Abstract
Background and Objectives: The treatment for X-linked hypophosphatemia (XLH) with phosphate and calcitriol can be complicated by secondary hyperparathyroidism and nephrocalcinosis. Furthermore, vitamin D and phosphate stimulate FGF23 production, the pathogenic factor causing XLH. We investigated in XLH patients: 1) whether treatment with the calcimimetic agent, cinacalcet, will block the rise in parathyroid hormone (PTH) caused by phosphate administration; and 2) whether treatment with oral phosphate and calcitriol increases FGF23 levels.
Design, Setting, Participants, and Measurements: Eight subjects with XLH were given a single oral dose of phosphate, followed the next day by combined treatment with phosphate and cinacalcet. Serum measurements of ionized calcium (Ca), phosphate, creatinine, intact PTH, 1,25(OH)2D, FGF23, and tubular threshold for phosphate/glomerular filtration rate (TP/GFR) were assessed in response to short-term treatment with phosphate and cinacalcet and compared with long-term administration of phosphate and calcitriol.
Results: Oral phosphate load increased serum phosphate, decreased ionized calcium, and increased PTH. Twenty-four hours later, FGF23 significantly increased and 1,25(OH)2D decreased. The concomitant administration of phosphate and cinacalcet resulted in further decrease in serum Ca2+ but suppression of PTH and greater increase in serum phosphate and TP/GFR. Chronic treatment with phosphate and calcitriol resulted in a smaller increment in serum phosphate and high serum FGF23.
Conclusions: Traditional therapy of XLH with phosphate and calcitriol elevates FGF23 and has the potential to stimulate PTH. Short-term treatment with cinacalcet suppresses PTH, leading to increase in TP/GFR and serum phosphate. Thus, long-term clinical studies are needed to investigate whether cinacalcet may be a useful adjuvant in the treatment of XLH, allowing the use of lower doses of phosphate and calcitriol.
X-linked hypophosphatemia (XLH) is caused by loss of function mutations in the endopeptidase PHEX, which results in the increased production and circulating levels of FGF23 (1–4). FGF23 inhibits renal tubular transport of phosphate and suppresses 1-α hydroxylation of 25(OH) vitamin D (5–7). The resulting hypophosphatemia and aberrant levels of serum 1,25(OH)2D levels lead to rickets/osteomalacia and growth retardation (8–10).
Currently, large oral doses of phosphate and 1,25(OH)2D (calcitriol) are the standard treatment of patients with XLH (8–10). Although this therapy is effective in raising serum phosphate and healing rickets and osteomalacia, it is often limited by nephrocalcinosis and secondary hyperparathyroidism, which in some patients may progress to tertiary hyperparathyroidism (9–13). Nephrocalcinosis is likely caused by combined effects of phosphaturia and vitamin D-mediated increased gastrointestinal calcium and phosphate absorption. The hyperparathyroidism is thought to be caused by the effect of high oral doses of phosphate to lower ionized calcium, which stimulates parathyroid hormone (PTH) through activation of the parathyroid gland calcium sensing receptor (CaR) (14–16). Finally, recent studies indicate that both dietary phosphate loading and administration of vitamin D can increase FGF23 levels (17–20), raising the possibility that the current treatment may further raise FGF23 levels, thereby creating a vicious cycle.
Calcimimetics may offer an alternative to combined treatment of XLH patients with calcitriol and oral phosphate. Calcimimetics are compounds that allosterically modulate the CaR in parathyroid chief cells (21), thereby enhancing its sensitivity to circulating serum calcium concentrations and consequently decreasing PTH secretion (22–24). Cinacalcet has recently been approved for treatment of secondary hyperparathyroidism in end-stage renal disease, where it was found to be effective in decreasing both PTH level and the calcium X phosphorus ion product (25–27). However, cinacalcet treatment of patients with less advanced chronic kidney disease results in increased serum phosphate levels, likely because of the loss of PTH-dependent phosphaturic activity (28). Although the effect of cinacalcet on FGF23 levels has not been studied, the observation that calcium does not appear to regulate FGF23 production in vitro suggests that calcimimetics may not regulate FGF23 levels (20). Furthermore, recently, Geller et al. (29), in their report on two patients with tumor-induced osteomalacia, found no acute effect of cinacalcet on serum FGF23 level. If so, by its suppressive effect on PTH, the addition of cinacalcet to the treatment regimen of XLH patients may have the advantage of raising serum phosphate levels in the face of lower doses of phosphate and calcitriol, thus decreasing the risks of nephrocalcinosis and secondary hyperparathyroidism.
