Abstract
Background and Objectives: Arginine vasopressin (AVP), an endogenous hormone with vasopressor properties, may be inadequately secreted during episodes of intradialytic hypotension (IDH).
Design, Setting, Participants, and Measurements: To evaluate this, we performed a prospective, observational pilot study of 20 chronic hemodialysis patients assessing the baseline AVP level and trend of AVP with ultrafiltration in patients with a diagnosis of IDH compared with patients without IDH. Ten symptomatic IDH patients and 10 controls were enrolled and matched for age, gender, and dialysis vintage. AVP levels were obtained hourly throughout the dialysis session and during hypotensive episodes.
Results: We observed that IDH patients experienced greater decreases in both systolic and diastolic blood pressure during the dialysis session despite equivalent ultrafiltration in both groups. AVP concentration did not increase in the IDH patients (5.0 ± 1.8) compared with controls (6.4 ± 6.0) (P = 0.5) despite hypotensive events.
Conclusions: This study suggests that symptomatic IDH patients are unable to mount an appropriate increase in AVP secretion in the setting of hypotension. These findings support the possibility of AVP as a mechanism driven therapy for patients with symptomatic IDH.
Intradialytic hypotension (IDH) is a major complication seen during hemodialysis, occurring in approximately 10% to 30% of treatments and is associated with significant morbidity and mortality (1). Cramps, nausea, vomiting, dizziness, and fatigue are common symptoms.
Life-threatening events, such as cerebrovascular accident, syncope, myocardial ischemia, and arrhythmia, may also occur. The etiology of hypotension occurring during dialysis is thought to be multifactorial: 1) autonomic dysfunction in uremia, 2) acute decreases in plasma osmolality, 3) decreased vascular reactivity to vasopressor agents and overproduction of vasodilators, and 4) underlying cardiac disease (2,3). In addition, common comorbidities associated with end-stage renal disease (ESRD), such as diabetes mellitus, are strongly associated with autonomic dysfunction, contributing to an increased risk for IDH (4–6). Also, neurally mediated vasopressor syncope could contribute. Current therapeutic modalities for managing IDH include sodium modeling, intravenous mannitol, midodrine, and cool dialysate; however, these measures have been used with variable success and associated risks (7–13).
Arginine vasopressin (AVP), an endogenous hormone synthesized in the hypothalamus and secreted by the posterior pituitary gland, is released in the setting of hyperosmolality or nonosmotic stimuli, including hypotension, hypovolemia, or nausea. AVP has been shown to increase in the setting of hypotension in septic or hypovolemic shock (14–16). In patients with autonomic insufficiency, however, AVP levels are inappropriately low to the level of hemodynamic compromise and likely related to baroreceptor dysfunction leading to decreased endogenous AVP synthesis and secretion (4,5,17). Thus, vasopressin insufficiency is thought to underlie or at the very least contribute to hypotension in these patients.
The role of vasopressin insufficiency as a cause of hypotension has also been briefly considered in ESRD patients. A study in 23 hemodialysis patients published more than 10 yr ago demonstrated vasopressin insufficiency as a possible mechanism of IDH in ESRD patients (18). A recent study also demonstrated a hemodynamic benefit with subpressor doses of vasopressin in 22 hypertensive patients during hemodialysis with ultrafiltration (19). Both studies shed important insight into the role of vasopressin in hemodynamic stability. However, the older study did not use a matched normotensive control group that would allow comparison of serum AVP levels in response to reductions in blood pressure (BP). Also, the more recent publication did not study IDH patients when they measured AVP levels or examined the hemodynamic response to vasopressin infusion.
This study was undertaken to evaluate the trend in AVP levels over the duration of hemodialysis in symptomatic patients with a diagnosis of IDH as compared with normotensive hemodialysis patients without IDH. In addition to verifying the data generated from the previously noted studies, we were particularly interested in the AVP response in IDH patients during frank hypotension. Understanding the serum AVP trend in symptomatic patients with IDH may assist with designing future clinical trials to treat this vexing problem.
Materials and Methods
The study was performed at the DaVita Chronic Dialysis Unit at Yale University (New Haven, CT). All patients gave informed consent to participate in the study. The study was approved by the Institutional Review Board of Yale University School of Medicine, and the authors adhered to the Declaration of Helsinki.
