Abstract
Background and objectives: Cardiovascular disease is an important cause of morbidity and death in kidney transplant recipients. This study examines the Framingham risk score's ability to predict cardiac and stroke events. Because cyclosporine and tacrolimus have different cardiovascular risk profiles, these agents were also examined.
Design, setting, participants, & measurements: A prospective cohort evaluation of 540 patients were followed for a median of 4.7 yr in an outpatient kidney transplant clinic. Baseline Framingham risk scores were calculated and all cardiovascular outcomes were collected.
Results: Rates per 100 patient-years were 1.79 for cardiac and 0.78 for stroke events. The ratio of observed-to-predicted cardiac events was 1.64-fold higher [95% confidence interval (CI) 1.19 to 2.94] for the cohort, 2.74-fold higher (95% CI 1.70 to 4.24) in patients age 45 to 60 with prior cardiac disease or diabetes mellitus, but not higher in other age subgroups. Stroke was not increased above predicted. Risk scores for cardiac (c = 0.65, P = 0.003) and stroke (c = 0.71, P = 0.004) events were modest predictors. 10-yr event scores for cardiac (9.3 versus 13.5%, P < 0.001) and stroke (7.1 versus 10.0%, P = 0.002) were lower for tacrolimus compared with cyclosporine-treated patients. However observed cardiac events were higher in tacrolimus recipients (2.50, 95% CI 1.09 to 5.90) in an adjusted Cox model.
Conclusions: Although risk scores are only modest predictors, patients with the highest event rates are easily identified. Treating high-risk patients with cardioprotective medications should remain a priority.
Cardiovascular disease (CVD) is responsible for a large proportion of the death and morbidity in the kidney transplant population (1,2). Two studies have previously examined the Framingham risk factor score to predict events in this population (3,4). These studies have found that the score underestimates event rates. The relatively poor predictive accuracy of the Framingham score, especially in the chronic kidney disease population, has generated interest in nontraditional risk factors such as anemia, indicators of inflammation, and other biomarkers (5–8). Unfortunately the clinical utility of these markers and the evidence that specific directed therapy is of benefit are limited if not controversial even in the general population where there has been more intense study (9–12). Nonetheless a large effort is expended in the clinic to achieve recommended BP and cholesterol targets and use cardioprotective medications [angiotensin converting enzyme inhibitors (ACEi) and/or angiotensin receptor blockers (ARB), β-blockers, statins, and acetylsalicylic acid (ASA)] (13,14). Identifying patients in whom more intense therapy is required would be useful, as combined use of these four classes of cardioprotective agents has the potential to reduce cardiac events rates by approximately 75% (15).
The primary objective analysis was to examine the ability of the Framingham risk score to predict actual events in a modern cohort of prevalent patients in the clinic. Historic cohorts were likely undertreated as evidence by higher BP in earlier decades (16,17). In addition to cardiac outcomes, stroke and other events (congestive heart failure, significant rhythm disturbances, and peripheral vascular disease) were examined in this cohort. Because the two calcineurin inhibitors, cyclosporine and tacrolimus, have different cardiovascular risk profiles, we also examined the effect of these agents on subsequent outcomes.
Materials and Methods
Population
All 540 prevalent adult (>age 18) kidney transplant recipients followed at our center with a functioning allograft for >6 mo were studied. Many of these patients (n = 439) were described in an earlier report and consisted of prevalent patients followed in the transplant clinic in July 2002 (13). These patients were followed prospectively from this time point onward. In addition, 101 new incident patients were added prospectively over a 3-yr period once they passed their 6-mo post-transplant time point. Permission for the study was granted by our institution's ethics review board.
Quantification of Cardiovascular Risk
The CVD risk calculator used was a Framingham study-based computer software package that calculated separate 10-yr event rates for cardiac and cerebral vascular events (18). The calculator also has been validated against event rates in patients with prior cardiac disease.
Event Definitions
Major cardiac events were defined as fatal and nonfatal myocardial infarction and invasive coronary artery therapy. Cerebral vascular events included nonfatal and fatal stoke and transient ischemic attacks. These events were confirmed from discharge summaries. Event rates such as new-onset angina were collected and analyzed along with other events. Congestive heart failure (CHF) required admission to hospital with a discharge diagnosis of CHF. Significant rhythm disturbances were ventricular tachycardia, atrial fibrillation, and need for a pacemaker. Significant peripheral vascular disease was defined as need for invasive therapy or conservatively managed limb gangrene.
