Abstract
Background and objectives: Renal biopsy in acute renal failure of unknown origin provides irreplaceable information for diagnosis, treatment, and prognosis. This study analyzed the frequency and clinicopathologic correlations of renal native biopsied acute renal failure in Spain during the period 1994 through 2006.
Design, setting, participants, & measurements: Acute renal failure was defined as a rapid deterioration of glomerular filtration rate, with or without oligoanuria or rapidly progressive renal insufficiency, including acute-on-chronic renal failure. Patients who were younger than 15 yr were considered children, those between 15 and 65 yr adults, and those >65 elderly.
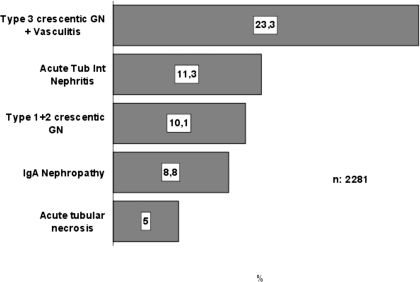
Results: Between 1994 and 2006, data on 14,190 native renal biopsies were collected from 112 renal units in Spain. Of these, 16.1% (2281 biopsies) were diagnosed with acute renal failure. The prevalence of the main clinical syndromes was different in the three age groups: Biopsy-confirmed acute renal failure in children was 5.7%, in adults was 12.5%, and in elderly increased significantly to 32.9%. The prevalence of biopsy-confirmed acute renal failure according to cause was as follows: Vasculitis, 23.3%; acute tubulointerstitial nephritis, 11.3%; and crescentic glomerulonephritis types 1 and 2, 10.1%. The prevalence of the different causes differed significantly according to age group.
Conclusions: The Spanish Registry of Glomerulonephritis provides useful information about renal histopathology in biopsy-confirmed acute renal failure. The prevalence of vasculitis and crescentic glomerulonephritis is high, especially in elderly patients. These data obtained from a national large registry highlight the value of renal biopsy in undetermined acute renal failure.
The study of the epidemiology of biopsy-confirmed renal disease provides useful information about the prevalence of renal disease and its clinical manifestations. Although there are several renal biopsy registries around the world, most describe the distribution of histopathologic findings, and very few analyze in detail the main clinical pictures that indicate renal biopsy, yet knowledge of the epidemiology of renal syndromes is of paramount importance in clinical nephrology.
The Spanish Registry of Glomerulonephritis has recorded individual patient data for all renal biopsies performed since 1994 (1,2). This information enables us to study the epidemiology of renal syndromes and histopathologic data. Renal biopsy in acute renal failure (ARF) of unknown origin provides irreplaceable information for diagnosis, treatment, and prognosis. In this report, we analyze the frequency and clinicopathologic correlations of renal native biopsied ARF in Spain during the period 1994 through 2006.
Materials and Methods
We analyzed the frequency and clinicopathologic correlations of ARF confirmed by native renal biopsy in Spain and the distribution of the different clinicopathologic findings according to age. Patients who were younger than 15 yr were classified as children, those between 15 and 65 yr as adults, and those older than 65 as elderly.
We analyzed the results of renal biopsies during the period 1994 through 2006 and completed one questionnaire for each patient. ARF was defined as a rapid deterioration of the GFR, with or without oligoanuria or rapidly progressive renal insufficiency, including acute-on-chronic renal failure. Nephrotic syndrome was defined as proteinuria >3.5/g per d/1.73 m2 and serum albumin <2.5 g/dl. Nephritic syndrome was defined as hematuria, hypertension, oliguria, edema, and reduced GFR. Asymptomatic urinary abnormalities were defined as proteinuria <3.5 g/d and/or hematuria with more than three red blood cells and no clinical manifestations, hypertension as BP >140/90 mmHg or antihypertensive treatment, and chronic renal failure as serum creatinine levels persistently >1.5 mg/dl. Each questionnaire recorded the following variables: Name, hospital, date of birth, gender, presence of arterial hypertension and/or antihypertensive treatment, serum creatinine (mg/dl), creatinine clearance (ml/min), proteinuria (g/d), urinary sediment, main defined clinical syndrome, study methods, and number of glomeruli obtained. Primary glomerulonephritis (GN) is classified into eight groups: Minimal-change disease; FSGS; proliferative endocapillary GN; crescentic GN (presence of crescents in >50% of glomeruli) types 1, 2, and 3; membranoproliferative types 1 and 2 GN; membranous GN, IgA nephropathy; and mesangioproliferative non-IgA nephropathy. Secondary GN has also been classified into eight groups: Fibrillary, lupus nephritis, collagenosis (scleroderma and other connective tissue diseases not included in another diagnosis), vasculitis, Goodpasture syndrome, cryoglobulinemic GN, amyloidosis, and light-chain nephropathy. Crescentic type 3 and pauci-immune rapidly progressive GN were included in the vasculitis group. Noninflammatory renal pathology has been classified into eight groups: Diabetic nephropathy, nephroangiosclerosis, atheroembolic disease, acute tubular necrosis, myeloma kidney, thrombotic microangiopathy, acute tubulointerstitial nephritis (ATIN), and chronic interstitial nephropathy. The remaining causes have been classified into three groups: Unclassified nephropathies, sclerotic kidney, and other miscellaneous pathologies. Biopsies of transplant kidneys were not studied (1,2). Testing for antineutrophil cytoplasmic autoantibodies (ANCA) and anti–glomerular basement membrane in serum was not requested in this study, and we did not collect outcome or follow-up information.