Study Protocol
To be eligible for study participation, patients were required to have ESRD and been on hemodialysis for more than 3 mo, be older than 18 yr, stable dry weight, and stable dialysis and medication regimen. Patients were included in the study if they had documented IDH, which was unresponsive to routine measures, such as dry weight adjustment, antihypertensive medication adjustment, and cool dialysate. A hypotensive “event” was defined as a systolic BP (SBP) decrease greater than 20 mmHg to levels less than 100 mmHg over 1 mo associated with hypotensive symptoms (nausea/vomiting, dizziness/lightheadedness, yawning, weakness). To be defined as an IDH patient, such events had to occur in greater than 50% of hemodialysis treatments over a 1-mo observation period. Control patients (hemodialysis patients without IDH) were selected by matching on the basis of age, sex, diabetic status, and dialysis vintage. Exclusion criteria were: patients in their first 3 mo of dialysis, active illness, severe congestive heart failure or cirrhosis, and unstable dry weight.
Patient Demographics
Ten male and 10 female patients were enrolled (Table 1). The mean age was 60 yr (range, 37 to 84 yr) and mean dialysis vintage was 58 mo. A total of 12 (60%) patients were diabetic; 80% patients were blacks. None of the patients received medications that would interfere with, stimulate, or suppress AVP synthesis or secretion.
Table 1.
Patient characteristics
| Characteristic | IDH Group (n = 10) | Control (n = 10) | P |
|---|---|---|---|
| Age, yr | 60.7 ± 13.3 | 59.4 ± 11.9 | 0.82 |
| Race, % black | 90 | 70 | 0.29 |
| % female | 50 | 50 | 1.0 |
| % diabetic | 60 | 60 | 1.0 |
| Dialysis vintage, mo | 58.7 ± 38.7 | 57.3 ± 49.3 | 0.94 |
| Duration HD, hours | 4.25 ± 0.67 | 3.75 ± 0.61 | 0.08 |
| History of HTN, % | 60 | 80 | 0.16 |
| History of CAD, % | 70 | 80 | 0.34 |
| History of LVH, % | 80 | 80 | 1.0 |
| Medications used, % | |||
| Beta blocker | 30 | 60 | 0.08 |
| ACE-I/ARB | 10 | 90 | 0.003 |
| CCB | 10 | 0 | 0.34 |
| History of PVD, % | 60 | 70 | 0.34 |
The continuous variables are presented as mean ± SD. IDH, intradialytic hypotension; HD, hemodialysis; HTN, hypertension; CAD, coronary artery disease; LVH, left ventricular hypertrophy; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; PVD, peripheral vascular disease.
Dialysis Treatment
All patients were studied during their regular hemodialysis treatment sessions. Routine hemodialysis was performed with high flux hollow fiber polysulfone dialyzers on Cobe Centry System 3 (Lakewood, CO) dialysis machines. All patients were dialyzed on a constant sodium bath at 140 mEq/L. The rest of the dialysis prescription was unchanged from baseline, including duration of dialysis, type of dialyzer, and medications administered. Ultrafiltration was performed to the target dry weight.
Hemodynamic Monitoring
Sitting SBP and diastolic BP (DBP) and pulse were measured in duplicate (via hemodialysis system) at 1-h intervals throughout the dialysis session via arm cuff. Because the study protocol required AVP concentrations to be sent during two separate treatments, the hemodynamic measurements were taken throughout both of the treatments as well. Symptomatic hypotensive events were treated in standard fashion with Trendelenburg positioning, cessation of ultrafiltration, and saline infusion if needed.
Laboratory Procedures
Blood samples were collected from the arterial line at the initiation of dialysis treatment and hourly throughout treatment for two hemodialysis sessions separated by at least 1 wk. Additional samples were taken during any hypotensive event, and all were sent for AVP levels and serum sodium concentration. Blood samples for AVP were collected in prechilled lavender top tubes, which were immediately centrifuged (within 10 min) for 10 min at 2000 rpm with plasma removed and immediately frozen at −20°C. Serum sodium concentrations were measured in standard fashion.
AVP Measurements
AVP was measured by double antibody RIA (Alpco Diagnostics, Salem, NH). All samples for AVP were run in duplicate for each time point and results were averaged. The assay range is between 1.25 and 80 pg/ml with sensitivity of 0.35 pg/ml. The intra-assay coefficient of variation was 4%.
