Abstract
Background and objectives: Early renal function decline begins before the onset of proteinuria in patients with type 1 diabetes. The association of elevated serum uric acid with advanced impaired renal function prompts an examination of its role in early renal function decline in patients before proteinuria develops.
Design, setting, participants, & measurements: Patients with type 1 diabetes and normoalbuminuria or microalbuminuria were recruited to the Second Joslin Kidney Study. A medical history and measurements of BP, hemoglobin A1c, albumin excretion rate, and serum concentrations of uric acid and cystatin C were obtained. Estimated glomerular filtration rate was measured by a cystatin C–based formula.
Results: We studied 364 patients with normoalbuminuria and 311 patients with microalbuminuria. Mean glomerular filtration rate in these groups was 119 and 99 ml/min, respectively. Mildly or moderately impaired renal function (<90 ml/min) was present in 10% of those with normoalbuminuria and 36% of those with microalbuminuria. In univariate and multivariate analyses, lower glomerular filtration rate was strongly and independently associated with higher serum uric acid and higher urinary albumin excretion rate, older age, and antihypertensive treatment.
Conclusions: Serum uric acid concentration in the high-normal range is associated with impaired renal function in patients with type 1 diabetes. Follow-up studies are needed to confirm that this level of serum uric acid is a risk factor for early renal function decline in type 1 diabetes and to determine whether its reduction would prevent the decline.
Diabetic nephropathy (DN) is a devastating late complication of diabetes that historically necessitated renal replacement for one of four patients with type 1 diabetes (1). Its earliest clinical sign is a slight elevation of urinary albumin excretion (microalbuminuria [MA]). Formerly, this leakage was believed to progress inexorably to gross proteinuria, which destroyed nephrons and led to ESRD (2); however, our recent follow-up study of a cohort of patients with type 1 diabetes and MA documented more diverse outcomes within a few years of its onset. One was reversion of MA to normoalbuminuria (NA) in approximately half of the cohort (3). Another was progressive renal function decline in one third of the cohort many years before the appearance of overt proteinuria (4). In the updated model of DN, MA is still an early sign but a nonspecific one. The precursor of ESRD, “progressive early renal function decline,” develops in only a subset of those with MA, and it starts many years earlier than has been assumed (4). Such radical revisions highlight our ignorance of the pathogenesis of early progressive renal function decline in type 1 diabetes, a stage that is readily detectable by serial measurement of cystatin C in serum (4).
Recently, renewed attention has been given to serum uric acid because of its association with cardiovascular and renal disease and hypertension (5). Hyperuricemia, defined as a serum uric acid >6.6 in women and >7.0 mg/dl in men, occurs in many renal diseases (5). The mechanisms underlying hyperuricemia as a result of reduced renal clearance of uric acid may involve a reduced GFR or dysfunctional handling of filtered uric acid by proximal tubules (6).
Furthermore, elevated serum uric acid itself may increase the risk for development of renal disease in both the general population and patients with diabetes. For example, in a community-based study of Japanese adults, hyperuricemia emerged as the only significant risk factor of renal failure besides age. Hyperuricemia was more strongly predictive than proteinuria (7). Among Taiwanese adults with type 2 diabetes, hyperuricemia was correlated with albumin excretion rate, and among Italian adults also with type 2 diabetes, it was associated with an increased risk for the development of overt nephropathy (8,9).
The goal of this study was to examine the association between serum uric acid and renal function in a large cohort of patients with type 1 diabetes. Patients were recruited because they had either NA or MA and comprise the Second Joslin Study on the Natural History of Microalbuminuria. Unlike previous studies, GFR and serum concentration of uric acid were normal in a majority of those enrolled. GFR was estimated from the serum concentration of cystatin C (cC-GFR), which has been shown to be a reliable index of renal function in patients with normal or elevated renal function (10).
Materials and Methods
Joslin Clinic Population
The study group was selected from the population attending the Joslin Clinic, a major center for the treatment of patients of all ages with type 1 or type 2 diabetes. The population is approximately 90% white, and most reside in eastern Massachusetts. Typically, patients come to the clinic within 5 yr of the diagnosis of diabetes, and a large proportion remain under the care of the clinic for decades. Demographic information and data generated during clinic visits are maintained in the Joslin electronic medical record system.