Data Presentation and Statistical Analyses
Descriptive data were presented as mean ± SD for continuous variables and percentages for dichotomous variables. Differences between groups were tested by χ2 test for dichotomous variables, by ANOVA for normally distributed continuous variables, and by nonparametric tests for skewed-distributed continuous variables. Significance was set at 5%. To analyze the ability of the Framingham scores to predict event rates, receiver-operating characteristic (ROC) curves and concordance statistics were calculated. Patients were censored at graft loss. Relative risk ratios for observed-to-predicted event rates were also calculated. A multivariate Cox proportional model was used to compare event rates between the two calcineurin inhibitors. Statistical analysis was performed using SPSS version 11.1.
Results
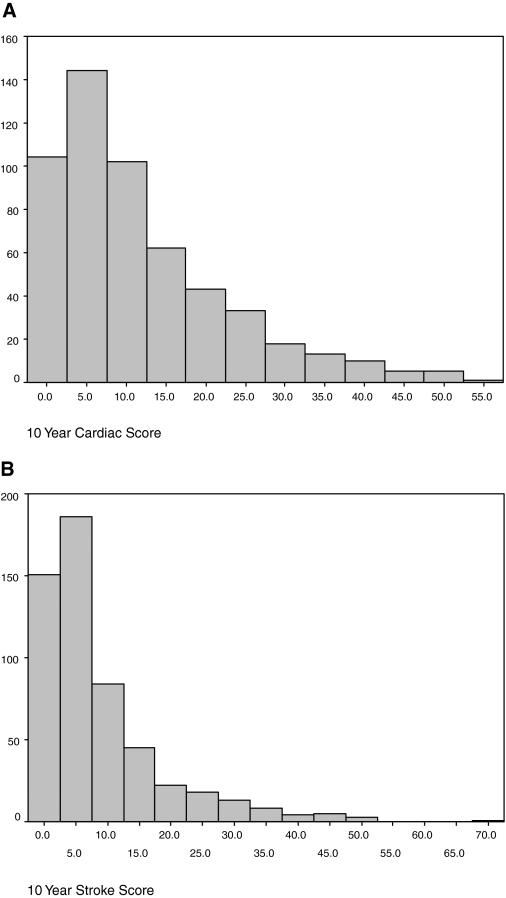
Tables 1 and 2 provide baseline descriptive characteristics, CVD risk factor, and medication use in our study population. The average patient at baseline was a middle-aged man transplanted approximately 6 yr ago and was treated with a calcineurin inhibitor, with 19% being steroid-free. Renal function was modest, with a mean creatinine clearance of 61 ml/min, and 31% having trace or greater proteinuria. Distributions of the Framingham 10-yr risk scores for cardiac disease and stroke are shown in Figures 1A and 1B. At baseline, systolic BP was >130 mmHg in 46% and LDL cholesterol was >2.5 mmol/L in 66% of patients.
Table 1.
Patient descriptors and medication use
| Age | 48 ± 12 yr (18–79; n = 440) |
| Gender | 59% (322 men) |
| Cadaver | 66% (355 cadaver) |
| Creatinine | 152 ± 65 μmol/l |
| CrCl | 61 ± 24 ml/min |
| Duration of function | 6.3 ± 6.6 yr |
| Duration of dialysis | 1.4 ± 1.8 yr |
| Body mass index | 28.6 ± 6.4 kg/m2 |
| Weight (kg) 0.5 to 31.3 yr | 81 ± 19 kg |
| Immunosuppression | |
| Cyclosporine | 51% |
| Tacrolimus | 36% |
| Mycophenolate mofetil | 38% |
| Azathioprine | 13% |
| Sirolimus | 13% |
| Prednisone | 81% |
| CVD drug use | |
| ACEi/ARB | 43% |
| β-blocker | 49% |
| Statin | 50% |
| ASA/other | 32% |
CrCl, creatinine clearance by Cockcroft-Gault; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; statin, HMG CoA reductase inhibitor; ASA, acetylsalicylic acid.
Table 2.