Statistical Analyses
All data are expressed as mean ± SD when values were normally distributed. Baseline characteristics, including age, gender, and ethnicity, were analyzed using a paired t test. P values of <0.05 were considered statistically significant. The effect of AVP on each of the parameters measured within the treatment and control group was evaluated by analysis of variance. Multiple comparisons analysis was performed using the SAS package for analysis (version 9.1, Cary, NC).
Results
Patient Characteristics
To ensure that endogenous vasopressin levels in the two study arms were not affected by any differences in baseline characteristics, the groups were matched for age within 10 yr, gender, and dialysis vintage within 24 mo. Table 1 illustrates the various patient characteristics and dialysis parameters. It is worth noting that all of the IDH patients had symptomatic declines in SBP to less than 90 mmHg during the observation period. There were no significant differences between baseline features of the IDH and control groups. All 20 patients completed the study. No complications occurred during the study period with any of the enrollments. All dialysis prescriptions remained the same as baseline, and no changes to medications were made.
Frequency and Timing of Hypotension in IDH
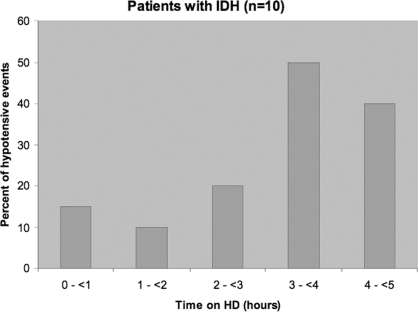
With BP measured hourly at two separate dialysis sessions, severe hypotension (symptomatic drop in BP >20 mmHg to SBP <100 mmHg) was noted in 9 of 10 (90%) of the IDH patients and 12 of 20 treatments (60%) performed in the IDH group. No control patients developed hypotensive episodes to this level. All patients were without symptoms of orthostasis before initiating dialysis and after completion of treatment. A greater proportion of patients in the IDH group experienced hypotension in the latter period of the dialysis session, particularly in hours 3 to 5 (Figure 1). All patients were dialyzed with standard ultrafiltration profile to their estimated dry weight.
Figure 1.
Timing of hypotensive episodes in patients with intradialytic hypotensive events during their treatments.
Difference in Systolic and Diastolic BP in IDH and Control Groups
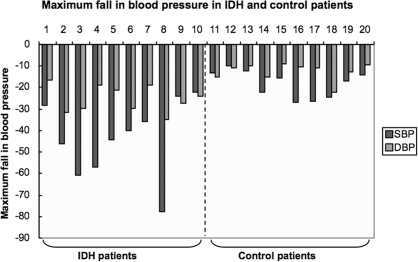
During the study period, the maximum decrease in BP throughout the dialysis sessions was substantially greater in the IDH group compared with the control group, as shown in Figure 2. This difference applied to both SBP and DBP. The mean decrease in SBP (IDH) was 43.1 ± 17 mmHg and DBP (IDH) was 25.5 ± 6.3 mmHg, whereas the mean change in SBP (control) was 17.8 ± 6.2 mmHg and DBP (control) was 12.1 ± 4.0 mmHg. The SBP and DBP differences between the two groups were statistically significant (P = 0.0004 and P < 0.0001, respectively). Despite the clear differences in both SBP and DBP between the two groups, there was no significant difference in ultrafiltration between IDH patients (3.38 L) and control patients (3.05 L, P = 0.56; Table 2).
Figure 2.
The magnitude of decline in BP (systolic and diastolic in mmHg) in patients with intradialytic hypotension and in control patients during the study. Patients 1 to 10 had intradialytic hypotension; patients 11 to 20 were the control patients.
Table 2.
Results
| Characteristic | IDH Group (n = 10) | Control (n = 10) | P |
|---|---|---|---|
| Vasopressin, pg/ml | 5.0 ± 1.8 | 6.4 ± 6.0 | 0.50 |
| Sodium, mmol/L | 135.7 ± 2.4 | 136.5 ± 2.6 | 0.47 |
| Ultrafiltration, L | 3.38 ± 0.97 | 3.05 ± 1.52 | 0.56 |
Values are mean ± SD. IDH, intradialytic hypotension.