During the period January 1, 2003, through December 31, 2004, patients who scheduled an appointment at the Joslin Clinic were screened in the data system for eligibility for this investigation, designated the Second Joslin Study on the Natural History of Microalbuminuria. Eligibility criteria included residence in Massachusetts, diabetes diagnosed before age 40 yr, treatment with insulin, current age 18 to 64 yr, diabetes duration 3 to 39 yr, and multiple measurements in the preceding 2-yr interval of hemoglobin A1c (HbA1c) and urinary albumin-to-creatinine ratio (ACR). For each patient, these laboratory measurements were summarized by their medians to characterize the current HbA1c and ACR status. Exclusion criteria were proteinuria (median ACR ≥250 for men and ≥355 μg/min for women), ESRD, or renal transplant. Because one of the aims of the Second Joslin Study is to identify risk factors that are associated with early renal function decline in the setting of hyperglycemia, patients with HbA1c <6.5% (near normoglycemia) were also excluded. Of the 14,500 residents of New England who attended the Joslin Clinic during the screening phase, these criteria identified 2667 eligible patients, including 2007 with NA (median ACR <17 for men and <25 for women) and 660 with MA (median ACR 17 to 250 for men and 25 to 355 for women) (11).
Enrollment and Examination
The Committee on Human Subjects of the Joslin Diabetes Center approved the protocol and informed consent procedures for the study. Our goal was to enroll and examine all eligible patients with MA and an equal number of eligible patients with NA. Study recruiters approached patients who were identified as eligible on their arrival at the clinic. After informing patients about the study and obtaining their written consent to participate, the recruiter (1) administered a structured interview that solicited the history of diabetes and its treatment, other health problems, and medications; (2) measured BP; and (3) obtained samples of blood and urine.
Enrollment continues for the Second Joslin Study on the Natural History of Microalbuminuria, but for this first report, the study group is restricted to those enrolled by the end of 2004. At that time, we had examined 465 (70%) of the 660 eligible patients with MA (26 refused participation, and 169 were missed during their visit because of scheduling conflicts) and 611 (30%) of the 2007 eligible patients with NA.
Refinement of Study Groups
The additional history of diabetes treatment and kidney disease obtained during the examination allowed refinement of the eligibility criteria for the study groups. Of the 1076 examined patients, we excluded 264 because of type 2 diabetes and 141 because of failure to meet eligibility criteria or presence of comorbidities such as hepatitis C, HIV, or kidney diseases unrelated to diabetes. The final study group included 675 participants: 311 with MA and 364 with NA.
cC-GFR
Cystatin C is a nonglycosylated basic protease inhibitor that is produced at a constant rate by all nucleated cells, freely filtered by the glomerulus, and stable in frozen serum or plasma (12,13). The validity of estimates of renal function that are based on cystatin C has been well demonstrated for patients with type 1 diabetes and elevated or normal GFR (10,14).
All serum samples were stored at −85°C until the day of assay and were analyzed for cystatin C concentration (Dade Behring Diagnostics, Deerfield, IL) on a BN Prospec System nephelometer (Dade Behring, Newark, DE). The assay's range of detection is 0.30 to 7.50 mg/L, and the reported reference range for young, healthy individuals is 0.53 to 0.95 mg/L. In our laboratory, the intraindividual coefficient of variation for patients with diabetes is 3.8 and 3.0% in samples from the lowest and highest quartiles of the cystatin C distribution, respectively (4). Furthermore, assay results for replicate aliquots of serum samples stored at −85°C for 6 mo or in the refrigerator for up to 2 wk were equivalent to results obtained on fresh samples.
The estimated GFR (cC-GFR ml/min) is the reciprocal of cystatin C (mg/L) multiplied by 86.7 and reduced by subtracting 4.2. This formula was recently developed by MacIsaac et al. (10) as a reliable estimate of GFR in patients with diabetes. Our method for measuring cystatin C was similar with respect to assay, equipment, and coefficient of variation as that reported by MacIsaac et al. (10).
Assessment of Exposure Variables
During the enrollment examination, two measurements of seated BP were taken 5 min apart with an automatic monitor (Omron Healthcare, Bannockburn, IL) and averaged to reduce variability. The medical history interview emphasized current and past use of medications (particularly angiotensin-converting enzyme inhibitors [ACEi], angiotensin II receptor blockers, and other antihypertensive drugs), and clinic records were examined to supplement the reported history and confirm dates of prescriptions. We also extracted all archived clinical laboratory measurements of HbA1c, ACR, and serum cholesterol. Details of these assays were described previously (15,16). ACR values were converted to albumin excretion rate (AER) according to a formula published previously (11). For characterizing patients’ recent exposures, repeated measures (AER, HbA1c, and cholesterol) were summarized by their median or mean.
Measurements of serum uric acid were run on thawed, vortexed, and centrifuged serum samples that had been stored in a freezer at −80°C. A timed end point method was used on a Beckman Coulter Synchron CX9. This system measures the change in absorbance at 520 nm and is directly proportional to the amount of uric acid in the sample (17,18). In our laboratory, the intraindividual coefficient of variation for patients with diabetes was 1.3 and 0.6% in samples from the lowest and highest quartiles of the uric acid distribution, respectively. To convert mg/dL to mmol/L uric acid, multiply by 0.059.