Statistical Analysis
Data were stored in a database (Microsoft Access; Microsoft Corp., Redmond, WA). Statistical analysis was by SPSS for Windows 10.0.6 (SPSS Systat Inc., Chicago, IL). The normal distribution was determined using the Kolmogorov-Smirnov test. The values are expressed as medians when the parameters did not follow a normal (Gaussian) distribution. The χ2 and Fisher exact test were used to compare qualitative variables. P < 0.05 (by two-tailed testing) was considered to indicate statistical significance.
Results
Between 1994 and 2006, we collected 14,190 native renal biopsies from 112 renal units in Spain, 99 of which had at least one case of ARF. The renal units are listed in the Acknowledgments.
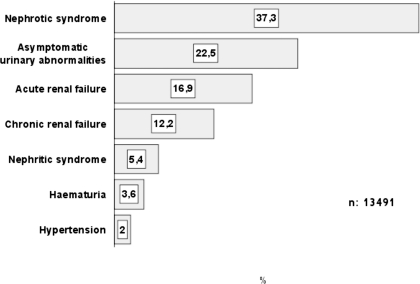
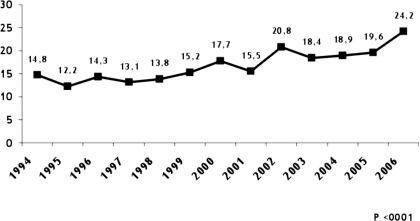
Sixteen percent (2281 biopsies) were diagnosed with ARF, which is the third indication for renal biopsy after nephrotic syndrome (35.4%) and asymptomatic urinary abnormalities (21.4%), as indicated in Figure 1. The prevalence of ARF over total syndromes as a main indication for renal biopsy increased significantly during the study period (P < 0.0001; Figure 2). The average incidence of ARF was 175 biopsies per year.
Figure 1.
Frequency of the main renal syndromes that required renal biopsy during the study period.
Figure 2.
Percentages of acute renal failure (ARF) as the main indication for renal biopsy during the study period.
The prevalence of the main clinical syndromes was statistically different in the three age groups. For children, ARF represented 5.7% of all renal syndromes, and for adults and elderly patients, this figure increased to 12.5 and 32.9%, respectively (Table 1). When we consider all biopsied ARF, the distribution according to age was also different: 1.9% in children, 52.8% in adults, and 45.3% in elderly patients.
Table 1.
Distribution of renal syndromes according to age: 1994 through 2006 dataa
| Age (yr) | Nephrotic Syndrome (%) | Nephritic Syndrome (%) | Asymptomatic Urinary Abnormalities (%) | Hypertension (%) | ARF (%) | Chronic Renal Failure (%) | Hematuria (%) |
|---|---|---|---|---|---|---|---|
| <15 (n = 748) | 47.3 | 6.8 | 22.9 | 0.3 | 5.7 | 3.5 | 13.5 |
| 15 to 65 (n = 9494) | 36.5 | 5.1 | 26.9 | 2.8 | 12.5 | 12.3 | 3.8 |
| >65 (n = 3111) | 36.8 | 5.8 | 9.1 | 0.8 | 32.9 | 13.9 | 0.7 |
| Total | 37.2 | 5.4 | 22.5 | 2.4 | 16.9 | 12.2 | 3.6 |
P < 0.0001 (χ2 test). ARF, acute renal failure.
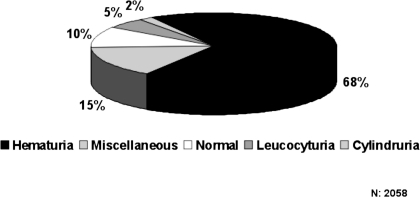
Ninety percent of the patients with biopsy-confirmed ARF had abnormal urinary sediment, mainly hematuria (Figure 3). Men predominated in every age group with an overall ratio of 1.5, and the prevalence of hypertension was high. The median age, proteinuria levels, number of glomeruli obtained, and statistical significance are shown in Table 2. The most important causes of ARF were secondary GN (32.5%), followed by nonglomerular pathology (30.4%), primary GN (28.8%), and other or unclassified nephropathies (8.3%).
Figure 3.
Urinary sediment.
Table 2.
Distribution of clinical data in biopsied ARF according to agea
| Age (yr) | Male/Female Ratio | Hypertension (%) | Age (yr) | Serum Creatinine (mg/dl) | GFR (ml/min) | Proteinuria (g/24 h) | No. of Glomeruli |
|---|---|---|---|---|---|---|---|
| <15 (n = 43) | 1.3 | 37.2 | 9 | 2.9 | 18.0 | 1.0 | 18 |
| 15 to 65 (n = 1191) | 1.7 | 53.0 | 50 | 5.0 | 16.0 | 1.7 | 12 |
| >65 (n = 1023) | 1.3 | 58.3 | 73 | 5.5 | 11.0 | 1.4 | 11 |
| Total | 1.5 | 53,1 | 63 | 5.1 | 13.1 | 1.5 | 12 |
| P | 0.0007b | <0.003b | – | <0.001c | <0.001c | <0.001c | <0.0001c |
Data are medians.
