Abstract
Background and objectives: Advanced glycation end products, known pro-inflammatory and pro-oxidative compounds that accumulate in patients with chronic kidney disease, may play a major role in their high prevalence of endothelial dysfunction and subsequent cardiovascular disease. This study examined the association of advanced glycation end product accumulation with cellular receptor for advanced glycation end product expression and endothelial dysfunction as well as the mechanisms of this association in chronic kidney disease.
Design, setting, participants, & measurements: A cross-sectional study was conducted of ambulatory patients without diabetes and with different stages of chronic kidney disease (n = 51), compared with gender- and age-matched healthy subjects. Fasting blood was obtained for measurement of advanced glycation end products and mRNA receptor for advanced glycation end product expression in peripheral blood mononuclear cells. Endothelial reactivity was assessed by the microcirculatory response to local ischemia (postocclusive reactive hyperemia) and local hyperthermia (thermal hyperemia). Sera were pooled and passed through affinity columns to separate advanced glycation end product–rich fractions, which were incubated with human aortic endothelial cells, with or without blockade of receptor for advanced glycation end product, to measure their effect on endothelial nitric oxide synthase.
Results: Glomerular filtration rate correlated with serum advanced glycation end product, mRNA receptor for advanced glycation end product levels, postocclusive reactive hyperemia, and thermal hyperemia. Serum advanced glycation end product correlated with receptor for advanced glycation end product and inversely with postocclusive reactive hyperemia. Advanced glycation end product–rich fractions from chronic kidney disease sera suppressed endothelial nitric oxide synthase expression of human aortic endothelial cells compared with sera from healthy subjects, an effect abrogated by receptor for advanced glycation end product blockade.
Conclusions: This study demonstrates for the first time an association of excess advanced glycation end product burden with increased peripheral blood mononuclear cell mRNA receptor for advanced glycation end product and in vivo endothelial dysfunction in patients with chronic kidney disease. Endothelial dysfunction in chronic kidney disease may be partly mediated by advanced glycation end product–induced inhibition of endothelial nitric oxide synthase through receptor for advanced glycation end product activation.
The epidemiologic association between chronic kidney disease (CKD) and cardiovascular disease (CVD) has been firmly established (1–3). Traditional CVD risk factors such as hypertension and hyperlipidemia do not fully explain the high prevalence of CVD in CKD (1). The recognition that inflammation, oxidative stress (OS), and endothelial dysfunction (ED) are common abnormalities in CVD has led to a detailed exploration of these pathways in search of new risk factors (4–8). Because renal failure is associated with increasing circulating levels of advanced glycation end products (AGE) (9) and these compounds are known to promote inflammation, OS, and ED, they are likely to play an important role in the pathogenesis of CVD in this population (10–12).
Numerous in vitro and animal studies have implicated AGE in the pathogenesis of CVD (10–12) via several receptor-independent and receptor-dependent mechanisms. For instance, AGE have direct influence on the structural integrity of the vessel wall and the underlying basement membranes in part induced by cross-linking of subendothelial matrix molecules, such as collagen, or by disruption of matrix–matrix and matrix–cell interactions (13,14). Other actions of AGE include nitric oxide (NO) quenching (15) and endothelial NO synthase (eNOS) inhibition (16), thereby adversely affecting vascular endothelium and its protective functions, particularly vascular relaxation. The direct mechanistic links between AGE and their endothelial effects have not been clearly delineated but may include enhanced expression and activity of receptor for AGE (RAGE) (17). In fact, expression of RAGE on peripheral blood monocytes has been found to be upregulated in patients without diabetes and with CKD (18).
The link among OS, inflammation, and ED as a new paradigm of atherosclerosis provides the rationale for a variety of studies, including flow-mediated vasodilation and reactive hyperemia of the microcirculation, gauging endothelial function as an index of the susceptibility of the vasculature to atherosclerosis (19–26). This noninvasive interrogation of the cutaneous microvasculature responses to both flow and thermal stimulations may offer a simple screening test for the presence of systemic ED (26). ED, defined by these noninvasive vascular tests, is a common finding in patients with CKD (22–25) and could reflect reduced NO generation (27) or impaired activity (16). Possible causes of NO deficiency are substrate (l-arginine) limitation as a result of perturbed renal biosynthesis of this amino acid or increased levels of circulating endogenous inhibitors of eNOS, such as asymmetric dimethylarginine, homocysteine, or AGE (26). Sera from patients with CKD as well as in vitro prepared AGE significantly suppress eNOS activity in cultured vascular endothelial cells (16,28), suggesting that the excess AGE in CKD may contribute to their ED.