Statistical Analyses
Analyses were performed in SAS 8.02 for Windows (SAS Institute, Cary, NC). Differences between groups were assessed using χ2 tests for categorical variables or t test for continuous variables, with an α level 0.05 for significance throughout.
Covariates that could be potential confounders were explored extensively through multivariate linear regression analysis. Covariates that were included in multivariate models were chosen when they were first found through univariate analysis to be significantly associated with cC-GFR in either the NA or MA group. HbA1c, body mass index (BMI), BP, gender, and medication use were particularly explored. Covariates were retained in the final model when independent effects on cC-GFR persisted after adjustment or when they altered parameter estimates by >10%. Results that suggested potential interactions between covariates were also explored.
Results
The Second Joslin Study on the Natural History of Microalbuminuria included 675 patients with type 1 diabetes grouped according to their AER during the 2-yr interval preceding the enrollment examination: 364 with NA and 311 with MA. The AER distributions (median [25th, 75th percentiles]) in these groups were 15 (11, 21) and 70 (46, 132) μg/min, respectively. Characteristics at enrollment are summarized in Table 1 according to AER group: NA or MA. The distributions of AER at this examination were unchanged from the preceding 2-yr interval. Men comprised slightly less than half of the NA and slightly more than half of the MA group. Age at diabetes diagnosis was the same in both groups, but diabetes duration was longer and current age was older in the MA group. Current cigarette smoking was not frequent, but the percentage was significantly higher in the MA group. BMI and systolic and diastolic BP were higher in the MA group, as were the proportions treated with renoprotective and antihypertensive agents. ACEi were the most commonly reported agents: 61% of the MA group and 26% of the NA group. Both HbA1c and the daily insulin dosage (U/kg) were higher in the MA group, as were serum cholesterol and the frequency of treatment with lipid-lowering agents. Serum uric acid was higher in the MA than NA group even though the value was in the normal range for the majority (94%).
Table 1.
Clinical and biochemical characteristics at examination of study participants according to presence of NA or MAa
| Characteristic at Examination | NA (n = 364) | MA (n = 311) | P |
|---|---|---|---|
| AER (μg/min; median [25th, 75th percentiles]) | 15.0 (11.4, 21.5) | 70.8 (45.8, 132.5) | b |
| Male (%) | 43 | 61 | <0.0001 |
| Age (yr; mean [SD]) | 38.7 (11.9) | 41.0 (11.9) | 0.01 |
| Age at diabetes diagnosis (yr; mean [SD]) | 18.6 (10.4) | 17.6 (10.1) | 0.24 |
| Diabetes duration (yr; mean [SD]) | 20.2 (9.4) | 23.4 (9.6) | <0.0001 |
| Current smoker (%) | 11 | 19 | 0.002 |
| BMI (kg/m2; mean [SD]) | 26.2 (4.0) | 27.4 (5.0) | 0.0004 |
| Systolic BP (mmHg; mean [SD]) | 119.4 (12.9) | 124.7 (13.8) | <0.0001 |
| Diastolic BP (mmHg; mean [SD]) | 71.8 (8.0) | 73.7 (8.2) | 0.004 |
| ACEi use (%) | 26 | 61 | <0.0001 |
| ARB use (%) | 4 | 15 | <0.0001 |
| Other antihypertensive agent use (%) | 12 | 22 | 0.0004 |
| HbA1c (%; mean [SD]) | 8.2 (1.2) | 8.5 (1.5) | 0.03 |
| Daily insulin (U/kg; mean [SD]) | 0.65 (0.25) | 0.71 (0.33) | 0.004 |
| Cholesterol (mg/dl; mean [SD]) | 178.7 (31.9) | 189.9 (36.8) | 0.0007 |
| Lipid-lowering agent use (%) | 28 | 36 | 0.025 |
| Uric acid (mg/dl; mean [SD]) | 4.2 (1.0) | 5.2 (1.5) | <0.0001 |
| cC-GFR (ml/min; mean [SD]) | 118.8 (24.1) | 98.7 (27.3) | <0.0001 |
| cC-GFR category (ml/min; %) | <0.0001 | ||
| ≥130 | 30 | 10 | |
| 90 to 129 | 61 | 54 | |
| 60 to 89 | 9 | 28 | |
| <60 | 1 | 8 |
ACEi, angiotensin-converting enzyme inhibitor; AER, albumin excretion rate; ARB, angiotensin receptor blocker; BMI, body mass index; DBP, diastolic BP; HbA1c, glycosylated hemoglobin; MA, microalbuminuria; NA, normoalbuminuria; SBP, systolic BP.
