Abstract
Background and objectives: Vascular access standards are predominantly based on older, single-center reports; however, the hemodialysis population has changed dramatically and primary arteriovenous fistula failure is a huge problem. This prospective, multicenter study used standardized definitions to analyze patency rates and potential risk factors that affect functional patency and late arteriovenous fistula functionality.
Design, setting, participants, & measurements: Eleven centers participated in a guidelines implementation program. All new permanent vascular accesses were included. Patency and functional patency, defined as access survival from creation and from first dialysis use, respectively, were calculated using Kaplan-Meier analysis. Risk factors for primary functional patency loss (intervention-free interval) and secondary failure (abandonment) were determined using regression models.
Results: A total of 491 arteriovenous fistulas were placed in 395 patients. Six-, 12-, and 18-mo secondary patency and functional patency were 75 ± 2.0, 70 ± 2.3, and 67 ± 2.7% and 90 ± 1.9, 88 ± 2.2, and 86 ± 2.7%, respectively. Primary failure rate was 40%. Thrombosis rate was 0.14 per patient-year. Diabetes and arteriovenous fistula surveillance were significantly associated with primary functional patency loss. Preoperative duplex was inversely related to secondary failure. The secondary failure rate per hospital varied from 0 to 39%.
Conclusions: This study showed a marked difference between patency and functional patency, likely to be explained by high primary failure rates. Hemodialysis patients with diabetes can be expected to have reduced primary functional patency rates, but if treated adequately, then arteriovenous fistula functionality can be maintained as long as in patients without diabetes.
The Kidney Disease Outcomes Quality Initiative (K/DOQI) standards promote the increased use of native vascular access because of superior patency rates and lower complication rates than grafts once established (1). These recommendations are predominantly based on single-center studies from the 1980s and early 1990s and on studies that excluded the phase between arteriovenous fistula (AVF) creation and cannulation from patency calculations (2); however, current hemodialysis patients are older, more often have diabetes (3), and more often have cardiovascular comorbidity (4,5). Moreover, fistulas have high primary failure (PF) rates (6), and failure to mature will increasingly challenge vascular access teams in meeting the K/DOQI goals (7). In patients with compromised forearm vessels, graft patency has been shown to be better than AVF patency (8); therefore, a renewed analysis of native vascular access patency rates is justified.
The Dialysis Outcomes and Practice Patterns Study (DOPPS), in which the Netherlands was not included, showed large differences in both national and regional vascular access placement policies (5,9). At the start of the new millennium, prevalent AVF use in the Netherlands was approximately 60% with a wide range (31 to 91%) (10); therefore, a multicenter guidelines implementation program, Care Improvement by Multidisciplinary approach for Increase of Native vascular access Obtainment (CIMINO), was initiated to increase AVF use in a proportion of the Dutch hemodialysis population. In addition, this prospective, multicenter, observational study was designed to learn more about both early and late functionality of the AVF. Recently, our group showed that hospital-specific aspects predominantly determine primary AVF failure (11).
The purpose of the analysis in this study was to compare AVF patency rates in 11 dialysis centers with K/DOQI standards using standardized definitions in a methodologically favorable study setup. Furthermore, we aimed to obtain insight on risk factors that affect functional patency rates and late AVF functionality.
