Abstract
Background and objectives: National Kidney Foundation Dialysis Outcomes Quality Initiative practice guidelines recommend serum albumin ≥4.0 g/dl for adults who are on hemodialysis. There is no established pediatric target for albumin and little evidence to support use of adult guidelines. This study examined the association between albumin and risk for death and hospitalization in adolescents who are on hemodialysis.
Design, setting, participants, & measurements: This retrospective cohort study linked data on patients aged 12 to 18 yr in 1999 and 2000 from the Centers for Medicare and Medicaid Services’ End Stage Renal Disease Clinical Performance Measures Project with 4-yr hospitalization and mortality records in the United States Renal Data System. Albumin was categorized as <3.5/3.2, ≥3.5/3.2 and <4.0/3.7, and ≥4.0/3.7 g/dl.
Results: Of 675 adolescents, 557 were hospitalized and 50 died. Albumin ≥4.0/3.7 g/dl was associated with male gender, Hispanic ethnicity, and higher hemoglobin level. Those with albumin ≥4.0/3.7 g/dl had fewer deaths per 100 patient-years and fewer hospitalizations per time at risk. In multivariate analysis, patients with albumin ≥4.0/3.7 g/dl had 57% decreased risk for death. Poisson regression showed progressive decrease in hospitalization risk as albumin level increased; however, confidence intervals were similar between albumin ≥4.0/3.7 g/dl and albumin ≥3.5/3.2 and <4.0/3.7 g/dl.
Conclusions: This study demonstrates decreased mortality and hospitalization risk with albumin ≥3.5/3.2 g/dl and suggests that adolescent hemodialysis patients who are able to achieve serum albumin ≥4.0/3.7 g/dl may have the lowest mortality risk.
The National Kidney Foundation Dialysis Outcomes Quality Initiative (DOQI) practice guidelines were first established in 1997 to create standards for dialysis care. The current outcome goal for serum albumin for adult maintenance dialysis patients is a predialysis or stabilized serum albumin equal to or greater than the lower limit of the normal range (4.0 g/dl for the bromcresol green [BCG] laboratory method) (1). There is no established pediatric outcome goal for serum albumin. For adults who are on hemodialysis (HD), serum albumin <4.0 g/dl has been shown to be a strong predictor of mortality (2–4). For children, serum albumin level <3.5/3.2 g/dl (BCG/bromcresol purple [BCP]) is generally considered the cutoff for hypoalbuminemia, although normal values for children 5 to 19 yr of age are serum albumin 4.0 to 5.3 g/dl (5). There is very little evidence to assess the relevance of adult target serum albumin levels in pediatric patients with ESRD.
Materials and Methods
For this retrospective cohort study, national data on all prevalent in-center HD patients who were aged 12 to 18 yr between October and December 1999 and October and December 2000 were obtained from the Centers for Medicare and Medicaid Services’ ESRD Clinical Performance Measures (CPM) Project. This information was then linked with 4-yr hospitalization and mortality records (October 1999 through November 2003) of Medicare-eligible HD patients in the US Renal Data System (USRDS) by unique USRDS identification numbers.
The ESRD CPM Project collects clinical data on dialysis patients to assess the quality of care in several areas, including serum albumin, anemia management, vascular access, and dialysis adequacy (6). In the ESRD CPM Project, serum albumin levels were recorded monthly during 3 mo of each year, October through December 1999 and 2000; up to three separate serum albumin measures were available for each patient per year. The monthly values were averaged and analyzed as means in each year. Mean albumin levels were categorized as <3.5/3.2, ≥3.5/3.2 and <4.0/3.7, and ≥4.0/3.7 g/dl (BCG/BCP).
Clinical characteristics that were obtained from the ESRD CPM data set were compared among the serum albumin groups. Age was considered as a continuous variable. The remaining covariates were examined as categorical variables, including mean hemoglobin level (<11 versus ≥11 g/dl), race (white versus other), Hispanic ethnicity (yes/no), gender, access type (catheter versus graft/fistula), and dialysis vintage (<180 versus ≥180 d). Mean serum ferritin level was classified as 100 to 500 versus <100 versus >500 ng/ml on the basis of the most recent KDOQI clinical practice recommendations for anemia in children (7). Body mass index (BMI)-for-age percentiles were calculated based on Centers for Disease Control and Prevention (CDC) gender-specific growth charts and then categorized by CDC classifications as fifth through 85th (healthy weight) versus less than fifth (underweight) versus >85th and <95th (risk of overweight) versus ≥95th percentile (overweight) (8). ESRD duration was categorized as <2 versus 2 to 5 versus > 5 yr. To adjust for possible differential urinary protein losses in nephrotic patients, ESRD cause was classified as nephrotic (using International Classification of Diseases, Ninth Revision codes 581.0 through 581.3, 581.8, 581.9, and 582.1) versus other. Mean single-pool Kt/V was calculated according to the Daugirdas II formula and categorized as <1.2 versus ≥1.2 (9). USRDS treatment history files were explored for any changes in treatment modality. Because a greater prevalence of low serum albumin in pediatric peritoneal dialysis patients versus HD patients has been reported, we adjusted for any treatment switching from peritoneal dialysis (PD) to HD by including a dichotomous variable for PD exposure during the time of the study (PD yes/no) (10).
Risk for death and hospitalization was analyzed by mean albumin level, categorized as noted previously. Death and hospitalization data were obtained from the USRDS patient and hospitalization files, respectively. Nonparametric Kaplan-Meier curves with log-rank tests and semiparametric Cox proportional hazard regression models were used to compare survival and time to first hospitalization, adjusting for the aforementioned covariates. Hospitalization risk was further analyzed as total claims per time at risk using Poisson regression. The start date for time at risk was entry date into the ESRD CPM cohort (October 1, 1999, or October 1, 2000). The last death date reported in the patient files was used as the end point for time at risk for both mortality and hospitalization analyses. A sensitivity analysis was performed excluding patients who were switched to PD during the study period. Additional analyses examined risk for hospitalization and mortality by cause and by Hispanic subgroups: Hispanic Mexican versus other Hispanic.