AER differs by design.
Mean cC-GFR was lower in the MA than NA group (98.7 versus 118.8 ml/min), although both were in the normal range. Hyperfiltration (cC-GFR ≥130 ml/min) was less frequent in the MA group and more frequent in NA (10 versus 30%). Conversely, impaired function (both mild [60 to 89 ml/min] and moderate [30 to 59 ml/min]) was more frequent in MA (28 and 8%, respectively) and less frequent in NA (9 and 1%, respectively).
The goal of this study was to identify factors that are associated with differences in renal function with the presence of MA presumed to be important; therefore, the first analysis was an examination of patient characteristics associated with cC-GFR performed separately in the NA and MA groups. Both groups were subdivided at the overall median cC-GFR, 109 ml/min, into low and high renal function groups (Table 2), resulting in a 40-ml/min difference between low and high cC-GFR subgroups in both the NA and MA groups.
Table 2.
Comparison of clinical and biochemical characteristics of study participants with low or high cC-GFR according to presence of NA or MAa
| Characteristics at Examination | NA
|
MA
|
||||
|---|---|---|---|---|---|---|
| cC-GFR >109 (n = 230) | cC-GFR <109 (n = 134) | P | cC-GFR >109 (n = 110) | cC-GFR <109 (n = 201) | P | |
| cC-GFR (ml/min; mean [SD]) | 132 (18) | 95 (12) | – | 126 (17) | 84 (19) | – |
| AER (μg/min; median [25th, 75th percentiles]) | 14 (10, 19) | 18 (13, 23) | <0.0001 | 54 (42, 99) | 85 (51, 148) | 0.0002 |
| Male (%) | 37 | 54 | 0.003 | 56 | 63 | 0.24 |
| Age at diabetes diagnosis (yr; mean [SD]) | 19 (10) | 19 (10) | 0.98 | 17 (11) | 18 (10) | 0.40 |
| Age (yr; mean [SD]) | 38 (11) | 40 (13) | 0.14 | 36 (12) | 44 (11) | <0.0001 |
| Diabetes duration (yr; mean [SD]) | 20 (9) | 21 (10) | 0.06 | 19 (10) | 26 (9) | <0.0001 |
| BMI (kg/m2; mean [SD]) | 25.8 (3.8) | 26.9 (4.4) | 0.01 | 27.2 (4.8) | 27.6 (5.1) | 0.49 |
| Systolic BP (mmHg; mean [SD]) | 119 (13) | 120 (14) | 0.36 | 123 (12) | 126 (15) | 0.07 |
| Diastolic BP (mmHg; mean [SD]) | 72 (9) | 72 (7) | 0.96 | 74 (9) | 74 (8) | 0.75 |
| ACEi use (%)b | 23 | 31 | 0.10 | 54 | 65 | 0.04 |
| ARB use (%)c | 7 | 2 | 0.01 | 12 | 17 | 0.22 |
| Antihypertensive agent use (%)d | 8 | 19 | 0.002 | 15 | 26 | 0.04 |
| HbA1c (%; mean [SD]) | 8.3 (1.3) | 8.1 (1.1) | 0.16 | 8.8 (1.8) | 8.3 (1.4) | 0.009 |
| Uric acid (mg/dl; mean [SD])e | 4.0 (0.9) | 4.6 (1.0) | <0.0001 | 4.5 (1.1) | 5.6 (1.6) | <0.0001 |
| Abnormal uric acid (%) | 0 | 1 | <0.0001 | 3 | 18 | <0.0001 |
Median cC-GFR for the whole cohort (109 ml/min) divided the NA and MA groups unequally. cC-GFR, GFR as measured by cystatin C–based formula.
The most commonly reported ACEi were lisinopril, enalapril, and quinapril.
The most commonly reported ARB were valsartan, losartan, and irbesartan.
Antihypertensive agents included medications other than ACEi or ARB that can alter BP. The majority reported use of diuretics. Other subcategories included calcium channel blockers and α and β blockers.
Median uric acid level for men was 5.0 mg/dL (range 2 to 11.4) and 3.9 mg/dL (range 1.8 to 9.3) for women. Values in excess of 7.0 mg/dL in men or 6.6 mg/dL in women are abnormal.
This 40-ml/min difference in cC-GFR in the MA group was accompanied by a large difference in AER, whereas the similar difference in the NA group was accompanied by a much smaller AER difference (although both differences were highly statistically significant). Gender was not associated with renal function in the MA group, but low cC-GFR was more common in men in the NA group. The age at diagnosis of diabetes was not associated with GFR in either group, but current age and diabetes duration (the difference between age and age at diagnosis) were inversely associated with low cC-GFR. Smoking was not associated with cC-GFR in either group. Low BMI was associated with high cC-GFR in the NA group, but BMI was not associated with cC-GFR in the MA group.
