Abstract
Background and objectives: The authors have previously shown that urine neutrophil gelatinase-associated lipocalin (NGAL), measured by a research ELISA, is an early predictive biomarker of acute kidney injury (AKI) after cardiopulmonary bypass (CPB). In this study, whether an NGAL immunoassay developed for a standardized clinical platform (ARCHITECT analyzer®, Abbott Diagnostics Division, Abbott Laboratories, Abbott Park, IL) can predict AKI after CPB was tested.
Design, setting, participants, & measurements: In a pilot study with 136 urine samples (NGAL range, 0.3 to 815 ng/ml) and 6 calibration standards (NGAL range, 0 to 1000 ng/ml), NGAL measurements by research ELISA and by the ARCHITECT® assay were highly correlated (r = 0.99). In a subsequent study, 196 children undergoing CPB were prospectively enrolled and serial urine NGAL measurements obtained by ARCHITECT® assay. The primary outcome was AKI, defined as a ≥50% increase in serum creatinine.
Results: AKI developed in 99 patients (51%), but the diagnosis using serum creatinine was delayed by 2 to 3 d after CPB. In contrast, mean urine NGAL levels increased 15-fold within 2 h and by 25-fold at 4 and 6 h after CPB. For the 2-h urine NGAL measurement, the area under the curve was 0.95, sensitivity was 0.82, and the specificity was 0.90 for prediction of AKI using a cutoff value of 100 ng/ml. The 2-h urine NGAL levels correlated with severity and duration of AKI, length of stay, dialysis requirement, and death.
Conclusions: Accurate measurements of urine NGAL are obtained using the ARCHITECT® platform. Urine NGAL is an early predictive biomarker of AKI severity after CPB.
Cardiopulmonary bypass (CPB) surgery is the most frequent major surgical procedure performed in hospitals worldwide, with well over a million operations undertaken each year in adults alone (1). Acute kidney injury (AKI), previously referred to as acute renal failure, is a frequent and serious complication encountered in 30% to 50% of subjects after CPB (2,3). AKI requiring dialysis occurs in up to 5% of these cases, in whom the mortality rate approaches 80%, and is the strongest independent risk factor for death after CPB, with an odds ratio of 7.9 (4). Even minor degrees of postoperative AKI, as manifest by only a 0.2 to 0.3 mg/dl rise in serum creatinine from baseline, predict a significant increase in short-term mortality (5,6). AKI after cardiac surgery is also associated with a number of adverse outcomes, including prolonged intensive care and hospital stay, dialysis dependency, diminished quality of life, and increased long-term mortality (7–9).
Clinical investigations have identified several risk factors associated with the development of AKI after CPB, the majority related to either impaired renal perfusion or decreased renal reserve, and have resulted in the development of clinical scoring systems for the prediction of AKI (10–13). However, these tools have not been validated across medical centers, and have focused primarily on identifying the small number of high-risk, dialysis-requiring subjects. Concomitant advances in the basic sciences have illuminated the pathogenesis of AKI and have paved the way for successful therapeutic approaches in animal models (14). However, translational research efforts for the identification of effective therapeutic strategies in humans with AKI have yielded disappointing results (2,15). A major reason for the failure to find an effective treatment in patients is the paucity of early biomarkers for AKI, akin to troponins in acute myocardial disease (16). The availability of effective biomarkers may lead to improved efforts at ameliorating the course of AKI or preventing further renal injury. However, in current clinical practice, the gold standard for identification and classification of AKI is dependent on serial serum creatinine measurements (17), which are especially unreliable during acute changes in kidney function (15,16).
We used a genome-wide interrogation strategy to identify kidney genes that are induced very early after AKI in animal models, whose protein products might serve as novel early biomarkers. We identified neutrophil gelatinase-associated lipocalin (NGAL) as one of the most upregulated genes in the kidney soon after ischemic injury (18–20). NGAL protein was also markedly induced in kidney tubule cells and easily detected in the plasma and urine in animal models of ischemic and nephrotoxic AKI (18–22). The expression of NGAL protein was also dramatically increased in kidney tubules of humans with ischemic, septic, and post-transplant AKI (23,24). Importantly, NGAL in the urine was found to be an early predictive biomarker of AKI in a variety of acute clinical settings (25). In a cohort of 20 subjects who developed AKI 2 to 3 d after cardiac surgery, urine NGAL measured by a research enzyme-linked immunosorbent assay (ELISA) was elevated within 2 to 6 h after CPB (16,26). Preliminary results using the research-based assay also suggest that urine NGAL measurements can predict AKI following contrast administration (27), kidney transplantation (28), hemolytic-uremic syndrome (29), lupus nephritis (30), and in critically ill subjects (31). The availability of a standardized clinical platform for NGAL measurements could revolutionize renal diagnostics, especially in intensive care situations (32). Therefore, the first objective of the present study was to determine whether an NGAL immunoassay developed for a standardized clinical platform (ARCHITECT® analyzer, Abbott Diagnostics) correlates with the research-based assay. The second objective was to determine the utility of the standardized NGAL immunoassay as a predictive biomarker of AKI after CPB in a large prospective cohort.