χ2 test.
Kruskal-Wallis nonparametric test.
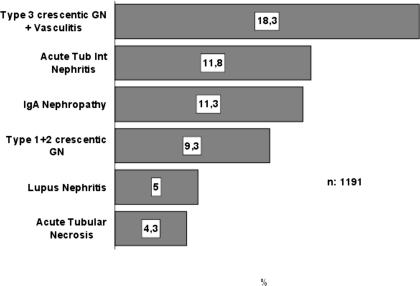
The histologic categories of the biopsies are listed in Table 3 and Figure 4. This distribution differs significantly in the three age groups (P < 0.001). Vasculitis (including pauci-immune crescentic or type 3 GN with or without angiitis) was the first cause (23.3%), followed by ATIN (11.3%) and crescentic GN types 1 and 2 (10.1%). Crescentic GN type 1 (anti–glomerular basement membrane antibodies) constituted 12.5% of all crescentic glomerular diseases; type 2 (immune complex related), 20.2%; and type 3, 67.2%. The percentage distribution of the different types of crescentic GN was similar in the three age groups (P = 0.11, data not shown). The prevalence of acute tubular necrosis in our registry is low: 5% of all renal biopsies, with minor differences in the three age groups (Table 3).
Table 3.
Distribution of histopathologic diagnoses in biopsied ARFa
| Diagnosis | Total(%)(n = 2281) | Age (yr)
|
||
|---|---|---|---|---|
| <15(%)(n = 43) | 15 to 65(%)(n = 1191) | >65(%)(n = 1023) | ||
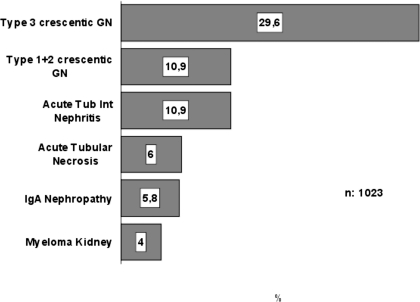
| Type 3 crescentic GN + vasculitis | 23.3 | 11.6 | 18.3 | 29.6 |
| Acute tubulointerstitial nephritis | 11.3 | 9.3 | 11.8 | 10.9 |
| Types 1 and 2 crescentic GN | 10.1 | 11.6 | 9.3 | 10.9 |
| IgA nephropathy | 8.8 | 11.6 | 11.3 | 6.0 |
| Acute tubular necrosis | 5.0 | 2.3 | 4.3 | 6.0 |
| Benign nephrosclerosis | 3.8 | – | 4.8 | 2.6 |
| Lupus nephritis | 3.3 | 4.7 | 5.0 | 1.4 |
| Others | 3.3 | 9.3 | 4.0 | 2.2 |
| Unclassifiable | 3.0 | 2.3 | 2.9 | 3.1 |
| Thrombotic microangiopathy | 2.8 | 20.9 | 3.4 | 1.2 |
| Light-chain cast nephropathy (myeloma kidney) | 2.8 | – | 1.8 | 4.0 |
| Membranoproliferative GN | 2.7 | – | 3.2 | 2.2 |
| Endocapillary GN | 2.5 | 4.7 | 2.1 | 2.8 |
| Chronic tubulointerstitial nephritis | 2.2 | – | 2.2 | 2.4 |
| FSGS | 2.1 | – | 2.6 | 1.4 |
| Sclerotic kidney | 2.1 | 4.7 | 2.1 | 1.9 |
| Amyloidosis | 2.0 | – | 1.5 | 2.4 |
| Anti–glomerular basement membrane antibody (Goodpasture disease) | 2.0 | – | 2.4 | 1.6 |
| Diabetic nephropathy | 1.8 | – | 1.7 | 2.0 |
| Non-IgA mesangial GN | 1.1 | 2.3 | 1.4 | 0.7 |
| Membranous nephropathy | 1.1 | – | 1.3 | 0.9 |
| Atheroembolism | 0.8 | – | 0.4 | 1.3 |
| Light-chain disease | 0.7 | – | 0.4 | 1.2 |
| Cryoglobulinemia | 0.7 | 2.3 | 0.8 | 0.6 |
| Minimal-change disease | 0.5 | 2.3 | 0.3 | 0.6 |
| Collagenosis | 0.4 | 0.4 | 0.3 | |
| Fibrillary GN | 0.1 | – | 0.2 | – |
GN, glomerulonephritis.
Figure 4.
Causes in all age groups. GN, glomerulonephritis.
In children (n = 43), the predominant conditions were thrombotic microangiopathy (20.9%), followed by crescentic GN types 1 and 2, IgA nephropathy, and vasculitis (11.6% each). The predominant conditions in adults (n = 1191) were vasculitis (18.3%), followed by ATIN (11.8%), IgA nephropathy (11.3%), and crescentic GN types 1 and 2 (9.3%; Table 3, Figure 5). This prevalence did not change during the study period.
Figure 5.
Causes of biopsy-confirmed ARF in adults.