For patients who had serum albumin data in both ESRD CPM Project study years, clustering by USRDS identification number was used to account for repeated measures. P < 0.05 was considered statistically significant. All data management and analysis were conducted using Stata 9.0 software (StataCorp, College Station, TX).
Results
Patient Characteristics
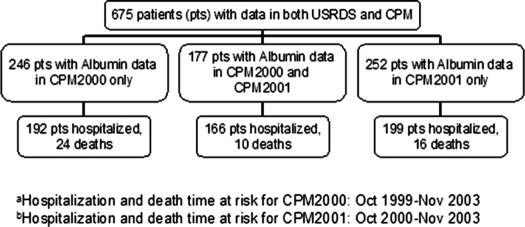
A total of 680 individual patients who were aged 12 to 18 yr were identified in the ESRD CPM project, and all had hospitalization and mortality data available in the USRDS database. Five patients were dropped from this merged data set secondary to transplantation or death within the first 2 mo of data collection. A total of 177 patients remained on HD from October 1999 through December 2000 and therefore had serum albumin information in 2 yr of ESRD CPM data collection (Table 1, Figure 1).
Table 1.
Clinical characteristics of cohort at study entry, mean serum albumin <3.5/3.2 versus ≥3.5/3.2 to 4.0/3/7 versus ≥4.0/3.7 g/dl (BCG/BCP), n = 675a
| Baseline Clinical Characteristic | Mean Serum Albumin
|
P | ||
|---|---|---|---|---|
| < 3.5/3.2 g/dl(n = 125) | ≥3.5/3.2 and <4.0/3/7 g/dl(n = 263) | ≥4.0/3.7 g/dl(n = 287) | ||
| Age (yr; mean [SD]) | 16 (1.7) | 15.8 (1.7) | 16.1 (1.6) | 0.217b |
| White race (n [%]) | 60 (48.0) | 116 (44.0) | 153 (53.0) | 0.096c |
| Male gender (n [%]) | 55 (44.0) | 132 (50.2) | 166 (58.0) | 0.021c,d |
| Hispanic ethnicity (n [%]) | 17 (13.6) | 58 (22) | 79 (27.5) | 0.030c,d |
| Nephrotic syndrome/FSGS (n [%]) | 29 (23.2) | 48 (18.3) | 43 (15.0) | 0.129c |
| HD access type: catheter (n [%]) | 74 (59.2) | 131 (49.8) | 111 (38.9) | 0.001c,d |
| Hemoglobin (g/dl; mean [SD]) | 10.1 (1.8) | 11 (1.6) | 11.5 (1.4) | 0.000b,d |
| Serum ferritin (ng/ml; mean [SD]) | 387 (469) | 274 (259) | 279 (285) | 0.005b,d |
| BMI (kg/m2; mean [SD]) | 20.2 (7.4) | 21.3 (7.6) | 21.1 (7.8) | 0.401b |
| spKt/V (mean [SD]) | 1.4 (0.4) | 1.5 (0.4) | 1.5 (0.4) | 0.096b |
| Dialysis vintage (d; mean [SD]) | 257 (590) | 540 (917) | 542 (759) | 0.002b,d |
| ESRD duration (d; mean [SD]) | 843 (1407) | 1072 (SD 1396) | 1054 (1401) | 0.283b |
| Transplant during study (n [%]) | 57 (45.6) | 121 (46.0) | 148 (51.6) | 0.342c |
| Peritoneal dialysis exposure (n [%]) | 10 (8.0) | 18 (6.8) | 12 (4.2) | 0.231c |
Bromcresol green/bromcresol purple (BCG/BCP) laboratory methods. BMI, body mass index; HD, hemodialysis; spKt/V, single-pool Kt/V.
ANOVA.
χ2
P < 0.05.
Figure 1.
Study population in USRDS/CPM linked dataset and grouping by time at risk and events.
Among the 675 included patients, the mean age was 16 yr (SD 1.66); 52% of patients were male, 49% were white, and 23% were Hispanic; 18% had nephrosis or FSGS as the cause of renal disease. The mean time since most recent HD initiation was 488 d (± 807). Mean time since first ESRD as documented in USRDS (2728 form) was 1022 d (± 1401). At time of study entry, 19% of patients had serum albumin <3.2/3.5 g/dl. Mean serum albumin in this pediatric adolescent cohort on HD was 3.77 g/dl (± 0.51); 47% had catheters for vascular access. Mean hemoglobin was 11.0 g/dl (± 1.61), and mean serum ferritin was 296 ng/ml (± 318). Mean BMI was 21 kg/m2 (± 7.7). Mean single-pool Kt/V was 1.48 (± 0.41). Forty (6%) patients were switched to PD for some portion of their follow-up time. A total of 326 (48%) patients were censored at transplantation; 15 (2%) patients were censored when they were lost to follow-up.
Patients with serum albumin ≥4.0/3.7 g/dl (BCG/BCP laboratory methods) were more likely to be male and Hispanic and more likely to have higher hemoglobin levels than those with serum albumin <3.5/3.2 g/dl. Patients with serum albumin <3.5/3.2 g/dl were more likely to have higher serum ferritin, catheters for vascular access, and shorter dialysis vintage.
During the designated follow-up period for hospitalization, 557 (83%) of the 675 included patients were hospitalized. The hospitalized cohort was composed of 358 patients with up to 4 yr of hospitalization time at risk (October 1999 through November 2003) and 199 patients who entered the study in the CPM2001 study year and thus had potentially 3 yr of hospitalization time at risk (October 2000 through November 2003).
There were 50 deaths during a 4-yr mortality follow-up period. Of those deaths, 24 occurred in patients with serum albumin data exclusively in CPM2000, 16 deaths occurred in patients with data exclusively in CPM2001, and 10 deaths occurred in patients with data from both collection periods. Known causes of death included 25 cardiovascular events and 11 deaths related to infection.