Materials and Methods
Guidelines Implementation Program
At the start of our program in 2003 (10), the Vascular Access Society (http://www.vascularaccesssociety.org) presented the most recently updated guidelines on vascular access care by means of 26 algorithms consisting of clearly structured flow charts supported by literature-based evidence and expert opinions (12,13). The recommendations of these “European guidelines” included (1) for nephrologists vein preservation; patient referral to vascular surgeon at least 6 mo before expected hemodialysis; performance of a standard preoperative duplex examination; and referral to ultrasound technician, surgeon, or radiologist in case of suspected inadequate maturation at 4 to 6 wk; (2) for vascular surgeons order of preference of access placement is (a) distal arm AVF, (b) proximal arm AVF, and (c) basilic vein transposition or graft insertion; artery and vein internal diameters both should be at least 2.0 mm, and end-to-side anastomosis is preferred over side-to-side; (3) for radiologists aggressive treatment of the failing and failed fistula; and (4) for dialysis unit a surveillance program including access flow measurements every 3 mo. Summaries of these guidelines (translated into Dutch) were provided to the centers, and vascular access teams were encouraged to adhere to these guidelines during the CIMINO program. In each center, a dedicated vascular access coordinator was appointed to register practice patterns in a newly developed Internet-linked database. This database contained information on medical history, medication use, preoperative duplex examination, surgery, and records of complications and interventions. In-center analysis of the database allowed participating physicians to evaluate their own practice patterns during the entire project. The entire database was available only to the coordinating center, the University Medical Center Utrecht. Newsletters went out regularly to update participants on progress of the CIMINO initiative.
Patients
Between May 2004 and July 2005, 11 vascular access centers in the middle part of the Netherlands, representing 1092 prevalent vascular accesses, started participation in this prospective, observational study (10). All hemodialysis patients or patients who had chronic renal failure and required a new permanent vascular access during this follow-up period were included.
Definitions
Coronary artery disease was defined as a history of coronary angioplasty, coronary bypass surgery, endovascular stenting, or myocardial infarction. Peripheral vascular disease (PVD) was defined as a history of angioplasty, surgical endarterectomy, endovascular stenting or bypass surgery of the iliac and/or femoral arteries, but also amputation as a result of peripheral artery occlusive disease. Cerebrovascular disease was defined as the same interventions in the carotid arteries and also included previous cerebrovascular accidents. Diabetes was defined as current use of hypoglycemic medication or use of insulin or when the diagnosis was recorded in a medical status.
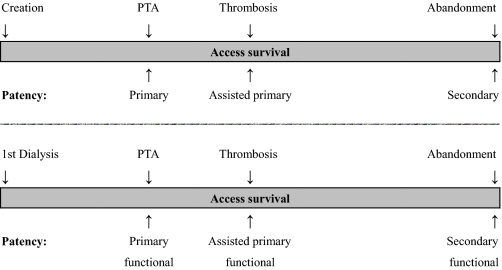
Primary patency (intervention-free access survival) was defined as the interval from time of access placement to any intervention designed to maintain or reestablish patency or to access thrombosis or the time of measurement of patency (14). Assisted primary patency (thrombosis-free access survival) was defined as the interval from time of access placement to access thrombosis or time of measurement of patency, including intervening manipulations (surgical or endovascular interventions) designed to maintain the functionality of a patent access (14). Secondary patency (access survival until abandonment) was defined as the interval from time of access placement to access abandonment or time of measurement of patency, including intervening manipulations (surgical or endovascular interventions) designed to reestablish the functionality of thrombosed access (14). The word “functional” was added to patency to indicate that patency interval started at date of first successful cannulation for hemodialysis treatment instead of date of access placement (Figure 1).
Figure 1.
Visual explanation of patency rates. Primary patency is the intervention-free access survival. Assisted primary patency is the thrombosis-free access survival. Secondary patency ends when the access is abandoned (14).
A functional AVF is an access that is able to deliver a flow rate of 350 to 400 ml/min without recirculation for the total duration of dialysis. A nonfunctional AVF is an access that is not being successfully used for hemodialysis, regardless of whether it is patent (14). Inadequate maturation was defined as insufficient access flow to maintain dialysis or the inability to cannulate an AVF, if required, at 6 wk after surgery.
PF was defined as an AVF that did not develop to maintain dialysis or thrombosed before the first successful cannulation for hemodialysis treatment, regardless of eventual AVF abandonment. This definition includes (1) inadequate maturation, (2) early thrombosis, (3) failure of first cannulation, and (4) other complications such as ischemia or infection. Secondary failure (SF) was defined as permanent failure of the AVF, after it had achieved adequacy for hemodialysis.