Notwithstanding all of the experimental data suggesting a major role for AGE in causing ED/CVD, the few human studies correlating serum AGE levels with cardiovascular outcome have given conflicting results. Two studies of hemodialysis patients who were followed for a variable period of time demonstrated that serum AGE levels were strong and independent predictors of mortality (29,30). Conversely, a study from Germany showed that high serum AGE levels at baseline were associated with better outcome in hemodialysis patients who were followed for 32 mo (31) and a group in Sweden showed that plasma pentosidine did not predict outcome of a group of patients who had ESRD and were identified close to the start of renal replacement therapy (32)
Despite the long-term postulation that AGE excess could lead to ED in CKD, no study has specifically described the relationship between circulating AGE levels and in vivo tests of endothelial function in these patients; therefore, we performed a study to examine this relationship in a group of patients with CKD and measured two widely known circulating AGE (ɛN-carboxymethyl-lysine [CML] and methyl-glyoxal [MG] derivatives) and the in vivo arteriolar vasodilatory response to both local ischemia and hyperthermia in a cohort of patients without diabetes and with CKD. Furthermore, we wanted to test whether the observed eNOS impairment in CKD could result from the excess of circulating AGE and whether this effect was mediated via RAGE. For this, we evaluated the effect of AGE from serum of patients with CKD on the eNOS expression of human aortic endothelial cells (HAEC) with or without blockade of RAGE expression. We also measured RAGE expression in peripheral mononuclear cells as a surrogate indicator of RAGE expression in endothelial cells. The findings suggest that CKD-related ED is due partly to excess accumulation of AGE, which activate RAGE to suppress eNOS in the vasculature.
Materials and Methods
Adult patients (n = 51) with CKD, stages 1 through 5, were recruited from the renal clinic of the Mount Sinai Medical Center (mean age 56.7 ± 16 yr [SD]; weight 74.4 ± 17 kg; and body mass index 27 ± 5.6). Patients with known diabetes or inflammatory/autoimmune diseases (e.g., lupus) were excluded. CKD was staged 1 through 5 according to Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines by using the Modification of Diet in Renal Disease (MDRD) GFR equation (33,34). Most patients were taking antihypertensive medications as well as other commonly used drugs in CKD, such as potassium binders, diuretics, iron replacement, and vitamin supplementation. Many patients were taking medications that could potentially affect endothelial function, including β blockers (n = 18 patients), calcium channel blockers (n = 21), angiotensin-converting enzyme inhibitors (n = 20), angiotensin receptor blockers (n = 10), and statins (n = 15). Detailed medical history, demographic data, a 3-d food record to estimate daily dietary AGE intake (35), a 24-h urine collection, and fasting blood samples were obtained and a noninvasive vascular test was performed (described in “Microcirculation Skin Blood Flow in Response to Ischemia and Hyperthermia” below). The Mount Sinai School of Medicine institutional review board approved the study protocol and informed consent.
Cause of CKD was determined by medical history and chart review and included 18 (35%) patients with hypertensive nephrosclerosis; 10 (20%) with FSGS; three (6%) with adult polycystic kidney disease; three (6%) with IgA nephropathy; six (12%) with chronic interstitial nephritis; three (6%) with chronic glomerulonephritis; four (8%) with unknown cause; and four (8%) each with membranous nephropathy, renal artery stenosis, minimal-change disease, and Alport syndrome. Data from a pool of healthy male and female subjects (n = 51), matched for gender and age (within ±4 yr) from another study (35), were used as control subjects.
Microcirculation Skin Blood Flow in Response to Ischemia and Hyperthermia
Measurement of microcirculatory blood flow was performed using Laser Doppler (PeriMed Corp., Stockholm, Sweden) (25,36,37) performed in the morning with participants fasting and resting in a comfortable chair. We measured reactive hyperemia in response to both transient ischemia and local heating.
After baseline recording for 2 min, transient ischemia was induced by using an occlusion cuff placed around the upper arm and inflating it to 20 mmHg above systolic BP for 3 min. The cuff was then rapidly released, and after 1 min, 2 min of recording was made. Postocclusive reactive hyperemia (PORH) was estimated from the percentage of increase in blood flow after hyperemia, compared with baseline values.
After 5 min of rest following PORH, a new baseline blood flow was recorded for 2 min. A special probe was heated to 43°C for the next 2 min over the skin, and recordings of the blood flow were made immediately after. Thermal hyperemia (TH) was estimated from the percentage increase of flow blood after hyperthermia, compared with baseline values.
Blood and Urinary Tests
Routine serum and urinary chemistry values were measured in the Mount Sinai Medical Center clinical laboratory. We measured two forms of circulating AGE: Serum total CML (sCML) and serum MG (sMG) derivatives. Serum samples were tested for CML (4G9 mAb; Alteon, Northvale, NJ) and for MG derivatives (MG3D11 mAb) by ELISA, as described previously (38). Plasma 8-isoprostane and vascular cellular adhesion molecule (VCAM-1) were measured using commercial ELISA kits (35). High-sensitivity C-reactive protein (hsCRP) was measured using nephelometry.