Serum Sodium Concentrations and AVP Levels
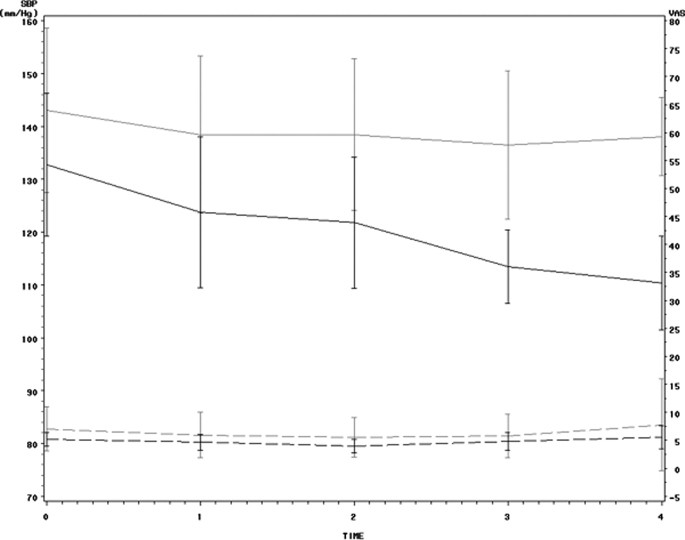
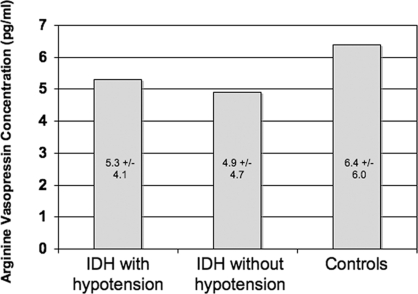
Mean serum sodium (Na+) concentration was similar in both the IDH and the control patients (Table 2). Mean AVP levels were 5.0 ± 1.8 pg/ml in the IDH group compared with 6.4 ± 6.0 pg/ml in the control group (Table 2). They were not statistically different (P = 0.50) despite significant differences in BP in the two groups (Figure 3). The AVP concentrations during the hypotensive events in the IDH patient treatments where the 12 significant hypotensive events occurred (5.3 ± 4.1 pg/ml) showed no statistical difference from the AVP levels in IDH patient treatments where no significant event occurred (4.9 ± 4.7 pg/ml, P = 0.18; Figure 4).
Figure 3.
This plot shows the means and 95% confidence intervals for the means across time for both systolic BP (solid lines) and vasopressin (dashed lines) in controls (gray) and intradialytic hypotension (black). The mean vasopressin levels remain similar despite the differences in systolic BP.
Figure 4.
Mean differences in vasopressin concentrations taken during “hypotensive events” during dialysis treatments in patients with intradialytic hypotension, and the mean arginine vasopressin levels in IDH patients who did not have hypotensive events. Also shown are mean arginine vasopressin concentrations in the control group.
Discussion
Vasopressin (AVP) is a well-recognized vasoconstrictor that has been exploited in the critical care setting for treatment of shock. However, it has not been routinely applied in clinical situations of hypotension, such as IDH in hemodialysis patients. Serum vasopressin response to hypotension that develops in hemodialysis patients compared with normotensive patients is not well established. Vasopressin release is regulated both by osmotic and nonosmotic stimuli. Nonosmotic stimuli consist primarily of hypotension, hypovolemia, pain, nausea, and hypoxia. In such scenarios, normal osmoregulation is not disrupted, but the relationship of vasopressin to osmolality is simply shifted to the left such that higher vasopressin levels are required to maintain normal osmolality (20–23). In hypotension or hypovolemia, baroreceptors, which are normally tonically inhibited, are deactivated, eliminating tonic inhibition and causing vasopressin release (in addition to other vasoconstrictors). The effect raises systemic resistance and elevates BP. IDH develops when these factors are dysfunctional because of a combination of autonomic dysfunction in uremia, decreased vascular reactivity to vasopressor agents, overproduction of vasodilators (nitric oxide, adrenomedullin), and underlying cardiac dysfunction (24,25).
Normal serum AVP concentration in an overnight, fasted hydrated human is less than 4 pg/ml. This hormone is metabolized by the hepatic and renal vasopressinases, and its half-life is approximately 10 to 35 min (26). Studies in patients with septic shock demonstrate that severe hypotension leads to an increase in vasopressin concentrations, sometimes as high as 500 pg/ml, to maintain adequate end-organ perfusion pressure (15). Nondialysis patients with severe autonomic dysfunction and orthostatic hypotension do not appropriately increase AVP concentrations in the setting of hypotension, perhaps representing a significant part of the underlying mechanism (4,6). Thus, it is possible that a similar pathomechanism might explain IDH that occurs in ESRD patients on hemodialysis.