Materials and Methods
Patients and Study Design
This investigation was approved by the institutional review board of the Cincinnati Children's Hospital Medical Center. All children undergoing elective CPB for surgical correction or palliation of congenital heart lesions between January 2004 and June 2006 were prospectively enrolled. We obtained written informed consent from the legal guardian of every participant before enrollment. Exclusion criteria included preexisting renal insufficiency, diabetes mellitus, peripheral vascular disease, and use of nephrotoxic drugs before or during the study period. Preexisting renal insufficiency was defined as a serum creatinine level that was greater than the 90th percentile for the child's age and gender. Patients with a history of potential nephrotoxin use (including aminoglycosides, angiotensin inhibitors, and nonsteroidal anti-inflammatory drugs) during the preoperative day or the first two postoperative days were excluded because of potential confounding effects on urinary NGAL measurements.
We used the Risk Adjustment for Congenital Heart Surgery 1 consensus-based scoring system to categorize the complexity of surgery (33) (Table 1). This method of risk stratification has emerged as a widely accepted tool for the evaluation of differences in outcomes of surgery for congenital heart disease.
Table 1.
Surgical procedures categorized by RACHS-1 scoring, as described by Jenkins et al. (33)
| No AKI (n = 97) | With AKI (n = 99) | |
|---|---|---|
| Category 1 | ||
| ASD closure | 25 | 0 |
| PAPVC repair | 7 | 0 |
| Category 2 | ||
| VSD closure | 22 | 3 |
| Pulmonary valvuloplasty/valve replacement | 5 | 4 |
| Pulmonary outflow tract augmentation | 8 | 4 |
| Subaortic stenosis resection | 3 | 2 |
| Tetralogy of Fallot repair | 9 | 7 |
| Bidirectional Glenn shunt | 6 | 4 |
| Partial AV canal repair | 5 | 1 |
| Category 3 | ||
| Complete AV canal repair | 2 | 10 |
| Fontan procedure | 1 | 20 |
| Aortic valve replacement | 1 | 4 |
| Aortoplasty | 0 | 4 |
| Mitral valvuloplasty | 0 | 5 |
| Tricuspid valvuloplasty | 0 | 2 |
| Tetralogy of Fallot/pulmonary atresia repair | 0 | 2 |
| Arterial switch operation | 0 | 4 |
| Right ventricle to PA conduit replacement | 3 | 7 |
| Aortic root replacement | 0 | 2 |
| Pulmonary artery reimplantation | 0 | 3 |
| Category 4 | ||
| Unifocalization for TOF/pulmonary atresia | 0 | 3 |
| Arterial switch operation with VSD closure | 0 | 2 |
| Rastelli operation | 0 | 3 |
| Category 6 | ||
| Norwood operation | 0 | 3 |
ASD, atrial septal defect; VSD, ventricular septal defect; PAPVC, partially anomalous pulmonary venous connection; AV, atrioventricular; TOF, tetralogy of Fallot; PA, pulmonary artery.
None of our subjects was in Category 5.
To obviate postoperative volume depletion and prerenal azotemia, all subjects received at least 80% of their maintenance fluid requirements during the first 24 h after surgery and 100% maintenance subsequently. We obtained spot urine samples at baseline and at frequent intervals after initiation of CPB. Urine samples were centrifuged at 2000 × g for 5 min, and the supernatants stored in aliquots at −80°C. Serum creatinine was measured by the hospital clinical laboratory at baseline and routinely monitored at least twice daily during the first 2 d after CPB and at least daily after the third postoperative day.
The primary outcome variable was the development of AKI, defined as a 50% or greater increase in serum creatinine from baseline. Other outcomes included percent change in serum creatinine, days in AKI, dialysis requirement, length of hospital stay, and mortality. Other variables we obtained included age, gender, ethnic origin, CPB time, previous heart surgery, and urine output.
In a pilot cross-sectional study, we measured NGAL concentrations with 136 urine samples (NGAL range, 0.3 to 815 ng/ml) and 6 calibration standards (NGAL range, 0 to 1000 ng/ml) to determine the correlation between the two assay methods described below. In a subsequent prospective study, serial urine samples from 196 children undergoing CPB were assayed for NGAL using the standardized clinical platform (ARCHITECT® analyzer, Abbott Diagnostics) to assess its ability to predict AKI and other adverse outcomes.
NGAL Analysis by Standardized Clinical Platform (ARCHITECT®)
The ARCHITECT® NGAL assay is currently under development. High affinity antibodies were generated toward distinct epitopes on NGAL and assay standards were prepared using human recombinant NGAL. The assay is a two-step (sandwich) assay using Chemiluminescent Microparticle Immunoassay technology. The ARCHITECT® assay had a functional sensitivity <2 ng/ml (20% coefficient of variation [CV], 95% confidence) and total CVs <5.0%.
