Abstract
Background and objectives: Development of new therapeutic strategies to improve long-term transplant outcomes requires improved understanding of the mechanisms by which these complications limit long-term transplant survival.
Design, setting, participants, & measurements: The association of acute rejection and new-onset diabetes was determined in the first posttransplantation year with the outcomes of transplant failure from any cause, death-censored graft loss, and death with a functioning graft in 27,707 adult recipients of first kidney-only transplants, with graft survival of at least 1 yr, performed between 1995 and 2002 in the United States.
Results: In multivariate analyses, patients who developed acute rejection or new-onset diabetes had a similar risk for transplant failure from any cause, but the mechanisms of transplant failure were different: Acute rejection was associated with death-censored graft loss but only weakly associated with death with a functioning graft. In contrast new-onset diabetes was not associated with death-censored graft loss but was associated with an increased risk for death with a functioning graft.
Conclusions: Acute rejection and new-onset diabetes have a similar impact on long-term transplant survival but lead to transplant failure through different mechanisms. The mechanisms by which new-onset diabetes leads to transplant failure should be prospectively studied. Targeted therapeutic strategies to minimize the impact of various early posttransplantation complications may lead to improved long-term outcomes.
Advances in immunosuppression have led to marked improvements in short-term but not long-term transplant survival (1–4). Maintenance immunosuppressive protocols are usually determined before transplantation on the basis of an assessment of immunologic risk, with the avoidance of acute rejection (AR) being the primary early therapeutic goal. Tailoring immunosuppressive protocols after the early posttransplantation period is now increasingly considered to improve long-term transplant survival (5–8). Most of these approaches have focused on improving allograft survival, without overtly considering all potential factors that may lead to transplant failure. It is now known that a number of early posttransplantation complications, in addition to AR, can limit long-term transplant survival by leading to either allograft failure or death with a functioning graft (9–14). Improved understanding of the relative impact of early posttransplantation complications and the mechanisms by which these complications lead to transplant failure would inform development of targeted therapeutic strategies to improve long-term transplant survival. In this analysis, we determined the association of two major early posttransplantation complications—AR and new-onset diabetes (NOD) occurring in the first posttransplantation year—with the outcomes of transplant failure from any cause, death-censored graft loss (DCGL), death with a functioning graft (DWFG), and overall patient survival including survival after transplant failure.
Materials and Methods
Data Source and Study Population
The data source for the study was the US Renal Data System. The study population included patients (≥18 yr of age) who received a first kidney-only transplant between January 1, 1995, and December 31, 2002, and had at least 1 yr of graft survival. The study population was further limited to patients with Medicare as the primary payer to ensure complete ascertainment of NOD from Medicare claims data. We excluded patients with a pretransplantation diagnosis of diabetes. Patients were identified as having pretransplantation diabetes when diabetes was recorded as the cause of ESRD or as comorbid condition or when there were any in-patient or outpatient Medicare claims for diabetes in the 12 mo before transplantation.
Definitions
We defined NOD from Medicare claims data according to a previously published and validated method (9,15–19). This method requires at least one in-patient claim or two outpatient Medicare claims for diabetes during the first posttransplantation year to establish a diagnosis of NOD (9,15–19). The date of onset of diabetes was assumed to be the date of the earliest claim. The specific International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic codes included 250, 250.x (x = 0 to 9), 250.0x, and 250.xy (y = 0 to 3). Patients were categorized into four categories on the basis of whether they developed AR or NOD in the first 12 mo after transplantation: (1) Neither AR nor NOD, (2) AR, (3) NOD, or (4) both AR and NOD.
Factors Associated with NOD and Acute Rejection
Separate multivariate logistic regression models were used to identify factors that were associated with NOD, AR, and both complications in combination during the first posttransplantation year. Factors that were considered for inclusion in these models were demographic and transplant-related factors associated with each outcome in univariate analyses.