Neither systolic nor diastolic BP was associated with cC-GFR in either the NA or MA group; however, use of renoprotective or antihypertensive drugs was associated with low cC-GFR in both.
Serum uric acid concentrations were inversely associated with cC-GFR in both the NA and MA groups. Elevations of serum uric acid into the abnormal range were present in very few patients, but such elevations were significantly more frequent in those with low cC-GFR (P < 0.0001).
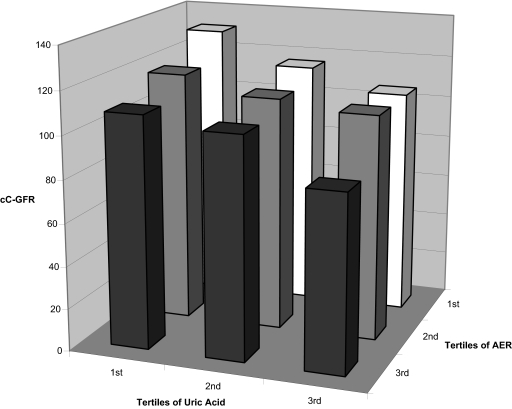
Thus, the two covariates most significantly associated with cC-GFR variation were urinary AER and serum uric acid levels. To examine their joint effects on variation of cC-GFR, we divided both into the tertiles of their distributions (Figure 1). Within each tertile of AER, mean cC-GFR decreased progressively with increasing serum uric acid. Similarly, within each tertile of serum uric acid, mean cC-GFR decreased progressively with increasing AER.
Figure 1.
Joint effects of tertiles of albumin excretion rate and serum uric acid on cC-GFR. Tertiles of AER are: <18.3, 18.4–46.8, and ≥46.9 μg/min. Tertiles of serum uric acid are: <4.0, 4.1–5.2, and ≥5.3 mg/dL.
Characteristics in Table 2 that were associated with cC-GFR in either the NA or MA group were examined in multiple regression models to evaluate their independent contributions. Age and diabetes duration increase in parallel; only age was included because it is associated with GFR in the general population. HbA1c, BMI, systolic BP, gender, and use of renoprotective or antihypertensive medications were identified as potential confounders. In particular, higher HbA1c was observed among those with higher cC-GFR in both normoalbuminuric and microalbuminuric groups. However, HbA1c was not correlated with uric acid (Spearman r = −0.09); neither was there a statistically significant difference in the magnitude of the cC-GFR and uric acid or cC-GFR and AER relationships when stratified by median HbA1c (data not shown). Therefore, the combined effect of HbA1c was entered into multivariate analysis. HbA1c, BMI, BP, and gender no longer had independent effects on cC-GFR and did not alter parameter estimates, so they were omitted from the final model. Among reported medications, only use of antihypertensive agents remained significantly related to cC-GFR. These included diuretics, calcium channel blockers, and α and β blockers. Diuretics had a stronger magnitude of effect on cC-GFR than other antihypertensive agents.
Mean cC-GFR according to each covariate retained in the model (R2 = 0.33) is shown in Table 3, unadjusted and adjusted for the other covariates. cC-GFR decreased steadily with increasing uric acid tertile and with increasing AER tertile. These effects were independent because they were minimally altered with adjustment for each other. The effect of age was different, decreasing significantly only after age 45 yr. The significant effect of treatment with antihypertensive agents, particularly use of diuretics, and lack of an effect of BP presumably illustrate a weakness of cross-sectional studies. Antihypertensive treatment in this instance is likely a proxy for exposure to high BP that has been recognized and treated by physicians. Such treatment may be a better indicator of long-term BP than the BP measured at the enrollment examination.
Table 3.
Mean cC-GFR according to significant covariates in multiple regression analysisa
| Parameter | Unadjusted Mean cC-GFR (ml/min) | Adjusted Mean cC-GFR (ml/min) | Overall Pc | P versus Reference Level |
|---|---|---|---|---|
| Uric acid tertile (mg/dl) | <0.0001 | |||
| first ≤4.0 | 122.6 | 117.4 | Reference | |
| second 4.1 to 5.2 | 110.3 | 108.1 | <0.0001 | |
| third ≥5.3 | 93.1 | 97.5 | <0.0001 | |
| AER tertile (mg/min) | <0.0001 | |||
| first ≤18.3 | 121.9 | 115.9 | Reference | |
| second 18.4 to 46.8 | 111.7 | 109.4 | 0.004 | |
| third ≥46.9 | 94.5 | 97.6 | <0.0001 | |
| Age tertile (yr) | <0.0001 | |||
| first ≤33 | 118.1 | 112.2 | Reference | |
| second 34 to 44 | 110.8 | 109.5 | 0.24 | |
| third ≥45 | 100.7 | 101.3 | <0.0001 | |
| Renoprotective or antihypertensive medication | 0.004 | |||
| none | 118.8 | 111.6 | Reference | |
| ACEi or ARB use only | 104.1 | 109.4 | 0.3 | |
| diuretic use | 89.5 | 100.4 | 0.002 | |
| other antihypertensive useb | 97.2 | 104.1 | 0.04 |
Model R2 = 0.33. HbA1c, BMI, SBP, and gender were dropped from the model because they were not statistically significant at α < 0.05.