In the current study, we examined if treatment with cinacalcet results in increased serum phosphate without stimulating elevations in PTH, and we assessed the effects of therapy with phosphate and calcitriol on FGF23, phosphate, and PTH levels in patients with XLH.
Design/Methods
The study was approved by the Pediatric Institutional Review Board, and informed consent was obtained from all participants and their guardians. Eight patients with the diagnosis of X-linked hypophosphatemic rickets (age, 6 to 19 yr; median age, 13 yr; 3 male and 5 female), followed in Children's Mercy Hospital Renal and Endocrine clinics, were enrolled in the study. There were three pairs of sibling, each with an afflicted mother, one girl with an afflicted father and one child with clinically healthy parents. All children had normal serum creatinine level for age and estimated glomerular filtration rate (GFR) >90 ml/min per 1.73 m2. All 8 were well controlled on regimen of phosphate and calcitriol. Radiographs showed healed rickets and serum alkaline phosphatase was normal in 4 and elevated up to 38% in the other 4. Two children had mild nephrocalcinosis. None had previous parathyroid surgery, and all had normal serum levels of PTH and calcium before the study.
Protocol
Patients underwent a run-in period for 7 d, during which time treatment with phosphate and calcitriol were discontinued. Patients were then evaluated in the Clinical Research Unit for 2 successive days with metabolic data collected in the morning over a 4-h period. On each morning of the study, the patients received a standard low caloric diet consisting of 2 pieces of toasts and jam, half a glass of nonfortified orange juice, and half a glass of water. During the 4 h in hospital observation period, they were given deionized water at 10% maintenance and a granola bar snack every 2 h.
On day 1, the patients received a single oral dose of phosphate (K-Phos M.F., Beach Pharmaceuticals, Tampa, FL) at 20 mg/kg. On day 2, each patient received the previous day phosphate dose plus cinacalcet (30 mg tablet for subjects weighing <30 kg and 60 mg for >30 kg). Blood was obtained for ionized Ca, phosphate, and intact PTH before treatment (time 0) and every 30 min after treatment for 4 h. Serum creatinine, FGF23 and 1,25 (OH)2 vitamin D were measured at times 0 and 4 h. A urine sample was also obtained before treatment and every 2 h after treatment for urinary Ca, phosphate, and creatinine measurements. Subjects were continuously monitored by a research nurse and vital signs and tolerance to therapy was assessed hourly.
After concluding the 2-d in-center study, patients were restarted on their prior treatment with phosphate and calcitriol in the range of 38 to 62 mg/kg per 24 h and 22 to 34 ng/kg per 24 h, respectively. Repeat assessments of blood chemistries were obtained 2 to 6 wk later. At follow-up, we measured ionized Ca, phosphate, creatinine, intact PTH, FGF23, and 1,25 (OH)2 vitamin D, and urine for Ca, phosphate, and creatinine in each patient. The blood and urine samples in the follow-up visit were obtained approximately 2 h after the patients took their morning medications. In addition, a single blood sample to measure FGF23 was obtained from 10 healthy controls (age, 7 to 16 yr; 3 male).
Biochemical Measurements
Serum samples for 1,25(OH)2 vitamin D and FGF23 were immediately frozen and kept at −70°C until batched analysis was conducted simultaneously on all samples. Other tests were conducted on the same day the sample was obtained. Intact PTH was analyzed by a solid-phase, two-site chemiluminescent enzyme-labeled immunometric assay (Immulite 2000, Diagnostic Products, Los Angeles, CA). Serum 1,25 OH vitamin D was analyzed by 1,25-dihydroxy vitamin D RIA (IDS Laboratories Tyne & Wear, United Kingdom, imported and distributed by IDS, Fountain Hills, AZ). Serum intact FGF23 was analyzed by ELISA Kit (Kainos Laboratories, Tokyo, Japan). All other tests were done by methods used routinely in the hospital clinical laboratory. From these data, random urine Ca/creatinine and the tubular threshold for phosphate (TP/GFR) were calculated (30).