NGAL Analysis by ELISA
The urine NGAL ELISA was performed using a commercially available assay (NGAL ELISA Kit 036; AntibodyShop, Grusbakken, Denmark) that specifically detects human NGAL. The assay was performed as per the manufacturer's protocol. Briefly, 100 μl of NGAL standards or diluted samples was applied on to the precoated microwells in duplicates. Microwells were then incubated for 1 h at room temperature and then washed with washing buffer. In succession, biotinylated NGAL antibody and HRP-streptavidin were incubated in the wells for 1 h each with shaking at 200 × g. TMB substrate was added for 10 min in the dark before adding stop solution. Finally, NGAL concentration was measured at 450 nm wavelength in each well with reference reading at 620 nm in blank wells. The intra-assay CV were 2.1% (range, 1.3% to 4.0%) and 3.0% (range, 1.2% to 4.0%) in urine and plasma, respectively. Interassay variation was 9.1% (range, 6.8% to 18.1%) and 8.2% (range, 2.2% to 11.2%) in urine and plasma, respectively. Urine creatinine was measured using quantitative colorimetric Microplate Assay Kit (Oxford Biomedical Research, Oxford, MA) to standardize urinary NGAL for changes in urine concentration. Urinary NGAL excretion is presented as the amount of urinary NGAL in ng per ml urine as well as in ng per mg of urine creatinine to correct for differences in NGAL due to urine dilution. All measurements were made in triplicate. The laboratory investigators were blinded to the sample sources and clinical outcomes until the end of the study.
Statistical Analysis
Statistical analysis for Table 2 was performed using SAS, version 9.2. Either a two-sample t test or Mann-Whitney rank sum test was used for continuous variables and χ2 or Fisher's exact test was used for categorical variables. The associations between NGAL and change in creatinine (Delta creat), duration of AKI (days), and hospital length of stay were assessed by Spearman Rank Order correlation analysis. Correlations between NGAL and AKI outcome, dialysis requirement, and death were performed using a logistic correlation. Multivariate stepwise multiple logistic regression analysis was undertaken to assess predictors of adverse clinical outcomes (duration of AKI and hospital length of stay). Potential independent predictor variables included age, gender, CPB time, and urinary NGAL values at 2, 4, and 6 h after CPB. The area under the curve (AUC) was calculated from a standard receiver-operating characteristic plot. Spearman Rank Order correlations were performed using the website www.wessa.net/rankcorr.wasp (v1.1.21-r4), and the logistic correlations were performed using JMP (v5.0.1a). An AUC of 0.5 is no better than expected by chance, whereas a value of 1.0 signifies a perfect biomarker. A P ≤ 0.05 was considered statistically significant.
Table 2.
Patient characteristics and clinical outcomes
| No AKI (n = 97) | AKI (n = 99) | P | |
|---|---|---|---|
| Age (yr) | 3.2 ± 0.4 | 4.8 ± 0.5 | NS |
| Males (%) | 55 | 50 | NS |
| Whites (%) | 86 | 88 | NS |
| Prior surgery (%) | 28 | 50 | <0.01 |
| RACHS-1 score | 1.6 ± 0.5 | 2.9 ± 0.6 | <0.001 |
| Bypass time (min) | 86 ± 3.5 | 138 ± 6 | <0.0001 |
| Urine output (ml/kg per h) | 1.6 ± 0.6 | 1.7 ± 0.9 | NS |
| Serum creatinine 0 h (mg/dl) | 0.39 ± 0.11 | 0.38 ± 0.11 | NS |
| Serum creatinine 24 h (mg/dl) | 0.39 ± 0.10 | 0.46 ± 0.18 | NS |
| Serum creatinine 48 h (mg/dl) | 0.41 ± 0.18 | 0.66 ± 0.22 | <0.01 |
| Serum creatinine 72 h (mg/dl) | 0.36 ± 0.10 | 0.67 ± 0.25 | <0.001 |
| Serum creatinine 96 h (mg/dl) | 0.35 ± 0.10 | 0.70 ± 0.34 | <0.001 |
| Delta creatinine (%) | 10.6 ± 3.2 | 102 ± 19 | <0.0001 |
| Duration of AKI (days) | 0 | 3.1 ± 0.7 | <0.0001 |
| Hospital stay (days) | 4.7 ± 0.7 | 10.6 ± 1.6 | <0.0001 |
| Dialysis (no.) | 0 | 4 | <0.001 |
| Deaths (no.) | 0 | 3 | <0.0001 |
NS, not significant. Values are mean ± SEM. RACHS-1 scoring was performed as described by (33). Delta creatinine represents the percent change in serum creatinine from baseline. Urine output during the first 24 h after surgery is shown.
Results
Verification of the Standardized Clinical Platform (ARCHITECT®)
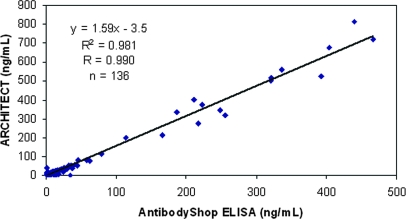
The cross-sectional pilot study was designed to verify the standardized clinical platform (ARCHITECT®) against the research-based NGAL ELISA assay. As shown in Figure 1, NGAL concentrations in 136 urine samples from subjects undergoing CPB (NGAL range, 0.3 to 815 ng/ml) and 6 calibration standards (NGAL range, 0 to 1000 ng/ml) determined by the two assays were highly correlated (r = 0.99), as shown in Figure 1. The slope difference observed between the assays is in large part due to differences in assay standardization between the assays. For commercialization, the ARCHITECT® assay will use highly purified, recombinant NGAL expressed in a mammalian system.
Figure 1.
Correlation between urine NGAL measurements obtained by ARCHITECT® platform and research-based NGAL ELISA assay (AntibodyShop).