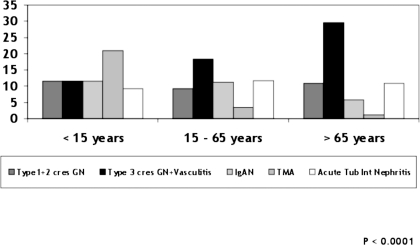
Finally, in elderly patients (n = 1023), there was an even greater prevalence of vasculitis that increased to 29.6%, followed by other forms of crescentic GN types 1 and 2 (10.9%) and ATIN (10.9%; Table 3, Figure 6). These values did not change during the study period. These results are summarized in Figure 7, which shows that the prevalence of vasculitis increased significantly in the three age groups: Thrombotic microangiopathy decreased in adults and elderly patients and ATIN was slightly lower in children but similar in adults and elderly patients.
Figure 6.
Causes of biopsy-confirmed ARF in elderly patients.
Figure 7.
Renal histopathology by age group.
Discussion
This observational study is the first description of the epidemiology of biopsy-confirmed ARF in native kidneys from a large national registry. Most of the patients have glomerular disease, and our analysis is made only for patients from whom biopsies have been taken because of clinical suspicion of GN or other renal diseases that merit histologic confirmation. Despite the biases common to national registries—biopsy studied by different pathologists, nonhomogeneous biopsy policy, and interpretation of histopathologic findings—the data offered by the Spanish Registry of Glomerulonephritis are representative of histologic findings in ARF. Moreover, the large number of renal biopsies (2281) and the long period of data collection (13 yr) reinforce the value of this database. In all renal syndromes, particularly in ARF, histopathologic findings are of paramount importance for correct diagnosis, prognosis, and even choosing therapy (3,4). Haas et al. (5) reported that in patients who were aged ≥60 yr and underwent renal biopsy, ARF was the main indication of biopsy in 24.3%; moreover, prebiopsy clinical diagnosis was correct in only 33% of cases, biopsy enabled a specific diagnosis to be reached in >90%, and a diagnosis offering the potential for improved outcomes with treatment was made in 73% of cases. The design of our study did not include outcome or follow-up data.
Curiously, the epidemiology of ARF has not been studied in detail. In one of the most interesting studies, Liaño et al. (6) reported 748 cases of ARF with an incidence of 209 cases per million people. The most frequent causes of ARF were acute tubular necrosis (45%), prerenal failure (21%), acute-onset chronic renal failure (12.7%), and obstructive ARF (10%). Although the number of renal biopsies performed was low (6.1% of all cases of ARF), it allowed us to diagnose several renal diseases by histopathology. The use of renal biopsy for patients with ARF is usually reserved for the following conditions: (1) absence of an obvious cause; (2) extrarenal manifestations compatible with a systemic disease; (3) proteinuria, hematuria, or cylindruria; (4) ARF of more than 3 wk duration or prolonged anuria; and (5) suspicion of a parenchymatous origin excluding acute tubular necrosis. In our patients, urinary sediment was normal in only 10% of cases and hematuria, cylindruria, and non-nephrotic proteinuria were present in most cases. Moreover, hypertension was more common than in other cases of ARF. Thus, we assumed that in ARF with hypertension, proteinuria, and altered urinary sediment (mainly hematuria) and after excluding obvious cases of acute deterioration of renal function, biopsy provided essential information on histopathology, pathogenesis, and classification (7,8). Although renal biopsy is much safer nowadays thanks to new biopsy guns and ultrasonic guidance, it is necessary to tailor indications and evaluate potentially severe risks (9).
Although there are many renal biopsy registries around the world, most describe the incidence and prevalence of histopathologic findings. Very few analyze in detail the main clinical syndromes that indicate renal biopsy. Renal biopsy registries in Australia (10), Denmark (11,12), Macedonia (13), Hungary (14), Thailand (15), Peru (16), Korea (17), China (18), New Zealand (19), Brazil (20), Saudi Arabia (21), and the Czech Republic (22) do not provide information about ARF as a separate clinical syndrome at the time of renal biopsy. In the Singaporean (23), Dutch (24), Uruguayan (25), and Japanese (26) registries, the clinical indication for renal biopsy was ARF in 7 to 13.6% of cases; however, the definition of clinical syndromes is not clear, and, in most cases, ARF is referred to as nephritic syndrome or rapidly progressive GN. These discrepancies may arise because the definition of ARF is problematic and a precise operational definition of ARF is not available (27). Thus, the term ARF was recently replaced by acute kidney injury, and ARF should be restricted to patients who have acute kidney injury and need renal replacement therapy (28). Moreover, the new Acute Kidney Injury Network has proposed uniform standards for diagnosis and classification, which were published in 2007 (29). Unfortunately, according to these standards, our definition of ARF is flawed, thus reflecting the problem of this type of definition in national registries during the study period (1994 to 2006). Although our consideration of ARF is more imprecise than the current definition, the results of our investigation are representative.