RAGE mRNA Expression in Peripheral Blood Mononuclear Cells
Peripheral blood mononuclear cells (PBMC) from 30-ml EDTA anticoagulated blood were isolated by Ficoll-Paque Plus gradient (American Biosciences, Uppsala, Sweden). Total RNA was extracted by Trizol (Molecular Probes, Eugene, OR). The extracted RNA had an OD280/260 ratio between 1.8 and 2.0. The total RNA was then reverse-transcribed using Superscript III RT (Invitrogen, Carlsbad, CA). Quantitative SYBR Green real-time PCR assay (39) was performed to measure expression of mRNA for RAGE. Briefly, 5 μl of template cDNA was added to a final volume of 10 μl containing 1× SYBR Green PCR master mix and 10 μM of the primers. Amplification was performed with 40 cycles of denaturation at 95°C for 15 s, annealing at 55°C for 20 s, and elongation at 72°C for 30 s. Sequences of the primers used for real-time PCR were as follows: Forward primer 5′-AGGAGCGTGCAGAACTGAAT-3′ and reverse primer 5′-TTGGCAAGGTGGGGTTATAC-3′. During thermal cycling, emission from each sample was recorded, and SDS software processed the raw fluorescence data to produce threshold cycle (Ct) values for each sample. The housekeeping genes β-actin and glyceraldehyde-3-phosphate dehydrogenase were used for internal normalization. The transcript copy number of target gene was determined on the basis of their Ct values (40).
In Vitro Tests
Isolation of AGE-Modified Serum Proteins by an AGE-Affinity Matrix.
Sera of patients with CKD stages 2, 3, and 5, as defined by K/DOQI guidelines (33), as well as sera from healthy subjects were obtained and pooled separately. AGE were fractionated from serum with the use of a Lysozyme (LZ)-linked matrix column as described by Mitsuhashi et al. (41). The LZ-linked column was prepared by conjugating LZ, a known AGE-binding protein, to cyanogen bromide-activated Sepharose 4B beads according to the manufacturer's instructions. A control column was prepared by conjugating BSA to Sepharose 4B beads following the same procedure. One-milliliter samples of sera were diluted 1:5 with PBS just before loading on LZ columns (bed volume 2 ml). After loading the diluted serum samples and collecting pass-through fractions, LZ columns were extensively washed with PBS. The LZ-bound fractions (0.5 ml per fraction) were eluted with 0.1 N NaOH and immediately neutralized with HCl.
Endothelial Cell Culture.
HAEC were purchased from ScienCell Research Laboratories (San Diego, CA). Cells were grown in endothelial cell medium with endothelial cell growth supplement and 5% FBS (ECM; ScienCell) at 37°C in 5% CO2. HAEC from passages 5 through 8 were used. These cells were incubated for 30 min, 24 h, or 48 h with the AGE-enriched fractions derived from serum described previously (50 μg protein/ml).
Transfection of Small Interfering RNA against RAGE in Endothelial Cells
Endothelial cells were transiently transfected using the Amaxa Nucleofection technology (Amaxa Biosystems, Gaithersburg, MD) (42). After optimization, we were able to achieve 70 to 80% transfection efficiency based on green fluorescence protein expression using Amaxa's Nucleofection kit and Program specific for primary culture of endothelial cells. We used a technique combining the Dharmacon (Chicago, IL) On Target plus SMARTpool small interfering (siRNA) and Amaxa RNAi Nucleofection kit to introduce siRNA successfully into endothelial cells. Two million cells grown to 70 to 80% confluence and 1.5 μg of siRNA were used for each nucleofection reaction. Cell survival after transfection was approximately 50 to 60%. Transfected endothelial cells were cultivated for 72 h. miRIDIAN microRNA Mimic Negative Control (CL) sequence based on Caenorhabditis elegans miRNA from Dharmacon Corp. was used as a negative experimental control in mammalian cells (5′-UCACAACCUCCUAGAAAGAGUAGA-3′). The specific siRNA targeting RAGE were purchased from Dharmacon Corp. (Dharmacon On Target plus SMARTpool siRNA). RAGE expression was reduced by 70 to 80% on the basis of Western blot analysis.
Transfected endothelial cells were cultured for 48 h and then incubated with serum-free medium for 12 h. Cells were stimulated with LZ-isolated AGE from normal serum or serum from CKD for 24 h at 37°C (50 μg protein/ml).
Western Blot
Endothelial cells were lysed with a buffer that contained 1% NP40, a protease inhibitor cocktail, and tyrosine and serine-threonine phosphorylation inhibitors. After determination of protein concentration, cell lysates were subjected to 8 to 12% SDS-PAGE before transfer to polyvinylidene difluoride membranes. Immunoblottings were performed using anti-eNOS (BD Biosciences Transduction Laboratories, San Jose, CA), anti-RAGE (Sigma, St. Louis, MO), or anti β-actin (Sigma) antibodies.