In the CIMINO program, vascular access teams were encouraged to perform regular access blood flow measurements for vascular access surveillance. For analyses in this study, vascular access surveillance was scored as positive when three or more access flow measurements were performed per access year.
Statistical Analyses
Results are shown as means ± SEM unless otherwise described. Kaplan-Meier survival analysis and the life-table method were used to calculate patency rates, and the log-rank test was used to compare patency rates.
Only the first created AVF per patient in this data set was used to determine relations between possible risk factors and AVF outcome. Risk factors for primary functional patency loss and for SF were determined using multivariate Cox proportional hazards models. The comparison of AVF failure rates across hospitals was done using a Cox proportional hazards model with dummy variables in which the largest hospital was considered as reference. Results are presented as hazard ratios (HR) with corresponding 95% confidence intervals (CI). Statistical significance was assumed at two-sided P < 0.05. Analyses were carried out using SPSS 12.0 (SPSS, Chicago, IL) and SigmaStat 3.11 (Systat Software, San Jose, CA) for Windows.
Results
From May 1, 2004, to May 1, 2006, a total of 649 permanent vascular accesses, representing all inclusions of CIMINO, were recorded in the database. This included 491 (76%) AVF in 395 patients. Of these patients, 80 received two AVFs during this observation period, 13 patients had three AVFs, and three patients had four AVFs. A total of 291 AVFs were created in the forearm, 198 in the upper arm, and two in the leg. Mean age was 64.6 ± 14.2 yr, and 62% were males. Baseline characteristics of the 395 patients are shown in Table 1.
Table 1.
Baseline characteristics of CIMINO patients (n = 395)a
| Characteristic | Value |
|---|---|
| No. of AVFs | 491 |
| No. of cannulated AVFs | 302 |
| Time to first cannulation in tertiles (d) | 0 to 44 |
| 44 to 69 | |
| >69 | |
| Age (yr; mean ± SD) | 64.6 ± 14.2 |
| Male gender (%) | 62 |
| RRT before AVF placement (%) | 55 |
| Coronary artery disease (%) | 23 |
| Peripheral vascular disease (%) | 10 |
| Cerebrovascular disease (%) | 12 |
| White ethnicity (%) | 78 |
| Current smoker (%) | 21 |
| BMI (kg/m2; mean ± SD) | 25.1 ± 4.5 |
| Diabetes (%) | 33 |
| Diabetes as primary cause of ESRD (%) | 17 |
AVF, arteriovenous fistula; BMI, body mass index; RRT, renal replacement therapy.
Total follow-up time from access placement to loss to follow-up, secondary AVF failure, or study end was 343.3 patient-years (i.e., 0.87 yr per patient). Follow-up time from first successful cannulation to loss to follow-up, secondary AVF failure, or study end was 204.8 patient-years (i.e., 0.72 functional years per patient).
Patency
Three-, 6-, 12-, and 18-mo patency rates and functional patency rates are depicted in Table 2 and Figure 2.
Table 2.
