Abstract
Background: While endovascular stent placement is the standard of care in most percutaneous coronary and peripheral artery intervention, its role in the salvage of thrombosed and stenotic hemodialysis access remains controversial.
Design, Setting, Participants, and Measurements: We compared the effects of stent versus angioplasty on primary patency rates in the treatment of stenotic arteriovenous fistulae (AVF) and arteriovenous grafts (AVGs). Moreover, we compared access flow (Qa) and urea reduction ratio (URR) between the two groups as a metric of the effect of stent placement versus angioplasty on dialysis delivery.
Results: Cox regression analysis revealed that the primary assisted AVG patency was significantly longer for the stent group compared with angioplasty, with a median survival of 138 versus 61 d, respectively (aHR = 0.17; 95% confidence interval, 0.07 to 0.39; P < 0.001). The primary AVG patency for stent versus angioplasty was 91% versus 80% at 30 d, 69% versus 24% at 90 d, and 25% versus 3% at 180 d, respectively. The primary assisted AVF patency did not differ significantly between the stent and angioplasty groups. In patients dialyzing via AVF, multiple regression analysis revealed that stent placement was associated with improved after intervention peak Qa, 1627.50 ml/min versus 911.00 ml/min (β = 0.494; P = 0.008), change in Qa from before to after intervention, 643.54 ml/min versus 195.35 ml/min (β = 0.464; P = 0.012), and change in URR from before to after intervention, 5.85% versus 0.733% (β = 0.389; P = 0.039).
Conclusions: Our results suggest that stent placement is associated with improved AVG primary assisted patency and improved AVF blood flow, which may significantly impact on dialysis adequacy.
Despite a multitude of recent theoretical advances, emerging clinical data, and well-articulated guidelines, vascular access remains the Achilles heel of hemodialysis (1). The most common cause of dysfunction or loss of the arteriovenous access is thrombosis in the low flow state (2). The patency of arteriovenous grafts (AVGs) and arteriovenous fistulae (AVF) is compromised mainly by intimal hyperplastic lesions, which usually develop in areas of turbulent flow. These lesions tend to occur at sites of artery-to-vein or graft-to-vein anastomoses (3–5). Numerous studies have demonstrated the effectiveness of balloon angioplasty in the treatment of stenotic lesions, but this procedure is associated with a high rate of recurrent stenosis. Early studies of angioplasty reported 6-mo primary patency rates of only 31% to 64% (5–7).
The limited success of intervention via angioplasty contributes to the inordinate amount of healthcare costs associated with end-stage renal disease and vascular access. The estimated total per person per year costs for hemodialysis access (grafts and fistulae) is greater than $9000, which is approximately a 40% increase from 1999 (8), at which time the annual cost of access related morbidity in the United States was estimated to be more than $1 billion (9). A large proportion of these costs encompass the morbidity associated with outpatient and inpatient procedures to salvage failed and failing access (8).
Although endovascular stent placement is the standard of care in most percutaneous coronary and peripheral artery disease interventions, its role in the salvage of thrombosed and stenotic hemodialysis access remains controversial. Percutaneous transluminal angioplasty (PTA) of hemodialysis access has been described since the 1980s (10,11). It offers an alternative to surgical revision (jump grafts, graft interposition, venous patch angioplasty, and open thrombectomy) without the considerable morbidity, length of stay, and costs typically associated with surgery (12,13). Endovascular stent placement, on the other hand, has been used in the management of hemodialysis access stenosis since 1988 (14,15). Stents have been used predominantly as a salvage to failed angioplasty (venous rupture, elastic recoil, rapidly recurrent stenosis after PTA, or residual stenosis >30%) or as adjunctive therapy. Multiple studies have compared stent versus angioplasty in terms of access patency with mixed results (16–21). In these studies, multiple confounders, such as a wide variety of stent types, the nature and locations of the stenotic lesions, the access configuration, the blood flow measurements, and the case mix, prevent the clinician from drawing definitive conclusions regarding the use of stent versus angioplasty (22). A recent observational cohort study by Maya and Allon addressed some of the aforementioned limitations and demonstrated improved primary and secondary graft patency after thrombectomy of AVGs (23).
The role of stent placement in the treatment of stenotic lesions (those not associated with thrombosis per se) in AVGs and AVF remains poorly defined. The objectives of our study are twofold: 1) to compare the effects of stent versus angioplasty on patency rates in the treatment of stenotic AVF and AVGs, controlling for age, gender, location of lesion, and stent type; and 2) to compare access flow (Qa) and urea reduction ratio (URR) between the two groups as a metric of the effect of stent placement versus angioplasty on dialysis delivery.