Other large national registries include detailed data on renal syndromes and allow comparison with other registries of the importance of renal biopsy in undetermined ARF. A survey by the Italian registry (30,31), which collected 15,461 biopsies, revealed the incidence of ARF as a clinical syndrome in the study of renal biopsies to be 9.2%. The most frequent cause of ARF was necrotizing vasculitis (20.1%) followed by crescentic GN (14%), ATIN (11.3%), and acute tubular necrosis (7.9%). In the French registry (32), ARF constitutes 20% of renal biopsy indications, with a decrease in the last period studied. In the United Arab Emirates, a study of renal diseases (33) revealed that biopsied ARF represents 3.5% of all ARF, although the low number of renal biopsies does not permit comparisons with other larger registries. In more recent reports, the frequency of ARF varied from 2.5 to 12.4%. In Romania (34), ARF was present in 12.4% of the population, although this percentage was significantly lower (9.2%) in patients who were younger than 60 yr than in patients who were older than 60 yr (26.6%). Brazilian data (35) showed that ARF affects 2.5% of the population and rapidly progressive GN affects 7.1%; when the two entities are taken as one, the real incidence of ARF is 9.6%. In the Portuguese renal biopsy registry, ARF affected 9.7% (36), and in the large Indian registry (37), ARF affected 9.3 and 5.2% during the two study periods (1971 to 1985 and 1986 to 2002); the decrease may be due to improved health care. In most of these investigations, ARF was preceded by nephrotic syndrome (35), urinary abnormalities (30), recurrent macrohematuria, or nephritic syndrome (34). In our registry (1,2), the main indication was nephrotic syndrome, although ARF constitutes the third indication for renal biopsy (16%). We also found in our database that ARF as a main indication of renal biopsy increased during the last few years of the study; this was probably because biopsy began to be indicated for elderly patients as a consequence of the increase in life expectancy. Thus, in our experience, the percentage of ARF as an indication for renal biopsy increases from 5.4% in children to 11.9% in adults and 31.6% in elderly patients. Many renal diseases are not diagnosed in elderly patients because of comorbidity and the results of serologic tests (ANCA and others), thus creating a new bias in our knowledge of the epidemiology of renal diseases in this age group.
When we analyze histopathology results, there is consensus on two points: (1) The different patterns according to the age group studied and (2) the elevated incidence of systemic or limited renal vasculitis, especially in the elderly. Thus, the report of the Italian National Registry of Renal Biopsies in Children (38) confirms that onset of ARF occurs at <15 yr in 5.3% of cases (very similar to our results) and that this is clearly preceded by isolated hematuria, proteinuria, and nephrotic syndrome. In this group of patients, the most frequent diseases are crescentic GN, ATIN, and hemolytic-uremic syndrome. Our results are similar, although we found that hemolytic-uremic syndrome was predominant in children. The Italian registry (30) reported that renal biopsy was indicated as a result of necrotizing vasculitis, chronic GN, acute tubulointerstitial nephritis, and acute tubular necrosis, in percentages similar to ours; however, that study made no distinctions between adult and elderly patients.
In our registry, crescentic proliferation (with or without angiitis) appears in one third (34.7%) of all renal biopsies; these findings are even more evident in the elderly (42.2%) and are consistent with those reported in elderly patients in the detailed investigations of Haas et al. (5) and Uezono et al. (39). When we consider the results of all renal biopsies in Spain during the same period, we see that crescentic GN and vasculitis take sixth place (6.8%), preceded by IgA nephropathy (14.5%), membranous nephropathy (10.6%), FSGS (9.4%), lupus nephritis (9.2%), and minimal-change disease (7.4) (1,2). In the Italian registry, there was an increase in the percentage of patients who were older than 65 yr and required renal biopsy (40). These findings highlight the role of crescentic proliferation in the acute deterioration of renal failure; however, it is widely known that pauci-immune crescentic GN is a form of renal limited vasculitis generally associated with ANCA. Here, the prompt diagnosis with renal biopsy is a key procedure when initiating immunosuppressive treatment to prevent irreversible glomerulosclerosis. In our registry, testing to determine the presence of ANCA was not requested to simplify the data collected and facilitate participation. The second cause of ARF is acute tubulointerstitial nephritis, which must be confirmed in severe cases by renal biopsy so that treatment with steroids and/or immunosuppressive drugs can be started. It is interesting that IgA nephropathy is a significant cause of biopsy-confirmed ARF in adults (approximately 11%), probably caused by hematuria and/or crescentic proliferation. Finally, it is also important to emphasize that the incidence of necrotizing crescentic GN has increased in recent years. In a UK study (41), pauci-immune rapidly progressive GN accounted for nearly 8% of ARF admissions. Apart from the clinical picture, crescentic GN is the third cause of renal disease, preceded only by membranous GN and IgA nephropathy (42); however, the spectrum of intrinsic ARF could be different in less developed countries (43).
The percentage of cases of acute tubular necrosis in our registry is very low (5% of all renal biopsies), because most cases are diagnosed with clinical data and we analyzed only the cases of biopsy-confirmed ARF; however, even profound acute tubular necrosis often has limited pathologic changes, so some cases with acute tubular necrosis might end up being classified under their underlying pathology, such as nephrosclerosis or diabetes. Nevertheless, several cases of acute allergic interstitial nephritis, atheroembolism, or another renal disease could have been confused with acute tubular necrosis. This fact stresses the importance of carefully indicating renal biopsy in “atypical” acute tubular necrosis and of developing updated guidelines for renal biopsy (44).
The Spanish Registry of Glomerulonephritis provides useful information about renal histopathology in biopsy-confirmed ARF. ARF associated with proteinuria and/or hematuria is produced by several renal diseases other than acute tubular necrosis, with important prognostic and therapeutic consequences. The prevalence of vasculitis and crescentic GN is high, especially in elderly patients. These data, obtained from a national large registry, stress the value of renal biopsy in undetermined ARF. The follow-up, prognosis, and outcome of this type of ARF must be studied in the future.