Primary, assisted primary and secondary patency rates at 3, 6, 12, and 18 mo with 95% CI and number of AVF at risk at the end of the interval (n)a
| Patency | 3 Mo (%) | 95% CI | n | 6 Mo (%) | 95% CI | n | 12 Mo (%) | 95% CI | n | 18 Mo (%) | 95% CI | n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patency rate from surgical AVF creation (n = 491 AVF) | ||||||||||||
| primary | 71 | 67 to 75 | 319 | 57 | 52 to 62 | 212 | 49 | 44 to 54 | 102 | 39 | 32 to 46 | 25 |
| assisted primary | 77 | 73 to 81 | 349 | 69 | 65 to 73 | 262 | 64 | 59 to 69 | 132 | 59 | 53 to 65 | 38 |
| secondary | 81 | 77 to 85 | 366 | 75 | 71 to 79 | 284 | 70 | 65 to 75 | 147 | 67 | 62 to 72 | 42 |
| Patency rate from first cannulation (n = 302 AVF) | ||||||||||||
| primary functional | 83 | 78 to 88 | 212 | 70 | 64 to 76 | 137 | 61 | 54 to 68 | 54 | 57 | 49 to 65 | 12 |
| assisted primary functional | 92 | 89 to 95 | 238 | 85 | 80 to 90 | 171 | 83 | 78 to 88 | 74 | 77 | 70 to 84 | 15 |
| secondary functional | 96 | 94 to 98 | 248 | 90 | 86 to 94 | 184 | 88 | 84 to 92 | 80 | 86 | 81 to 91 | 15 |
Patency starts with 491 AVFs at risk at T = 0, functional patency starts with 302 AVFs at T = 0. CI, confidence interval.
Figure 2.
Primary and secondary patency from fistula creation with number of patients at risk. Note the significant number of early complications that led to approximately 20% fistula abandonment within 3 mo.
Primary AVF Function
Of 491 fistulas placed (Figure 3), the fistula outcomes were indeterminate in 63, as a result of death (n = 16), transplantation (n = 3), loss to follow-up (n = 4), and predialytic phase (n = 40). Of the remaining 428 fistulas with known outcomes, 170 (40%) had a PF. Of these, 44 were subsequently salvaged and cannulated for dialysis. Thus, a total of 302 (258 with primary success + 44 with salvage after PF) were used successfully for dialysis. PF salvaging required 14 Percutaneous Transluminal Angioplasty (PTA) procedures, 14 surgical interventions, five antibiotic treatments, and extension of the maturation period in the remaining AVF.
Figure 3.
Diagram of primary AVF function. Of the 428 fistulas with known outcomes, 170 (40%) had a primary failure; 302 primary successful and salvaged fistulas were successfully used for hemodialysis.
Complications in Successfully Used AVFs
At the end of the follow-up period, 302 AVFs had been used for hemodialysis treatment in 285 patients. Thrombosis occurred 29 times (0.14 per patient-year). Eight patients received antibiotics for AVF infection. Two ischemic events required surgical intervention: One in a forearm AVF and one in an upper arm AVF. A total of 49 PTA procedures (0.24 per patient-year) and 40 surgical revisions (including the two procedures for ischemia; 0.20 per patient-year) were performed to salvage fistulas (0.29 procedures per fistula). Eventually, 31 AVF were abandoned in 27 patients.
Risk Factors for Functional Patency Loss
In univariate analyses, female gender (HR 1.40; 95% CI 0.91 to 2.14), age ≥65 yr (HR 1.53; 95% CI 0.995 to 2.37), presence of PVD (HR 0.47; 95% CI 0.17 to 1.28), diabetes (HR 1.56; 95% CI 1.01 to 2.40), and AVF surveillance (HR 2.33; 95% CI 1.51 to 3.60) were related to primary functional patency loss. P values of renal replacement therapy before access cannulation, coronary artery disease, ethnicity, body mass index ≥30 kg/m2, and fistula location were >0.15 in univariate analyses. Only preoperative duplex examination was associated with SF (HR 0.33; 95% CI 0.15 to 0.72).
On multivariate survival analysis, diabetes (HR 1.66; 95% CI 1.06 to 2.59) and AVF surveillance (HR 2.35; 95% CI 1.51 to 3.65) were significantly related to primary functional patency loss. Female gender (HR 1.52; 95% CI 0.99 to 2.34) and age ≥65 yr (HR 1.49; 95% CI 0.96 to 2.31) were borderline significantly related to primary functional patency loss.