Cardiovascular risk factors, prevalent cardiovascular disease, and predicted Framingham risk scores
| Current smoker | 20%(108/532a) |
| Left ventricular hypertrophy | 20%(102/513a) |
| Diabetes mellitus | |
| Pre | 20% |
| Post | 31% |
| Systolic BP (mmHg) | 133 ± 17 |
| Diastolic BP (mmHg) | 78 ± 10 |
| Total cholesterol (mmol/L) | 5.2 ± 1.1 |
| HDL cholesterol (mmol/L) | 1.3 ± 0.4 |
| LDL cholesterol (mmol/L) | 3.0 ± 0.9 |
| Proteinuria (>negative) | 31% |
| Albumin (g/L) | 38 ± 4 |
| Hemoglobin (g/L) | 130 ± 16 |
| 10-yr cardiac risk score | 11.7 ± 11.0 |
| 10-yr stroke risk score | 8.7 ± 9.8 |
| Any cardiovascular diseaseb | 28% |
| Angina | 10.9% |
| Myocardial infarction | 11.3% |
| Congestive heart failure | 9.6% |
| Coronary bypass surgery/angioplasty | 7.2% |
| Peripheral vascular disease | 4.9% |
| Stroke | 4.6% |
| Atrial fibrillation | 3.0% |
Information was missing in some patients. Those without information on smoking or EKG were assumed to be nonsmokers in calculating cardiovascular disease risk (13).
An individual may have more than one cardiovascular disease.
Figure 1.
(A) Distribution of 10-yr Framingham cardiac risk scores, and (B) distribution of 10-yr Framingham stroke risk scores.
Patients were followed for a median of 4.73 yr. A description of all cardiovascular events is shown in Table 3. First event rates per 100 patient-yr for the entire cohort were 1.79 (n = 38), 0.77 (n = 16), and 4.1 (n = 92) for cardiac, stroke, and all events (cardiac, stroke, and all other events). Graft loss (including death with function) and total mortality per 100 patient-yr were 4.00 (n = 89) and 2.26 (n = 50), respectively. The concordance statistics comparing actual events to the risk scores were 0.646 (95% CI 0.573 to 0.720, P = 0.003) for major cardiac events, 0.713 (95% CI 0.598 to 0.827, P = 0.004) for stroke, and 0.701 (95% CI 0.65 to 0.752, P < 0.001) for all events (for all events the 10-yr cardiac and stroke score was summed). Surprisingly, the risks scores were better predictors of subsequent CHF (c = 0.689, P < 0.001 and c = 0.731, P < 0.001 for the cardiac and stroke scores, respectively).
Table 3.
Total cardiovascular events
| Cardiac | |
| All first major events | 38 |
| Myocardial infarction | 23 |
| Coronary bypass surgery or angioplasty | 14 |
| Coronary death | 11 |
| Angina | 6 |
| Cerebral Vascular | |
| All events | 16 |
| Nonfatal | 11 |
| Fatal | 2 |
| Transient ischemic attack | 3 |
| Other | |
| Congestive heart failure | 31 (7 fatal) |
| Peripheral vascular diseases | 6 |
| Rhythm | |
| Atrial fibrillation | 4 |
| Pacemaker | 4 |
| Ventricular tachycardia | 2 |
There were multiple events in 25 patients.
Overall the Framingham risk score underestimated observed cardiac events but not stroke. The observed-to-predicted event rate ratios in the entire cohort were 1.64 (95% CI 1.19 to 2.94) and 0.96 (95% CI 0.59 to 1.55) for cardiac and stroke, respectively. Stroke was not overestimated in any population subgroup; however, several subgroups demonstrated significant overestimates for cardiac events. Pretransplant diabetes mellitus (2.12, 95% CI 1.27 to 3.44, 13 events in 109 patients at risk) and prior cardiac disease (2.00, 95% CI 1.32 to 2.94, 20 events in 151 patients at risk) had significantly higher observed event rates relative to the predicted events. Patients age >60 (1.1, 95% CI 0.61 to 1.94, 10 events in 119 patients at risk) were not at increased risk. Patients age <45 without diabetes mellitus (1.2, 95% CI 0.42 to 3.33, three events in 161 patients at risk) were not at increased risk. Patients between ages 45 and 60 without prior diabetes mellitus or cardiac history were not at increased risk (1.22, 95% CI 0.57 to 2.55, 6 events in 128 patients at risk). However, patients with ages between 45 and 60 with either diabetes mellitus or prior cardiac disease had event rates that were much higher than predicted (2.74, 95% CI 1.70 to 4.24, 15 events in 95 patients at risk).
Combined ACE inhibitor (or ARB), ASA, β-blocker, and statin use was explored in the cohort. Overall only 41 of the entire population (7.6%), 37 of the population age >45, and 25 of the patients age 45 to 60 (26%) who had diabetes mellitus or prior cardiac history, were on all four medications. Overall 147 of the entire population (27%), 125 of the population age >45 (37%), and 53 of the patients age 45 to 60 (55%) who had diabetes mellitus or prior cardiac history, were on three of the four medications.