Bivariate Analysis: Survival
In bivariate analysis, there were 4.98 deaths per 100 patient-years among patients with serum albumin <3.5/3.2 g/dl compared with 3.29 deaths among those with serum albumin ≥3.5/3.2 and <4.0/3.7 g/dl and 2.07 deaths in those with serum albumin ≥4.0/3.7 g/dl (P = 0.062) (Table 2). Patients who died tended to be older (16.4 yr of age for those who died versus 15.9 yr of age for those who survived; P = 0.04) and tended to have lower mean hemoglobin (10.6 g/dl for those who died versus 11.1 g/dl for those who survived; P = 0.05).
Table 2.
Bivariate analysis: Risk for death and hospitalization by mean serum albumin category, n = 675a
| Parameter | Mean Serum Albumin
|
P | ||
|---|---|---|---|---|
| < 3.5/3.2 g/dl(n = 125) | ≥3.5/3.2 and <4.0/3/7 g/dl(n = 263) | ≥4.0/3.7 g/dl(n = 287) | ||
| Deaths per 100 patient-years | 4.98 | 3.29 | 2.07 | 0.062b |
| Hospitalizations per year at risk (SD) | 2.25 | 1.69 | 1.60 | 0.007c,d |
| Mean time to first hospitalization (d) | 246 | 290 | 306 | 0.087b |
BCG/BCP laboratory methods.
Log rank.
ANOVA.
P < 0.05.
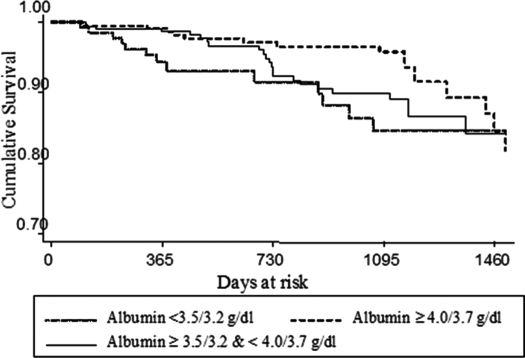
Kaplan-Meier curves and log-rank testing were also used to assess survival risk. Log-rank testing did not demonstrate a statistically significant difference in survival by differential mean serum albumin levels at 1500 d (P = 0.06); however, the survival curve did suggest that the higher serum albumin groupings had improved survival up to at least 1095 d, or 3 yr (Figure 2).
Figure 2.
Kaplan-Meier survival curve by serum albumin categories.
Bivariate Analysis: Hospitalization
Patients with serum albumin <3.5/3.2 g/dl had 2.25 hospitalizations per time at risk versus 1.69 hospitalizations for patients with serum albumin 3.5/3.2 and < 4.0/3.7 g/dl and 1.60 hospitalizations for patients with serum albumin ≥4.0/3.7 g/dl (P = 0.007). The mean time to first hospitalization also increased as serum albumin level increased, from 246 to 306 d; however, this was not statistically significant (P = 0.087) (Table 2). Of the categorical covariates, nonwhite race was associated with increased risk for hospitalization (P = 0.034, χ2). In addition, all 40 patients who received PD at some time during the study were hospitalized. By t testing, several covariates were associated with differential risk for hospitalization. Mean hemoglobin level in patients who were hospitalized was 10.9 versus 11.5 g/dl in those who were not hospitalized (P = 0.0004). Hospitalized patients also had higher mean ferritin levels (310 versus 230 ng/ml in those who were not hospitalized; P = 0.021), had ESRD for a longer period of time (1123 versus 543 d; P = 0.000), and had longer dialysis vintage (539 versus 250 d in those who were not hospitalized; P = 0.004).
We also examined more closely patients with repeated measures. Of the 177 patients with mean serum albumin measures in both ESRD CPM Project years, there were 10 deaths and 166 hospitalizations. A total of 116 (66%) patients had the same albumin level in both years, 43 (24%) patients experienced an increase in serum albumin level, and 18 (10%) patients had a serum albumin level decrease. Of the patients who died, only one had persistently low serum albumin levels <3.5/3.2 g/dl and one had a serum albumin drop from 3.5 to 4.0 g/dl to <3.5/3.2 g/dl. Among the patients who had repeated measures and were hospitalized, 18 (10%) had persistently low serum albumin and there was no detectable difference by χ2 analysis between patients who had constant serum albumin levels versus increased versus decreased levels.
Multivariate Analysis: Survival
Cox proportional hazard regression models were constructed with adjustment for the aforementioned covariates. The full model with all covariates demonstrated decreased risk for death (hazard ratio [HR] 0.43; 95% confidence interval [CI] 0.19 to 0.96) for patients with serum albumin ≥4.0/3.7 g/dl versus patients with serum albumin <3.5/3.2 g/dl. Test of trend showed a 33% decrease in risk for death as serum albumin grouping increased from <3.5/3.2 to ≥4.0/3.7 g/dl (HR 0.67; 95% CI 0.46 to 0.97). By Cox modeling, the only covariate that was independently associated with risk for death in both crude and adjusted models was mean hemoglobin; there was a 60% decreased risk for death for hemoglobin ≥11 versus <11 g/dl (HR 0.40; 95% CI 0.20 to 0.78). Mean dialysis vintage was also associated with differential risk for mortality; for those who were on dialysis ≥180 d, mortality risk was more than two-fold higher (HR 2.26; 95% CI 1.11 to 4.59; Table 3).
Table 3.