Statistical Analyses
The Kolmogorov-Smirnov goodness-of-fit test was used to determine whether a variable had a normal distribution. Because hsCRP had a skewed distribution, its values were used log-transformed in the analyses. Data are presented as means ± SD, except for hsCRP, which is reported as median and range in Table 1. Differences of means between groups were analyzed by unpaired t test or ANOVA (followed by Bonferroni correction for multiple comparisons), depending on the number of groups. Correlation analyses between the different variables were examined by Pearson correlation coefficient. Multiple regression analysis was performed to examine the variables that were independently associated with GFR, serum AGE, RAGE, and measures of endothelial reactivity. Significant differences were defined as those at P < 0.05 and were based on two-sided tests. Data analysis used the SPSS 14.0 for Windows (SPSS, Chicago, IL).
Table 1.
Circulating levels of AGE, markers of inflammation and oxidative stress, and tests of endothelial function in patients with CKD and healthy control subjectsa
| Parameter | CKD (n = 51) | Control (n = 51) | P |
|---|---|---|---|
| Age (yr) | 57 ± 16 | 58 ± 14 | NS |
| Weight (kg) | 74 ± 17 | 72 ± 15 | 0.862 |
| Dietary AGE (AGE Eq/d) | 13 ± 6 | 15 ± 9 | 0.456 |
| GFR (ml/min per 1.73 m2) | 41 ± 36 | 116 ± 44 | 0.000 |
| sCML (U/ml) | 19 ± 12 | 11 ± 4 | 0.004 |
| sMG (pmol/ml) | 2.0 ± 0.3 | 0.8 ± 0.3 | 0.000 |
| hsCRP (mg/L) | 8 ± 11 | 3 ± 5 | 0.032 |
| sVCAM-1 (ng/ml) | 1121 ± 303 | 809 ± 237 | 0.008 |
| 8-isoprostane (ng/ml) | 317.0 ± 129.5 | 222.0 ± 82.0 | 0.000 |
| RAGE (mRNA copy number) | 706 ± 382 | 399 ± 318 | 0.000 |
| PORH (%) | 261 ± 163 | 385 ± 186 | 0.007 |
| TH (%) | 832 ± 546 | 984 ± 352 | 0.172 |
AGE, advanced glycation end products; CKD, chronic kidney disease; hsCRP, high-sensitivity C-reactive protein; PORH, postocclusive reactive hyperemia; RAGE, receptor for AGE; sCML, serum εN-carboxymethyl-lysine; sMG, serum methyl-glyoxal; sVCAM-1, serum vascular cellular adhesion molecule-1; TH, thermal hyperemia
Results
Circulating AGE and PBMC RAGE mRNA Expression Are Increased in CKD
As previously noted, patients with CKD had significantly higher levels of circulating AGE (sCML and sMG), hsCRP, VCAM-1, and 8-isoprostane, as compared with healthy subjects (Table 1). In addition, PBMC RAGE mRNA expression was significantly higher in patients with CKD, compared with healthy subjects (P = 0.000).
The average daily dietary AGE intake in patients with CKD was not significantly different from that in healthy control subjects (Table 1). Moreover, dietary AGE intake was not associated with any of the other parameters measured (data not shown).
Endothelial Reactivity Is Impaired in CKD
The in vivo endothelial reactivity was assessed by response to both ischemia (PORH) and hyperthermia (TH) and found to be decreased in patients with CKD, as compared with healthy control subjects, although only PORH differences reached statistical significance (Table 1). When patients with CKD and healthy control subjects were analyzed together and separated by MDRD GFR above or below 80 ml/min per 1.73 m2, the differences in endothelial reactivity became very pronounced. Individuals with GFR >80 ml/min per 1.73 m2 had significantly higher PORH (423 ± 189) and TH (1383 ± 558) than those with GFR <80 ml/min per 1.73 m2 (250 ± 165; P = 0.004 and 712 ± 366; P = 0.001, respectively). Age had a significant effect on both PORH (r = −0.378, P = 0.014) and TH (r = −0.466, P = 0.002), but gender did not.
Decreased GFR Is Associated with Increased AGE, Increased PBMC RAGE mRNA, and Decreased Endothelial Reactivity in CKD
GFR was assessed both by measuring endogenous creatinine clearance and by the MDRD GFR equation (34). The correlation between both methods was excellent (r = 0.92, P = 0.000), and all of the subsequent analyses are reported for MDRD GFR.
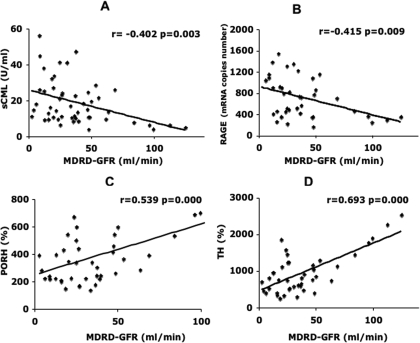
In patients with CKD, GFR correlated inversely with sCML (r = −0.402, P = 0.003; Figure 1A) and with PBMC RAGE mRNA (r = −0.415, P = 0.009; Figure 1B). GFR correlated directly and significantly with the two in vivo measures of endothelial reactivity, response to ischemia (PORH; r = 0.539, P = 0.000; Figure 1C) and response to hyperthermia (TH; r = 0.693, P = 0.000; Figure 1D). No significant correlation was found between GFR and circulating levels of VCAM-1, hsCRP, and 8-isoprostanes among patients with CKD.