Materials and Methods
Design and Population
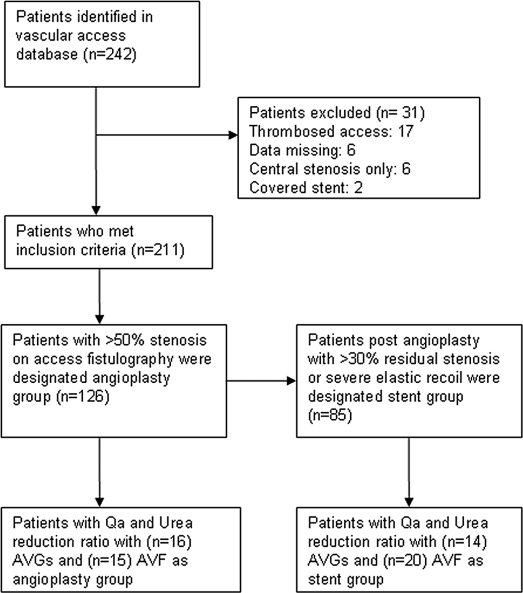
A prospective computerized database was used to identify 242 vascular access interventions with stent and angioplasty during a 2.5-yr period from January 1, 2005 to June 30, 2007. Patients were included in the sample if they had a hemodialysis access intervention for stenosis within the University of Wisconsin Hospitals and Clinics, which is a tertiary referral center for the region. Patients who were lost to follow-up, transferred to another facility, died, were transplanted, or changed dialysis modality were not included. A total of 31 patients were excluded: 11 in the stent group and 20 in the angioplasty group. Two patients were excluded for covered stent placement, 17 were excluded for an intervention in the setting of a thrombosed AVF or AVG, 6 were excluded for central stenosis without a peripheral lesion, and 6 were excluded for missing data fields (e.g., unknown medical record number). Those patients who received hemodialysis at our local center, as part of our primary end-stage renal disease care program, were included in the blood flow measurement and analysis (Figure 1).
Figure 1.
Strategy for group allocation. Qa, access flow; AVGs, arteriovenous grafts; AVF, arteriovenous fistulae.
After applying the inclusion and exclusion criteria described above to the original 242 patients identified, a total of 211 (87.2%) stent and matched angioplasty controls (112 AVF and 99 AVG) were entered in the analysis. Baseline clinical characteristics collected included age, gender, access type, diabetic status, and location of the lesion. Complications were classified according to the guidelines stipulated by the most recent position statement by the American Society of Diagnostic and Interventional Nephrology (24). We compared primary assisted patency matched for age, gender, diabetic status, location of lesion, and type of access between the two groups. A total of 85 stents and 126 angioplasty procedures were done in the 2.5-yr study period.
We measured access blood flow before and after intervention in a subset of patients among the 211 who received dialysis therapy at our local, tertiary hospital affiliated unit. The before intervention Qa, peak Qa, and change in Qa were analyzed for both AVF (n = 35) and AVG (n = 30). In addition, we calculated the before and after intervention URR in the subset of patients who were followed in our local hemodialysis center. The before intervention URR, peak URR, and change in URR were analyzed separately for AVGs and AVF.
This Health Insurance Portability and Accountability Act–compliant study was approved by the University of Wisconsin Hospitals and Clinics Institutional Review Board.
Technical Description
All referrals for intervention were based on standard Kidney Disease Outcomes Quality Initiative (KDOQI)/Fistula First published criteria, which may include one or a combination of the following: physical examination abnormalities, poor blood flows, elevated venous/arterial pressures, increased bleeding, inability to cannulate, decrease in Transonic HD02 Hemodialysis Monitor (Transonic Systems, Ithaca, NY) access flows, or decrease in dialysis adequacy measured by Kt/V or URR (25,26).
Arteriovenous fistulae or grafts were initially cannulated with a micropuncture needle, after which a 4-French introducer was inserted over a wire into the lumen of the AVF or AVG. Diluted contrast was injected under fluoroscopy to localize the stenotic lesion. Intervention was performed if a lesion greater than 50% was detected, at which point a 6- or 7-French sheath was exchanged over a wire depending on location and size of the lesion (7,27–29). A guidewire was then introduced into the access and manipulated under fluoroscopic guidance across the lesion followed by an angioplasty balloon (Powerflex, Cordis, Miami, FL) and inflated via automated device (Everest 20 or 30, Medtronic, Minneapolis, MN) to 20 to 30 atm as described previously (17,30–32). If residual stenosis more than 30% or severe elastic recoil was detected on after intervention fistulography, a nitinol, self-expanding stent (SMART, Cordis) was deployed in the area of the lesion, as described previously (33). Stent length and diameter were selected to exceed the maximal vessel diameter by 1- to 2-mm based on overlay images.