Statistical Analysis
Two-tailed unpaired T test, one-way ANOVA, Randomized Complete Block Design, and Random Intercept Mixed Linear Model were used for statistical analysis. A P value <0.05 was considered statistically significant.
Results
Baseline Values
Consistent with the diagnosis of XLH, subjects at baseline (time 0) (Table 1, period A) had hypophosphatemia, inappropriately normal serum 1,25(OH)2D levels and elevated circulating FGF23 levels (147 ± 90 in subjects versus 32 ± 14 pg/ml in controls; P < 0.01), and decreased renal threshold for phosphate reabsorption (TP/GFR). Serum creatinine remained unchanged throughout the study.
Table 1.
Blood and urine biochemical and hormonal variables in 8 XLH subjects treated on day 1 with phosphate alone, on day 2 with phosphate + cinacalcet, and at follow-up after chronic treatment with phosphate + calcitriol
| Treatment | Oral Phosphate: Day 1
|
Oral Phosphate + Cinacalcet: Day 2
|
Follow-up: Period E (oral phosphate + calcitriol) | Normal Range | ||
|---|---|---|---|---|---|---|
| Period A: 0 h | Period B: 4 h | Period C: 0 h | Period D: 4 h | |||
| Ca2+ (mmol/L) | 1.28 ± 0.03 | 1.23 ± 0.04 | 1.26 ± 0.04 | 1.13 ± 0.06c | 1.24 ± 0.07 | 1.13–1.37 |
| Phosphate (mg/dl) | 2.1 ± 0.4 | 3.1 ± 0.4b | 2.2 ± 0.5 | 3.4 ± 0.3e | 2.9 ± 0.6b | 2.9–4.9 |
| PTH (pg/ml) | 36 ± 19 | 53 ± 13c | 34 ± 6 | 23 ± 8c | 33 ± 15 | 7–75 |
| FGF23 (pg/ml) | 147 ± 90 | 149 ± 67 | 192 ± 120d | 221 ± 91d | 247 ± 195d | 9–53 |
| 1,25 vitamin D (pmol/L) | 67 ± 35 | 75 ± 44 | 13 ± 21d | 17 ± 25d | 14 ± 6d | 35–84 |
| TP/GFR (mg/dl) | 1.70 ± 0.38 | 1.81 ± 0.42 | 1.73 ± 0.36 | 2.48 ± 0.39c | 1.86 ± 0.71 | 2.8–4.7 |
| Ca/creatininea (mg/mg) | 0.12 ± 0.08 | 0.06 ± 0.04 | 0.13 ± 0.08 | 0.10 ± 0.08 | 0.14 ± 0.05 | <0.20 |
Measured in 6 subjects.
P < 0.05 versus A, C.
P < 0.05 versus all other groups.
P < 0.05 versus A, B.
P < 0.05 versus A, C, E (P = 0.06 versus B).