Figure 1.
Decreased GFR is associated with increased circulating advanced glycation end products (AGE), increased peripheral blood mononuclear cell (PBMC) receptor for AE (RAGE) mRNA, and decrease endothelial reactivity. (A and B) Relationship between Modification of Diet n Renal Disease (MDRD) GFR and serum ɛN-carboxymethyl-lysine (sCML; A) and RAGE (B). (C and D) Relationship between MDRD GFR and postocclusive reactive hyperemia (PORH; C) and thermal hyperemia (TH; D), both indices of endothelial reactivity. All correlation analyses were performed using Pearson coefficient.
Circulating AGE Correlate Directly with PBMC RAGE mRNA Expression and Inversely with Endothelial Reactivity in CKD
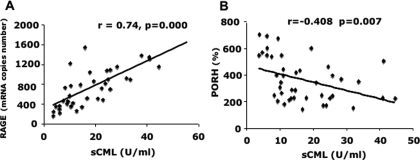
In patients with CKD, circulating CML levels correlated directly with RAGE mRNA (r = 0.737, P = 0.00; Figure 2A), sMG (r = 0.374, P = 0.016), 8-isoprostane (r = 0.419, P = 0.005), and VCAM-1 (r = 0.379, P = 0.013) and inversely with PORH (r = −0.408, P = 0.007; Figure 2B). A trend of an inverse correlation between sCML and TH was also noted (r = −0.285, P = 0.067). In a stepwise multiple regression analysis including sCML, GFR, age, and PORH, GFR was found to be an independent determinant of PORH. RAGE mRNA levels were found to correlate negatively with PORH (r = −0.334, P = 0.046) and TH (r = −0.332, P = 0.048). In a stepwise multiple regression analysis including RAGE, sCML, and PORH, sCML was found to be an independent determinant of PORH.
Figure 2.
Increased circulating AGE are associated with increased RAGE and decreased in vivo endothelial reactivity. (A) Relationship between sCML and RAGE. (B) Relationship between sCML and PORH. All correlation analyses were performed using Pearson coefficient.
AGE Isolated from CKD Sera Suppress eNOS Protein Expression in HAEC
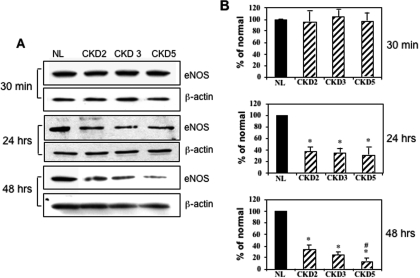
To determine whether there was a direct relationship between excess circulating AGE levels and impaired endothelial reactivity, we studied the effect of AGE-rich fractions from the sera of patients with CKD or healthy subjects on eNOS expression of HAEC. eNOS expression was not affected at 30 min of incubation but was markedly inhibited at 24 and 48 h of incubation with AGE-rich fractions from pooled sera of patients with CKD stages 2, 3, and 5, compared with pooled sera from healthy subjects (Figure 3A). Figure 3B shows the corresponding quantitative plots of these blots (each one is an average of three experiments). After 48 h of incubation, sera that were obtained from patients with CKD stage 5 showed significantly more eNOS expression inhibition than sera from patients with CKD stage 2 (Figure 3).
Figure 3.
Effect of AGE-rich extracts from serum of patients with chronic kidney disease (CKD) on cultured human aortic endothelial cells (HAEC). HAEC were incubated with AGE that were isolated from normal individuals (NL) or patients with CKD stage 2, 3, or 5 for 30 min, 24 h, and 48 h (50 μg protein/ml). After stimulation, cells were lysed for Western blot analysis using anti–endothelial nitric oxide synthase (anti-eNOS) antibody and normalized with β-actin. The representative Western blots of three independent experiments are shown in A, and the corresponding quantitative plots are shown in B. *Significantly different from normal; #significantly different from CKD2.
AGE-Induced Suppression of eNOS Expression Is Prevented by Blockade of RAGE Expression
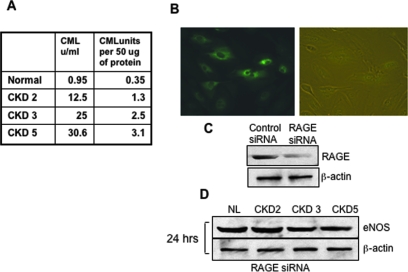
Figure 4A shows the concentration of AGE (CML) in each one of the AGE fractions used. To test whether AGE-induced eNOS suppression was mediated via RAGE, we studied the effect of the same AGE-rich fractions as described already on eNOS expression of HAEC transfected with siRNA against RAGE after incubation for 24 h. Figure 4B shows representative photographs of pmaxGFP-transfected HAEC under light and epifluorescence microscopy. Figure 4C shows Western blots documenting RAGE protein expression in HAEC transfected with either control siRNA or siRAGE. The previously noted eNOS inhibition at 24 h (Figure 3A) was almost completely abrogated by blockade of RAGE expression (Figure 4D), indicating that RAGE contributed significantly to this effect.