NGAL as a Predictor of AKI and Other Adverse Outcomes
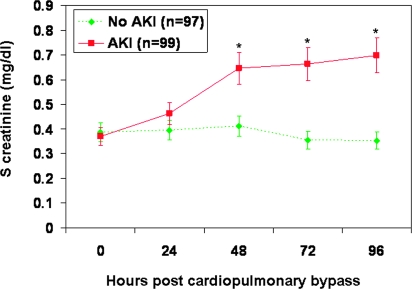
In a subsequent prospective study, serial urine samples from 196 children who met the inclusion and exclusion criteria were assayed for NGAL using the ARCHITECT® platform to assess its ability to predict AKI and other adverse outcomes. Ninety-nine patients (51%) met the criteria for AKI within a 3-d period. However, the increase in serum creatinine by 50% or greater from baseline was delayed by 1 to 3 d after CPB, as shown in Figure 2. Of these 99 patients, the increase in serum creatinine was noted in 7 patients (7.1%) at 24 h after CPB, in 60 patients (60.1%) at 48 h, and in 32 patients (32.1%) at 72 h. The increase in serum creatinine was sustained for at least 4 d after CPB, suggesting the presence of intrinsic AKI rather than a prerenal etiology. Based on this primary outcome, we classified subjects into those with and without AKI. No differences were noted between the two groups with respect to age, gender, race, urine output, hypertension, baseline use of angiotensin converting enzyme inhibitors, or baseline serum creatinine levels (Table 2). In patients who developed AKI, the duration of CPB was significantly longer, the complexity of surgery was significantly greater by Risk Adjustment for Congenital Heart Surgery 1 scoring, and the clinical outcomes were significantly worse. The serum creatinine rose by a greater percentage in the AKI group, and both length of hospitalization and mortality rate were significantly higher (Table 2). Four patients required dialysis, and there were three deaths, all in the AKI group.
Figure 2.
Serum creatinine measurements obtained at various time points post-CPB. AKI, acute kidney injury, defined as a 50% increase in serum creatinine from baseline. Values are mean ± SEM and are shown in Table 2. *P < 0.05 comparing AKI versus no AKI groups.
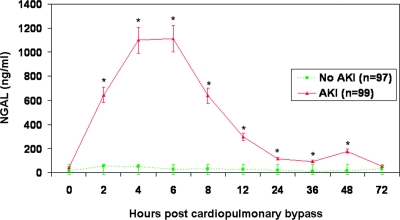
By ARCHITECT® assay, the absolute urine NGAL measurements at baseline were comparable in the two groups (Table 3). In the non-AKI group, there was a small but statistically significant increase in urine NGAL at 2 and 4 h after CPB, which normalized back to baseline levels at later time points. In marked contrast, in subjects who subsequently developed AKI, there was a robust 15-fold increase in urine NGAL at 2 h after CPB, which was even further accentuated at the 4 h (25-fold increase) and 6 h (26-fold increase) time points (Table 3; Figure 3). In the AKI group, a statistically significant increase in urine NGAL from baseline was apparent up to 48 h after CPB. Similar findings were recorded when the urine NGAL measurements were corrected for urinary creatinine concentrations (Table 4).
Table 3.
Urine NGAL measurements in ng/ml at various time points after cardiopulmonary bypass
| Time After CPB (h) | No AKI (n = 97): NGAL, ng/ml (mean ± SEM) | AKI (n = 99): NGAL, ng/ml (mean ± SEM) | No AKI (n = 97): NGAL, ng/ml [median (range)] | AKI (n = 99): NGAL, ng/ml [median (range)] | P | AUC |
|---|---|---|---|---|---|---|
| 0 | 14.8 ± 2.7 | 43.6 ± 14.2 | 5.7 (0.1–138) | 9.3 (0.6–976) | 0.09 | 0.59 |
| 2 | 53.0 ± 20.7 | 646.3 ± 69.8 | 4.0 (0.2–1557) | 457 (3.6–2778) | <0.0001 | 0.93 |
| 4 | 50.6 ± 14.4 | 1098.8 ± 87.1 | 8.2 (0.9–814) | 1080 (3.4–2580) | <0.0001 | 0.96 |
| 6 | 28.3 ± 6.9 | 1113.4 ± 88.8 | 8.4 (1.5–422) | 948 (20–2841) | <0.0001 | 0.98 |
| 8 | 30.5 ± 8.9 | 638.7 ± 69.8 | 7.6 (0.9–652) | 444 (3.3–2571) | <0.0001 | 0.94 |
| 12 | 26.9 ± 5.3 | 300.2 ± 42.7 | 9.5 (0.6–322) | 170 (2.8–2346) | <0.0001 | 0.88 |
| 24 | 20.8 ± 4.7 | 116.0 ± 28.0 | 7.6 (0.3–353) | 25 (2.8–1900) | 0.0005 | 0.76 |
| 36 | 11.2 ± 1.8 | 92.8 ± 39.8 | 9.1 (1.0–58) | 16 (0.5–2581) | 0.001 | 0.69 |
| 48 | 16.3 ± 2.9 | 176.6 ± 54.9 | 7.8 (0.5–128) | 22 (1.0–2809) | 0.005 | 0.72 |
| 72 | 30.9 ± 13.7 | 53.2 ± 23.3 | 7.7 (1.8–220) | 7.9 (0.6–558) | 0.5 | 0.51 |
At each time point, the calculated area under the curve (AUC) for prediction of AKI is also shown.
Figure 3.