Cox proportional hazard: Crude and adjusted risks for mortality for each covariate, including mean serum albumin <3.5/3.2 versus ≥3.5/3.2 and <4.0/3/7 versus ≥4.0/3.7 g/dl, n = 675a
| Covariates | HR, Crude (95% CI) | HR, Adjusted (95% CI) |
|---|---|---|
| Serum albumin ≥3.5/3.2 and < 4.0/3/7 versus <3.5/3.2 g/dl | 0.66 (0.33 to 1.33) | 0.74 (0.35 to 1.60) |
| Serum albumin ≥4.0/3.7 versus <3.5/3.2 g/dl | 0.42 (0.20 to 0.89)b | 0.43 (0.19 to 0.96)b |
| Age (yr) | 1.11 (0.91 to 1.34) | 1.16 (0.94 to 1.43) |
| White race | 1.21 (0.70 to 2.11) | 1.30 (0.69 to 2.45) |
| Male gender | 1.00 (0.58 to 1.71) | 1.03 (0.57 to 1.87) |
| Hispanic ethnicity | 1.06 (0.55 to 2.03) | 1.29 (0.60 to 2.74) |
| Nephrotic syndrome/FSGS | 0.57 (0.25 to 1.34) | 0.56 (0.22 to 1.41) |
| HD access type: catheter | 1.23 (0.77 to 1.98) | 1.23 (0.73 to 2.07) |
| Mean hemoglobin ≥11 versus < 11 g/dl | 0.40 (0.22 to 0.71)b | 0.40 (0.20 to 0.78)b |
| Mean serum ferritin (ng/ml) | ||
| 100 to 500 | Reference | Reference |
| <100 | 0.89 (0.43 to 1.84) | 0.86 (0.38 to 1.96) |
| >500 | 1.49 (0.76 to 2.92) | 1.37 (0.70 to 2.69) |
| Mean BMI-for-age percentile | ||
| 5th through 85th | Reference | Reference |
| <5th | 1.90 (0.92 to 3.92) | 2.05 (0.98 to 4.31) |
| ≥85th through 95th | 0.59 (0.18 to 1.95) | 0.55 (0.15 to 2.01) |
| ≥95th | 1.50 (0.70 to 3.25) | 1.54 (0.67 to 3.51) |
| Mean spKt/V ≥1.2 versus <1.2 | 1.08 (0.60 to 1.98) | 0.97 (0.50 to 1.86) |
| Mean dialysis vintage ≥180 versus <180 d | 1.29 (0.71 to 2.36) | 2.26 (1.11 to 4.59)b |
| Mean ESRD duration (yr) | ||
| <2 | Reference | Reference |
| 2 to 5 | 0.86 (0.44 to 1.69) | 0.81 (0.42 to 1.57) |
| ≥5 | 0.70 (0.34 to 1.43) | 0.60 (0.28 to 1.27) |
| PD exposure | 0.96 (0.34 to 2.72) | 1.12 (0.38 to 3.35) |
CI, confidence interval; HR, hazard ratio; PD, peritoneal dialysis.
P < 0.05
Because there were multiple covariates and only 50 deaths, we constructed several different models (Table 4). Because hemoglobin was independently associated with mortality risk, a model that included only serum albumin level and hemoglobin was constructed. Two sensitivity analyses were performed; one excluded any patients who received a transplant during the study, because these patients may have represented a healthier subset of the total population. The second sensitivity analysis excluded 40 patients who were switched to PD during the study. Overall, the point estimates suggested a 23 to 38% lower risk for mortality for those with mean serum albumin ≥3.5/3.2 ad <4.0/3.7 g/dl versus serum albumin <3.5/3.2 g/dl, although these results were not statistically significant for any of the models. For serum albumin ≥4.0/3.7 versus <3.5/3.2 g/dl, there was a 57 to 62% decreased risk for mortality that was statistically significant in the crude, full, demographic and dialysis models. The statistical significance of these findings was mitigated in the remaining models.
Table 4.
Cox proportional hazard: Mortality risk by albumin level
| Parameter | Serum Albumin ≥3.5/3.2 and <4.0/3/7 versus <3.5/3.2 g/dl | Serum Albumin ≥4.0/3.7 versus <3.5/3.2 g/dl |
|---|---|---|
| Crude model | 0.66 (0.33 to 1.33) | 0.42 (0.20 to 0.89)f |
| Full modela | 0.74 (0.33 to 1.60) | 0.43 (0.19 to 0.96)f |
| Demographic modelb | 0.65 (0.32 to 1.30) | 0.38 (0.18 to 0.82)f |
| Dialysis modelc | 0.62 (0.30 to 1.27) | 0.42 (0.20 to 0.87)f |
| Nutritional modeld | 0.77 (0.36 to 1.65) | 0.54 (0.24 to 1.21) |
| Anemia modele | 0.76 (0.37 to 1.54) | 0.54 (0.24 to 1.18) |
Adjusted for age, race, gender, Hispanic ethnicity, nephrotic syndrome/FSGS, vascular access type, mean hemoglobin, mean serum ferritin level, BMI-for-age percentile grouping, spKt/V, dialysis vintage, ESRD duration, and PD exposure.
Adjusted for age, race, gender, and Hispanic ethnicity.
Adjusted for vascular access type, mean spKt/V, dialysis vintage, ESRD duration, and PD exposure.
Adjusted for nephrotic syndrome/FSGS, mean hemoglobin, mean serum ferritin level, and BMI-for-age percentile grouping.
Adjusted for anemia only.
P < 0.05.
Multivariate Analysis: Hospitalization
Hospitalization was examined by hospital claims per time at risk using Poisson regression. In the crude model, serum albumin ≥4.0/3.7 g/dl was associated with decreased risk for hospitalization (incidence rate ratio [IRR] 0.71; 95% CI 0.55 to 0.90) as was serum albumin ≥3.5/3.2 to 4.0/3.7 g/dl (IRR 0.78; 95% CI 0.60 to 0.99; Table 5); when adjusted for the previously mentioned covariates, these results were no longer statistically significant. In the crude model, patients who were Hispanic had a 39% decreased risk for hospitalization per time at risk than patients who were non-Hispanic (IRR 0.61; 95% CI 0.50 to 0.75). Because there have been published reports demonstrating differences in inflammation and albuminuria in Mexican Americans, we examined the Hispanic subgroups Hispanic Mexican and Hispanic other in the full model (11,12). Hispanic Mexicans had a statistically significant decreased risk for hospitalization versus non-Hispanic patients (IRR 0.74; 95% CI 0.59 to 0.93). There was no statistically significant difference in risk for hospitalization between other Hispanic versus non-Hispanic patients. Besides Hispanic ethnicity, the only other covariate that was statistically significant in the full model was PD. Patients who were switched to PD at some time during the study demonstrated a two-fold increased risk for hospitalization versus those who were not on PD (IRR 2.01; 95% CI 1.39 to 2.90). We also performed several analyses to examine different combinations of covariates. Of the models detailed in Table 6, the demographic, dialysis, and Hispanic subgroup models demonstrated a statistically significant association between increasing serum albumin level and decreasing risk for hospitalization. Although, across models, serum albumin ≥4.0/3.7 g/dl (versus albumin <3.5/3.2 g/dl) showed lower point estimates for risk for hospitalization compared with albumin ≥3.5/3.2 and <4.0/3.7 g/dl (versus albumin <3.5/3.2 g/dl), the CI in both groupings were fairly similar.