Dialysis Facility Factors
Primary functional patency loss varied from 14 to 69% among the centers. Primary functional patency rates were not significantly different among the 11 hospitals (P = 0.052 for 302 AVFs; P = 0.060 for 285 patients). The SF rate per hospital varied from 0 to 39% (Table 3). Secondary functional patency rates were different among the 11 hospitals (P < 0.01 for 302 AVFs; P = 0.010 for 285 patients).
Table 3.
Patient characteristics and failure rates per hospitala
| Characteristic | Hospital
|
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| No. of patients | 35 | 53 | 27 | 13 | 22 | 37 | 10 | 34 | 21 | 11 | 22 | 285 |
| Male gender (%) | 69 | 59 | 63 | 69 | 59 | 68 | 50 | 77 | 57 | 82 | 59 | 65 |
| Age ≥65 yr (%) | 51 | 55 | 59 | 54 | 73 | 62 | 70 | 56 | 52 | 27 | 41 | 55 |
| CAD (%) | 33 | 23 | 15 | 31 | 14 | 22 | 40 | 29 | 33 | 27 | 18 | 25 |
| PVD (%) | 6 | 8 | 7 | 23 | 9 | 3 | 30 | 9 | 10 | 18 | 5 | 9 |
| Diabetes (%) | 20 | 43 | 44 | 46 | 36 | 27 | 50 | 21 | 33 | 9 | 14 | 31 |
| BMI ≥30 kg/m2 (%) | 3 | 17 | 8 | 0 | 19 | 11 | 20 | 24 | 15 | 0 | 14 | 13 |
| RRT before cannulation (%) | 69 | 74 | 82 | 69 | 59 | 51 | 80 | 85 | 76 | 91 | 73 | 72 |
| Forearm AVF (%) | 66 | 42 | 33 | 85 | 46 | 76 | 50 | 62 | 62 | 36 | 64 | 56 |
| Preoperative duplex (%) | 54 | 96 | 93 | 0 | 73 | 30 | 50 | 65 | 62 | 82 | 23 | 62 |
| AVF surveillance (%) | 20 | 43 | 63 | 15 | 45 | 22 | 40 | 47 | 86 | 20 | 64 | 42 |
| Primary failure rate (%) (11) | 24 | 38 | 50 | 43 | 39 | 8 | 24 | 46 | 13 | 35 | 32 | 33 |
| PFP loss (%) | 37 | 28 | 48 | 69 | 32 | 14 | 20 | 27 | 29 | 27 | 23 | 31 |
| Secondary failure rate (%) | 17 | 6 | 7 | 39 | 5 | 3 | 0 | 9 | 0 | 9 | 23 | 10 |
CAD, coronary artery disease; PVD, peripheral vascular disease; PFP, primary functional patency.
Compared with the largest center, SF was significantly increased in centers 4 and 11 (HR 6.35 [95% CI 1.52 to 26.58] and HR 4.55 [95% CI 1.08 to 19.18], respectively). When preoperative duplex was entered in the multivariate model, none of the centers had an increased risk for SF. In a multivariate model with female gender, age ≥65 yr, PVD, diabetes, and AVF surveillance, the risk for primary functional patency loss was increased only for hospital 4 (HR 3.51; 95% CI 1.48 to 8.33) when compared with the largest center.
Discussion
In this prospective, multicenter study, we have shown that hemodialysis AVF patency and functional patency are markedly different. This difference seems to be caused by high PF rates. After adjustment for potential risk factors, primary functional patency was decreased only in patients with diabetes. SF rate among participating hospitals varied from 0 to 39% and was not related to patient characteristics or cardiovascular risk factors.
The thrombosis rate at 0.14 episodes per patient-year at risk was well below current outcome goals (0.25 per patient-year) (1). Regarding the multicenter character of this study, the K/DOQI goal seems to be more than reasonable.