Association of NOD and AR with Posttransplantation Outcomes
The univariate association of NOD and AR with transplant failure from any cause, DCGL, and DWFG was determined by the Kaplan-Meier method. A Cox multivariate regression model was used to determine the independent association of NOD and AR with each outcome. Survival time in these models was calculated from the date of transplantation until the outcome of interest or December 31, 2004, whichever occurred first. In addition to our primary exposure variables of interest (NOD and AR), the following variables were tested for inclusion in the model: Patient demographics (age at transplantation, gender, race, cause of ESRD, duration of dialysis before transplantation, body mass index [BMI], and hepatitis C sero-status), transplant characteristics (donor source, HLA mismatch, panel-reactive antibody [PRA], transplant era, induction immunosuppression, and maintenance immunosuppression [medications prescribed at the time of hospital discharge after transplantation]). Variables were entered into the model when they met the proportional hazards (PH) assumption. The PH assumption was tested using log-negative-log plots of the within-group survivorship probabilities versus log time. Patients with missing covariate information were coded as “missing” for that covariate and included in the multivariate models. Subgroup analyses were performed for patients who were at increased risk for AR (black patients, sensitized patients [PRA >30%]), and patients at increased risk for cardiovascular disease [age >60 yr, patients with pretransplantation BMI >30 kg/m2]). Using a similar analysis, we determined the association of AR and NOD with overall patient survival including survival after transplant failure. In this analysis, patients were followed from the date of transplantation until death, repeat transplantation, or end of follow-up (December 31, 2004). All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC), and figures were produced using S-Plus 7.0 (Insightful Corp, Seattle, WA).
Results
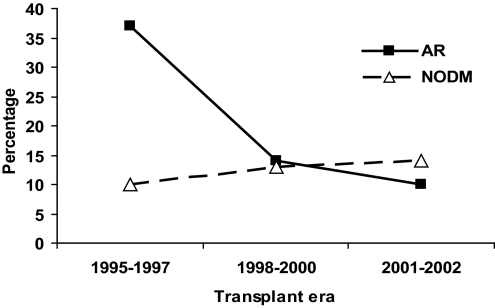
Among the 51,184, adult recipients of a first kidney-only transplant who had Medicare as their primary payer, we excluded 19,586 patients who had pretransplantation diabetes and 3891 patients who had transplant survival of <1 yr. The demographic characteristics of the 27,707 study patients are shown in Table 1. Included patients were younger (mean [SD]) 45.8 yr [14.1] versus 47.1 yr [12.5]), more likely to be of black race (29 versus 21%), and more likely to be recipients of deceased-donor organs (71 versus 63%) than excluded patients (data not shown). During the first posttransplantation year, 2598 (9.3%) of the study patients developed NOD, 5111 (18.5%) developed AR, 747 (2.7%) developed both AR and NOD, and 19,251 (69.5%) developed neither AR nor NOD. The incidence of AR fell from 37% during 1995 to 1997 to 10% between 2001 and 2002 (Figure 1). In comparison, the incidence of NOD increased from 10% during 1995 to 1997 to 14% between 2001 and 2002 (Figure 1). The incidence of both complications in combination was low in each era (3.7, 2.4, and 1.8% in 1995 to 1997, 1998 to 2000, 2001 to 2002, respectively).
Table 1.