Figure 4.
AGE-induced suppression of eNOS expression is prevented by blockade of RAGE expression. (A) This table shows the concentration of AGE, measured as CML, in each of the fractions used. (B) Representative photographs of pmaxGFP-transfected HAEC under light and epifluorescence microscopy. Transient transfection of endothelial cells with a small interfering RNA (siRNA) for RAGE or the control oligo was carried out using the Amaxa Nucleofection Device (see the Materials and Methods section). Transfection efficiency was measured by the percentage of cells expressing green fluorescence protein (GFP) after transfection with pmaxGFP. (C) Western blot documenting RAGE protein expression in HAEC transfected with either control siRNA or siRAGE. (D) eNOS expression after incubation for 24 h of sera-extracted AGE with HAEC transfected with siRNA (50 μg protein/ml).
Discussion
This study documents for the first time that excess circulating levels of AGE are associated with increased PBMC RAGE mRNA expression and in vivo ED in a cross-section of patients without diabetes and with CKD. Moreover, AGE-rich fractions isolated from the serum of these patients markedly inhibited eNOS expression in cultured HAEC, an effect largely prevented by blockade of RAGE expression. Thus, the progressive accumulation of AGE as renal function deteriorates may play a major role in the ED preceding CVD in patients with CKD.
Because ED is an early marker of atherosclerosis, these findings may provide a causal mechanism linking the excess body AGE burden and the high prevalence of CVD in patients with CKD, although the presence of other mechanisms cannot be excluded. AGE have previously been shown to have significant direct effects on endothelial cells, including increased expression and release of VCAM-1 (17,43), tissue factor (44), and a host of procoagulant and vasoactive factors and cytokines (45). A decrease of eNOS activity has been a prominent response to AGE demonstrated both in vitro and in vivo (16,27). Marked impairment in endothelium-dependent vasodilation was prevented by treatment with aminoguanidine, an inhibitor of AGE formation, in streptozotocin-induced diabetic rats and in normal rats that were administered an injection of AGE-albumin (11). Recently, we showed that a single oral AGE load causes significant acute impairment in flow-mediated vasodilation in healthy individuals and patients with diabetes (46); however, the involvement of eNOS was not determined in these studies (46).
We demonstrated that serum-derived AGE caused the inhibition of eNOS on cultured HAEC and that this inhibition involved RAGE, but the exact pathways leading to this effect were not elucidated in this study. Many AGE effects are mediated by RAGE. AGE–RAGE interaction stimulates the production of reactive oxygen species, which in turn activate a range of signaling pathways, including NF-κB that affects the transcriptional activation of numerous cytokines and adhesion molecules, many of which are closely linked to inflammation and atherosclerosis (10,12,47). We are not aware of any studies demonstrating that activation of NF-κB leads to suppression of eNOS. Our data suggest that RAGE activation may favor atherosclerosis not only by mediating inflammation but also by its effect on vascular response through NO production. Whether these two pathways cross-talk cannot be established with these data. The finding of increased RAGE expression in PBMC supports the presence of a state of increased intracellular oxidative stress, already suggested by increased circulating levels of CML and 8-isoprostanes (35). Ideally, we would have liked to measure RAGE expression in endothelial cells derived from our patients, but this is very difficult to do in human studies; therefore, we used PBMC as surrogate indicators of vascular endothelium because both of them express RAGE and are exposed to the same intravascular AGE load.
The association of progressive impairment in renal function with a parallel reduction in endothelium-dependent vasodilation was previously described in patients with CKD (24), but our direct evaluation of cutaneous vasodilation in response to both local ischemia and local hyperthermia adds significant information. We did not find a correlation between levels of VCAM-1, a circulating marker of ED, and PORH or TH, direct test of endothelial function.
The absence of significant changes in markers of inflammation such as hsCRP with the severity of renal function agrees with the work of Oberg et al. (48), but it is in contrast with the findings by several other authors (49,50). Of interest, circulating AGE and PBMC RAGE expression, both “inflammatory factors,” increased with worsening renal function, and further inquiry may support their use as predictors of disease.
We did not observe any association between dietary AGE intake and circulating AGE or vascular reactivity parameters. We previously showed that a low-AGE diet reduces circulating AGE and CRP levels in dialysis patients; therefore, we strongly believe that dietary AGE are major players in the pathogenesis of the increased inflammation/oxidative stress state of CKD (51). The lack of demonstrable effects of dietary AGE on any of the parameters measured in this study may simply reflect the overwhelming effect of CKD on these parameters.
The main limitations of this study are the relatively small number of participants and the limitations inherent to any cross-sectional design, specifically that a single sample at a certain point in time may fail to reflect the natural course of the process being studied. The presence of different comorbid conditions in a CKD population using multiple drugs represents an additional limitation in the interpretation of the data.