Other antihypertensive use included calcium channel blockers and α and β blocking agents.
Overall P relates to adjusted mean cC-GFR.
Discussion
This examination of renal function in a large group of patients with type 1 diabetes and NA or MA has documented large variation in GFR, as estimated by serum cystatin C. It was above normal (>130 ml/min) in 20% and below normal (<90 ml/min) in 18%. Renal function decreased progressively with increasing urinary AER and increasing serum uric acid, even within their normal ranges. It was lower after age 45 yr and in those who were using antihypertensive agents. It was not associated with the HbA1c or BP measured at the enrollment examination.
It has been assumed that progressive renal function decline in patients with type 1 diabetes followed the development of overt proteinuria (2). Recent studies indicate that decline might begin earlier (19,20). Our recent follow-up study of a large cohort of patients with type 1 diabetes and MA showed clearly that progressive renal function decline begins in one third of these patients while their AER is in the low range of MA (4). This present study, although cross-sectional in design, is consistent with the initiation of declining GFR even while the AER is in the high NA range. This finding supports our new model of DN in which the precursor of ESRD, progressive renal function decline, develops very early, perhaps as early as when the AER is in the high NA range. Rising urinary AER, rather than preceding GFR decline, merely parallels the decline (4,21).
The association of renal function with serum levels of uric acid is a new finding in several regards. First, the concentrations of uric acid over which this association is evident include much of the normal range. The distribution of concentrations in this group of patients with type 1 diabetes approximates that in the Framingham Heart Study. The median uric acid concentrations in men and women, respectively, in that study were 5.5 and 4.1 mg/dL and in our patients were 5.0 and 3.9 mg/dL (22). Second, in patients whose renal function was still within the normal or mildly impaired range, a clear dose-response relationship was evident between increasing serum uric acid and decreasing cC-GFR. This relationship was robust against adjustments for AER and other covariates.
Serum uric acid concentration is associated with abnormal urinary albumin excretion in both individuals with and without diabetes (8,23). In patients with type 2 diabetes, serum uric acid concentration is associated with renal function as estimated by creatinine clearance; however, unlike this study, a large proportion of those patients had impaired renal function (9). According to a community-based study of Japanese adults, hyperuricemia and age were the only significant predictors of the risk for renal failure. Hyperuricemia has stronger predictive ability than proteinuria (7).
Our finding that even high-normal values of serum uric acid may reduce renal function is in agreement with results from experiments in rats. In rats rendered mildly hyperuricemic by being fed a urate oxidase inhibitor, an afferent arteriolopathy developed and was accompanied in some cases by an increase in BP (24). Also in rats with a five-sixths nephrectomy, progressive renal failure developed when they were made hyperuricemic (25).
The mechanisms underlying the association between high-normal values of serum uric acid and diminished renal function in patients with type 1 diabetes are not clear. Serum uric acid can function as a proinflammatory molecule with the capacity to act as both a pro-oxidant and an antioxidant (5). As a result, cyclooxygenase-2, specific mitogen-activated protein kinases, inflammatory mediators including C-reactive protein and monocyte chemoattractant protein-1, and the renin-angiotensin system are upregulated, and endothelial nitric oxide production may be inhibited (25–29). Damage to vascular endothelium (30) may be initiated in preglomerular vessels and result in injury to the renal interstitium (31). Rats that were rendered hyperuricemic developed increased cellularity of the afferent arteriole that decreased luminal diameter and produced renal ischemia. Glomerulosclerosis and tubulointerstitial fibrosis were also observed (32).