Patients had their access blood flow and URR measured by Transonic HD02 from day 1 to day 30 after intervention and then routinely as part of our access monitoring program once per month (25,26).
Statistical Analyses
Baseline clinical characteristics were compared between both groups using χ2 analysis and T tests. To address the first objective of our study, Cox regression was used to generate access survival curves and adjusted hazard ratios between groups with censoring to the end of the study period.
To address the second objective, T tests were used to determine the difference in Qa and URR between stent and angioplasty groups. We considered a significance level of P < 0.10 in the univariate analysis to determine subsequent entry into multivariate regression models adjusted for age, diabetic status, gender, and location of the lesion.
Results
The clinical characteristics of the patients treated with stent placement and the angioplasty control group are compared in Table 1. The two treatment groups did not differ significantly in age, gender, race, or diabetic status. The proportion of the stent group that consisted of AVG (51.80%) was higher than that of the AVF group (42.9%, P < 0.05). The stent group was also less likely to have an inflow lesion as the primary cause of the problem (n = 13, 15.30%) compared with the angioplasty group (n = 34, 26.9%, P = 0.02).
Table 1.
Baseline characteristics of stent versus angioplasty groups
| Stent, n = 85 (%) | Angioplasty, n = 126 (%) | Statistical Significance, P | |
|---|---|---|---|
| Age, yr (mean ± SD) | 62.5 ± 18.1 | 63.9 ± 14.7 | 0.55 |
| AVG | 44 (51.80) | 54 (42.9) | <0.05a |
| Diabetes mellitus | 43 (50.60) | 63 (50.0) | 0.11 |
| Gender, % female | 28 (32.9) | 41 (32.5) | 0.12 |
| Location of lesion, % inflow | 13 (15.30) | 34 (26.9) | 0.02a |
Statistically significant (χ2 analysis).
The technical success of the intervention was judged radiographically as per the Society of Cardiovascular and Interventional Radiology guidelines (33). Complications did not differ between groups. Complications were 1.6% in the angioplasty group and 2.4% in the stent group. There were two grade 1 hematomas in the stent group and one grade 1 hematoma and one grade 3 hematoma in the angioplasty group.
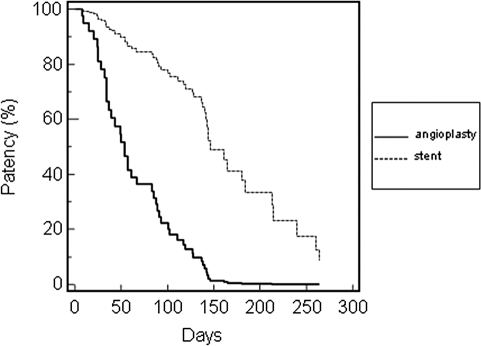
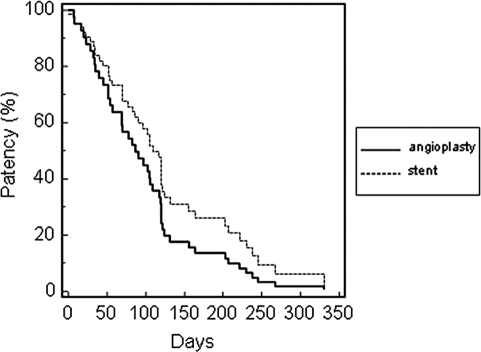
Cox regression analysis adjusted for age, gender, diabetic status, and location of lesion is shown for AVG and AVF (Table 2). The primary assisted AVG patency adjusted for age, gender, diabetic status, and location of lesion was significantly longer for the stent group compared with angioplasty, with a median survival of 138 versus 61 d, respectively (aHR = 0.17; 95% confidence interval, 0.07 to 0.39; P < 0.001; Figure 2). The primary patency was 91% versus 80% at 30 d, 69% versus 24% at 90 d, and 25% versus 3% at 180 d, respectively. Assisted or secondary AVG patency (time from initial referral to permanent access failure) was not assessed because of the exclusion of thrombectomy-associated interventions from the study. The primary assisted AVF patency adjusted for age, gender, diabetic status, and location of lesion did not differ significantly between the stent and angioplasty groups (Figure 3).
Table 2.