Table 5.
Poisson regression: Crude and adjusted risks for hospitalization for each covariate, including mean serum albumin <3.5/3.2 versus ≥3.5/3.2 and <4.0/3/7 versus ≥4.0/3.7 g/dl, n = 675
| Covariate | HR, Crude (95% CI) | HR, Adjusted (95% CI) |
|---|---|---|
| Serum albumin ≥3.5/3.2 and <4.0/3/7 versus <3.5/3.2 g/dl | 0.78 (0.60 to 0.99)a | 0.85 (0.65 to 1.12) |
| Serum albumin ≥4.0/3.7 versus <3.5/3.2 g/dl | 0.71 (0.55 to 0.90)a | 0.84 (0.64 to 1.11) |
| Age (yr) | 1.02 (0.97 to 1.07) | 1.05 (0.99 to 1.11) |
| White race | 0.89 (0.74 to 1.06) | 0.95 (0.79 to 1.15) |
| Male gender | 0.97 (0.81 to 1.16) | 1.04 (0.84 to 1.27) |
| Hispanic ethnicity | 0.61 (0.50 to 0.75)a | 0.65 (0.52 to 0.80)a |
| Hispanic Mexican versus non-Hispanic | 0.74 (0.59 to 0.93)a | 0.75 (0.60 to 0.93)a |
| Nephrotic syndrome/FSGS | 0.89 (0.72 to 1.11) | 0.89 (0.70 to 1.13) |
| HD access type: catheter | 1.12 (0.95 to 1.32) | 1.07 (0.87 to 1.32) |
| Mean hemoglobin <11 versus ≥11 g/dl | 0.92 (0.88 to 0.97)a | 0.95 (0.90 to 1.01) |
| Mean serum ferritin | ||
| 100 to 500 ng/ml | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) |
| <100 ng/ml | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) |
| >500 ng/ml | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) |
| Mean BMI-for-age percentile | ||
| 5th through 85th | 0.99 (0.95 to 1.03) | 0.99 (0.95 to 0.99) |
| <5th | 0.99 (0.95 to 1.03) | 0.99 (0.95 to 0.99) |
| ≥85th through 95th | 0.99 (0.95 to 1.03) | 0.99 (0.95 to 0.99) |
| ≥95th | 0.99 (0.95 to 1.03) | 0.99 (0.95 to 0.99) |
| Mean spKt/V <1.2 versus ≥1.2 | 1.06 (0.87 to 1.30) | 0.95 (0.66 to 1.35) |
| Mean dialysis vintage <180 versus ≥180 d | 1.00 (0.99 to 1.00) | 1.00 (0.99 to 1.00) |
| Mean ESRD duration (yr) | ||
| <2 | 0.99 (0.99 to 1.00) | 0.99 (0.99 to 1.00) |
| 2 to 5 | 0.99 (0.99 to 1.00) | 0.99 (0.99 to 1.00) |
| ≥5 | 0.99 (0.99 to 1.00) | 0.99 (0.99 to 1.00) |
| PD exposure | 1.97 (1.50 to 2.60)a | 2.01 (1.39 to 2.90)a |
| Received transplant during study | 1.00 (0.85 to 1.19) | 1.14 (0.94 to 1.37) |
P < 0.05.
Table 6.
Poisson regression: Hospitalization risk by mean serum albumin level
| Parameter | Serum Albumin ≥3.5/3.2 and <4.0/3/7 versus <3.5/3.2 g/dl | Serum Albumin ≥4.0/3.7 versus <3.5/3.2 g/dl |
|---|---|---|
| Crude model | 0.78 (0.60 to 0.99)g | 0.71 (0.55 to 0.90)g |
| Full modela | 0.85 (0.65 to 1.12) | 0.84 (0.64 to 1.11) |
| Demographic modelb | 0.80 (0.63 to 1.03) | 0.76 (0.60 to 0.98)g |
| Dialysis modelc | 0.76 (0.58 to 0.98)g | 0.72 (0.56 to 0.92)g |
| Nutritional modeld | 0.91 (0.69 to 1.20) | 0.84 (0.63 to 1.11) |
| Hispanic subgroup modele | 0.76 (0.60 to 0.98)g | 0.69 (0.54 to 0.89)g |
| HD only modelf | 0.88 (0.67 to 1.15) | 0.83 (0.62 to 1.11) |
Adjusted for age, race, gender, Hispanic ethnicity, nephrotic syndrome/FSGS, vascular access type, mean hemoglobin, mean serum ferritin level, BMI-for-age percentile grouping, spKt/V, dialysis vintage, ESRD duration, and PD exposure.
Adjusted for age, race, gender, and Hispanic ethnicity.
Adjusted for vascular access type, mean spKt/V, dialysis vintage, ESRD duration, and PD exposure.
Adjusted for nephrotic syndrome/FSGS, mean hemoglobin, mean serum ferritin level, BMI-for-age percentile grouping.
Adjusted for Hispanic Mexican versus non-Hispanic.
Full model excluding any patients who received PD during study.
P < 0.05.