Effects of Oral Phosphate Treatment
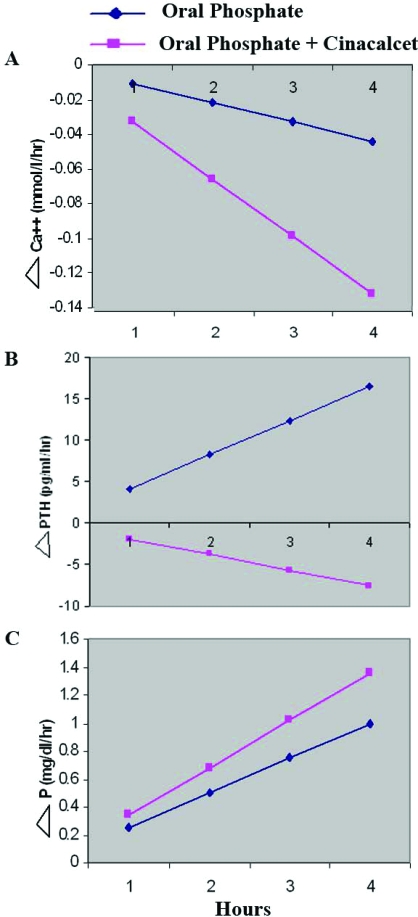
Oral phosphate administration resulted in a time-dependent increase in serum phosphate, any increase in serum PTH, and a trend toward reducing serum calcium (Figure 1). At 4 h after oral phosphate treatment (Table 1, period B), we observed statistically significant increases in both serum phosphate (3.1 ± 0.4 mg/dl versus 2.1 ± 0.4 mg/dl, respectively) and PTH (53 ± 13 versus 36 ± 19 pg/ml, respectively), and statistically insignificant decrease in ionized calcium and increase in TP/GFR. The changes in serum phosphate, calcium, and PTH values were transient, returning to values not significantly different from baseline by the beginning of day 2 (Table 1, period C). Similarly, there was no difference in TP/GFR (1.70 ± 0.38 versus 1.73 ± 0.36 mg/dl). FGF23 levels were unchanged from baseline at 4 h but were significantly increased 24 h after phosphate administration (192 ± 120 versus 147 ± 90 pg/ml), and 1, 25 (OH)2D levels, unchanged at 4 h, were reduced at 24 h after phosphate therapy (13 ± 21 versus 67 ± 35 pmol/L). With phosphate administration, urine Ca/creatinine ratio (because of a technical error measured in 6 subjects) decreased, albeit not significantly, and returned to baseline the next morning.
Figure 1.
Effect of P loading alone (day 1) compared with P + cinacalcet (day 2) on serum (A) ionized calcium, (B) PTH, and (C) phosphate, in 8 subjects with XLH. Blood samples were obtained every 30 minutes. Data are shown using Random Intercept Mixed Linear Model. The change in ionized calcium was −0.011 during the first day and −0.033 mmol/L per hour during the second day (P = 0.0001, 95% CI −0.032 to −0.014). The change in PTH was 4.1 during the first day and −1.9 pg/ml per hour on the second day (P = 0.001, 95% CI −9.6 to −2.5). The change in phosphorus was 0.25 during the first day and 0.34 mg/dl per hour during the second day (P = 0.088, 95% CI −0.013 to 0.195).
Effect of Cinacalcet and Oral Phosphate
As shown in Figure 1, compared with day 1, oral phosphate in combination with cinacalcet resulted in a significant time-dependent greater reduction in serum ionized calcium concentration. However, this treatment resulted in suppression of PTH that was associated with a greater increase in serum phosphate levels. Four hours after combination treatment with oral phosphate and a single oral dose of cinacalcet, the serum phosphate was higher than baseline (3.4 ± 0.3 versus 2.2 ± 0.05 mg/dl) and trended (P = 0.06) to be higher than the value of 3.1 ± 0.4 obtained 4 h after administration of phosphate alone (Table 1, periods D, C, and B, respectively). This increase in phosphate was associated with a reduction in PTH (23 ± 8 versus 53 ± 13 pg/ml) and an increase in TP/GFR (2.48 ± 0.39 versus 1.81 ± 0.42 mg/dl) compared with these parameters after phosphate administration in the absence of cinacalcet. As shown in Table 1, at the end of day 2, serum Ca2+ concentration of 1.13 ± 0.06 was lower than at the end of day 1, 1.23 ± 0.04 (P < 0.01) and on follow-up 1.24 ± 0.07 mmol/L (P < 0.005). Urine Ca/creatinine ratio decreased, not significantly, as in day 1. No patient experienced tetany or was found to have latent tetany throughout the study.