Cox regression analysis of factors associated with access patency
| AVG
|
AVF
|
|||||
|---|---|---|---|---|---|---|
| aHR | 95% CI | Statistical Significance, P | aHR | 95% CI | Statistical Significance, P | |
| Stent | 0.17 | 0.07, 0.39 | <0.001 | 0.66 | 0.34, 1.26 | 0.21 |
| Age | 0.99 | 0.97, 1.01 | 0.40 | 0.99 | 0.98, 1.01 | 0.35 |
| Female | 0.36 | 0.18, 0.72 | 0.004 | 1.49 | 0.63, 3.48 | 0.36 |
| Diabetes mellitus | 0.47 | 0.26, 0.87 | 0.02 | 1.36 | 0.72, 2.58 | 0.35 |
| Location of lesion, % inflow | 1.25 | 0.33, 4.78 | 0.74 | 0.90 | 0.45, 1.79 | 0.76 |
Figure 2.
Primary patency rates of arteriovenous graft (AVG).
Figure 3.
Primary patency rates of arteriovenous fistula (AVF).
In the subset of patients dialyzing via AVG, patients receiving stents were less likely to be diabetic (P = 0.008 by χ2 analysis) and more likely to have an inflow lesion (P = 0.014 by χ2 analysis). Of those who had blood flow data recorded (n = 30), stent placement was not associated with improved blood flow (Table 3). In patients dialyzing via AVF who had blood flow data recorded (n = 35), multiple regression analysis revealed that stent placement was associated with improved peak Qa after intervention (β = 0.494; P = 0.008), change in Qa from before to after intervention (β = 0.464; P = 0.012), and change in URR from before to after intervention (β = 0.389; P = 0.039; Table 4).
Table 3.
Blood flow (Qa) and urea reduction ratio (URR) comparison between stent versus angioplasty groups for AVG
| Stent, n = 14 (SD) | Angioplasty, n = 16 (SD) | Univariate Analysis, P | |
|---|---|---|---|
| Age, yr | 70.2 (9.1) | 68.6 (14.3) | 0.71 |
| Diabetes mellitus, % | 14.3 | 62.5 | 0.008a |
| Gender, % female | 71.4 | 37.5 | 0.06 |
| Location of lesion, % inflow | 35.7 | 0 | 0.014a |
| Qa before intervention | 803.9 (286.7) | 854.4 (520.0) | 0.74 |
| Peak Qa after intervention | 1138.2 (309.6) | 1092.2 (446.9) | 0.75 |
| Change in Qa | 334.3 (316.8) | 237.8 (592.7) | 0.57 |
| URR before intervention | 69.1 (5.8) | 68.7 (10.0) | 0.89 |
| Peak URR after intervention | 71.3 (4.0) | 71.2 (11.3) | 0.97 |
| Change in URR | 2.1 (3.3) | 2.6 (8.5) | 0.83 |
Statistically significant (χ2 analysis).
Table 4.
Blood flow (Qa) and urea reduction ratio (URR) comparison between stent versus angioplasty groups for AVF
| Stent, n = 20 (SD) | Angioplasty, n = 15 (SD) | Univariate Analysis, P | Multivariate Analysis, P | |
|---|---|---|---|---|
| Age, yr | 62.6 (16.2) | 63.9 (11.2) | 0.78 | — |
| Diabetes mellitus, % | 30.0 | 33.3 | 0.28 | — |
| Gender, % female | 55.0 | 13.3 | 0.0119a | — |
| Location of lesion, % inflow | 25.0 | 13.3 | 0.24 | — |
| Qa before intervention | 767.5 (379.2) | 760.9 (398.9) | 0.96 | 0.95 |
| Peak Qa after intervention | 1627.5 (808.0) | 911.0 (455.5) | 0.0023b | 0.008c |
| Change in Qa | 643.5 (714.3) | 195.4 (455.5) | 0.0016b | 0.012c |
| URR before intervention | 68.4 (6.5) | 73.4 (8.1) | 0.06 | 0.40 |
| Peak URR after intervention | 74.2 (9.2) | 74.1 (3.4) | 0.96 | 0.93 |
| Change in URR | 5.9 (8.1) | 0.7 (6.7) | 0.0502 | 0.039c |
Statistically significant (χ2 analysis).
Statistically significant (T test based on P < 0.10 a priori for inclusion in multivariable meta-regression).
Statistically significant, using multivariable meta-regression, adjusted for age, diabetes mellitus, gender, and location of lesion.