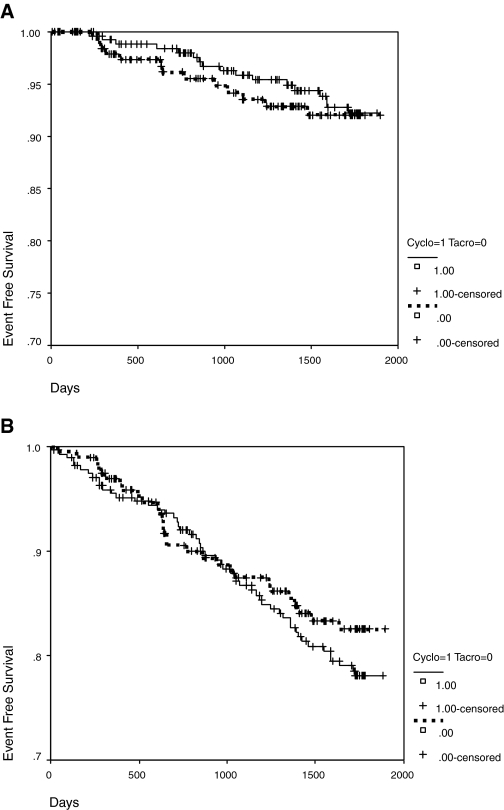
Figures 2A and 2B show that there were no significant differences for observed major cardiac (P = 0.71) or all-event rates (P = 0.36) between the two calcineurin inhibitors. However 10-yr event rates predicted for cardiac (9.3 versus 13.5%, P < 0.001) and stroke (7.1 versus 10.0%, P = 0.002) were lower for tacrolimus compared with cyclosporine-treated patients. Table 4 shows that there were significant differences between these two subcohorts for baseline covariates. After adjusting for patient age, gender, left ventricular hypertrophy by EKG, duration of transplant function, donor source, and diabetes mellitus before transplantation the analysis found hazard ratios for tacrolimus (relative to cyclosporine) to be 2.50 (95% CI 1.09 to 5.90, P = 0.036) for major cardiac events, 1.55 (95% CI 0.80 to 3.01, P = 0.198) for combined cardiac and stroke events, and 1.30 (95% CI 0.78 to 2.17, P = 0.320) for all events. In the tacrolimus-treated patients with new-onset diabetes mellitus there were only three events in 38 patients (7.9%), whereas in the cyclosporine-treated patients with new-onset diabetes mellitus there were six events in 44 patients (13.6%).
Figure 2.
(A) Event-free survival stratified by calcineurin inhibitor use for major cardiac events. (B) Event-free survival stratified by calcineurin inhibitor use for all events (cardiac, stroke, peripheral vascular disease and congestive heart failure).
Table 4.
Baseline characteristics of the cyclosporine and tacrolimus subgroups
| Variable | Tacrolimus (n = 197) | Cyclosporine (n = 273) | Probability |
|---|---|---|---|
| Age | 46 ± 12 | 51 ± 13 | <0.001 |
| Male gender % (n) | 53.8 (106) | 62.6 (171) | 0.055 |
| Deceased donor (%) | 66.5 (131) | 66.7 (182) | 0.969 |
| Duration transplant | 2.6 ± 2.6 | 6.9 ± 5.0 | <0.001 |
| Any cardiovascular disease | 22.8 (45) | 30.8 (84) | 0.058 |
| Diabetes mellitus pretreatment | 25.4 (50) | 17.6 (48) | 0.04 |
| New-onset diabetes mellitus | 19.3 (38) | 16.1 (44) | 0.372 |
| EKG left ventricular hypertrophy | 13.3 (26/195) | 22.7 (58/256) | 0.012 |
| Body mass index | 28.4 ± 6.1 | 30.0 ± 6.6 | 0.363 |
| Smoking | 23.9 (47) | 17.9 (48) | 0.116 |
| Systolic BP | 131 ± 17 | 135 ± 17 | 0.043 |
| Diastolic BP | 78 ± 9 | 78 ± 10 | 0.517 |
| Total cholesterol | 4.83 ± 1.02 | 5.40 ± 1.15 | <0.001 |
| LDL | 2.69 ± 0.81 | 2.93 ± 0.93 | <0.001 |
| Albumin | 38 ± 4 | 38 ± 4 | 0.419 |
| Hemoglobin | 132 ± 17 | 129 ± 16 | 0.041 |
| Creatinine clearance | 67 ± 25 | 57 ± 21 | <0.001 |
| Dipstick >negative | 27.7 (54) | 31.0 (83) | 0.447 |
| ASA | 29.4 (58) | 33.7 (92) | 0.33 |
| β-blocker | 50.5 (99) | 49.8 (136) | 0.926 |
| ACEi/ARB | 36.0 (71) | 48.7 (133) | 0.006 |
| Statin | 42.1 (83) | 58.2 (159) | 0.001 |
Discussion
The principal finding of this analysis is that the Framingham risk scores for cardiac and stroke are only moderate predictors in a prevalent cohort of patients being treated in the transplant clinic with the cardiac scores tending to underestimate risk. However the biggest underestimation of cardiac risk is in patients between the ages of 45 and 60 with prior diabetes mellitus or prior cardiac disease. It may simply be that those who develop kidney failure at a younger age and have prior cardiac disease and/or diabetes mellitus are a subgroup with more malignant cardiac but not cerebral vascular disease.