Patency Rates
A significant proportion of the AVFs encounter PF during the first weeks after surgery (6,11,15); however, when patency rates are calculated starting at the day of first cannulation, primary failed AVFs are not included. To prevent confusion and incorrect comparisons, we discriminated patency from functional patency as reported by Sidawy et al. (14). Functional patency started when a vascular access had been successfully used for hemodialysis treatment for the first time; patency started at the day of surgical AVF creation. Whereas primary AVF failure was extensively studied and reported previously by our group (11), we focused on aspects of functional patency in this study.
Primary functional patency was similar to rates in the current literature (16). Our 18-mo secondary functional patency was somewhat higher at 86% (median >960 d) versus 77%. The difference may be explained by the fact that more than half of the reports used in the review by Huber et al. (16) were published before the appearance of the first K/DOQI guidelines, and surveillance programs and preventive stenosis correction were not common practice yet. In contrast, 18-mo secondary patency (from creation date) was 67%. The long-term difference of approximately 20% seems to be caused by significant PF rates. In this study, 23% of the AVFs were abandoned. Thus, after adequate maturation that results in successful initiation of HD treatment, only a few fistulas are abandoned (Table 2). Consequently, reduction of PF is likely to result in greatest patency improvements. Of note, Lok et al. (17) recently developed a scoring system to stratify a patient's risk for failure of AVF maturation.
Diabetes was identified as a risk factor associated with primary functional patency loss (HR 1.66) but not with SF (18). These results indicate that patients with diabetes may encounter more complications during fistula life, but, if treated adequately, then functionality can be maintained as long as in patients without diabetes, regardless of the anatomic location of the anastomosis (19). Although a larger sample size may have resulted in a significantly increased risk for primary functional patency loss in aging or female hemodialysis patients, older patients and women were not at significantly increased risk for fistula abandonment in our population (20). All other factors including obesity or cardiovascular disease did not reduce functional AVF survival (21,22).
Adoption of access blood flow surveillance is known to result in increased intervention rates (23). Consequently, an increased risk for primary functional patency loss can be expected in patients who receive vascular access surveillance. Although surveillance led to a decreased risk for SF in univariate analyses, no significance was achieved (HR 0.73; 95% CI 0.31 to 1.74). These findings are in conformity with work of others (24).
Preoperative duplex examination may result in increased AVF prevalence and adequacy for dialysis (25); however, the relation between preoperative duplex and long-term AVF outcomes has not been reported before. High SF rates were observed in two centers where few patients received preoperative duplex examination. To what extent these center-specific outcomes reflect actual increased risk for SF remains unclear.
Dialysis Facility Factors
SF rate varied from 0 to 39% among the hospitals that participated in CIMINO, resulting in significant difference of secondary functional patency rates (log rank test P < 0.01 for 302 AVF; P = 0.010 for 285 patients). One hospital had an increased risk for primary functional patency loss, but two hospitals had an increased risk for SF. Although this study was not designed to identify dialysis facility factors of SF in detail, surgical factors are less likely to be involved. Indeed, Prischl et al. (26) suggested that the surgeon who created the fistula was involved in patency, but these differences were predominantly generated during the first months after fistula creation. In this study, only successfully used AVF were analyzed. Practice factors such as negligent shunt surveillance (dialysis unit), delayed action to detected stenoses (nephrologists), or inadequate PTA/surgery procedures (radiologist/vascular surgeon) may have contributed to these findings. Further in-center analysis can be useful to improve secondary functional patency rates, but, obviously, the multidisciplinary character of complication handling requires a well-functioning vascular access team (27).
Limitations
Fistulas are preferred over grafts because of superior long-term patency. Follow-up time in our study was limited to 18 mo. In expanded polytetrafluoroethylene grafts, 6-, 12-, and 18-mo secondary functional patencies are approximately 76, 65, and 55%, respectively (16). When primary graft failures (approximately 10%) are also included, secondary graft patency from date of creation is likely to decrease slightly, expecting fistula survival to be superior from 12 mo on (Table 2). Extra follow-up time is required to obtain further insight into long-term AVF patency, but fewer interventions can be expected in fistulas compared with grafts (28).