Urine NGAL measurements obtained by ARCHITECT® assay at various time points post-CPB. AKI, acute kidney injury, defined as a 50% increase in serum creatinine from baseline. Values are mean ± SEM and are shown in Table 3. *P < 0.05 comparing AKI versus no AKI groups.
Table 4.
Urine NGAL measurements in ng/mg creatinine at various time points after cardiopulmonary bypass
| Time After CPB (h) | No AKI (n = 97): NGAL, ng/mg Cr (mean ±SEM) | AKI (n = 99): NGAL, ng/mg Cr (mean ±SEM) | No AKI (n = 97): NGAL, ng/mg Cr [median (range)] | AKI (n = 99): NGAL, ng/mg Cr [median (range)] | P | AUC |
|---|---|---|---|---|---|---|
| 0 | 19.7 ± 3.1 | 88.8 ± 43.3 | 7.7 (0.1–140) | 9.8 (1.0–3487) | 0.07 | 0.57 |
| 2 | 79.10 ± 25.3 | 1850.2 ± 197.1 | 9.7 (0.6–1622) | 1369 (6.0–10148) | <0.0001 | 0.95 |
| 4 | 128.2 ± 37.7 | 3578.2 ± 329.4 | 27 (1.8–2904) | 3336 (6.0–15782) | <0.0001 | 0.96 |
| 6 | 59.9 ± 11.3 | 2827.4 ± 222.0 | 22 (1.5–693) | 2431 (48–7784) | <0.0001 | 0.98 |
| 8 | 53.0 ± 11.0 | 1507.7 ± 175.8 | 17 (1.0–724) | 906 (6.3–5698) | <0.0001 | 0.95 |
| 12 | 39.5 ± 7.0 | 590.3 ± 101.9 | 13 (1.3–425) | 248 (3.9–6174) | <0.0001 | 0.87 |
| 24 | 38.2 ± 8.2 | 173.9 ± 41.7 | 14 (0.7–535) | 48 (1.9–2836) | 0.004 | 0.75 |
| 36 | 24.8 ± 3.9 | 248.9 ± 114.4 | 20 (2.5–142) | 35 (1.0–7592) | 0.022 | 0.68 |
| 48 | 40.2 ± 8.6 | 345.0 ± 129.1 | 16 (0.9–336) | 40 (1.5–6442) | 0.02 | 0.68 |
At each time point, the calculated area under the curve (AUC) for prediction of AKI is also shown.
To assess the relationship between urinary NGAL and AKI severity, we compared NGAL values in the entire AKI cohort with those that developed early AKI (≥50% increase in serum creatinine at 24 h after CPB) or required dialysis. In both scenarios, urinary NGAL levels were found to be significantly higher in subjects who had either an earlier rise in serum creatinine or needed renal replacement therapy (Table 5). This difference was seen in the 2 h urine NGAL levels and persisted in the 4 and 6 h NGAL measurements.
Table 5.
Urine NGAL measurements (mean ± SEM) in ng/mg creatinine at various time points after cardiopulmonary bypass
| NGAL (ng/mg creatinine) | Baseline | 2 h | 4 h | 6 h |
|---|---|---|---|---|
| Entire AKI (n = 99) | 89 ± 43 | 1850 ± 197 | 3578 ± 329 | 2827 ± 222 |
| Early AKI (n = 7) | 52 ± 34 | 3689 ± 294a | 4821 ± 298a | 4240 ± 166a |
| Dialysis (n = 4) | 45 ± 31 | 5081 ± 320a | 5170 ± 350a | 3914 ± 224a |
Subjects who developed early AKI (≥ 50% increase in serum creatinine from baseline within 24 h after surgery) or dialysis requirement were compared with those in the entire AKI cohort.
P < 0.05 versus entire AKI cohort.
To assess independent predictors for the development of AKI in the entire cohort, multivariate logistic regression was performed. All variables that were found by univariate analysis to display a P < 0.1 were entered into the model. The most powerful independent predictors of AKI were the 6 h urine NGAL measurements in ng/ml (R2 = 0.68, P < 0.0001) and in ng/mg creatinine (R2 = 0.75, P < 0.0001). Age, gender, and race were not independent predictors of AKI.
To test the hypothesis that urine NGAL levels measured soon after CPB could be used to predict eventual clinical outcomes, the Spearman Rank Order correlation analysis or logistic regression analysis was performed as appropriate. The results are illustrated in Table 6. The 2, 4, and 6 h urine NGAL levels strongly correlated with percent change in serum creatinine, duration of AKI, and length of hospital stay (all P < 0.0001). The 2 h urine NGAL levels also correlated with subsequent dialysis requirement (r = 0.48, P = 0.01) as well as mortality (r = 0.53, P = 0.01).
Table 6.