Kasiske et al. showed that the Framingham equation for recipients without overt CVD predicted event rates that were somewhat lower than observed (3). Some of the traditional risk factors (in particular diabetes mellitus) appeared to have more weight in the transplant compared with the general population. A more recent report also showed the equation underestimated risk but only in “high risk” patients (4). Our findings are consistent with these two reports. Patients with diabetes mellitus and prior cardiac disease were populations with high observed-to-predicted event rates. However, neither of the prior studies examined concordance statistics in their datasets nor examined the Framingham stroke score. The concordance statistic for cardiac disease in our study was of similar magnitude to a large male community population with chronic kidney disease from observational cohort trials but considerably lower than the Framingham population (5). In this study by Weiner et al., event rates observed for chronic kidney disease patients were about 2-fold higher than predicted. They also showed that prediction concordance scores in the kidney disease population improved significantly (from 0.60 to 0.68 for men and 0.73 to 0.81 for women) by refitting the traditional risk variables (age, diabetes, smoking, cholesterol, and BP) with population-specific coefficients (5). In an analysis of 27 studies with 71,727 patients, Brindle et al. found that the Framingham risk score ranged from an overestimate (2.87, 95% CI 2.87 1.91 to 4.31) of event rates in low-risk patients to an underestimate risk of event rates (0.43, 95% CI 0.27 to 0.67) in high-risk patients (19). The high-risk patients in these studies were those with hypertension and left ventricular hypertrophy and those with diabetes mellitus. These findings underscore the notion that traditional risk factors remain very important and likely have different relative importance depending on the population studied despite the relative low predictability of the original Framingham equation.
The implication of the low predictive value of the risk score is that aggressive BP control and lipid control is not necessary. This would be a mistake. There is relatively strong evidence for statin use in the transplant population (20). Cohort studies support importance of BP control for cardiac and renal protection (16,17). The added benefit of ACE inhibitors is under study in the transplant population and was strongly supported in studies of chronic kidney disease (21). ASA might be worthy of formal study in the transplant population yet is already well established for primary and secondary prevention in other populations (22,23). βblockers have also not been studied rigorously in the kidney transplant population, but are of proven benefit after an ischemic cardiac event and in patients with chronic kidney disease undergoing noncardiac surgery (24,25). Given that the patients with diabetes mellitus and prior cardiovascular disease are the ones at highest risk, aggressive traditional risk factor reduction is essential. In this study almost 75% of the patients at high risk were not on all four cardioprotective medications (ACEi/ARB, β-blocker, ASA, and statin) and only slightly more than half were on three of the four medications, implying important potential for improvement (15). In his review, Yusuf estimates that each of the four cardioprotective agents reduces risk independently by 25 to 30% (15). In addition to greater medication use, this study shows that there is considerable room for improvement in the areas of BP and lipid control (46% of the population had a systolic BP >130 mmHg and 65% had LDL cholesterol >2.5 mmol/L).