This study is limited in its ability to detect significant predictors of SF as a result of the small number of SF (n = 27). In addition, the number of primary functional failures is such that the magnitude of the relation between a predictor and primary functional failure should be considerable to become statistically significant as a result of restricted precision of the estimate.
Conclusions
A total of 76% of the vascular accesses in our prospective database were native AVFs. Using recently suggested standardized definitions, we showed a marked difference between patency and functional patency that can be explained by high PF rates. Hemodialysis patients with diabetes can be expected to have reduced primary functional patency rates, but, if treated adequately, then AVF functionality should be maintained as long as in patients without diabetes.
Disclosures
None.
Acknowledgments
H.J.T.H. is supported by a grant of the Dutch Kidney Foundation (KB 25).
Members of the CIMINO study group include the following: Dr. Y.C. Schrama, Dr. C.H. Wittens, H. Aerts, RN, T. van Westen, RN (St. Franciscus Gasthuis, Rotterdam); Dr. J.R. Beukhof, A. de Groot, RN, S. Temmink, RN (Isala Clinics, Zwolle); E. van Wijk, RN (University Medical Center Utrecht, Utrecht); Dr. A.A. Hollander, Dr. J.G. Olsman, T. Kokx, RN, C. Boeren, RN (Jeroen Bosch Hospital,'s-Hertogenbosch); Dr. B.C. van Jaarsveld, Dr. S.K. Nagesser, M. Baars, RN (Dianet & Diakonessenhuis, Utrecht); Dr. K.J. Parlevliet, A. van de Kaaden, RN (Rijnstate Hospital, Arnhem); Prof. M.J. Nube, J. Wijnker, RN (Medical Center Alkmaar); Dr. M.G. Betjes, L. Chardon, RN, M. Konings, RN (Erasmus Medical Center, Rotterdam); Dr. C.J. Doorenbos, Dr. C.G. Vermeij, M. Voskamp, RN (Deventer Hospital, Deventer); Dr. M.A. van den Dorpel, Dr. A.A. de Smet, L. Steegman, RN (Medical Center Rijnmond Zuid, Rotterdam); Dr. M.A. ten Dam, Dr. W.B. Barendregt, Dr. P.H. Haarbrink, F. Dastnaei, RN, A. Jilisen, RN (Canisius Wilhelmina Hospital, Nijmegen).
Cees Haaring (Department of Radiology, UMC Utrecht) is gratefully acknowledged for excellent work on database setup and maintenance. We gratefully acknowledge the contribution of the CIMINO members to the project.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.NKF-K/DOQI clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl 1]: S248–S273, 2006 [DOI] [PubMed] [Google Scholar]
- 2.NKF-K/DOQI clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl 1]: S176–S247, 2006 [DOI] [PubMed] [Google Scholar]
- 3.US Renal Data System: USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006
- 4.Rostand SG, Gretes JC, Kirk KA, Rutsky EA, Andreoli TE: Ischemic heart disease in patients with uremia undergoing maintenance hemodialysis. Kidney Int 16: 600–611, 1979 [DOI] [PubMed] [Google Scholar]
- 5.Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, Wolfe RA, Goodkin DA, Held PJ: Vascular access use in Europe and the United States: Results from the DOPPS. Kidney Int 61: 305–316, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Patel ST, Hughes J, Mills JL Sr: Failure of arteriovenous fistula maturation: An unintended consequence of exceeding dialysis outcome quality Initiative guidelines for hemodialysis access. J Vasc Surg 38: 439–445, discussion 445, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Rooijens PP, Burgmans JP, Yo TI, Hop WC, de Smet AA, van den Dorpel MA, Fritschy WM, de Groot HG, Burger H, Tordoir JH: Autogenous radial-cephalic or prosthetic brachial-antecubital forearm loop AVF in patients with compromised vessels? A randomized, multicenter study of the patency of primary hemodialysis access. J Vasc Surg 42: 481–486, discussion 487, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Mendelssohn DC, Ethier J, Elder SJ, Saran R, Port FK, Pisoni RL: Haemodialysis vascular access problems in Canada: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS II). Nephrol Dial Transplant 21: 