Conclusions
We have demonstrated an association among impaired renal function, increased circulating levels of AGE, increased expression of RAGE in PBMC, and decreased endothelial reactivity. AGE-rich extracts from sera of patients with CKD inhibited eNOS expression, an effect in part mediated by RAGE. These findings support the view that as renal disease progresses, excessive AGE are poised to impair endothelial function. Thus, AGE are important mediators in the pathogenesis of CVD in patients with CKD.
Disclosures
None.
Acknowledgments
This work was supported by the National Institute on Aging (MERIT AG-23188 and AG-09453, H.V.) and by the National Institute of Research Resources, MO1-RR-00071, awarded to the General Clinical Research Center at Mount Sinai School of Medicine.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Weiner DE, Tabatabai S, Tighiouart H, Elsayed E, Bansal N, Griffith J, Salem DN, Levey AS: Cardiovascular outcomes and all-cause mortality: Exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis 48: 392–401, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Norlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA: Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 351: 1285–1295, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Ross R: Atherosclerosis: An inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM: The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62: 1524–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Stenvinkel P, Pecoits-Filho R, Lindholm B: Coronary artery disease in end-stage renal disease: No longer a simple plumbing problem. J Am Soc Nephrol 14: 1927–1939, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Kaysen GA: The microinflammatory state in uremia: Causes and potential consequences. J Am Soc Nephrol 12: 1549–1557, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Thambyrajah J, Landray MJ, McGlynn FJ, Jones HJ, Baigent C, Kendall MJ, Townend JN, Wheeler DC: Abnormalities of endothelial function in patients with predialysis renal failure. Heart 83: 205–209, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss MF, Erhard P, Kader-Attia FA, Wu YC, Deoreo PB, Araki A, Glomb MA, Monnier VM: Mechanisms for the formation of glycoxidation products in end-stage renal disease. Kidney Int 57: 2571–2585, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Goldin A, Beckman JA, Schmidt AM, Creager MA: Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 114: 597–605, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, Bucala R: Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: A model for diabetic and aging complications. Proc Natl Acad Sci U S A 89: 12043–12047, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park l, Raman KG, Lee KJ, Lu Y, Ferran LJ, Chow WS, Stern D, Schmidt AM: Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 4: 1025–1031, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka S, Avigad G, Brodsky B, Eikenberry EF: Glycation induces expansion of the molecular packing of collagen. J Mol Biol 203: 495–505, 1988 [DOI] [PubMed] [Google Scholar]
- 14.Haitoglou CS, Tsilibary EC, Brownlee M, Charonis AS: Altered cellular interactions between endothelial cells and nonenzymatically glycosylated laminin/type IV collagen. J Biol Chem 267: 12404–12407, 1992 [PubMed] [Google Scholar]
- 15.Bucala R, Tracey KJ, Cerami A: Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest 87: 432–438, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu B, Chibber R, Ruggiero D, Kohner E, Ritter J, Ferro A: Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. FASEB J 17: 1289–1291, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D: Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice: A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest 96: 1395–1403, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou FF, Ren H, Owen WF, Guo ZJ, Chen PY, Schmidt AM, Miyata T, Zhang X: Enhanced expression of receptor for advanced glycation end products in chronic kidney disease. J Am Soc Nephrol 15: 1889–1896, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Vita JA, Keaney JF Jr: Endothelial function: A barometer for cardiovascular risk? Circulation 106: 640–642, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R, International Brachial Artery Reactivity Task Force: Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilatation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Rundek T, Hundle R, Ratchford E, Ramas R, Sciacca R, Di Tullio MR, Boden-Albala B, Miyake Y, Elkind MS, Sacco RL, Homma S: Endothelial dysfunction is associated with carotid plaque: A cross-sectional study from the population based Northern Manhattan Study. BMC Cardiovasc Disord 6: 35, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dogra G, Irish A, Chan D, Watts G: Insulin resistance, inflammation and blood pressure determine vascular dysfunction in CKD. Am J Kidney Dis 48: 926–934, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Annuk M, Lind L, Linde T, Fellström B: Impaired endothelium-dependent vasodilatation in renal failure in humans. Nephrol Dial Transplant 16: 302–306, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Ghiadoni L, Cupisti A, Huang Y, Mattei P, Cardinal H, Favilla S, Rindi P, Barsotti G, Taddei S, Salvetti A: Endothelial dysfunction and oxidative stress in chronic renal failure. J Nephrol 17: 512–519, 2004 [PubMed] [Google Scholar]
- 25.Kruger A, Stewart J, Sahityani R, O'Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS: Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: Correlation with cardiovascular risk. Kidney Int 70: 157–164, 2006 [DOI] [PubMed] [Google Scholar]