Disclosures
None.
Acknowledgments
The following are the participating renal units: Álava: Hospital Santiago Apóstol, Txagorritxu Ospitalea; Albacete: Hospital General de Albacete; Alicante: Hospital de Villajoyosa-Benidorm, Hospital General de Elda, Hospital General Universitario de Alicante, Hospital General Universitario de Elche, Hospital Virgen de los Lirios, Hospital Perpetuo Socorro; Almería: Hospital Torrecárdenas; Asturias: Hospital Central de Asturias, Hospital Central de Asturias (Infantil), Hospital Valle Nalón; Ávila: Hospital Nuestra Señora de Sonsoles; Badajoz: Hospital Universitario Infanta Cristina; Baleares: H. Son Llatzer, Hospital Son Dureta, Policlínica Miramar; Barcelona: Clínica Quiront, Consorci Hospitalari del Parc Taulí, Fundación Puigvert, H. Althaia, Hospital Clinic i Provincial, Hospital de Bellvitge, Hospital de la Cruz Roja, Hospital de Terrassa, Hospital del Mar, Hospital General Vall d'Hebrón, Hospital San Joan de Deu, Hospital Universitari Germans Trias i Pujol; Burgos: Hospital General Yagüe; Cáceres: Complejo Hospitalario San Pedro de Alcántara; Cádiz: Hospital de Jerez, Hospital Puerta del Mar, Hospital Universitario de Puerto Real; Cantabria: Hospital Universitario Marqués de Valdecilla; Castellón: Hospital General de Castellón; Ciudad Real: Hospital General de Ciudad Real; Córdoba: Complejo Hospitalario Universitario Reina Sofía; Coruña, A: Complejo Hospitalario Universitario de Santiago; Hospital Universitario Juan Canalejo; Cuenca: Hospital Virgen de la Luz; Girona: Hospital Dr. Josep Trueta; Granada: Ciudad Sanitaria Virgen de la Nieves, Hospital Universitario de Granada; Guadalajara: Hospital General Universitario de Guadalajara; Guipúzcoa: Hospital Nuestra Sra. de la Antigua; Huelva: Hospital General Juan Ramón Jiménez; Huesca: Hospital San Jorge; Jaén: Complejo Hospitalario Princesa de España, Hospital General de Especialidades Ciudad de Jaén; Las Palmas de Gran Canaria: Hospital Nuestra Sra. del Pino, Hospital Universitario Insular de Las Palmas; León: Hospital Virgen Blanca; Lleida: Hospital Arnau de Vilanova; Lugo: Complejo Hospitalario Xeral Calde; Madrid: Clínica Puerta de Hierro, Clínica Ruber, Fundación Hospital Alcorcón, Fundación Jiménez Díaz, Hospital 12 de Octubre (Adultos), Hospital del Aire, Hospital del Niño Jesús, Hospital General Universitario Gregorio Marañón (Adultos), Hospital General Universitario Gregorio Marañón (Infantil), Hospital Militar Gómez Ulla, Hospital Universitario de Getafe, Hospital Universitario de la Princesa, Hospital Universitario La Paz, Hospital Universitario La Paz (Infantil), Hospital Universitario Severo Ochoa; Málaga: Hospital Carlos Haya (Adultos), Hospital Carlos Haya (Infantil), Hospital Clínico Universitario Virgen de la Victoria, Hospital Regional de Málaga (Infantil); Murcia: Hospital Universitario Virgen de la Arrixaca; Navarra: Hospital de Navarra; Ourense: Hospital Cristal Piñor; Palencia: Hospital General Río Carrión; Pontevedra: Complejo Hospitalario Xeral Cíes, Hospital Montecelo, Hospital Provincial de Pontevedra; Rioja, La: Hospital San Millán y San Pedro; Salamanca: Hospital Clínico Universitario de Salamanca; Segovia: Hospital General de Segovia; Sevilla: Hospital General Virgen del Rocío, Hospital Universitario Virgen Macarena, Hospital Virgen del Rocío (Infantil); Soria: Hospital General de Soria; Tarragona: Hospital Joan XXIII; Tenerife: Complejo Hospitalario Nuestra Sra. de la Candelaria, Hospital Nuestra Sra. de la Candelaria (Infantil), Hospital Universitario de Canarias; Teruel: Hospital Obispo Polanco; Toledo: Hospital Virgen de la Salud; Valencia: Hospital Clínico Universitario de Valencia, Hospital Dr. Peset Aleixandre, Hospital General Universitari, Hospital San Francesc de Borja, Hospital Universitario La Fe (Adultos), Hospital Universitario La Fe (Infantil); Valladolid: Hospital del Río Ortega, Hospital Universitario de Valladolid; Vizcaya: Hospital de Basurto, Hospital de Cruces, Hospital de Cruces (Infantil), Hospital de Galdakao; Zamora: Hospital Virgen de la Concha; Zaragoza: Hospital Clínico Universitario de Zaragoza, Hospital Miguel Servet, Hospital Miguel Servet (Infantil).
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Renal Biopsies in Acute Kidney Injury: Who Are We Missing?” on pages 647–648.