Data on the vasopressin response in dialysis patients are scant and varied. No studies have specifically examined AVP concentrations in patients who carry the diagnosis of IDH. These patients routinely develop severe hypotension. One would expect an intact baroreceptor circuit to increase AVP synthesis and secretion to hypotension. An abnormally low response would suggest that vasopressor insufficiency contributes to hypotension in these patients. One small study of 5 normotensive hemodialysis patients measured hemodynamic profiles (cardiac output, systemic vascular resistance [SVR], and BP) during a routine hemodialysis treatment (27). In this group, gradual decreases in BP were associated with increasing SVR levels. AVP levels increased and correlated with increased SVR. Another study examined 15 normotensive hemodialysis patients and found that mean AVP concentrations rose as blood volume decreased with ultrafiltration (28). In the present study, AVP concentrations increased significantly in one patient who developed hypotension. The rest of the patients were normotensive or hypertensive and had very small, if any, rises in AVP concentrations.
A study of 6 normotensive hemodialysis patients found no changes in AVP concentrations during ultrafiltration, except in one hypotensive patient whose concentrations increased from 5.5 to 18 pg/ml (29). This is considered an appropriate response in normotensive patients who are excessively ultrafiltered. There was a small correlation between serum osmolarity and rising AVP concentrations, demonstrating that the effect of osmolality on AVP secretion is intact in hemodialysis patients. Similar results were noted in one study that evaluated the effect of volume removal on vasoactive hormones in 16 patients on dialysis (30). After hemodialysis, the blood volume had decreased significantly; however, vasopressin levels were not significantly changed after dialysis in the normotensive patients. Similarly, other studies document that volume removal during hemodialysis does not increase plasma AVP concentrations in otherwise normotensive patients (28).
Over a decade ago, Friess et al. demonstrated insufficient vasopressin secretion in patients with IDH (18). Six patients with nausea and hypotension manifested increased AVP levels during hemodialysis and were thus considered “control” patients. This article clearly identified vasopressin insufficiency as a potentially important cause of IDH, but the findings were not subsequently verified. Also, a true matched control group was not used in the study. More recently, in an important observation by van der Zee et al., subpressor doses of intravenous vasopressin provided hemodynamic benefit and prevented hypotension during dialysis with ultrafiltration in 22 hypertensive hemodialysis patients (19). They also measured serum AVP levels in 10 normotensive patients during hemodialysis, but the significance of these values is difficult to interpret because they were not from IDH patients. Indeed, the AVP levels in their normotensive patients were similar to the AVP levels measured in our non-IDH control patients. In view of these data, we hypothesized that symptomatic IDH patients may have an aberrant AVP response to hypotension. Thus, we set out to examine the endogenous AVP response to hypotension in both IDH patients and a matched normotensive control group.
The data from our pilot study echo the findings of the previously described studies, in that the normotensive or hypertensive control hemodialysis patients did not develop incremental rises in AVP concentrations with ultrafiltration during hemodialysis. In our IDH patients who developed significant hypotensive episodes (mean decrease in SPB/DBP = 43/25 mmHg), one would expect an increase in AVP concentration in response to severe hypotension if AVP secretion was intact. Despite these significant decreases in BP, our IDH patients showed no increase in AVP concentrations. This may represent a mechanism by which IDH patients with autonomic dysfunction (or some form of baroreceptor defect) do not appropriately produce compensatory vasoconstrictor hormones to thwart hypotension. Even AVP concentrations obtained specifically during significant hypotensive episodes did not increase as would normally be expected. Thus, our data also suggest vasopressin insufficiency as a mechanism underlying IDH, confirming the findings of Friess et al. (18).
The sodium bath was kept constant during all hemodialysis treatments to eliminate the effect of osmolality on AVP concentration. As seen in Table 2, there was no difference in serum sodium concentrations between the two groups. Previous studies demonstrate that neither hemodialysis nor ultrafiltration prevents a proportionate increase in AVP concentrations during AVP infusion (19). This suggests that AVP concentration is not affected by hemodialysis and ultrafiltration, so AVP removal cannot explain the results found in this study. Several mechanisms for the occurrence of vasopressin deficiency may be considered in this symptomatic IDH population. They include early secretion and depletion of vasopressin stores or decreased endogenous production, autonomic dysfunction, increased nitric oxide production, and elevated norepinephrine levels (31). Which of these mechanisms plays the most important is currently unknown.