Patient characteristics (n = 27,707)a
| Variable | Value |
|---|---|
| Age at time of transplantation (yr; mean [SD]) | 45.8 (14.1) |
| Age at transplant >60 yr (%) | 18.7 |
| Male (%) | 59.3 |
| Race (%) | |
| white | 64.9 |
| black | 29.0 |
| other | 6.1 |
| Cause of ESRD (%) | |
| glomerulonephritis | 40.0 |
| hypertension | 26.7 |
| polycystic disease | 10.3 |
| other | 23.0 |
| Duration of dialysis (yr; median [Q1 to Q3]) | 2.6 (1.2 to 4.1) |
| BMI (%; kg/m2) | |
| <30 | 79.9 |
| >30 | 20.1 |
| Hepatitis C positive (%) | 5.3 |
| Deceased-donor recipient (%) | 70.8 |
| Preemptive transplant (%) | 6.1 |
| HLA mismatch (%) | |
| 0 | 5.3 |
| 1 to 3 | 42.8 |
| 4 to 6 | 51.9 |
| PRA >30 (%) | 11.9 |
| Transplantation year (%) | |
| 1995 to 1997 | 35.8 |
| 1998 to 2000 | 35.9 |
| 2001 to 2002 | 28.3 |
| Type of CNI prescribed at hospital discharge (%) | |
| cyclosporine | 60.5 |
| tacrolimus | 32.4 |
| none | 7.1 |
| Type of antimetabolite prescribed at hospital discharge | |
| MMF | 67.6 |
| azathioprine | 18.3 |
| none | 14.1 |
| Sirolimus prescribed at hospital discharge (%) | 8.3 |
| Induction therapy at time of transplantation (%) | |
| none | 53.7 |
| depleting | 24.6 |
| nondepleting | 21.7 |
BMI, body mass index; CNI, calcineurin inhibitor; MMF, mycophenolate mofetil; PRA, panel reactive antibody; Q1 to Q3, quartile 1 to quartile 3.
Figure 1.
Rate of acute rejection (AR) and new-onset diabetes (NODM) in first posttransplantation year by transplant era.
Table 2 shows the independent association of various factors with the development of NOD, AR, and both complications in combination, during the first posttransplantation year. Age, black race, obesity (BMI >30), hepatitis C, tacrolimus, sirolimus, later transplant era, and high HLA mismatch were associated with NOD. Younger age, male gender, black race, high HLA mismatch, cyclosporine, mycophenolate mofetil, induction with depleting antibodies, and earlier transplant era were associated with AR. Black race, obesity, deceased donor type, and higher HLA mismatch were associated with the development of both complications, AR and NOD, in combination.
Table 2.
Factors associated with NOD, AR, or both complications in combination during the first posttransplantation yeara
| Parameter | OR | 95% CI |
|---|---|---|
| NOD (n = 2598) | ||
| age (yr) | ||
| 18 to 45 | 0.48 | 0.44 to 0.53 |
| 41 to 59 (reference) | 1.00 | |
| >60 | 1.33 | 1.21 to 1.46 |
| black race | 1.58 | 1.45 to 1.73 |
| BMI >30 kg/m2 | 1.76 | 1.60 to 1.94 |
| hepatitis C | 1.82 | 1.59 to 2.09 |
| tacrolimus (reference cyclosporine) | 1.51 | 1.38 to 1.65 |
| sirolimus (reference no sirolimus) | 1.29 | 1.12 to 1.49 |
| azathioprine (reference MMF) | 1.11 | 0.98 to 1.25 |
| transplant era | ||
| 1995 to 1997 | 1.00 | |
| 1998 to 2000 | 1.27 | 1.14 to 1.41 |
| 2001 to 2002 | 1.18 | 1.04 to 1.33 |
| HLA mismatch | ||
| 0 | 1.00 | |
| 1 to 3 | 1.25 | 1.02 to 1.54 |
| 4 to 6 | 1.34 | 1.09 to 1.64 |
| AR (n = 5111) | ||
| age at transplantation (yr) | ||
| 18 to 44 | 1.23 | 1.15 to 1.32 |
| 45 to 59 | 1.00 | |
| ≥60 | 0.86 | 0.78 to 0.94 |