References
- 1.Rivera F, López-Gómez JM, Pérez-García R: Frequency of renal pathology in Spain 1994–1999. Nephrol Dial Transplant 17: 1594–1602, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Rivera F, López-Gómez JM, Pérez García R: Clinicopathological correlations of renal pathology in Spain. Kidney Int 66: 898–904, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Risler T, Braun N, Ittel TH: Multi-centre glomerulonephritis registry: Basis for optimised therapy. Kidney Blood Press Res 20: 184–187, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Andreucci VE, Fuiano G, Stanziale P, Andreucci M: Role of renal biopsy in the diagnosis and prognosis of acute renal failure. Kidney Int Suppl 66: S91–S95, 1998 [PubMed] [Google Scholar]
- 5.Haas M, Spargo BH, Wit EJ, Meehan SM: Etiologies and outcome of acute renal insufficiency in older adults: A renal biopsy study of 259 cases. Am J Kidney Dis 35: 433–447, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Liaño F, Pascual J: Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 50: 811–818, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Richards NT, Darby S, Howie AJ, Adu D, Michael J: Knowledge of renal histology alters patient management in over 40% of cases. Nephrol Dial Transplant 9: 1255–1259, 1994 [PubMed] [Google Scholar]
- 8.Fuiano G, Mazza G, Comi N, Caglioti A, De Nicola L, Iodice C, Andreucci M, Andreucci VE: Current indications for renal biopsy: A questionnaire-based survey. Am J Kidney Dis 35: 448–457, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Solez K, Racusen LC: Role of the renal biopsy in acute renal failure. Contrib Nephrol 132: 68–75, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Briganti EM, Dowling J, Finlay M, Hill PA, Jones CL, Kincaid-Smith PS, Sinclair R, McNeil JJ, Atkins RC: The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant 16: 1364–1367, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Heaf J: The Danish Renal Biopsy Register. Kidney Int 66: 895–897, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Heaf J, Løkkegaard H, Larsen S: The epidemiology and prognosis of glomerulonephritis in Denmark 1985–1997. Nephrol Dial Transplant 14: 1889–1897, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Polenakovic MH, Grcevska L, Dzikova S: The incidence of biopsy-proven primary glomerulonephritis in the Republic of Macedonia: Long-term follow-up. Nephrol Dial Transplant 18[Suppl 5]: v26–v27, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Sipiczki T, Ondrik Z, Abraham G, Pokorny G, Turi S, Sonkodi S, Kemeny E, Ivanyi B: The incidence of renal diseases as diagnosed by biopsy in Hungary. Orv Hetil 145: 1373–1379, 2004 [PubMed] [Google Scholar]
- 15.Parichatikanond P, Chawanasuntorapoj R, Shayakul C, Choensuchon B, Vasuvattakul S, Vareesangthip K, Chanchairujira T, Sritippayawan S, Vongwiwatana A, Premasathian N, Kiattisunthorn K, Larpkitkachorn R, Ongajyooth L: An analysis of 3,555 cases of renal biopsy in Thailand. J Med Assoc Thai 89[Suppl 2]: S106–S111, 2006 [PubMed] [Google Scholar]
- 16.Hurtado A, Escudero E, Stromquist CS, Urcia J, Hurtado ME, Gretch D, Watts D, Russell K, Asato C, Johnson RJ: Distinct patterns of glomerular disease in Lima, Peru. Clin Nephrol 53: 325–332, 2000 [PubMed] [Google Scholar]
- 17.Choi IJ, Jeong HJ, Han DS, Lee JS, Choi KH, Kang SW, Ha SK, Lee HY, Kim PK: An analysis of 4514 cases of renal biopsy in Korea. Yonsei Med J 42: 247–254, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Li LS, Liu ZH: Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13 519 renal biopsies. Kidney Int 66: 920–923, 2004 [DOI] [PubMed] [Google Scholar]
- 19.The New Zealand Glomerulonephritis Study: Introductory report. Clin Nephrol 31: 239–246, 1989 [PubMed] [Google Scholar]
- 20.Bahiense-Oliveira M, Saldanha LB, Mota EL, Penna DO, Barros RT, Romao-Junior JE: Primary glomerular diseases in Brazil (1979–1999): Is the frequency of focal and segmental glomerulosclerosis increasing? Clin Nephrol 61: 90–97, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Mitwalli AH, Wakeel JSA, Mohaya SSA, Malik HG, Abu-Aisha H, Hassan OS, Akhtar M: Pattern of glomerular disease in Saudi Arabia. Am J Kidney Dis 27: 797–802, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Rychlík I, Janèová E, Tesaø V, Kolsky A, Lácha J, Stejskal J, Stejskalová A, Dušek J, Herout V: The Czech registry of renal biopsies: Occurrence of renal diseases in the years 1994–2000. Nephrol Dial Transplant 19: 3040–3049, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Woo KT, Chiang GS, Pall A, Tan PH, Lau YK, Chin YM: The changing pattern of glomerulonephritis in Singapore over the past two decades. Clin Nephrol 52: 96–102, 1999 [PubMed] [Google Scholar]