Limitations
The small number of patients enrolled in the study limits the generalizability of the study results. A larger number of patients may have allowed differences in vasopressin concentrations to be more apparent between the IDH and control groups. Also, a greater number of symptomatic hypotensive events in the IDH group would provide more power to the study results. Our study did not have a positive control (non-IDH patient) with a hypotensive episode to assess AVP response. Inducing hypotension in a normotensive control patient would not have been feasible from study protocol limitations. However, other studies have shown this in select patients.
The AVP concentrations were clustered within a low range for both study groups. Vasopressin secretion may have been truly suppressed to a similar degree in both study arms. Alternatively, the assay may have not been sensitive enough to detect a significant difference among the samples, although it was tested with control vials in varying concentrations of AVP from 0 to 80 pg/ml and found to be accurate. Although no medications were altered in this study, patients in the IDH group were on midodrine for BP support. It is doubtful, however, that this drug decreased vasopressin release in these patients, as they still developed hypotension. Future studies may consider investigating vasopressin release in the setting of hypotension in untreated IDH patients.
Conclusion
It is therefore possible that the inappropriately low vasopressin concentrations may, indeed, represent a novel mechanism for IDH that could have therapeutic implications. Intravenous vasopressin, as recently shown in non-IDH patients (19), and perhaps intranasal vasopressin administration, may improve hemodynamic stability in an otherwise difficult to treat population.
Disclosures
None.
Acknowledgments
This work was supported by the NIH/NCRR/GCRC Program Grant #M01-RR00125 and we are grateful for the statistical help of James Dzuria from the GCRC. We appreciate the professional staff at the New Haven, CT DaVita dialysis unit for their support during the study. A portion of the data in this study was presented at the 2007 NKF clinical meeting in abstract form.
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Shoji T, Tsubakihara Y, Fujii M, Imai E: Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 66: 1212–1220, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Armengol NE CAA, Bono Illa M, Calls Ginesta J, Gaya Bertran J, Rivera Fillat DR: Vasoactive hormones in uraemic patients with chronic hypotension. Nephrol Dial Transplant 12: 321–324, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Cases ACE: Chronic hypotension in the dialysis patient. J Nephrol 15: 331–335, 2002 [PubMed] [Google Scholar]
- 4.Sato K, Kimura T, Ota K, Shoji M, Ohta M, Yamamoto T, Funyu T, Abe K: Changes in plasma vasopressin levels and cardiovascular function due to postural changes in diabetic neuropathy. Tohoku J Exp Med 177: 49–60, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Cignarelli M, De Pergola G, Paternostro A, Corso M, Cospite MR, Centaro GM, Giorgino R: Arginine-vasopressin response to supine-erect posture change: an index for evaluation of the integrity of the afferent component of baroregulatory system in diabetic neuropathy. Diabete Metab 12: 28–33, 1986 [PubMed] [Google Scholar]
- 6.Saad CI, Ribeiro AB, Zanella MT, Mulinari RA, Gavras I, Gavras H: The role of vasopressin in blood pressure maintenance in diabetic orthostatic hypotension. Hypertension 11: I217–I221, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Alappan R, Cruz D, Abu-Alfa AK, Mahnensmith R, Perazella MA: Treatment of severe intradialytic hypotension with the addition of high dialysate calcium concentration to midodrine and/or cool dialysate. Am J Kidney Dis 37: 294–299, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Cruz DN, Mahnensmith RL, Brickel HM, Perazella MA: Midodrine is effective and safe therapy for intradialytic hypotension over 8 months of follow-up. Clin Nephrol 50: 101–107, 1998 [PubMed] [Google Scholar]