- 26.O'Riordan E, Chen J, Brodsky SV, Smirnova I, Li H, Goligorsky MS: Endothelial cell dysfunction: The syndrome in making. Kidney Int 67: 1654–1658, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Baylis C: Arginine, arginine analogs and nitric oxide production in chronic kidney disease. Nat Clin Pract Nephrol 2: 209–220, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao S, Wagner L, Schmidt RJ, Baylis C: Circulating endothelial nitric oxide synthase inhibitory factor in some patients with chronic renal disease. Kidney Int 59: 1466–1472, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner Z, Molnar M, Molnar GA, Tamasko M, Laczy B, Wagner L, Csiky B, Heidlenad A, Nagy J, Wittmann I: Serum carboxymethyllysine predicts mortality in hemodialysis patients. Am J Kidney Dis 47: 294–300, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Roberts MA, Thomas MC, Fernando D, Macmillan N, Power DA, Ierino FL: Low molecular weight advanced glycation endproducts predict mortality in asymptomatic patients receiving chronic hemodialysis. Nephrol Dial Transplant 21: 1611–1617, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Schwedler SB, Metzger T, Schinzel R, Wanner C: Advanced glycation end products and mortality in hemodialysis patients. Kidney Int 62: 301–310, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Suliman ME, Heimburger O, Barany P, Anderstam B, Pecoits-Filho R, Rodriguez Ayala E, Qureshi AR, Fehrman-Ekholm I, Lindholm B, Stenvinkel P: Plasma pentosidine is associated with inflammation and malnutrition in end-stage renal disease patients starting on dialysis therapy. J Am Soc Nephrol 14: 1614–1622, 2003 [DOI] [PubMed] [Google Scholar]
- 33.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 34.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H: Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress and aging. J Gerontol Med Sci 62: 427–433, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stirban A, Negrean M, Stratmann B, Gawlowski T, Horstmann T, Gotting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J, Vlassara H, Tschoepe D: Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care 29: 2064–2071, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Kubli S, Waeber B, Dalle-Ave A, Feihl F: Reproducibility of laser Doppler imaging of skin blood flow as a tool to assess endothelial function. J Cardiovasc Pharmacol 36: 640–648, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Makita Z, Vlassara H, Cerami A, Bucala R: Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem 267: 5133–5138, 1992 [PubMed] [Google Scholar]
- 39.Watanabe M, Kohdera U, Kino M, Haruta T, Nukuzuma S, Suga T, Akiyoshi K, Ito M, Suga S, Komada Y: Detection of adenovirus DNA in clinical samples by SYBR Green real-time polymerase chain reaction assay. Pediatr Int 47: 286–291, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Valasek MA, Repa JJ: The power of real-time PCR. Adv Physiol Educ 29: 151–159, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Mitsuhashi T, Li YM, Fishbane S, Vlassara H: Depletion of reactive advanced glycation end products from diabetic serum using a lysozyme-linked matrix. J Clin Invest 15: 847–854, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuang PY, Yu Q, Fang W, Uribarri J, He J: Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int 72: 965–976, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai W, He JC, Zhu L, Peppa M, Lu C, Uribarri J, Vlassara H: High levels of dietary advanced glycation end products transform low-density lipoprotein into a potent redox-sensitive mitogen-activated protein kinase stimulant in diabetic patients. Circulation 110: 285–291, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Bierhaus A, Illmer T, Kasper M, Luther T, Quehenberger P, Trischler H, Wahl P, Ziegler R, Muller M, Nawroth PP: Advanced glycation end products (AGE)-mediated induction of tissue factor in cultured endothelial cells is dependent on RAGE. Circulation 96: 2262–2271, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Pertynska-Marczewska M, Kiriakidis S, Wait R, Beech J, Feldmann M, Paleolog EM: Advanced glycation end products upregulate angiogenic and pro-inflammatory cytokine production in human monocyte/macrophage. Cytokine 28: 35–47, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Uribarri J, Stirban A, Sander D, Cai W, Negrean M, Buenting CE, Koschinsky T, Vlassara H: Single oral challenge by advanced glycation end products acutely impairs endothelial function in diabetic and nondiabetic subjects. Diabetes Care 30: 2579–2582, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Ayala E, Anderstam B, Suliman M, Seeberger A, Heimburger O, Lindholm B, Stenvinkel P: Enhanced RAGE-mediated NFκB stimulation in inflamed hemodialysis patients. Atherosclerosis 180: 333–340, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J: Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 65: 1009–1016, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Tong M, Carrero JJ, Qureshi R, Anderstam B, Heimburger O, Barany P, Axelsson J, Alvestrand A, Stenvinkel P, Lindholm B, Suliman ME: Plasma pentraxin 3 in patients with chronic kidney disease: Associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol 2: 889–897, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Shlipak MG, Katz R, Cushman M, Sarnak MJ, Stehman-Breen C, Psaty BM, Siscovich D, Tracy RP: Cystatin-C and inflammatory markers in the ambulatory elderly. Am J Med 118: 1416, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Peppa M, Uribarri J, Cai W, Lu M, Vlassara H: Glycoxidation and inflammation in renal failure patients. Am J Kidney Dis 43: 690–695, 2004 [DOI] [PubMed] [Google Scholar]