Discussion
Our study suggests that stent placement is associated with superior AVG patency compared with angioplasty alone. Furthermore, our study suggests that stent placement is associated with improved AVF flow rates and URR compared with PTA, although no difference in patency rate was observed. Across a large sample of patients over a 2.5-yr period, we observed a significant improvement in stent patency rates within the adjusted AVG subset over a period of 180 d with a median survival of 138 versus 61 d compared with PTA. The primary assisted patency rate of the AVF subset did not differ between stent or PTA.
In the last decade, there have only been a handful of well-designed clinical trials comparing stent versus angioplasty in hemodialysis access patency. In 1993, Beathard demonstrated, in a prospective randomized trial of 58 patients with 50% or greater stenosis, that the Gianturco Rosch Z metallic stent conferred no advantage in duration of patency at 30, 60, 90, 180 or 360 d than percutaneous angioplasty (20). He concluded that the Gianturco stent was of no value for graft-vein stenosis that occurred in polytetrafluoroethylene (PTFE) dialysis access. Later, Quinn et al. showed, in their randomized study, that primary and secondary patency for PTA versus stents was comparable (18). In their study of 87 prospective patients over a 3-yr period, the primary patency rates for PTA at 60, 180, and 360 d were 55%, 31%, and 10%, respectively, and for stents were 36%, 27%, and 11%, respectively. The differences in patency were not statistically significant, which also held true for assisted primary and secondary patency. This study used a variety of stents (Gianturco, Palmaz, and Wallstent), which the author points out as a limitation to his results (18). Finally, Hoffer et al. demonstrated, in their prospective randomized trial, primary patency rates for PTA at 30, 60, 180, and 360 d of 89%, 53%, 23%, and 7%, respectively, and for stents, 81%, 56%, 12% and 0%, respectively. These authors used Wallstents exclusively and concluded that despite a significant increase in cost, there was no advantage to stent placement compared with conventional angioplasty (19). We suggest that perhaps the controversy in efficacy between stent and angioplasty is a result of the stent design used in the majority of studies. Metallic stents, as mentioned in the previous studies, have inherent properties that may contribute to loss of distensibility, increased fracture rate, or migration, especially in the setting of repeated puncture for dialysis access (34,35). These stents may also have poor wall contact and radial strength, which is also a risk factor for in-stent restenosis (35,36).
Our study compared with the aforementioned study (19) used exclusively nitinol SMART (shape memory alloy recoverable technology) stents, which is a nickel, titanium alloy material that has been used to salvage difficult hemodialysis access stenoses and central venous occlusions in hemodialysis patients (17,23,37). Its advantage to the metallic stents is its shape memory and superelasticity, which is not affected by external forces or repeated interventions (38,39). It also allows equal distribution of wall contact, maintaining radial strength, without embedding or anchoring into the vessel (35,40,41). Vogel and Parise demonstrated the improved performance of nitinol stents, showing an increase in mean primary patency time from 2.5 mo with angioplasty to 10.6 mo after nitinol SMART stent (17). The same authors again showed, in a prospective, nonrandomized trial in 60 patients with dysfunctional upper extremity AVGs, a significant decrease in restenosis in the stent group compared with angioplasty (7% versus 16%; P = 0.001) and an improvement in mean primary graft patency (5.6 versus 8.2 mo; P = 0.05) (21).
More recently, Maya and Allon (23) evaluated whether graft patency after thrombectomy is improved by placement of a nitinol stent compared with PTA alone. The primary graft patency was significantly longer for the stent group (median survival, 85 versus 27 d; P = 0.02) and secondary patency was also longer for the stent group (median survival, 1215 versus 46 d; P = 0.049) (23). Our study extends the seminal work of Maya and Allon by describing a patency benefit to stent placement versus PTA in nonthrombosed AVG.
Our observation that stents are associated with improved patency in grafts but not fistulae, suggesting that the biology of native AVF is substantially different from AVGs, is quite intriguing but certainly not new. It is known that grafts are at higher risk for recurrent stenosis and thrombosis compared with fistulae. On the other hand, AVF have higher primary failure rates plagued by a significant proportion that fail to mature (42). Our results also suggest that diabetes and female gender may confer an independent patency benefit in the AVG group. Although it is well documented that female gender, peripheral vascular disease, age, and race are independently associated with primary fistula failure (43–45), variables associated with AVG failure are less well defined. It has been previously shown that female gender may confer a survival benefit in PTFE grafts compared with males (46,47). The evidence on diabetes, however, as a risk factor for arteriovenous graft patency is sparse (48). Our observation again highlights the important differences between the biology of AVF and AVG, implying that previous observations about one access modality may not necessarily apply to the other. Roy-Chaudhury et al. have eloquently elucidated the difference in biology between the AVF and AVG: the significant adventitial angiogenesis and migration of macrophages is observed solely in the perigraft region of PTFE (40). Perhaps, in addition, this observation may imply that the national mandate to place AVF preferentially in all patients needs to be examined more closely.