- 24.Tiebosch AT, Wolters J, Frederik PF, van der Wiel TW, Zeppenfeldt E, van Breda Vriesman PJ: Epidemiology of idiopathic glomerular disease: A prospective study. Kidney Int 32: 112–116, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Mazzuchi N, Acosta N, Caorsi H, Schwedt E, Di Martino LA, Mautone M, Gadola L, Petraglia A, Noboa O, Programa de Prevencion y Tratamiento de las Glomerulopatias: Frequency of diagnosis and clinic presentation of glomerulopathies in Uruguay. Nefrologia 25: 113–120, 2005 [PubMed] [Google Scholar]
- 26.Iseki K, Miyasato F, Uehara H, Tokuyama K, Toma S, Nishime K, Yoshi S, Shiohira Y, Oura T, Tozawa M, Fukiyama K: Outcome study of renal biopsy patients in Okinawa, Japan. Kidney Int 66: 914–919, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Biesen WV, Vanholder R, Lameire N: Defining acute renal failure: RIFLE and beyond. Clin J Am Soc Nephrol 1: 1314–1319, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Molitoris BA, Levin A, Warnock DG, Joannidis M, Mehta RL, Kellum JA, Ronco C, Shah SV, on behalf of the Acute Kidney Injury Network working group: Improving outcomes of acute kidney injury: Report of an initiative. Nat Clin Pract Nephrol 3: 439–442, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, and the Acute Kidney Injury Network: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schena FP, Italian Group of Renal Immunopathology: Survey of the Italian Registry of Renal Biopsies: Frequency of the renal diseases for 7 consecutive years. Nephrol Dial Transplant 12: 418–426, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Gesualdo L, Di Palma AM, Morrone LF, Strippoli GF, Schena FP, Italian Immunopathology Group, Italian Society of Nephrology: The Italian experience of the national registry of renal biopsies. Kidney Int 66: 890–894, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Simon P, Ramée MP, Boulahrouz R, Stanescu C, Charasse C, Seng Ang K, Leonetti F, Cam G, Laruelle E, Autuly V, Rioux N: Epidemiologic data of primary glomerular diseases in western France. Kidney Int 66: 905–908, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Yahya TM, Pingle A, Boobes Y, Pingle S: Analysis of 490 kidney biopsies: Data from the United Arab Emirates Renal Diseases Registry. J Nephrol 11: 148–150, 1998 [PubMed] [Google Scholar]
- 34.Covic A, Schiller A, Volovat C, Gluhovschi G, Gusbeth-Tatomir P, Petrica L, Caruntu ID, Bozdog G, Velciov S, Trandafirescu V, Bob F, Gluhovschi C: Epidemiology of renal disease in Romania: A 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant 21: 419–424, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Malafronte P, Mastroianni-Kirsztajn G, Betônico GN, Romao JE Jr, Alves MA, Carvalho F, Neto OM, Cadaval RA, Bérgamo RR, Woronik V, Sens YA, Marrocos MS, Barros RT: Paulista registry of glomerulonephritis: 5-Year data report. Nephrol Dial Transplant 21: 3098–3105, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Carvalho E, Faria MdS, Nunes JPL, Sampaio S, Valbuena C: Renal diseases: A 27-year renal biopsy study. J Nephrol 19: 500–507, 2006 [PubMed] [Google Scholar]
- 37.Narasimhan B, Chacko B, John GT, Korula A, Kirubakaran MG, Jacob CK: Characterization of kidney lesions in Indian adults: towards a renal biopsy registry. J Nephrol 19: 205–210, 2006 [PubMed] [Google Scholar]
- 38.Coppo R, Gianoglio B, Porcellini MG, Maringhini S: Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal Biopsies in Children). Group of Renal Immunopathology of the Italian Society of Pediatric Nephrology and Group of Renal Immunopathology of the Italian Society of Nephrology. Nephrol Dial Transplant 13: 293–297, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Uezono S, Hara S, Sato Y, Komatsu H, Ikeda N, Shimao Y, Hayashi T, Asada Y, Fujimoto S, Eto T: Renal biopsy in elderly patients: A clinicopathological analysis. Ren Fail 28: 549–555, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Stratta P, Segoloni GP, Canavese C, Sandri L, Mazzucco G, Roccatello D, Manganaro M, Vercellone A: Incidence of biopsy-proven primary glomerulonephritis in an Italian province. Am J Kidney Dis 27: 631–639, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Hedger N, Stevens J, Drey N, Walker S, Roderick P: Incidence and outcome of pauci-immune rapidly progressive glomerulonephritis in Wessex, UK: A 10-year retrospective study. Nephrol Dial Transplant 15: 1593–1599, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Vendemia F, Gesualdo L, Schena FP, D'Amico G: Renal Immunopathology Study Group of the Italian Society of Nephrology: Epidemiology of primary glomerulonephritis in the elderly. Report from the Italian Registry of Renal Biopsy. J Nephrol 14: 340–352, 2001 [PubMed] [Google Scholar]
- 43.Prakash J, Sen D, Kumar NS, Kumar H, Tripathi LK, Saxena RK: Acute renal failure due to intrinsic renal diseases: Review of 1122 cases. Ren Fail 25: 225–233, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Cagnoli L, Italian Society of Nephrology: Instructions and implementations for percutaneous renal biopsy: Guidelines for the therapy of glomerular nephropathies. G Ital Nefrol 20[Suppl 24]: S3–S47, 2003 [PubMed] [Google Scholar]