Achieving cardiovascular risk targets and medication retention are important concerns. Although patient reluctance, medication cost, and side effects are important barriers, there still is a significant gap in physician prescribing in our transplant population (13). Clinical inertia is the failure to intensify or add medications when indicated and this has been demonstrated to be quite high for hypertension in our kidney transplant population. In only 36% of clinic encounters did physicians adjust medications to reduce the BP in patients presenting with elevated BP (26). This phenomena is well described in the general population in which rates of clinical inertia remain very high (27). There may be strategies to improve clinical inertia in the clinic by increasing BP measurement certainty, but this requires further rigorous study (26). We have also shown that although patients are prescribed cardioprotective medications, the drugs are stopped over time in a significant proportion of patients (28). For example, in our kidney transplant population over a 2-yr period, 66 new patients were started on a statin; however, 19 patients previously on the medication had their statin stopped, often for reasons that were not clear. Poor adherence to statins, β-blockers, and ACE inhibitors in patients suffering their first cardiac event has also been reported in the nontransplant population, where discontinuation rates approach 40% within the first year of follow-up (29). Strategies to improve therapeutic intervention and improve retention deserve further study.
The study confirmed that patients treated with tacrolimus have better cardiovascular risk profiles compared with cyclosporine-treated patients (30,31,32). Cyclosporine patients in our study had higher cholesterols and systolic BP, as expected. In addition, cyclosporine patients had poorer renal function and lower hemoglobin levels. Despite these differences, tacrolimus-treated patients did not have fewer cardiac events. One plausible explanation was that new-onset diabetes mellitus in the tacrolimus-treated patients was not only more frequent but also more malignant than the same disease process in cyclosporine-treated patients and therefore responsible for the excess events. However, there were in fact fewer events in the subgroup of tacrolimus-treated, new-onset diabetic patients. Patients on cyclosporine were more likely to receive ACEi/ARBs and ASA. These may have afforded some additional protection not detected by BP and cholesterol levels. Given that there were no differences when all events were considered, the significant finding of fewer hard cardiac endpoints in cyclosporine-treated patients may simply be a type 1 statistical error. In a randomized trial in patients with chronic allograft nephropathy, Waid found fewer cardiovascular events in patients converted to tacrolimus compared with those remaining on tacrolimus (32). However a breakdown into the types of events was not provided in their report. Nonetheless, the implication of our findings is that conversion to tacrolimus to reduce cardiovascular risk may not readily translate into a reduction in observed events. On the other hand, relatively more cardioprotective medications will be needed in cyclosporine-treated patients.
In keeping with other reports, CHF was a frequent event. Rigatto et al. reported high de novo CHF rates in their kidney transplant population (33). Significant predictors were age, sex, diabetes mellitus, BP, and anemia. Surprisingly, in our cohort the Framingham cardiac and stroke risk scores were predictive of CHF events to a greater extent than ischemic events. Because most of the predictors in the Rigatto study are also variables in the risk equations, and most of the patients (21 of 31) who developed CHF in our study had prior cardiovascular events, the observation that the risk scores predicted CHF should not be unexpected. Although the scores were not designed to predict CHF events, they may help predict those most likely to develop CHF and therefore are of added clinical utility in the transplant clinic.
The limitations of this study are the relatively small sample size and small number of major cardiac and stroke events. This limited our ability to test individual traditional or nontraditional risk factors and to consider a new prediction model. Others are in the process of developing a kidney transplant-specific instrument, but this will require large external samples for validation (34). The Framingham risk equation was also developed and validated for those without prior cardiac disease. The computerized software that was used in this study is proprietary, well validated for patients without prior cardiac history, but less well validated in those with a prior cardiac history (18). The presence of more overt disease and possibly a greater prevalence of covert disease in chronic kidney disease populations may be the reasons why cardiac events are not as easily predicted and are underestimated in this population. In addition, our study endpoints are not exactly the same as the original Framingham population endpoints. In our study, as well as the other kidney transplant reports, angina alone was not used as an endpoint (3) nor were follow-up electrocardiograms evaluated for the presence of silent myocardial infarction (3,4). Study endpoints have also differed in re-evaluations of the Framingham risk score in the general population studies referred to earlier (5,19). The risk calculator assumed that the combined endpoints of fatal and nonfatal coronary artery disease and revascularization would be equivalent to the Framingham endpoints of fatal and nonfatal myocardial infarction, angina, and coronary insufficiency (18). The study also used prevalent patients >6 mo post-transplantation rather than incident patients and there may be a survival bias for patients transplanted many years earlier. However, the study by Meier-Kriesche et al. showed that event rates are fairly stable after 6 mo (35). The concept that the four cardioprotective medications will independently improve cardiac outcomes in the transplant population is also not known (15). Ideally, examining differences between calcineurin inhibitors is best dealt with in a de novo randomized clinical trial. A meta-analysis of past studies (if the data are available) or an analysis of cardiovascular outcomes from the DIRECT trial would be more informative and unbiased (36).