Additional Analyses
We examined the relationship between serum albumin level and cause of death using bivariate analysis and χ2 testing. In our study, 25 of 50 deaths were due to cardiovascular reasons and 11 were due to infectious causes: 39% of patients with serum albumin <3.5/3.2 g/dl had a cardiovascular-related death (n = 5) versus 55% of patients with serum albumin 3.5/3.2 to 4.0/3.7 g/dl (n = 12) versus 53% of patients with serum albumin ≥4.0/3.7 g/dl (n = 8; P = 0.625). Fifteen percent of patients with serum albumin <3.5/3.2 g/dl died as a result of infection (n = 2) versus 27% of those with serum albumin 3.5/3.2 to 4.0/3.7 g/dl (n = 6) versus 20% of those with serum albumin ≥4.0/3.7 g/dl (n = 3; P = 0.697).
We also examined whether being hospitalized for infectious or cardiovascular reasons might vary risk for hospitalization. Ninety-one of 557 (16%) patients who were hospitalized were hospitalized for cardiovascular causes, and 273 (49%) of 557 patients who were hospitalized were hospitalized for infectious causes (not mutually exclusive). Of patients who were hospitalized, those with serum albumin 3.5/3.2 to 4.0/3.7 g/dl (versus <3.5/3.2 g/dl) were 32% less likely to be hospitalized for a cardiovascular reason (IRR 0.68; 95% CI 0.38 to 1.20), and those with serum albumin ≥4.0/3.7 g/dl (versus <3.5/3.2 g/dl) were 45% less likely to be hospitalized for a cardiovascular cause (IRR 0.55; 95% CI 0.30 to 0.99). When hospitalizations for infectious reasons were examined, the risk for hospitalization was similar for both serum albumin 3.5/3.2 to 4.0/3.7 g/dl (IRR 0.78; 95% CI 0.60 to 1.01) and serum albumin ≥4.0/3.7 g/dl (IRR 0.78; 95% CI 0.60 to 1.02) versus those with serum albumin <3.5/3.2 g/dl.
Discussion
Achieving a serum albumin level equal to or greater than the lower limit of the normal range (4.0 g/dl for the BCG method) is recommended by the National Kidney Foundation as an outcome goal for adult maintenance dialysis patients. No evidence-based recommendations for serum albumin exist for children with ESRD. Only one previous study to our knowledge has examined the association between hypoalbuminemia and mortality risk in pediatric patients with ESRD; Wong et al. (13) examined the association between serum albumin obtained 45 d before dialysis initiation and death among incident pediatric patients. The authors found a 1.90 increased risk for mortality (95% CI 1.16 to 3.10) among patients with serum albumin <3.5 g/dl versus those with serum albumin ≥3.5 g/dl in up to 3 yr of follow-up. We set out to investigate whether subcategories of serum albumin levels were associated with differential risk for mortality and hospitalization among all prevalent HD pediatric patients and to investigate the influence of multiple confounders on this relationship.
For mortality, we found that patients with serum albumin ≥4.0/3.7 g/dl had fewer deaths per 100 patient-years than patients with serum albumin 3.5/3.2 to 4.0/3.7 g/dl and serum albumin <3.5/3.2 g/dl. In Cox regression, across multiple models, serum albumin ≥4.0/3.7 g/dl was associated with a 57 to 62% decreased risk for mortality compared with serum albumin <3.5/3.2 g/dl. Test of trend demonstrated a 33% decrease in risk for death as serum albumin grouping increased from <3.5/3.2 to ≥4.0/3.7 g/dl (HR 0.67; 95% CI 0.46 to 0.97). Point estimates were fairly consistent across multiple models.
Mean dialysis vintage ≥180 d was an independent predictor of mortality. This finding is not unexpected, because patients who have been on dialysis longer are likely to be sicker and more chronically malnourished and have chronic inflammation, etc. In addition, patients who were on HD for <180 d may have been placed on HD as a temporary measure (e.g., while awaiting transplantation).
Hemoglobin was a strong independent predictor of mortality. We previously reported the relationship between anemia and mortality in this same adolescent HD cohort with adjustment for a different set of covariates (14). In our previous reported data, we found that hemoglobin ≥11 g/dl was associated with decreased risk for death (HR 0.38; 95% CI 0.20 to 0.72) in multivariate analysis. Serum albumin level ≥3.5/3.2 g/dl was also associated with both improved survival and decreased risk for hospitalization in crude analyses, but these findings were mitigated in adjusted analyses. These results are consistent with previous literature on adult patients with chronic kidney disease (2). Madore et al. (15) retrospectively examined 21,899 patients who were receiving HD and found that “of all of the variables examined …, albumin was the most closely associated with hemoglobin concentration.” The authors postulated that visceral protein deficiency manifested as hypoalbuminemia might contribute to ineffective erythropoiesis and subsequent anemia. Recently, another study of 82 adult patients who were on HD demonstrated that serum albumin concentration was an important predictor of both baseline hemoglobin and erythropoietin sensitivity (16). The authors found that after controlling for erythropoietin dosage and multiple other covariates, the only factor that explained hemoglobin variation was serum albumin. The rate of change in hemoglobin was increased 0.49 g/dl per mo for each 1-g/dl per mo increase in albumin. The authors suggested that both serum albumin and hemoglobin may be markers of overall health status, with declines in both markers occurring with episodes of infection, increased inflammation, oxidative stress, and malnutrition. Clearly, there is an important interaction between anemia and hypoalbuminemia, and further studies to examine causality are needed.
It is interesting that the Kaplan-Meier survival curve demonstrated improved survival in patients with albumin ≥4.0/3.7 g/dl up to 3 yr after onset of the study. At 4 yr, the survival curves for each serum albumin grouping became similar. This result may reflect that as patients are further out from the time of serum albumin measurement, serum albumin becomes a less effective predictor of survival.