Correlation of NGAL urine measurements (ng/ml) at 2, 4, and 6 h after CPB surgery with independent clinical factors
| Comparison parameter | Hour | Correlation Type | R | P | AUC |
|---|---|---|---|---|---|
| NGAL versus Δ creatinine (%) | 2 | Nonparametric | 0.66 | <0.0001 | — |
| NGAL versus LOS (days) | 2 | Nonparametric | 0.42 | <0.0001 | — |
| NGAL versus days in AKI | 2 | Nonparametric | 0.73 | <0.0001 | — |
| NGAL versus dialysis | 2 | Logistic | 0.38 | 0.01 | 0.86 |
| NGAL versus death | 2 | Logistic | 0.53 | 0.01 | 0.91 |
| NGAL versus Δ creatinine (%) | 4 | Nonparametric | 0.68 | <0.0001 | — |
| NGAL versus LOS (days) | 4 | Nonparametric | 0.44 | <0.0001 | — |
| NGAL versus days in AKI | 4 | Nonparametric | 0.77 | <0.0001 | — |
| NGAL versus dialysis | 4 | Logistic | 0.30 | 0.1 (NS) | 0.80 |
| NGAL versus death | 4 | Logistic | 0.33 | 0.1 (NS) | 0.81 |
| NGAL versus Δ creatinine (%) | 6 | Nonparametric | 0.72 | <0.0001 | — |
| NGAL versus LOS (days) | 6 | Nonparametric | 0.49 | <0.0001 | — |
| NGAL versus days in AKI | 6 | Nonparametric | 0.80 | <0.0001 | — |
| NGAL versus dialysis | 6 | Logistic | 0.45 | 0.06 (NS) | 0.90 |
| NGAL versus death | 6 | Logistic | 0.15 | 0.48 (NS) | 0.67 |
LOS, length of hospital stay (days); Δ creatinine, percent change in serum creatinine from baseline; AUC, area under the curve values from a typical ROC plot (for predicting dialysis, n = 4; and death, n = 3); —, not applicable; NS, not significant. The predictive modeling for death and dialysis events were limited due to the size of the study.
To assess the utility of urine NGAL as a predictor of adverse clinical outcomes, a multivariate analysis was undertaken with duration of AKI and hospital length of stay as outcome variables and age, gender, CPB time, and NGAL levels as input variables. The 2 h NGAL value was found to be a strong independent predictor of duration of AKI (P < 0.0001). The 4 h NGAL measurement was a strong indicator of both duration of AKI (P < 0.0001) and length of stay (P = 0.0007). Similarly, the 6 h NGAL measurement was a powerful indicator of both duration of AKI (P < 0.0001) and length of stay (P < 0.0001).
To assess the utility of urine NGAL measurements at varying cutoff values to predict AKI, conventional receiver-operating characteristic curves were generated, and the AUCs calculated. Table 7 lists the derived sensitivities and specificities at different cutoff concentrations, and the calculated AUCs at each time point are shown in Table 3. For urine NGAL at 2 h after CPB, sensitivity and specificity were optimal at the 100 ng/ml cutoff, with an AUC of 0.93 for the prediction of AKI, indicative of an excellent biomarker. Using a similar approach, the 2 h urine NGAL was also an excellent biomarker for the prediction of dialysis requirement (AUC = 0.86) and mortality (AUC = 0.91). However, the number of events was small (n = 4 for dialysis requirement and n = 3 for mortality).
Table 7.
Derived sensitivities and specificities at various time points after CPB at various urine NGAL cutoffs
| NGAL Cutoff (ng/ml) | 2 h Sensitivity | 2 h Specificity | 4 h Sensitivity | 4 h Specificity | 6 h Sensitivity | 6 h Specificity |
|---|---|---|---|---|---|---|
| 50 | 0.89 | 0.85 | 0.95 | 0.83 | 0.92 | 0.91 |
| 100 | 0.82 | 0.90 | 0.91 | 0.91 | 0.89 | 0.95 |
| 150 | 0.79 | 0.92 | 0.90 | 0.92 | 0.88 | 0.95 |
| 200 | 0.73 | 0.94 | 0.89 | 0.95 | 0.87 | 0.95 |
| 250 | 0.67 | 0.94 | 0.85 | 0.95 | 0.87 | 0.98 |
| 300 | 0.63 | 0.97 | 0.79 | 0.95 | 0.81 | 0.99 |
| 350 | 0.61 | 0.97 | 0.76 | 0.96 | 0.77 | 0.99 |
| 400 | 0.59 | 0.97 | 0.72 | 0.96 | 0.76 | 0.99 |
Discussion
We have shown that accurate measurements of urine NGAL are obtained using a standardized clinical platform. Urine NGAL measured by this platform represents an early predictive biomarker of AKI severity after CPB.
Serum creatinine is an inadequate marker for AKI (25). First, substantial losses of glomerular filtration rate (GFR) may occur before an increase in serum creatinine will be measured. Second, serum creatinine does not accurately depict kidney function until a steady state has been reached, which may require several days. Although animal studies have shown that AKI can be prevented and/or treated by several maneuvers, these must be instituted very early after the insult, well before the rise in serum creatinine. Our study indicates that monitoring of urine NGAL levels can provide a very early warning to providers of clinical care. The urine NGAL levels measured by the ARCHITECT® assay at several early time points after CPB were excellent biomarkers for the subsequent development of AKI and its complications. This assay is easy to perform with no manual pretreatment steps, a first result available within 35 min, and it requires only 150 μl of urine.