Effect of Standard Treatment With Phosphate and Calcitriol
The standard treatment with oral phosphate and calcitriol resulted in higher serum phosphate concentration (Table 1, period E) compared with no treatment (Table 1, periods A and C). The effects of phosphate to suppress serum 1,25(OH)2D persisted in the presence of concurrent calcitriol therapy (Table 1, periods E and C). Concomitant therapy with calcitriol prevented the phosphate-mediated increase in PTH but did not suppress the PTH levels to the same degree as occurred with the addition of cinacalcet (Table 1, periods E and D). Consequently, the TP/GFR was unchanged from baseline in XLH patients treated with combined phosphate and calcitriol (Table 1, periods E and A), whereas TP/GFR was increased when cinacalcet was added to phosphate therapy (Table 1, period D), presumably because of the greater suppression of PTH.
Discussion
Inactivating mutations of PHEX, a cell surface endopeptidase, is the cause of XLH (1). PHEX mutations result in increased production by bone of the phosphaturic hormone FGF23, which causes phosphaturia and suppresses circulating 1,25(OH)2 vitamin D (5,6). Similar to previous studies, which found that most XLH patients had elevated serum FGF23 levels (2–4), baseline FGF23 levels were 4 to 8 times higher in all 8 of our XLH patients than in controls (Table 1). Consistent with excess FGF23, we observed the characteristic biochemical features of XLH of hypophosphatemia, low TP/GFR, normal serum Ca2+ and PTH concentrations, and normal 1,25(OH)2 vitamin D concentration, albeit low for the degree of hypophosphatemia (8–10) in all 8 patients at baseline.
The current standard of care for treating patients with XLH consists of oral phosphate supplements and calcitriol (10). This therapy was empirically developed before the understanding of the genetic and molecular basis of this disease and was directed toward treating the three major manifestations of XLH, namely, hypophosphatemia, aberrant vitamin D levels, and rickets/osteomalacia. We found that oral phosphate therapy only transiently raised serum phosphate levels, and in a time course as described previously by others (18,31,32) and as related to the observation that phosphate increases bone cells FGF23 mRNA (33) also increased serum concentrations of FGF23, the principal factor causing the hypophosphatemia and suppression of 1,25(OH)2 D, thereby creating a positive feedback loop (Table 1). On the other hand, combined use of oral phosphate and calcitriol resulted in a more sustained increase in serum phosphate levels compared with oral phosphate alone and prevented phosphate-mediated elevations in PTH levels (Table 1). Neither phosphate supplementation nor calcitriol corrected the renal phosphate wasting as evidenced by the persistently low TP/GFR (Table 1), which is consistent with the recent findings that calcitriol stimulates FGF23 production in animal models and humans, and the related finding that dietary phosphate supplementation can also increase circulating FGF23 levels in animals and humans (17,19,34–36). Treatment with oral phosphate and calcitriol, rather than targeting the renal phosphate leak in XLH, corrects serum phosphate by increasing phosphate absorption from the gastrointestinal tract. This may explain why treatment of XLH with combinations of oral phosphate and calcitriol is associated with nephrocalcinosis (9,11,13,37).
The failure of phosphate and calcitriol to correct the primary biochemical abnormality in XLH and the potential of this treatment to cause nephrocalcinosis and secondary hyperparathyroidism in these patients suggests that other approaches are needed that do not exacerbate the elevated FGF23 levels and that limit the potential sequelae of phosphate loading and calcitriol.
Because FGF23 causes phosphaturia by inhibiting sodium-dependent phosphate transport in the proximal tubule, a more optimal therapy might be to reduce FGF23 levels or to inhibit its end-organ effects. At present, agents that suppress FGF23 production or specifically block its actions to inhibit renal phosphate absorption and 1,25(OH)2 vitamin D production are not available. However, PTH is a potent inhibitor of sodium-dependent phosphate uptake in the proximal tubule through suppression of the Na-P cotransporter (38,39). Consequently, we reasoned that a primary reduction in PTH might provide a means to offset the primary effects of FGF23 on the proximal tubule. Calcimimetics, which directly target the calcium receptor on the parathyroid glands, provide the ability to reduce serum PTH levels, without the administration of calcium or calcitriol (22,23).