Nevertheless, in our AVF subgroup, we demonstrated that peak Qa and the change in Qa were improved after stent placement compared with PTA. Moreover, we saw this translate into an improved change in URR from before to after intervention, yet there was no difference in patency rates. The increase in Qa and URRs is encouraging because there is a paucity of information on the efficacy of stents in AVF; and given these preliminary data on Qa and URR, more studies need to be conducted to confirm our findings and demonstrate whether this translates into a long-term benefit. Our study demonstrated a dissociation between patency rates and Qa/URR in both AVGs and AVF. One plausible explanation as to why patency rates and Qa/URR do not correlate may be a difference in the pathogenesis and interplay of a complex variety of cytokines on frequently accessed AVF and AVG. Also, although the Transonic HD02 is the gold standard for access flow measurements, its accuracy may be compromised in a variety of circumstances, such as operator differences, flow/dilution sensor malfunction, timing and amount of saline introduced, and hemodynamic status of patients. We recognized this as an inherent variable in our Qa measurements, which may partially explain the differences between AVF and AVGs.
As with all retrospective analyses, there are limitations. Although we adjusted for variables known to affect access patency, there are confounding factors we did not take into account. Patients’ demographic characteristics, hemodynamic status, coronary artery disease, dialysis vintage, previous interventions and accesses, access age, and medications may all play critical roles in the pathogenesis and pathology of vascular access stenosis and thrombosis. Another potential limitation to interpreting the results of this study is that AVGs required a higher proportion of stents compared with AVF. This may merely imply, however, that the AVG-associated lesions are inherently more difficult to treat. Furthermore, inflow lesions were substantially less frequent in the stent group. Likely, this is because arterial inflow stents are usually not placed in brachial artery anastomotic lesions to avoid blood flow compromise to the distal extremity in the event of stent failure. We recognize these limitations and acknowledge that more robust randomized comparative trials need to confirm the findings of our study. Nevertheless, in an attempt to mitigate some of the aforementioned limitations, we standardized our stent type, controlled for location of the lesion, and analyzed access type separately, controlling for potential confounders with Cox regression. In addition, our study is the first to follow monthly Qa and URRs as a parameter of improved dialysis adequacy after intervention. It is also the largest subgroup of patients studied with exclusively nitinol stent technology in peripheral dialysis access stenosis.
Conclusion
Improving the survival of AVGs and AVF in hemodialysis patients has been the focus of increasing research in the last decade. Not until recently have the benefits of endovascular stent placement been demonstrated in hemodialysis access grafts (21,23). Our results suggest that in AVGs not associated with thrombosis per se, stent placement may confer a patency benefit. In a subset of failed AVF, stent placement may improve access blood flow in the short term, which may in turn translate to improved dialysis efficiency. Important conclusions that can be drawn from our data are that AVGs and AVF respond differently to stent intervention, that there may be more to vascular access outcomes than patency alone, and that future studies performed by interventionalists should attempt to merge patency data with surrogate markers of dialysis delivery whenever possible. Until prospective, randomized controlled trials are conducted that control for the type of access, type of lesion, patient characteristics, and previous history of intervention, and take quality of delivered hemodialysis into account, the issue of stent versus angioplasty will continue to generate controversy.
Disclosures
None.
Acknowledgments
The authors thank Wisconsin Dialysis, Inc., for its support and assistance with dialysis flow data.