This study shows that the Framingham risk score is inadequate for our population. Nonetheless, patients at the highest risk are easily identified (diabetes mellitus and prior cardiac disease). Although this suggests that pretransplant care in this subpopulation may be deficient and that future study of novel biomarkers will be needed, we are still left with improving the outcomes of those presently in our clinics. Greater attention to cardiovascular risk factor control and increased use of cardioprotective medication may provide substantial benefit to our recipients.
Disclosures
None.
Acknowledgments
Funding for the study was provided in part by Astellas Pharma Canada, Inc. The results of this study were presented in part at the American Society of Nephrology Annual Meeting in San Francisco, CA, November 2 to November 5, 2007.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK: Long-term survival in renal transplant recipients with graft function. Kidney Int 57: 307–313, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ: Cardiovascular disease after renal transplantation. J Am Soc Nephrol 7: 158–165, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Chakkera HA, Roel J: Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol 11: 1735–1743, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Ducloux D, Kazory A, Chalopin JM: Predicting coronary heart disease in renal transplant recipients: A prospective study. Kidney Int 66: 441–447, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ: The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol 50: 217–224, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Cheung AK, Sarnak MJ, Yan G, Dwyer JT, Heyka RJ, Rocco MV, Teehan BP, Levey AS: Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int 58: 353–362, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Longenecker JC, Coresh J, Powe NR, Levey AS, Fink NE, Martin A, Klag MJ: Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: The CHOICE study. J Am Soc Nephrol 13: 1918–1927, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Roberts MA, Hare DL, Ratnaike S, Ierino FL: Cardiovascular biomarkers in CKD: Pathophysiology and implications for clinical management of cardiac disease. Am J Kidney Dis 48: 341–360, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D’Agostino RB, Vasan RS: Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 355: 2631–2639, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Wilson PW, Nam BH, Pencina M, D’Agostino RB Sr, Benjamin EJ, O’Donnell CJ: C-reactive protein and risk of cardiovascular disease in men and women from the Framingham Heart Study. Arch Intern Med 165: 2473–2478 2005 [DOI] [PubMed] [Google Scholar]
- 11.May A, Wang TJ: Evaluating the role of biomarkers for cardiovascular risk prediction: Focus on CRP, BNP and urinary microalbumin, Expert Rev Mol Diagn 7: 793–804, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Folsom AR, Chambless LE, Ballantyne CM, Coresh J, Heiss G, Wu KK, Boerwinkle E, Mosley TH Jr, Sorlie P, Diao G, Sharrett AR: An assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: The atherosclerosis risk in communities study. Arch Intern Med 166: 1368–1373, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kiberd B, Keough-Ryan T, Panek R: Cardiovascular disease reduction in the outpatient kidney transplant clinic. Am J Transplant 3: 1393–1399, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Bostom AD, Brown RS Jr, Chavers BM, Coffman TM, Cosio FG, Culver K, Curtis JJ, Danovitch GM, Everson GT, First MR, Garvey C, Grimm R, Hertz MI, Hricik DE, Hunsicker LG, Ibrahim H, Kasiske BL, Kennedy M, Klag M, Knatterud ME, Kobashigawa J, Lake JR, Light JA, Matas AJ, McDiarmid SV, Miller LW, Payne WD, Rosenson R, Sutherland DE, Tejani A, Textor S, Valantine HA, Wiesner RH: Prevention of post-transplant cardiovascular disease: Report and recommendations of an ad hoc group. Am J Transplant 2: 491–500, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S: Two decades of progress in preventing vascular disease. Lancet 360: 2–3, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Opelz G, Döhler B: Collaborative Transplant Study. Improved long-term outcomes after renal transplantation associated with blood pressure control. Am J Transplant 5: 2725–2731, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Kasiske BL, Anjum S, Shah R, Skogen J, Kandaswamy C, Danielson B, O'shaughnessy EA, Dahl DC, Silkensen JR, Sahadevan M, Snyder JJ: Hypertension after kidney transplantation. Am J Kidney Dis 43: 1071–1081, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Hingorani AD, Vallance P: A simple computer program for guiding management of cardiovascular risk factors and prescribing. BMJ 318: 101–105, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brindle P, Beswick A, Fahey T, Ebrahim S: Accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: A systematic review. Heart 92: 1752–1759, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holdas H, Fellström B, Cole E, Nyberg G, Olsson AG, Pedersen TR, Madsen S, Grönhagen-Riska C, Neumayer HH, Maes B, Ambühl P, Hartmann A, Staffler B, Jardine AG: Assessment of LEscol in Renal Transplantation (ALERT) Study Investigators. Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: The ALERT extension study. Am J Transplant 5: 2929–2936, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Knoll GA, Cantarovitch M, Cole E, Gill J, Gourishankar S, Holland D, Kiberd B, Muirhead N, Prasad R, Tibbles LA, Treleaven D, Fergusson D: The Canadian ACE-inhibitor trial to improve renal outcomes and patient survival in kidney transplantation study design. Nephrol Dial Transplant 23: 354–358, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Collaborative Group of the Primary Prevention Project. Low dose aspirin and vitamin E in people at cardiovascular risk: A randomized trial in general practice. Lancet 357: 89–95, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Antiplatelet Trialists’ Collaboration: Secondary prevention of vascular disease by prolonged antiplatelet treatment. BMJ 296: 320–331, 1988 [PMC free article] [PubMed] [Google Scholar]
- 24.Freemantle N, Cleland J, Young P, Mason J, Harrison J: Beta blockade after myocardial infarction: Systematic review and meta regression analysis. BMJ 318: 1730–1737, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welten GM, Chonchol M, Hoeks SE, Schouten O, Bax JJ, Dunkelgrün M, van Gestel YR, Feringa HH, van Domburg RT, Poldermans D: Beta-blockers improve outcomes in kidney disease patients having noncardiac vascular surgery. Kidney Int 72: 1527–1534, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kiberd J, Panek R, Kiberd B: Strategies to reduce clinical inertia in hypertensive kidney transplant recipients. BMC Nephrol 8: 10, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okonofua EC, Simpson KN, Jesri A, Rehman SU, Durkalski VL, Egan BM. Therapeutic inertia is an impediment to achieving the Healthy People 2010 blood pressure control goals. Hypertension 47: 345–351, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kiberd B, Keough-Ryan T, Kiberd M, Panek R: Cardiovascular risk reduction in the transplant clinic: Running harder to stay in the same place [Abstract]. J Am Soc Nephrol 15: 878A, 2004 [Google Scholar]
- 29.Blackburn DF, Dobson RT, Blackburn JL, Wilson TW, Stang MR, Semchuk WM: Adherence to statins, beta-blockers and angiotensin-converting enzyme inhibitors after a first cardiovascular event: A retrospective cohort study. Can J Cardiol 21: 485–488, 2005 [PubMed] [Google Scholar]
- 30.Krämer BK, Montagnino G, Del Castillo D, Margreiter R, Sperschneider H, Olbricht CJ, Krüger B, Ortuño J, Köhler H, Kunzendorf U, Stummvoll HK, Tabernero JM, Mühlbacher F, Rivero M, Arias M: European Tacrolimus vs Cyclosporin Microemulsion Renal Transplantation Study Group. Efficacy and safety of tacrolimus compared with cyclosporin A microemulsion in renal transplantation: 2-year follow-up results. Nephrol Dial Transplant 20: 968–973, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, Moons P, Borm G, Hilbrands LB: Conversion from cyclosporine to tacrolimus improves quality-of-life indices, renal graft function and cardiovascular risk profile. Am J Transplant 4: 937–945, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Waid T: CRAF Study Group. Tacrolimus as secondary intervention vs. cyclosporine continuation in patients at risk for chronic renal allograft failure. Clin Transplant 19: 573–580, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Rigatto C, Parfrey P, Foley R, Negrijn C, Tribula C, Jeffery J: Congestive heart failure in renal transplant recipients: risk factors, outcomes, and relationship with ischemic heart disease. J Am Soc Nephrol 13: 1084–1090, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Fellstrom BC, Holdas H, Jardine A, Holme I: Cardiovascular risk calculator in renal transplantation [Abstract]. J Am Soc Nephrol 18: 434, 2007 [Google Scholar]
- 35.Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B: Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant 4: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Vincenti F, Tuncer M, Castagneto M, Klinger M, Friman S, Scheuermann EH, Wiecek A, Russ GR, Martinek A, Nonnast-Daniel B; DIRECT Study Group. Prospective, multicenter, randomized trial to compare incidence of new-onset diabetes mellitus and glucose metabolism in patients receiving cyclosporine microemulsion versus tacrolimus after de novo kidney transplantation. Transplant Proc 37: 1001–1004, 2005 [DOI] [PubMed] [Google Scholar]