This study lends substantial support to our previous findings in a small cohort of 20 subjects who developed AKI 2 to 3 d after cardiac surgery, in whom both urine and plasma NGAL measured by a research ELISA was elevated within 2 to 6 h after CPB (16). Human NGAL, a member of the lipocalin superfamily, was initially described as a 25 kD protein covalently bound to gelatinase in neutrophils (34). NGAL expression is induced in injured epithelia, including lung, colon, and especially kidney (18–25). Emerging experimental and clinical evidence indicates that, in the early phases of AKI from diverse etiologies, NGAL accumulates within two distinct pools, namely, a renal and a systemic pool. Gene expression studies in AKI have clearly demonstrated rapid and massive upregulation of NGAL mRNA in the thick ascending limb of Henle's loop and the collecting ducts, with resultant synthesis of NGAL protein in the distal nephron (the renal pool) and secretion into the urine where it comprises the major fraction of urinary NGAL (35,36). In addition, AKI results in increased NGAL mRNA expression in distant organs, especially the liver and lung, and the overexpressed NGAL protein is most likely released into the circulation and constitutes the systemic pool (35–37).
Our study has several strengths. First, we prospectively recruited a relatively homogeneous cohort of pediatric subjects in whom the only obvious etiology for AKI would be the result of CPB. These patients comprise an ideal and important population for the study of AKI biomarkers because they do not exhibit common comorbid variables that complicate similar studies in adults, such as diabetes, hypertension, atherosclerosis, and nephrotoxin use (38). Second, all subjects started with normal kidney function, and the study design allowed for the precise temporal definition of altered urine NGAL concentrations and a direct comparison with subsequent changes in serum creatinine. Our results clearly indicate that urine NGAL is a powerful early biomarker of AKI that precedes the increase in serum creatinine by several hours to days. The magnitude of rise supports the notion that urine NGAL is a highly discriminatory biomarker with a wide dynamic range and cutoff values that allow for easy risk stratification. Third, this is the first example of how a standardized clinical laboratory platform (ARCHITECT®) may be useful for predicting AKI using urine samples. The majority of biomarkers of AKI described thus far have been measured in the urine (25). Urinary diagnostics have several advantages, including the noninvasive nature of sample collection and the reduced number of interfering proteins. Fourth, the results obtained using the ARCHITECT® platform were independent of changes in urinary concentration and were equally applicable after correction for urine creatinine measurements.
This study has important limitations. First, it is a single-center study of pediatric subjects with congenital heart defects undergoing elective CPB. Our results, although provocative and of clear statistical significance, will certainly need to be validated in a larger randomized prospective trial, including adults with the usual confounding variables and comorbid conditions that normally accumulate with increasing age. Second, ours was a cohort with normal kidney function at recruitment, and it will be important to confirm our findings in documented high-risk settings, such as preexisting kidney dysfunction, diabetes mellitus, and concomitant nephrotoxic drug use. Third, it is acknowledged that, although all recruited subjects had normal serum creatinine measurements before surgery, an estimation of GFR to document normal kidney function was not made. However, estimation of GFR in children using serum creatinine or creatinine-based equations is fraught with imprecision (39). Fourth, in addition to NGAL, simultaneous examination of other urinary biomarkers as potential predictors of AKI may be informative (25). It is possible that a collection of other strategically selected candidates along with NGAL will prove of value for early and rapid diagnosis of AKI.
What is the potential clinical utility of our findings? Ideally, an early elevation in urine NGAL would trigger an immediate paradigm shift in the clinical management of the patient. At the very least, clinicians informed of such a situation would be aware of the potential for development of clinical AKI, and biomarkers, such as NGAL, may add substantively to existing clinical scoring systems for AKI prediction. Such patients would deserve closer monitoring with respect to blood pressure, urine output, and renal perfusion. Every effort to monitor postoperative intravascular status and to optimize hydration and renal perfusion would be deployed. These subjects would benefit from the diligent avoidance of nephrotoxins. The ability to predict which patients will develop AKI after CPB could enable early initiation of interventions to change the dismal outcomes associated with this all-too-common clinical problem. For example, earlier intervention with renal replacement therapy may be strongly considered for subjects with elevated biomarker levels who are developing fluid overload but will not display increased serum creatinine for several days due to hemodilution and time required for reestablishment of a steady state. The availability of promising early biomarkers may enable the timely initiation of interventions, such as atrial natriuretic peptide (40) and insulin-like growth factor (41) that have been successful in smaller, phase II level efficacy studies but not in larger phase III trials. Possibly, these interventions would have been successful if they were initiated at the onset of AKI (as determined by predictive biomarkers) rather than waiting several days for serum creatinine to rise. In addition, animal studies have identified, and continue to reveal, novel therapies, such as growth factors, anti-apoptotic, anti-inflammatory, and anti-oxidant approaches that are effective in early AKI, before the rise in serum creatinine (14). The availability of a standardized commercial platform for urinary NGAL determination will enable these safe and highly promising agents to be investigated in humans with AKI.
Disclosures
None.
Acknowledgments
This work was supported in part by a restricted research grant from Abbott Diagnostics. P.D. is supported by grants from the NIH/NIDDK (RO1-DK53289, P50-DK52612, R21-DK070163), a Grant-in-Aid from the American Heart Association Ohio Valley Affiliate, and a Translational Research Initiative Grant from Cincinnati Children's Hospital Medical Center.
Abbott Diagnostics has signed an exclusive licensing agreement with Cincinnati Children's Hospital and Columbia University for developing urine NGAL as a biomarker of acute renal failure.
The authors thank our patients and their families for their participation.