For hospitalization analysis, we found that patients with serum albumin ≥4.0/3.7 g/dl had fewer hospitalizations per time at risk (P = 0.007). They also had longer mean time to hospitalization; however, this result was not statistically significant. Poisson regression showed progressive decrease in risk for hospitalization as serum albumin level increased; however, CI were quite similar between patients with serum albumin >4.0/3.7 g/dl and patients with serum albumin 3.5/3.2 to 4.0/3.7 g/dl (Tables 5 and 6). All models demonstrated decreased risk for hospitalization for both groupings versus patients with serum albumin <3.5/3.2 g/dl. Of the covariates examined, patients who were of Hispanic Mexican ethnicity and those with hemoglobin ≥11 g/dl were at decreased risk for hospitalization. It is not surprising that those who are less anemic are at lower risk for being hospitalized. The differing point estimates between Hispanic Mexican and other Hispanic patients were surprising, however. Previous literature showed increased C-reactive protein (CRP) and microalbuminuria in Hispanic Mexican patients; therefore, we expected increased hospitalization risk in this Hispanic subgroup (11,12). It is possible that we are missing claims for Hispanic Mexican patients. In 2004, the CDC produced a report entitled “Access to Health Care Among Hispanic/Latino Children: United States, 1998–2001” (17). This report found that of five Hispanic subgroups examined, Mexican children were most likely to lack health insurance coverage and least likely to have a usual place to go for health care. Thus, our finding may reflect lack of access to health care rather than intrinsic biologic or ethnic differences. Atkinson et al. (18) examined associations between ethnicity and intermediate outcomes of anemia, hypoalbuminemia, and clearance parameters in pediatric ESRD patients in the CPM cohort from 2004 through 2005; the authors found that Hispanic patients were significantly more likely to achieve mean serum albumin ≥4.0/3.7 g/dl (BCG/BCP) compared with non-Hispanic white patients and were as likely to achieve clearance and hemoglobin targets. They did not, however, examine Hispanic subgroups. Further study into ethnic disparities in ESRD outcomes is needed.
Serum albumin is commonly used as a proxy of nutritional status as well as a marker of inflammation (19). In pediatric ESRD, markers of inflammation and malnutrition are still being defined. Cengiz et al. (20) demonstrated higher CRP, serum ferritin, and erythrocyte sedimentation rate and lower serum albumin and serum transferrin in 27 children who were on HD versus 20 healthy pediatric control subjects. Goldstein et al. (21) showed elevated cytokines in 13 children who were receiving maintenance HD versus published age-matched control subjects. Brem et al. (22) demonstrated an inverse relationship between serum ferritin and serum albumin in 39 pediatric patients with ESRD. In our analysis, besides serum albumin, we used serum ferritin as a surrogate for inflammation. Serum ferritin is an acute-phase reactant and may also reflect inflammation, malnutrition, or infection. Although serum ferritin is an imperfect marker of inflammation, low levels have been shown to correlate well with iron deficiency and high levels are more likely to correlate with inflammation (23). Thus, for multivariate analysis, we classified serum ferritin levels into three categories: 100 to 500 versus <100 versus >500 ng/ml. Patients with serum albumin <3.5/3.2 g/dl in our study had higher mean serum ferritin levels (Table 1). Categorical serum ferritin levels did not demonstrate a statistically significant association with mortality in our Cox regression analyses; however, the point estimates did suggest decreased risk for mortality in patients with serum ferritin <100 ng/ml and increased risk for mortality in those with serum ferritin >500 ng/ml (versus ferritin 100 to 500 ng/ml; Table 3). In Poisson regression, serum ferritin was not predictive of hospitalization risk. We did not have data on other inflammatory markers, such as transferrin, CRP, or specific cytokines. We also did not have data on factors such as prealbumin, acid-base status, IGF-1, cholesterol, phosphorus, or intact parathyroid hormone, which have all been linked with nutritional aberration. It is also possible that serum ferritin was higher in patients who had active infection; however, we were unable to discern statistically significant differences between serum albumin groupings and hospitalization or death as a result of infectious causes.
Wong et al. (24) examined anthropometric measures and risk for death in children with ESRD using USRDS data and found that decreases in growth velocity and height SD score were associated with increased risk for death. They suggested that poor growth might be a reflection of an abnormal growth hormone and insulin growth factor axis, which might promote cardiovascular disease and subsequently cardiac death. It has also been reported that hypoalbuminemia can induce a hypercoagulable and atherogenic state (25). We included BMI as a surrogate for nutrition; however, elevated BMI may also have reflected volume overload. We found no association between BMI and mortality or hospitalization. We also examined the association between cardiac death and serum albumin level in bivariate analysis; we performed a secondary analysis that looked at deaths and hospitalizations identified as being due to cardiovascular causes. We detected no difference in cardiovascular deaths by serum albumin level; however, we did detect a 45% lower risk for hospitalization for cardiovascular causes in patients with serum albumin >4.0/3.7 g/dl and a 32% lower risk in patients with serum albumin 3.5/3.2 to 4.0/3.7 g/dl.
We also adjusted for dialysis prescription (i.e., Kt/V). Brem et al. (26) demonstrated a strong inverse relationship between Kt/V and serum albumin level in 53 pediatric dialysis patients over 18 mo. In our population, mean Kt/V values were similar for patients in all serum albumin groupings (Table 1), and we were unable to detect any association between dialysis adequacy and mortality or hospitalization.
The ESRD CPM Projects include all prevalent pediatric HD patients in US dialysis centers, and thus this data analysis examines the largest, most comprehensive data set available for this population. Our data analyses demonstrated statistically significant differences between risk for mortality and serum albumin levels. For mortality, analyses consistently showed the lowest risk at serum albumin ≥4.0/3.7 g/dl. For hospitalization, risk was similar for both serum albumin ≥4.0/3.7 g/dl and serum albumin 3.5/3.2 to 4.0/3.7 g/dl but clearly lower than risk at serum albumin level <3.5/3.2 g/dl. This retrospective cohort study cannot discern cause and effect; it does, however, support adult data demonstrating decreased risk for mortality and hospitalization with serum albumin ≥3.5/3.2 g/dl and suggests that patients who are able to achieve a serum albumin ≥4.0/3.7 g/dl may have the lowest risk for mortality and hospitalization. It is most likely that patients who are able to achieve this highest serum albumin level are also the patients with less anemia, less chronic inflammation, and better overall health status. Our results call particular attention to the strong relationship between serum albumin and anemia and the need for more basic science research to elucidate this complex interaction.
Disclosures
None.