Portions of this manuscript were presented as an oral presentation at the 53rd Annual ASAIO conference, Chicago, IL, June 7–9, 2007.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Fan PY, Schwab SJ: Vascular access: concepts for the 1990s. J Am Soc Nephrol 3: 1–11, 1992 [DOI] [PubMed] [Google Scholar]
- 2.White JJ, Bander SJ, Schwab SJ: Is percutaneous transluminal angioplasty an effective intervention for arteriovenous graft stenosis? Semin Dial 18: 190–192, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Swedberg SH, Brown BG, Sigley R, Wight TN, Gordon D, Nicholls SC: Intimal fibromuscular hyperplasia at the venous anastomosis of PTFE grafts in hemodialysis patients. Circulation 80: 1726–1736, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Saeed M, Newman GE, McCann RL, Sussman SK, Braun SD, Dunnick NR: Stenoses in dialysis fistulas: treatment with percutaneous angioplasty. Radiology 164: 693–697, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Kanterman RY, Vesely TM, Pilgram TK, Guy BW, Windus DW, Picus D: Dialysis access grafts: anatomic location of venous stenosis and results of angioplasty. Radiology 195: 135–139, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Glanz S, Gordon DH, Butt KM, Hong J, Lipkowitz GS: The role of percutaneous angioplasty in the management of chronic hemodialysis fistulas. Ann Surg 206: 777–781, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beathard GA: Percutaneous transluminal angioplasty in the treatment of vascular access stenosis. Kidney Int 42: 1390–1397, 1992 [DOI] [PubMed] [Google Scholar]
- 8.United States Renal Data System: Annual data report, 2006. Available at: http://www.usrds.org/adr.htm. Accessed online June 22, 2007
- 9.Feldman HI, Kobrin S, Wasserstein A: Hemodialysis vascular access morbidity. J Am Soc Nephrol 7: 523–535, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Novelline RA: Percutaneous transluminal angioplasty: newer applications. AJR Am J Roentgenol 135: 983–988, 1980 [DOI] [PubMed] [Google Scholar]
- 11.Glanz S, Gordon D, Butt KM, Hong J, Adamson R, Sclafani SJ: Dialysis access fistulas: treatment of stenoses by transluminal angioplasty. Radiology 152: 637–642, 1984 [DOI] [PubMed] [Google Scholar]
- 12.McCutcheon B, Weatherford D, Maxwell G, Hamann MS, Stiles A: A preliminary investigation of balloon angioplasty versus surgical treatment of thrombosed dialysis access grafts. Am Surg 69: 663–667, 2003 [PubMed] [Google Scholar]
- 13.Yehia M, McDonald M, Walker R: The management and outcome of occluded haemodialysis access: a retrospective audit. N Z Med J 115: 1–5, 2002 [PubMed] [Google Scholar]
- 14.Zollikofer CL, Antonucci F, Stuckmann G, Mattias P, Brühlmann WF, Salomonowitz EK: Use of the Wallstent in the venous system including hemodialysis-related stenoses. Cardiovasc Intervent Radiol 15: 334–341, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Günther RW, Vorwerk D, Klose KC, Bohndorf K, Kistler D, Mann H, Sieberth HG: Self-expanding stents for the treatment of a long venous stenosis in a dialysis shunt: case report. Cardiovasc Intervent Radiol 12: 29–31, 1989 [DOI] [PubMed] [Google Scholar]
- 16.Patel RI, Peck SH, Cooper SG, Epstein DM, Sofocleous CT, Schur I, Falk A: Patency of Wallstents placed across the venous anastomosis of hemodialysis grafts after percutaneous recanalization. Radiology 209: 365–370, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Vogel PM, Parise C: SMART stent for salvage of hemodialysis access grafts. J Vasc Interv Radiol 15: 1051–1060, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Quinn SF, Schuman ES, Demlow TA, Standage BA, Ragsdale JW, Green GS, Sheley RC: Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenoses in patients undergoing hemodialysis: intermediate results. J Vasc Interv Radiol 6: 851–855, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Hoffer EK, Sultan S, Herskowitz MM, Daniels ID, Sclafani SJA: Prospective randomized trial of a metallic intravascular stent in hemodialysis graft maintenance. J Vasc Interv Radiol 8: 965–973, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Beathard GA: Gianturco self-expanding stent in the treatment of stenosis in dialysis access grafts. Kidney Int 43: 872–877, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Vogel PM, Parise C: Comparison of SMART stent placement for arteriovenous graft salvage versus successful graft PTA. J Vasc Interv Radiol 16: 1619–1626, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Beathard GA: Is percutaneous transluminal angioplasty an effective intervention for arteriovenous graft stenosis? Semin Dial 18: 194–196, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Maya ID, Allon M: Outcomes of thrombosed arteriovenous grafts: comparison of stents vs angioplasty. Kidney Int 69: 934–937, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Vesely TM, Beathard G, Ash S, Hoggard J, Schon D, for the ASDIN Clinical Practice Committee: A position statement from the American Society of Diagnostic and Interventional Nephrology. Semin Dial 20: 359–364, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Vascular Access Work Group: Clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl]: S248–S273, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Wijnen