Similar histologic changes occurred in humans with gouty nephropathy (33). Lowering serum uric acid concentration with allopurinol attenuated these histologic and functional changes, but this effect may in part be due to reduced oxidative stress (5). The use of allopurinol to slow renal disease progression was tested in a clinical trial involving 54 hyperuricemic patients with mild to moderate chronic renal disease (34). Allopurinol significantly reduced serum uric acid from 9.75 ± 1.18 to 5.88 ± 1.01 mg/dL. After 1 yr, only four (16%) of 25 treated patients had either a >40% increase in serum creatinine or developed ESRD compared with 12 (46%) of 26 in the control group. Agents, such as probenecid, that reduce serum uric acid concentration by fostering increased uric acid excretion are used primarily in patients with preserved renal function (35). Whether either of these drugs can prevent or retard progressive early renal function decline in patients with type 1 diabetes and MA remains to be tested.
Age and diabetes duration increase in parallel, and both are significantly associated with variation in renal function, particularly among those with MA. Whether to attribute this effect to aging or to a specific exposure in a diabetic milieu is unclear. Because GFR is known to decline with age in individuals without diabetes, we modeled the effect in terms of age (36); however, the mechanisms underlying this effect are not clear. Diabetes duration is associated with significant accumulation of glomerular lesions even in the absence of any elevated AER (37,38).
Because of the cross-sectional design of this study, caution must be used in the interpretation of results. The effect of serum uric acid concentration on renal function seems very strong, but confirmation must be obtained in a follow-up study before a clinical trial is planned. Also, neither HbA1c nor BP retained independent effects on renal function or altered the parameter estimate for the uric acid and cC-GFR relationship in multivariate analysis. The unexpected absence of an effect of HbA1c or BP on renal function must be seen in the context of the design. Both are risk factors for declining renal function identified in previous studies (4,39,40); however, the HbA1c and BP measured in this study may have been influenced by intensified physician and patient efforts prompted by the diagnosis of MA or hypertension. Supporting evidence of a stricter treatment protocol was seen in the patients who were treated with antihypertensive agents and had lower average HbA1C (data not shown). Furthermore, past HbA1c measurements must have been higher because, in an analysis of a subgroup of patients with very long follow-up, a high average HbA1c over the long term is associated with low cC-GFR (data not shown).
Thiazide diuretics reportedly increase whereas losartan and ACEi lower uric acid levels (41–43). In our study, uric acid levels were significantly higher among patients who reported use of thiazide diuretics but use did not confound the relationship between uric acid and cC-GFR. No other significant associations were found between renoprotective medications and uric acid after multivariate adjustment. Use of antihypertensive medications is a proxy for elevated BP, and a longitudinal study might illuminate an expected association between BP and cC-GFR. If we had had comparable past measurements of the BP that prompted treatment with antihypertensive agents as we did for HbA1c, then the expected association with BP may have been confirmed. Finally, our study was carried out in patients with type 1 diabetes, and the generalizability of the findings to patients with type 2 diabetes is uncertain.
Disclosures
None.
Acknowledgments
This study was supported by National Institutes of Health grant DK41526. E.T.R. was supported by AHRQ T32, Child Health Services Research Training Program, grant HS00063. M.A.N. was supported by American Diabetes Association mentor-based fellowship 7-03-MN-28.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Krolewski M, Eggers PW, Warram JH: Magnitude of end-stage renal disease in IDDM: A 35 year follow-up study. Kidney Int 50: 2041–2046, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Parving HH, Mauer M, Ritz E: Diabetic nephropathy. In: Brenner and Rector's The Kidney, 7th Ed., edited by Brenner BM, Elsevier, 2004, pp 1777–1818
- 3.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS: Regression of microalbuminuria in type 1 diabetes. N Engl J Med 348: 2285–2293, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS: Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 18: 1353–1361, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M: Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 41: 1183–1190, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Perez-Ruiz F, Calabozo M, Erauskin GG, Ruibal A, Herrero-Beites AM: Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum 47: 610–613, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, Takishita S: Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res 24: 691–697, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Tseng C-H: Correlation of uric acid and urinary albumin excretion rate in patients with type 2 diabetes mellitus in Taiwan. Kidney Int 68: 796–801, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G: Hypouricemia and hyperuricemia in type 2 diabetes: two different phenotypes. Eur J Clin Invest 31: 318–321, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Macisaac RJ, Tsalamandris C, Thomas MC, Premaratne E, Panagiotopoulos S, Smith TJ, Poon A, Jenkins MA, Ratnaike SI, Power DA, Jerums G: Estimating glomerular filtration rate in diabetes: A comparison of cystatin-C- and creatinine-based methods. Diabetologia 49: 1686–1689, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Warram JH, Gearin G, Laffel L, Krolewski AS: Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol 7: 930–937, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Vinge E, Lindergard B, Nilsson-Ehle P, Grubb A: Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 59: 587–592, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Newman DJ: Cystatin C. Ann Clin Biochem 39: 89–104, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Tan GD, Lewis AV, James TJ, Altmann P, Taylor RP, Levy JC: Clinical usefulness of cystatin C for the estimation of glomerular filtration rate in type 1 diabetes: Reproducibility and accuracy compared with standard measures and iohexol clearance. Diabetes Care 25: 2004–2009, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH: Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med 332: 1251–1255, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Ficociello LH, Perkins BH, Silva KH, Finkelstein DM, Ignatowska-Switalska H, Gaciong Z, Cupples LA, Aschengrau A, Warram JH, Krolewski AS: Determinants of progression from microalbuminuria to proteinuria in type 1 diabetes patients treated with ACE Inhibitors. Clin J Am Soc Nephrol 2: 461–469, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Fossati P, Prencipe L, Berti G: Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem 26: 227–231, 1980 [PubMed] [Google Scholar]
- 18. Beckman-Coulter Synchron CX System(s) Chemistry Information Sheet 389812AB: Uric Acid, Beckman-Coulter, Fullerton, CA, November 2005
- 19.Rudberg S, Osterby R: Decreasing glomerular filtration rate: An indicator of more advanced diabetic glomerulopathy in the early course of microalbuminuria in IDDM adolescents? Nephrol Dial Transplant 12: 1149–1154, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Bangstad HJ, Osterby R, Rudberg S, Hartmann A, Brabrand K, Hanssen KF: Kidney function and glomerulopathy over 8 years in young patients with type I (insulin-dependent) diabetes mellitus and microalbuminuria. Diabetologia 45: 253–261, 2002 [DOI] [PubMed] [Google Scholar]
- 21.de Boer IH, Steffes MW: Glomerular filtration rate and albuminuria: Twin manifestation of nephropathy in diabetes. J Am Soc Nephrol 18: 1036–1037, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Sundstrom J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS: Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 45: 28–33, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Lee JE, Kim YG, Choi YH, Huh W, Kim DJ, Oh HY: Serum uric acid is associated with microalbuminuria in prehypertension. Hypertension 47: 962–967, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ: Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38: 1101–1106, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ: A role for uric acid in the progression of renal disease. J Am Soc Nephrol 13: 2888–2897, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Coutinho Tde A, Turner ST, Peyser PA, Bielak LF, Sheedy PF 2nd, Kullo IJ: Associations of serum uric acid with markers of inflammation, metabolic syndrome, and subclinical coronary atherosclerosis. Am J Hypertens 20: 83–89, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kang DH, Park SK, Lee IK, Johnson RJ: Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 16: 3553–3562, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ: Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 41: 1287–1293, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Perlstein TS, Gumieniak O, Hopkins PN, Murphey LJ, Brown NJ, Williams GH, Hollenberg NK, Fisher ND: Uric acid and the state of the intrarenal renin-angiotensin system in humans. Kidney Int 66: 1465–1470, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Hayden MR and Tyagi SC: Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus—The urate redox shuttle. Nutr Metab 1: 10, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B: Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med 346: 913–923, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa T, Mazzali M, Kang DH, Kanellis J, Watanabe S, Sanchez-Lozada LG, Rodriguez-Iturbe B, Herrera-Acosta J, Johnson RJ: Hyperuricemia causes glomerular hypertrophy in the rat. Am J Nephrol 23: 2–7, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Talbott JH, Terplan KL: The kidney in gout. Medicine 39: 405–467, 1960 [PubMed] [Google Scholar]
- 34.Siu YP, Leung KT, Tong MK, Kwan TH: Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 47: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Terkeltaub R, Bushinsky DA, Becker MA: Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics. Arthritis Res Ther 8[Suppl 1]: 1–9, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epstein M: Aging and the kidney. J Am Soc Nephrol 7: 1106–1122, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Lane PH, Steffes MW, Mauer SM: Glomerular structure in IDDM women with low glomerular filtration rate and normal urinary albumin excretion. Diabetes 41: 581–586, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Caramori ML, Fioretto P, Mauer M: Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: An indicator of more advanced glomerular lesions. Diabetes 52: 1036–1040, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Mogensen CE: Microalbuminuria, blood pressure and diabetic renal disease: Origin and development of ideas. Diabetologia 42: 263–285, 1999 [DOI] [PubMed] [Google Scholar]
- 40.The Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Sica DA, Schoolwerth AC: Part 1: Uric acid and losartan. Curr Opin Nephrol Hypertens 11: 475–482, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Dang A, Zhang Y, Liu G, Chen G, Song W, Wang B: Effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia in Chinese population. J Hum Hypertens 20: 45–50, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Burnier M, Roch-Ramel F, Brunner HR: Renal effects of angiotensin II receptor blockade in normotensive subjects. Kidney Int 49: 1787–1790, 1996 [DOI] [PubMed] [Google Scholar]