- 9.Cruz DN, Mahnensmith RL, Perazella MA: Intradialytic hypotension: is midodrine beneficial in symptomatic hemodialysis patients? Am J Kidney Dis 30: 772–779, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Hoeben H, Abu-Alfa AK, Mahnensmith R, Perazella MA: Hemodynamics in patients with intradialytic hypotension treated with cool dialysate or midodrine. Am J Kidney Dis 39: 102–107, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Perazella MA: Efficacy and safety of midodrine in the treatment of dialysis-associated hypotension. Expert Opin Drug Saf 2: 37–47, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Perazella MA: Pharmacologic options available to treat symptomatic intradialytic hypotension. Am J Kidney Dis 38[Suppl]: S26–S36, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Perazella MA: Approach to patients with intradialytic hypotension: a focus on therapeutic options. Semin Dialysis 12: 175–181, 1999 [Google Scholar]
- 14.Delmas ALM, Rousseauu S, Albanese J, Martin C: Clinical review: vasopressin and terlipressin in septic shock patients. Cric Care 9: 212–222, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes CL, Patel BM, Russell JA, Walley KR: Physiology of vasopressin relevant to management of septic shock. Chest 120: 989–1002, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Jochberger S, Mayr VD, Luckner G, Wenzel V, Ulmer H, Schmid S, Knotzer H, Pajk W, Hasibeder W, Friesenecker B, Mayr AJ, Dunser MW: Serum vasopressin concentrations in critically ill patients. Crit Care Med 34: 293–299, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Reid W, Ewing DJ, Lightman SL, Eadington D, Williams TD, Roulston JE, Clarke BF: Vasopressin secretion in diabetic subjects with and without autonomic neuropathy: responses to osmotic and postural stimulation. Clin Sci (Lond) 77: 589–597, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Friess U, Rascher W, Ritz E, Gross P: Failure of arginine-vasopressin and other pressor hormones to increase in severe recurrent dialysis hypotension. Nephrol Dial Transplant 10: 1421–1427, 1995 [PubMed] [Google Scholar]
- 19.van der Zee S, Thompson A, Zimmerman R, Lin J, Huan Y, Braskett M, Sciacca RR, Landry DW, Oliver JA: Vasopressin administration facilitates fluid removal during hemodialysis. Kidney Int 71: 318–324, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Czaczkes JW: The effect of various states of hydration and the plasma concentration on the turnover of antidiuretic hormone in mammals. J Clin Invest 43: 1649–1658, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes CL, Granton JT: Vasopressin and the cardiovascular system: part 2. Clinical physiology. Crit Care 427–434, 2004 [DOI] [PMC free article] [PubMed]
- 22.Holmes CL, Granton JT: Vasopressin and the cardiovascular system: part 1. Receptor physiology. Crit Care 7: 427–434, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes CL, Russell JA: Vasopressin. Semin Respir Crit Care Med 25: 705–711, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Cases A, Esforzado N, Lario S, Vera M, Lopez-Pedret J, Rivera-Fillat F, Jimenez W: Increased plasma adrenomedullin levels in hemodialysis patients with sustained hypotension. Kidney Int 57: 664–670, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Iwanaga K, Takamura N, Abe Y, Zhaojia Y, Shinzato K, Hosoda H, Kangawa K, Ohtsuru A, Kohno S, Yamashita S, Aoyagi K: Plasma concentrations of adrenomedullin and ghrelin in hemodialysis patients with sustained and episodic hypotension. Endocr J 52: 23–28, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Goldsmith SR: The effect of moderate hypotension on vasopressin levels in normal humans. Am J Med Sci 298: 295–298, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Nakayama M, Yamada K, Nakano H, Miura Y, Tuchida H, Kawaguchi Y: Stimulated secretion of arginine vasopressin during hemodialysis in patients with hemodialysis hypotension. Nephron 79: 488–489, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Heintz B, Reiners K, Gladziwa U, Kirsten R, Nelson K, Wieland D, Riehl J, Mann H, Sieberth HG: Response of vasoactive substances to reduction of blood volume during hemodialysis in hypotensive patients. Clin Nephrol 39: 198–204, 1993 [PubMed] [Google Scholar]
- 29.Fasanella d'Amore T WJ, Waeber B, Nussberger J, Brunner HR: Response of plasma vasopressin to changes in extracellular volume an/or plasma osmolality in patients on maintenance hemodialysis. Clin Nephrol 23: 299–302, 1985 [PubMed] [Google Scholar]
- 30.Katzarski KS, Randmaa I, Bergstrom J: Influence of hemodialysis on intravascular volume and vasoactive hormones. Clin Nephrol 52: 304–311, 1999 [PubMed] [Google Scholar]
- 31.Lin SH, Chu P, Yu FC, Diang LK, Lin YF: Increased nitric oxide production in hypotensive hemodialysis patients. ASAIO J 42: M895–M899, 1996 [DOI] [PubMed] [Google Scholar]