E, Essers S, van Meijel G: Comparison between two on-line reversed line position hemodialysis vascular access flow measurement techniques: saline dilution and thermodilution. ASAIO J 52: 410–415, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Gray RJ, Sacks D, Martin LG, Trerotola SO: Reporting standards for percutaneous interventions in dialysis access. J Vasc Interv Radiol 14[Suppl]: S433–S442, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Safa AA, Valji K, Roberts AC, Ziegler TW, Hye RJ, Oglevie SB: Detection and treatment of dysfunctional hemodialysis access grafts: effect of a surveillance program on graft patency and the incidence of thrombosis. Radiology 199: 653–657, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Turmel-Rodrigues L, Pengloan J, Blanchier D, Abaza M, Birmelé B, Haillot O, Blanchard D: Insufficient dialysis shunts: improved long-term patency rates with close hemodynamic monitoring, repeated percutaneous balloon angioplasty, and stent placement. Radiology 187: 273–278, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Clark TW, Hirsch DA, Jindal KJ, Veugelers PJ, LeBlanc J: Outcome and prognostic factors of restenosis after percutaneous treatment of native hemodialysis fistulas. J Vasc Interv Radiol 13: 51–59, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Clark TW, Cohen RA, Kwak A, Markmann JF, Stavropoulos SW, Patel AA, Soulen MC, Mondschein JI, Kobrin S, Shlansky-Goldberg RD, Trerotola SO: Salvage of nonmaturing native fistulas by using angioplasty. Radiology 242: 286–292, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Schwab SJ, Raymond JR, Saeed M, Newman GE, Dennis PA, Bollinger RR: Prevention of hemodialysis fistula thrombosis: early detection of venous stenoses. Kidney Int 36: 707–711, 1989 [DOI] [PubMed] [Google Scholar]
- 33.Aruny JE, Lewis CA, Cardella JF, Cole PE, Davis A, Drooz AT, Grassi CJ, Gray RJ, Husted JW, Jones MT, McCowan TC, Meranze SG, Van Moore A, Neithamer CD, Oglevie SB, Omary RA, Patel NH, Rholl KS, Roberts AC, Sacks D, Sanchez O, Silverstein MI, Singh H, Swan TL, Towbin RB, Trerotola SO, Bakal CW; Society of Interventional Radiology Standards of Practice Committee: Quality improvement guidelines for percutaneous management of the thrombosed or dysfunctional dialysis access: Standards of Practice Committee of the Society of Cardiovascular and Interventional Radiology. J Vasc Interv Radiol 10: 491–498, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Zaleski GX, Funaki B, Rosenblum J, Theoharis J, Leef J: Metallic stents deployed in synthetic arteriovenous hemodialysis grafts. AJR Am J Roentgenol 176: 1515–1519, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Clark TW: Nitinol stents in hemodialysis access. J Vasc Interv Radiol 15: 1037–1040, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Rogers C, Edelman ER: Endovascular stent design dictates experimental restenosis and thrombosis. Circulation 91: 2995–3001, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Naoum JJ, Irwin C, Hunter GC: The use of covered nitinol, stents to salvage dialysis grafts after multiple failures. Vasc Endovascular Surg 40: 275–279, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Rajan DK, Saluja JS: Use of nitinol stents following recanalization of central venous occlusions in hemodialysis patients. Cardiovasc Intervent Radiol 30: 662–667, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Shabalovskaya SA: On the nature of the biocompatibility and on medical applications of NiTi shape memory and superelastic alloys. Biomed Mater Eng 6: 267–289, 1996 [PubMed] [Google Scholar]
- 40.Roy-Chaudhury P, Sukhatme VP, Cheung AK: Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol 17: 1112–1127, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Duda SH, Wiskirchen J, Tepe G, Bitzer M, Kaulich TW, Stoeckel D, Claussen CD: Physical properties of endovascular stents: an experimental comparison. J Vasc Interv Radiol 11: 645–654, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Rayner HC, Pisoni RL, Gillespie BW, Goodkin DA, Akiba T, Akizawa T, Saito A, Young EW, Port FK; Dialysis Outcomes and Practice Patterns Study: Creation, cannulation and survival of arteriovenous fistulae: data from the Dialysis Outcomes and Practice Patterns Study. Kidney Int 63: 323–330, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Allon M: Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Miller CD, Robbin ML, Allon M: Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int 63: 346–352, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Yevzlin AS, Conley EL, Sanchez RJ, Young HN, Becker BN: Vascular access outcomes and medication use: a USRDS study. Semin Dial 19: 535–539, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Astor BC, Coresh J, Powe NR, Eustace JA, Klag MJ: Relation between gender and vascular access complications in hemodialysis patients. Am J Kidney Dis 36: 1126–1134, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Lilly RZ, Carlton D, Barker J, Saddekni S, Hamrick K, Oser R, Westfall AO, Allon M: Predictors of arteriovenous graft patency after radiologic intervention in hemodialysis patients. Am J Kidney Dis 37: 945–953, 2001 [DOI] [PubMed] [Google Scholar]