721–728, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Huijbregts HJ, Bots ML, Moll FL, Blankestijn PJ: Accelerated increase of arteriovenous fistula use in haemodialysis centres: Results of the multicentre CIMINO initiative. Nephrol Dial Transplant 22: 2595–2600, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Huijbregts HJ, Bots ML, Moll FL, Blankestijn PJ: Hospital specific aspects predominantly determine primary failure of hemodialysis arteriovenous fistulas. J Vasc Surg 45: 962–967, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Bakran A, Mickley V, Passlick-Deetjen J: Management of the Renal Patient: Clinical Algorithms on Vascular Access for Haemodialysis, Lengerich, Pabst Science Publishers, 2003
- 13.Tordoir JH, Mickley V: European guidelines for vascular access: Clinical algorithms on vascular access for haemodialysis. EDTNA ERCA J 29: 131–136, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Sidawy AN, Gray R, Besarab A, Henry M, Ascher E, Silva M Jr, Miller A, Scher L, Trerotola S, Gregory RT, Rutherford RB, Kent KC: Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg 35: 603–610, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Rooijens PP, Tordoir JH, Stijnen T, Burgmans JP, Smet de AA, Yo TI: Radiocephalic wrist arteriovenous fistula for hemodialysis: Meta-analysis indicates a high primary failure rate. Eur J Vasc Endovasc Surg 28: 583–589, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Huber TS, Carter JW, Carter RL, Seeger JM: Patency of autogenous and polytetrafluoroethylene upper extremity arteriovenous hemodialysis accesses: A systematic review. J Vasc Surg 38: 1005–1011, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Konner K: Primary vascular access in diabetic patients: An audit. Nephrol Dial Transplant 15: 1317–1325, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Hakaim AG, Nalbandian M, Scott T: Superior maturation and patency of primary brachiocephalic and transposed basilic vein arteriovenous fistulae in patients with diabetes. J Vasc Surg 27: 154–157, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV: Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int 67: 2462–2469, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Ravani P, Brunori G, Mandolfo S, Cancarini G, Imbasciati E, Marcelli D, Malberti F: Cardiovascular comorbidity and late referral impact arteriovenous fistula survival: A prospective multicenter study. J Am Soc Nephrol 15: 204–209, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kats M, Hawxby AM, Barker J, Allon M: Impact of obesity on arteriovenous fistula outcomes in dialysis patients. Kidney Int 71: 39–43, 2007 [DOI] [PubMed] [Google Scholar]
- 23.McCarley P, Wingard RL, Shyr Y, Pettus W, Hakim RM, Ikizler TA: Vascular access blood flow monitoring reduces access morbidity and costs. Kidney Int 60: 1164–1172, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Tessitore N, Lipari G, Poli A, Bedogna V, Baggio E, Loschiavo C, Mansueto G, Lupo A: Can blood flow surveillance and pre-emptive repair of subclinical stenosis prolong the useful life of arteriovenous fistulae? A randomized controlled study. Nephrol Dial Transplant 19: 2325–2333, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML: Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60: 2013–2020, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Prischl FC, Kirchgatterer A, Brandstatter E, Wallner M, Baldinger C, Roithinger FX, Kramar R: Parameters of prognostic relevance to the patency of vascular access in hemodialysis patients. J Am Soc Nephrol 6: 1613–1618, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Huijbregts HJ, Blankestijn PJ: Dialysis access-guidelines for current practice. Eur J Vasc Endovasc Surg 31: 284–287, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM: Superiority of autogenous arteriovenous hemodialysis access: Maintenance of function with fewer secondary interventions. Ann Vasc Surg 18: 66–73, 2004 [DOI] [PubMed] [Google Scholar]