| female gender | 0.87 | 0.82 to 0.93 |
| black race | 1.25 | 1.16 to 1.34 |
| transplant era | ||
| 1995 to 1997 (reference) | 1.00 | |
| 1998 to 2000 | 0.27 | 0.25 to 0.29 |
| 2001 to 2002 | 0.19 | 0.17 to 0.21 |
| HLA mismatch | ||
| 0 | 1.00 | |
| 1 to 3 | 1.39 | 1.19 to 1.63 |
| 4 to 6 | 1.56 | 1.33 to 1.83 |
| sirolimus (reference no sirolimus) | 1.04 | 0.90 to 1.18 |
| azathioprine (reference MMF) | 0.68 | 0.62 to 0.74 |
| tacrolimus (reference cyclosporine) | 0.75 | 0.69 to 0.82 |
| antibody induction | ||
| none | 1.00 | |
| depleting antibody | 1.17 | 1.09 to 1.26 |
| nondepleting | 0.94 | 0.85 to 1.03 |
| Both NOD and AR (n = 747) | ||
| age (yr) | ||
| 18 to 45 | 0.61 | 0.52 to 0.73 |
| 41 to 59 (reference) | 1.00 | |
| >60 | 1.19 | 0.98 to 1.45 |
| black race | 1.79 | 1.51 to 2.12 |
| BMI >30 kg/m2 | 2.08 | 1.73 to 2.51 |
| hepatitis C | 2.08 | 1.62 to 2.69 |
| deceased donor | 1.39 | 1.14 to 1.72 |
| tacrolimus (reference cyclosporine) | 1.22 | 1.01 to 1.47 |
| azathioprine (reference MMF) | 0.80 | 0.65 to 1.00 |
| transplant era | ||
| 1995 to 1997 | 1.00 | |
| 1998 to 2000 | 0.43 | 0.35 to 0.52 |
| 2001 to 2002 | 0.26 | 0.20 to 0.33 |
| HLA mismatch | ||
| 0 | 1.00 | |
| 1 to 3 | 2.35 | 1.36 to 4.05 |
| 4 to 6 | 2.63 | 1.53 to 4.53 |
Only significant factors and immunosuppressant medications are shown. Other factors included in the model for new-onset diabetes (NOD): Gender, cause of ESRD, duration of dialysis exposure, PRA, and antibody induction. Factors included in the model for acute rejection (AR) also included cause of ESRD, BMI, donor source, hepatitis C, transplant era, duration of pretransplantation dialysis, and PRA. Other factors included in the model for AR and NOD were gender, cause of ESRD, duration of dialysis exposure, PRA, and antibody induction. CI, confidence interval; OR, odds ratio.
Association of AR and NOD with Transplant Failure from Any Cause
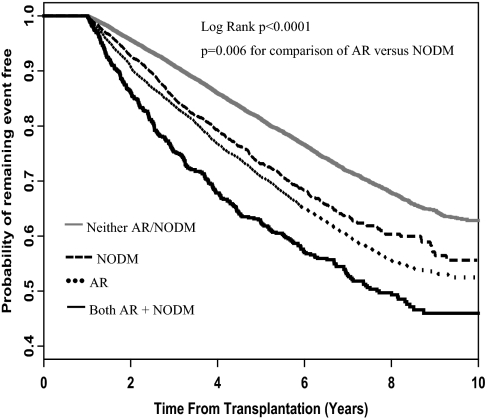
Patients who developed both AR and NOD had the shortest time to transplant failure from any cause, followed by patients who had AR and patients who had NOD, whereas patients who developed neither complication had the longest time to transplant failure from any cause (Figure 2). Although the time to transplant failure seemed similar for patients who had AR and patients who had NOD, there was a statistically significant difference in survival between these two groups (P = 0.006, log-rank test comparing AR and NOD groups).
Figure 2.
Time to transplant failure from any cause.
In multivariate analysis (Table 3), the risk for transplant failure from any cause was highest for patients who developed both NOD and AR, followed by patients with AR and patients with NOD. When directly compared, patients with AR had a higher risk for graft loss from any cause than patients who had NOD (hazard ratio [HR] 1.15; 95% confidence interval [CI] 1.05 to 1.27; P = 0.003). The associations of NOD and AR with graft loss from any cause were similar in subgroup analyses among black patients, sensitized patients, patients who were older than 60 yr, and those with BMI >30 (data not shown).