Acknowledgments
S.A. was supported during the time of this research by an National Institute of Diabetes and Digestive and Kidney Diseases Renal Disease Epidemiology Training Grant (5 T32 DK07732), Johns Hopkins University, Bloomberg School of Public Health. S.F. receives support for this research through an R21 grant, R21 DK 064313-01.
This work was presented in part in abstract form at the American Society of Pediatric Nephrology conference; San Francisco, CA, May 1–4, 2004; and the annual meeting of the American Society of Nephrology; October 29 through November 1, 2004; St. Louis, MO.
Published online ahead of print. Publication date available at www.cjasn.org.
The views expressed in this article are those of the authors and do not necessarily reflect official policy of the Centers for Medicare and Medicaid Services or the US government.
References
- 1.Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 35: S1–S140, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Owen WF Jr, Lew NL, Liu Y, Lowrie EG, Lazarus JM: The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 329: 1001–1006, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Goldwasser P, Mittman N, Antignani A, Burrell D, Michel MA, Collier J, Avram MM: Predictors of mortality in hemodialysis patients. J Am Soc Nephrol 3: 1613–1622, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Phillips A, Shaper AG, Whincup PH: Association between serum albumin and mortality from cardiovascular disease, cancer and other causes. Lancet 2: 1434–1436, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Meites S, ed.: Pediatric Clinical Chemistry, Reference (Normal) Values, 3rd Ed., Washington, DC, American Association for Clinical Chemistry, 1989
- 6.Sugarman JR, Frederick PR, Frankenfield DL, Owen WF Jr, McClellan WM: Developing clinical performance measures based on the Dialysis Outcomes Quality Initiative Clinical Practice Guidelines: Process, outcomes, and implications. Am J Kidney Dis 42: 806–812, 2003 [DOI] [PubMed] [Google Scholar]
- 7.KDOQI clinical practice recommendations for anemia in children with chronic kidney disease. Am J Kidney Dis 47[Suppl 3]: 86–108, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL: CDC growth charts: United States. Adv Data (314): 1–27, 2000 [PubMed] [Google Scholar]
- 9.Daugirdas JT: Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J Am Soc Nephrol 4: 1205–1213, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Brem AS, Lambert C, Hill C, Kitsen J, Shemin DG: Prevalence of protein malnutrition in children maintained on peritoneal dialysis. Pediatr Nephrol 17: 527–530, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Giles WH, Myers GL, Rifai N, Ridker PM, Mannino DM: C-reactive protein concentration distribution among US children and young adults: Findings from the National Health and Nutrition Examination Survey, 1999–2000. Clin Chem 49: 1353–1357, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Jones CA, Francis ME, Eberhardt MS, Chavers B, Coresh J, Engelgau M, Kusek JW, Byrd-Holt D, Narayan KM, Herman WH, Jones CP, Salive M, Agodoa LY: Microalbuminuria in the US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 39: 445–459, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Wong CS, Hingorani S, Gillen DL, Sherrard DJ, Watkins SL, Brandt JR, Ball A, Stehman-Breen CO: Hypoalbuminemia and risk of death in pediatric patients with end-stage renal disease. Kidney Int 61: 630–637, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Amaral S, Hwang W, Fivush B, Neu A, Frankenfield D, Furth S: Association of mortality and hospitalization with achievement of adult hemoglobin targets in adolescents maintained on hemodialysis. J Am Soc Nephrol 17: 2878–2885, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Madore F, Lowrie EG, Brugnara C, Lew NL, Lazarus JM, Bridges K, Owen WF: Anemia in hemodialysis patients: Variables affecting this outcome predictor. J Am Soc Nephrol 8: 1921–1929, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Davis JL, Smith L: Serum albumin is strongly associated with erythropoietin sensitivity in hemodialysis patients. Clin J Am Soc Nephrol 3: 98–104, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott G, Ni H: Access to health care among Hispanic/Latino Children: United States, 1998–2001. Adv Data (344): 1–20, 2004 [PubMed] [Google Scholar]
- 18.Atkinson MA, Neu AM, Fivush BA, Frankenfield DL: Ethnic disparity in outcomes for pediatric peritoneal dialysis patients in the ESRD Clinical Performance Measures Project. Pediatr Nephrol 22: 1939–1946, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Owen WF, Lowrie EG: C-reactive protein as an outcome predictor for maintenance hemodialysis patients. Kidney Int 54: 627–636, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Cengiz N, Baskin E, Agras PI, Sezgin N, Saatci U: Relationship between chronic inflammation and cardiovascular risk factors in children on maintenance hemodialysis. Transplant Proc 37: 2915–2917, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Goldstein SL, Currier H, Watters L, Hempe JM, Sheth RD, Silverstein D: Acute and chronic inflammation in pediatric patients receiving hemodialysis. J Pediatr 143: 653–657, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Brem AS, Lambert C, Hill C, Kitsen J, Shemin DG: Clinical morbidity in pediatric dialysis patients: Data from the Network 1 Clinical Indicators Project. Pediatr Nephrol 16: 854–857, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Kalantar-Zadeh K, Lee G: The fascinating but deceptive ferritin: To measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol 1[Suppl 1]: S9–S18, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Wong CS, Gipson DS, Gillen DL, Emerson S, Koepsell T, Sherrard DJ, Watkins SL, Stehman-Breen C: Anthropometric measures and risk of death in children with end-stage renal disease. Am J Kidney Dis 36: 811–819, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Aguilera A, Sanchez-Tomero JA, Bajo MA, Ruiz-Caravaca ML, Alvarez V, del Peso G, Herranz A, Cuesta MV, Castro MJ, Selgas R: Malnutrition-inflammation syndrome is associated with endothelial dysfunction in peritoneal dialysis patients. Adv Perit Dial 19: 240–245, 2003 [PubMed] [Google Scholar]
- 26.Brem AS, Lambert C, Hill C, Kitsen J, Shemin DG: Outcome data on pediatric dialysis patients from the end-stage renal disease clinical indicators project. Am J Kidney Dis 36: 310–317, 2000 [DOI] [PubMed] [Google Scholar]