We found that the administration of cinacalcet in combination with oral phosphate had salutary effects on the biochemical abnormalities in XLH (Table 1; Figure 1). In this regard, cinacalcet resulted in reduction in PTH that was associated with an increase in TP/GFR and serum phosphate that exceeded values obtained with oral phosphate alone or with the traditional combination of phosphate and calcitriol therapy (Table 1). This expected physiologic increase in TP/GFR and serum phosphate in response to PTH suppression by calcimimetics has been reported in other patients with intact or partially impaired kidney function (28). Although low TP/GFR in XLH patients is the result of high FGF23 levels, Alon and Chan (40) previously demonstrated that renal phosphate absorption can be independently modulated, albeit not fully corrected, by reduction of PTH activity. This was recently similarly also shown in 2 patients with tumor-induced osteomalacia, also caused by elevated circulating levels of FGF23, in whom PTH, normal at baseline, was suppressed with cinacalcet (29).
A cinacalcet-mediated decrease in serum PTH (Figure 1A) was accompanied by a significant reduction in serum ionized Ca concentration (Table 1). This mild, asymptomatic hypocalcemia, after the administration of calcimimetics, has been observed by others (41,42). Cinacalcet was well tolerated in our patients, and none demonstrated tetany or latent tetany. Because of the design of our study (i.e., no data collection in the morning of the third day), we could not assess the independent effect of cinacalcet on serum FGF23 and 1,25(OH)2D levels in patients with XLH; however, the major contributors to increments in FGF23 appeared to be phosphate and calcitriol (Table 1). Albeit only in 2 patients, Geller et al. (29) recently reported no acute effect of cinacalcet on serum FGF23 level.
Our study has several limitations. First, we administered less daily phosphate than currently recommended. Recommended therapeutic oral doses of phosphate for treatment of XLH typically are in the range of 40 to 100 mg/kg per day divided into four doses (10), whereas we provided our patients with a single dose of 20 mg/kg. Nevertheless, the administration of this single dose of phosphate on day 1 resulted in a moderate increase in serum phosphate (Table 1; Figure 1C), mild decrease in serum ionized Ca (Table 1, Figure 1A), and a significant increase in serum PTH (Table 1; Figure 1B). The higher dose of phosphate used in clinical practice would be expected to have more significant effects on these parameters. This indeed may indicate the need for even more careful attention to serum ionized Ca concentration when higher doses of phosphate combined with a calcimimetic agent are given. Second, we studied a relatively small number of patients, and our results need to be corroborated by additional studies.
A hypothetical concern when using calcimimetics is potential increase in calciuria resulting from the stimulation of the tubular calcium sensing receptor. However, Bushinsky et al. (43) in the rat and Shoback et al. (24) in humans did not find calcimimetics to increase calciuria. Similarly, the use of cinacalcet in our patients did not result in an increase in urine calcium probably because of the decrease in serum ionized Ca concentration.
Conclusion
The results of our acute study with phosphate and cinacalcet, combined with a short-term follow-up of standard therapy with phosphate and calcitriol, provides new insights into current and potential future management of XLH. The traditional treatment with phosphate and calcitriol, although successful in preventing treatment-induced elevation in PTH, was less effective in raising serum phosphate, possibly because of the increase in FGF23. In contrast, the use of cinacalcet suppressed PTH and increased serum phosphate concentration. Although the long-term effect of cinacalcet on serum FGF23 remains to be determined, this therapeutic profile might result in higher serum phosphate levels and allow for less oral phosphate and calcitriol supplementation. The use of calcimimetics in the longitudinal treatment regimen of XLH patients might minimize the risk of secondary hyperparathyroidism, hypercalcemia, hypercalciuria, and nephrocalcinosis because of high-dose phosphate and calcitriol therapy (8,10,13). Indeed, as recently shown by Geller et al. (29) in 2 patients with tumor-induced osteomalacia, the adjuvant use of cinacalcet allowed the use of lower doses of both phosphate and calcitriol. Long-term studies are needed to investigate whether the addition of cinacalcet to the treatment protocol of XLH will improve the clinical manifestations of this disorder while minimizing secondary hyperparathyroidism and nephrocalcinosis, the common adverse effects of current therapy.