Table 3.
Risk for graft lossa
| Patient Group | Transplant Failure from any Cause (HR [95% CI]) | Death-Censored Graft Loss (Return to Dialysis or Repeat Transplantation; HR [95% CI]) | Death with a Functioning Graft (HR [95% CI]) |
|---|---|---|---|
| Neither AR nor NOD | 1.00 | 1.00 | 1.00 |
| AR only | 1.43 (1.35 to 1.52) | 1.60 (1.49 to 1.72) | 1.15 (1.04 to 1.27) |
| NOM only | 1.24 (1.14 to 1.35) | 1.12 (0.99 to 1.26) | 1.41 (1.25 to 1.59) |
| Both AR and NOD | 1.66 (1.48 to 1.87) | 1.86 (1.61 to 2.15) | 1.43 (1.18 to 1.73) |
In addition to NOD and AR, the following variables were tested for inclusion in the model: Patient demographics (age at transplantation, gender, race, cause of ESRD, duration of dialysis before transplantation, BMI, hepatitis C sero-status) and transplant characteristics (donor source, HLA mismatch, PRA, transplant era, induction immunosuppression, and maintenance immunosuppression [medications prescribed at the time of hospital discharge after transplantation]).
Association of AR and NOD with DCGL or DWFG
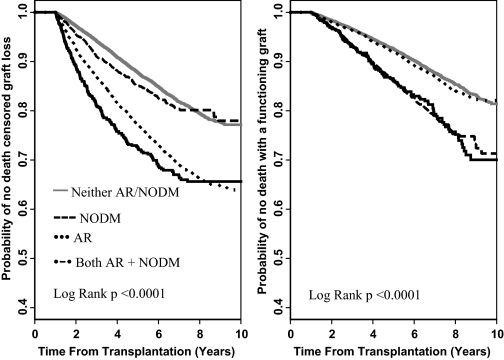
To understand further the mechanisms of transplant failure in patients with AR and NOD during the first posttransplantation year, we determined the association of these complications with DCGL (return to dialysis or transplant failure) and DWFG. The cause of transplant failure was markedly different in patients who developed AR versus NOD (Figure 3). Patients who developed AR during the first posttransplantation year had markedly shorter time to DCGL but did not seem to be at increased risk for DWFG. In fact, the time to DWFG in patients with AR was the same as that among patients who developed neither complication during the first posttransplantation year (Figure 3). In contrast, patients who developed NOD did not seem to be at increased risk for DCGL but had a much shorter time to DWFG than the reference group of patients who developed neither AR nor NOD (Figure 3).
Figure 3.
Time to death censored graft loss and death with a functioning graft.
In multivariate analysis (Table 3), the risk for DCGL was highest in patients with both AR and NOD, followed by patients with AR and patients with NOD (Table 3). We directly compared the risk for DCGL in patients who developed AR or NOD. Patients with AR had a higher risk for DCGL than patients who had NOD (HR 1.43; 95% CI 1.26 to 1.52; P < 0.0001). In multivariate analysis, the risk for DWFG was primarily associated with NOD, whereas AR was only weakly associated with this outcome (Table 3). We directly compared the risk for DWFG in patients who developed NOD or AR. Patients who developed NOD had a higher risk for DWFG compared with patients who had AR (HR 1.22; 95% CI 1.06 to 1.40; P = 0.006).
