- Lo JC, Zhao X, Scuteri A, Brockwell S, Sowers MR 2006 The association of genetic polymorphisms in sex hormone biosynthesis and action with insulin sensitivity and diabetes mellitus in women at midlife. Am J Med 119(Suppl 1):S69–S78 [DOI] [PubMed] [Google Scholar]
- Grenman S, Ronnemaa T, Irjala K, Kaihola HL, Gronroos M 1986 Gonadotropin, cortisol and prolactin levels in healthy, massively obese women: correlation with abdominal fat cell size and effect of weight reduction. J Clin Endocrinol Metab 63:1257–1261 [DOI] [PubMed] [Google Scholar]
- Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, Gibbs K, Polotsky HN, Feng S, Isaac B, Santoro N 2007 Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab 92:2468–2473 [DOI] [PubMed] [Google Scholar]
- Polotsky AJ, Hailpern S, Skurnick J, Lo J, Sternfeld B, Santoro N, Adolescent obesity exerts a detrimental effect on lifetime parity, independent of adult body mass: baseline data from the Study of Women’s Health Across the Nation (SWAN). Presented at the 57th Annual Meeting of the Society for Gynecologic Investigation, Reno Nevada, 2007, Reproductive Sciences 14:225A (Abstract 595) [Google Scholar]