Disclosures
U.S.A., R.L.-O., W.V.M., J.S., and S.L.: none; L.D.Q. serves as a consultant and on the speakers bureau of Amgen.
Acknowledgments
This work was supported in part by grants from Katharine B. Richardson Associates and the Sam and Helen Kaplan research fund in Pediatric Nephrology, and the Eric McClure Research Fund in Pediatric Bone and Mineral Diseases and by National Institutes of Health Grant RO1-AR45955 from NIAMS (L.D.Q., S.L.) and National Institutes of Health Grant P20 RR-17708 from the NCRR (S.L.).
The authors thank Steve Simon, PhD, and Connie Haney, RN, for their valuable assistance.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets: the HYP Consortium. Nat Genet 11: 130–136, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, TakeuchiY, Fujita T, Nakahara K, Yamashita T, Fukumoto S: Increased circulatory level of biologically active full-length FGF23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87: 4957–4960, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Jonsson KB, Zahradnik R, Larsson T, Whithe KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H: Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348: 1656–1663, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Weber TJ, Liu S, Indridason OS, Quarles LD: Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res 18: 1227–1234, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T: Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 98: 6500–6506, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T: FGF23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: 429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD: Pathogenic role of FGF23 in Hyp mice. Am J Physiol Endrocrinol Metab 291: E38–E49, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Baroncelli GI, Bertelloni S, Sodini F, Galli L, Vanacore T, Fiore L, Saggese G: Genetic advances, biochemical and clinical features and critical approach to treatment of patients with X-linked hypophosphatemic rickets. Pediatr Endocrinol Rev 1: 361–379, 2004 [PubMed] [Google Scholar]
- 9.Cho HY, Lee BH, Kang JH, Ha IS, Cheong HI, Choi Y: A clinical and molecular genetic study of hypophosphatemic rickets in children. Pediatr Res 58: 329–333, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Alon US: Hypophosphatemic vitamin D-resistant rickets. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 6th ed, Washington, DC, American Society for Bone and Mineral Research, 2006: 342–345
- 11.Alon U, Donaldson DL, Hellerstein S, Warady BA, Harris DJ: Metabolic and histologic investigation of the nature of nephrocalcinosis in children with hypophosphatemic rickets and in the Hyp mouse. J Pediatr 120: 899–905, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Alon US, Monzavi R, Lilien M, Rasoulpour M, Geffner ME, Yadin O: Hypertension in hypophosphatemic rickets: role of secondary hyperparathyroidism. Pediatr Nephrol 18: 155–158, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Makitie O, Doria A, Kooh SW, Cole WG, Daneman A, Sochett E: Early treatment improves growth and biochemical and radiographic outcome in X-linked hypophosphatemic rickets. J Clin Endocrinol Metab 88: 3591–3597, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Almaden Y, Hernandez A, Torregrosa V, Canalejo A, Sabate L, Fernandez Cruz L, Campistol JM, Torres A, Rodriguez M: High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol 9: 1845–1852, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Savio RM, Gosnell JE, Posen S, Reeve TS, Delbridge LW: Parathyroidectomy for tertiary hyperparathyroidism associated with X-linked dominant hypophosphatemic rickets. Arch Surg 139: 218–222, 2004 [DOI] [PubMed] [Google Scholar]
- 16.McHenry CR, Mostafavi D, Murphy TA: Tertiary hyperparathyroidism attributable to long-term oral phosphate therapy. Endocr Pract 12: 294–298, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA: Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146: 5358–5364, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Burnett SM, Gunawardene SC, Bringhurst FB, Jüppner H, Lee H, Finkelstein FS: Regulation of C-terminal and intact FGF23 by dietary phosphate in men and women. J Bone Miner Res 21: 1187–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Collins MT, Lindsay JR, Jain A, Kelly MH, Cutler CM, Weinstein LS, Liu J, Fedarko NS, Winer KK: Fibroblast growth factor-23 is regulated by 1α,25-dihydroxyvitamin D. J Bone Miner Res 20: 1944–1950, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD: Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17: 1305–1315, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hammerland LG, Garrett JE, Hung BC, Levinthal C, Nemeth EF: Allosteric activation of the Ca2+ receptor expressed in Xenopus laevis oocytes by NPS 467 or NPS 568. Mol Pharmacol 53: 1083–1088, 1998 [PubMed] [Google Scholar]