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Albert MA, Antman EM: Preoperative evaluation for cardiac surgery. In Cohn LH, Edmunds LH Jr (eds): Cardiac Surgery in the Adult, New York, McGraw-Hill, 2003, pp 235–248
- 2.Haase M, Haase-Fielitz A, Bagshaw SM, Ronco C, Bellomo R: Cardiopulmonary bypass-associated acute kidney injury: a pigment nephropathy? Contrib Nephrol 156: 340–353, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Rosner MH, Okusa MD: Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 1: 19–32, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J: Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 104: 343–348, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Lassning A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Thakar CV, Worley S, Arrigain S, Yared J-P, Paganini EP: Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int 67: 1112–1119, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT: Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med 128: 194–203, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Lok CE, Austin PC, Wanh H, Tu JV: Impact of renal insufficiency on short- and long-term outcomes after cardiac surgery. Am Heart J 148: 430–438, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Loef BG, Epema AH, Smilde TB, Henning RH, Ebels T, Navis G, Stegemean CA: Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol 16: 195–200, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Mehta RL: Acute renal failure and cardiac surgery: Marching in place or moving ahead? J Am Soc Nephrol 16: 12–14, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Thakar CV, Arrigain S, Worley S, Yared J-P, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16: 162–168, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Mehta RH, Grab JD, O'Brien SM, Bridges CR, Gammie JS, Haan CK, Ferguson TB, Peterson ED, for the Society of Thoracic Surgeons National Cardiac Surgery Database Investigators: Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation 114: 2208–2216, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT, Beattie WS: Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA 297: 1801–1809, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Jo SK, Rosner MH, Okusa MD: Pharmacologic treatment of acute kidney injury: Why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol 2: 356–365, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P: Acute renal failure: Definition, outcome measures, animal models, fluid therapy and information technology needs. The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P: Differential gene expression following early renal ischemia-reperfusion. Kidney Int 63: 1714–1724, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of NGAL as a novel urinary biomarker for ischemic injury. J Am Soc Nephrol 4: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Devarajan P, Mishra J, Supavekin S, Patterson LT, Potter SS: Gene expression in early ischemic renal injury: Clues towards pathogenesis, biomarker discovery and novel therapeutics. Mol Genet Metab 80: 365–376, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL): A novel urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol 24: 307–315, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P: Amelioration of ischemic acute renal injury by NGAL. J Am Soc Nephrol 15: 3073–3082, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Mori K, Lee HT, Rapoport D, Drexler I, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J: Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia. J Clin Invest 115: 610–621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, Devarajan P: Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol 21: 856–863, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Devarajan P: Emerging biomarkers of acute kidney injury. Contrib Nephrol 156: 203–212, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL: Urinary IL-18 is an early predictive biomarker of AKI after cardiac surgery. Kidney Int 70: 199–203, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH, Ma Q, Bennett M, Mitsnefes M, Devarajan P: NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol 22: 2089–2095, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P: Urine NGAL and IL-18 are predictive biomarkers for DGF following kidney transplantation. Am J Transplant 6: 1639–1645, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Trachtman H, Christen E, Cnaan A, Patrick J, Mai V, Mishra J, Jain A, Bullington N, Devarajan P: Urinary NGAL in D+HUS: A novel marker of renal injury. Pediatr Nephrol 21: 989–994, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Brunner HI, Mueller M, Rutherford C, Passo MH, Witte D, Grom A, Mishra J, Devarajan P: Urinary NGAL as a biomarker of nephritis in childhood-onset SLE. Arthritis Rheum 54: 2577–2584, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Zappitelli M, Washburn KM, Arikan AA, Loftis L, Ma Q, Devarajan P, Parikh CR, Goldstein SL: Urine NGAL is an early marker of acute kidney injury in critically ill children: A prospective cohort study. Crit Care 11: R84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honore PM, Joannes-Boyau O, Boer W: The early biomarker of acute kidney injury: In search of the Holy Grail. Intensive Care Med 33: 1866–1868, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI: Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 123: 110–118, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Xu S, Venge P: Lipocalins as biomarkers of disease. Biochim Biophys Acta 1482: 298–307, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Ott KM, Mori K, Kalandadze A, Li JY, Paragas N, Nicholas T, Devarajan P, Barasch J: NGAL-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens 15: 442–449, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J: Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18: 407–413, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Liu Q, Nilsen-Hamilton M: Identification of a new acute phase protein. J Biol Chem 270: 22565–22570, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Goldstein SL: Pediatric acute kidney injury: It's time for real progress. Pediatr Nephrol 21: 891–895, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Filler G, Foster J, Acker A, Lepage N, Akbari A, Ehrich JH: The Cockcroft-Gault formula should not be used in children. Kidney Int 67: 2321–2324, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Allgren RL, Marbury TC, Rahman SN, Weisberg LS, Fenves AZ, Lafayette RA, Sweet RM, Genter FC, Kurnik BR, Conger JD, Sayegh MH: Anaritide in acute tubular necrosis: Auriculin Anaritide Acute Renal Failure Study Group. N Engl J Med 336: 828–834, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Hirschberg R, Kopple J, Lipsett P, Benjamin E, Minei J, Albertson T, Munger M, Metzler M, Zaloga G, Murray M, Lowry S, Conger J, McKeown W, O'Shea M, Baughman R, Wood K, Haupt M, Kaiser R, Simms H, Warnock D, Summer W, Hintz R, Myers B, Haenftling K, Capra W, et al: Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int: 55: 2423–2432, 1999 [DOI] [PubMed] [Google Scholar]