Association of AR and NOD with Overall Patient Survival, Including Survival after Transplant Failure
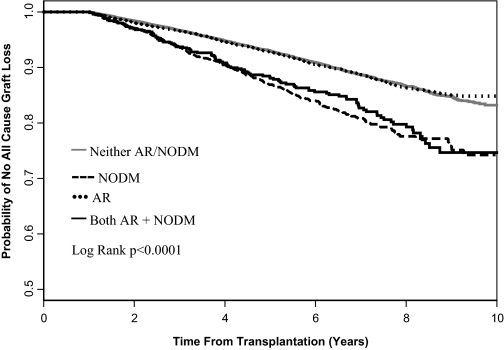
Patients who developed NOD had decreased survival compared with patients who developed AR (Figure 4). The survival of patients with AR was similar to that for patients who developed neither complication (Figure 4). The 5-yr patient survival was 86.7% in patients with NOD and 92.5% in patients with AR (log rank P < 0.0001). Patients who developed both NOD and AR had similar survival to that among patients with NOD (Figure 4).
Figure 4.
Patient survival, including survival on dialysis after transplant failure.
In multivariate analysis, patients with NOD had a relative risk for death of 1.38 (95% CI 1.22 to 1.55; P < 0.0001) compared with the reference group of patients who developed neither NOD nor AR. In contrast, patients with AR were not at an increased risk for death (HR 1.10; 95% CI 0.99 to 1.21; P = 0.07). When directly compared, patients who developed NOD had a higher relative risk for death compared with patients who developed AR (HR 1.26; 95% CI 1.09 to 1.45; P < 0.002). In subgroup analyses of black patients, sensitized patients (PRA >30%), patients who were older than 60 yr, and patients with BMI >30, the associations of AR and NOD with patient survival were similar to that in the overall study population (data not shown).
Discussion
Individualization of immunosuppressive regimens and targeted use of nonimmunosuppressive therapies that are based on a patient's risk profile may be an important strategy to improve long-term transplant outcomes; however, few studies have determined the relative risk for different posttransplantation complications or elucidated the mechanisms by which these complications lead to transplant failure to permit development of individualized therapeutic strategies. In this study, we determined the risk for specific posttransplantation outcomes associated with two of the major recognized early posttransplantation complications, AR and NOD. We demonstrated that both complications are important risk factors for transplant failure but that AR and NOD seem to lead to transplant failure through different mechanisms. AR was primarily associated with an increased risk for DCGL, whereas NOD was associated only with DWFG. These findings should be confirmed in prospective studies but suggest that different therapeutic approaches may be warranted for patients who develop AR or NOD during the first posttransplantation year.
After the first posttransplantation year, it is often clinically evident that patients develop a unique constellation of risk factors that can limit long-term transplant survival. Uniquely, our analysis provides information about the long-term risk for transplant failure after patients have successfully passed through the first posttransplantation year, when prevention of AR is the paramount clinical issue. Although NOD is a widely recognized posttransplantation complication (9,10,20–25) and guidelines for its management have been developed (26,27), it is not clear how aggressively this complication is managed. Increased understanding of the importance of NOD relative to more familiar posttransplantation complications such as AR and the mechanisms by which NOD leads to transplant failure should lead to improved management of this complication. We believe that the value of our analysis is that it highlights the importance of both complications rather than suggest that one complication is more important than the other. Definitive conclusions regarding the relative impact of AR compared with NOD cannot be made from our retrospective study.
Our findings differ from those of Kasiske et al. (9), who reported a similar risk for DCGL and DWFG in patients who developed NOD. Importantly, AR was not included as a potential confounder in that analysis (9). The mechanisms by which NOD might contribute to DCGL are not clear. The hypothesis that NOD may be acting as a surrogate marker for AR has been proposed to explain the association of NOD with DCGL (9). For example, patients who develop AR may be exposed to higher dosages of steroids and thus may develop NOD; alternatively, patients who develop NOD may have their immunosuppressive medications reduced to improve blood glucose control, thereby precipitating AR. This hypothesis is not supported by our study, in which relatively few patients developed both AR and NOD in combination. We found NOD to be primarily associated with DWFG, presumably through increased cardiovascular disease (9,21,28,29). Importantly, patients who developed NOD in our analysis were older and more likely to be obese (Table 2), and thus some of these patients may have had either unrecognized diabetes or had a significant burden of preexisting cardiovascular disease. Our analysis stresses the need for prospective studies to understand further the mechanisms by which NOD leads to transplant failure.