- 22.Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, Balandrin MF: Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A 95: 4040–4045, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemeth EF, Bennett SA: Tricking the parathyroid gland with novel calcimimetic agents. Nephrol Dial Transplant 13: 1923–1925, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Shoback DM, Bilezikian JP, Turner SA, McCary LC, Guo MD, Peacock M: The calcimimetic cinacalcet normalizes serum calcium in subjects with primary hyperparathyroidism. J Clin Endocrinol Metab 88: 5644–5649, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drueke TB, Goodman WG: Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Harris RZ, Padhi D, Marbury TC, Noveck RJ, Salfi M, Sullivan JT: Pharmacokinetics, pharmacodynamics, and safety of cinacalcet hydrochloride in hemodialysis patients at doses up to 200 mg once daily. Am J Kidney Dis 44: 1070–1076, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Goodman WG: Calcimimetic agents and secondary hyperparathyroidism: rationale for use and results from clinical trials. Pediatr Nephrol 18: 1206–1210, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Srinivas TR, Schold JD, Womer KL, Kaplan B, Howard RJ, Bucc CM, Meier-Kriesche HU: Improvement in hypercalcemia with cinacalcet after kidney transplantation. Clin J Am Soc Neprol 1: 323–326, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Geller JL, Khosravi A, Kelly MH, Riminucci M, Adams JS, Collins MT: Clinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res 22: 931–937, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Alon U, Hellerstein S: Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr Nephrol 8: 250–251, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Schiavi SC: Fibroblast growth factor 23: the making of a hormone. Kidney Int 69: 425–427, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Nagano N, Miyata S, Kobayashi N, Wakita S, Yamashita T, Wada M: Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rates. Kidney Int 69: 531–537, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Mirams M, Robinson BG, Mason RS, Nelson AE: Bone as a source of FGF23: regulation by phosphate? Bone 35: 1192–1199, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK: 1apha 25-dihydrovitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289: G1036–G1042, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N: Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 280: 2543–2549, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Yu X, Sabbagh Y, Davis SI, Demay MB, White KE: Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone 36: 971–977, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Verge CF, Lam A, Simpson JM, Cowell CT, Hoeard NJ, Silink M: Effects of therapy in X-linked hypophosphatemic rickets. N Engl J Med 325: 1843–1848, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Agus ZS, Puschett JB, Senesky D, Goldberg M: Mode of action of parathyroid hormone and cyclic adenosine 3′,5′-monophosphate on renal tubular phosphate reabsorption in the dog. J Clin Invest 50: 617–626, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Favus MJ, Bushinsky DA, Lemann JJ: Regulation of calcium, magnesium, and phosphate metabolism. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 6th ed, Washington, DC, American Society for Bone and Mineral Research, 2006: 76–83
- 40.Alon U, Chan JC: Effects of parathyroid hormone and 1,25-dihydroxyvitamin D3 on tubular handling of phosphate in hypophosphatemic rickets. J Clin Endocrinol Metab 58: 671–675, 1984 [DOI] [PubMed] [Google Scholar]
- 41.Chertow CM, Blumenthal S, Turner S, Roppolo M, Stern L, Chi EM, Reed J: Cinacalcet hydrochloride (Sensipar) in hemodialysis patients on active vitamin-D derivatives with controlled PTH and elevated calcium x phosphate. Clin J Am Soc Nephrol 1: 305–312, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Quarles LD: Cincalcet HCI: a novel treatment for secondary hyperparathyroidism in stage 5 chronic kidney disease. Kidney Int 68[Suppl]: S24–S28, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Bushinsky DA, Laplante K, Asplin JR: Effect of cinacalcet on urine calcium excretion and supersaturation in genetic hypercalciuric stone-forming rats. Kidney Int 69: 1586–1592, 2006 [DOI] [PubMed] [Google Scholar]