To determine the overall impact of each posttransplantation complication on patient survival, we performed an additional survival analysis including deaths after transplant failure, a period when patients are known to be at high risk for morbidity and mortality (30–32). Specifically, this approach avoids potential underestimation of the risk for death in patients with AR, who had a higher rate of DCGL. Even with the inclusion of deaths after transplant failure, we failed to find an association of AR with patient survival. This is consistent with our previous work in which AR was not associated with survival after transplant failure (30). Although previous studies reported an association between AR and survival (33,34), there are few analyses in the current era to compare our findings to. It is possible that earlier detection and improved management of AR in the current era, including improved immunosuppression and prophylaxis for infection, have led to a decrease in the morbidity previously associated with AR. The need to consider patient outcomes after transplant failure has been highlighted in a number of studies (30–32) and is further justified by the fact that transplant failure is now a leading cause of dialysis initiation in the United States (3).
We found that the incidence of NOD in the current era surpassed that of AR (14 versus 10%, respectively). The temporal rise in the incidence of NOD, coupled with the dramatic decrease in AR, suggests that the reduction of AR attributable to changes in immunosuppression has been achieved at a cost of increased NOD. This may in part explain why recent analyses have failed to show an improvement in long-term graft survival despite the reduction in AR (2). Consistent with other analyses (35,36), we found that both tacrolimus and sirolimus were associated with NOD. In addition, the changing demographics of transplant recipients (e.g., increased transplantation of older patients) may contribute to the rise in NOD. Similar to other analyses (9,37,38), we were able to identify a number of factors associated with NOD. Better methods to predict NOD before transplantation and development of targeted strategies to decrease the incidence of this complication are needed.
We defined NOD from Medicare claims data according to previously published and validated methods (9,15–19). It is important to note that that this criterion is not the same as the “gold standard” established by the American Diabetes Association and World Health Organization, which requires laboratory results and patient symptoms (26,27) that are not available in the US Renal Data System. The definition used in this study has been validated in a number of studies that consistently found a high level of accuracy and concordance between cases that were identified by this method and the American Diabetes Association/World Health Organization criteria (16,18,19). Our definition has a sensitivity of 0.75, a specificity of 0.97, and a positive predictive value of 0.88 compared with self-reported diabetes (16).
When interpreting the results of this study, readers should consider the inherent limitations of retrospective analyses of administrative data sets, including nonrandom assignment of patients to different immunosuppression protocols and possibly a reporting bias of posttransplantation complications. The study involved American patients who had Medicare as the primary payer, which may limit the applicability of our findings to other patient populations. In addition, the identification of NOD from Medicare claims data likely underestimates the incidence of NOD. Patients who were included in this study had graft survival of at least 1 yr. Because the focus of this study is on long-term transplant outcomes, the exclusion of patients with graft loss in the first posttransplantation year is justified; however, readers should note that the exclusion of patients with early graft loss may lead to underestimation of the association of AR with graft and patient survival. Similarly, the impact of NOD and AR that occurred after the first posttransplantation year is not captured in this analysis.
Conclusions
Both NOD and AR are important risk factors for long-term transplant failure. The incidence of NOD now exceeds that of AR. NOD and AR seem to act through different mechanisms: AR primarily leads to DCGL, whereas NOD primarily leads to DWFG. Different therapeutic approaches may be warranted for patients who develop these complications during the first posttransplantation year. Prospective studies should be performed to elucidate further the mechanisms by which NOD leads to transplant failure. Newer therapeutic strategies should be developed with the aim of maintaining low AR rates without the observed increase in NOD.
Disclosures
None.
Acknowledgments
J.S.G. is supported by the Michael Smith Foundation for Health Research. O.J. is supported by a grant from the Canadian Institute of Health Research and the Michael Smith Foundation for Health Research.
This work was presented in abstract form at the American Transplant Congress; San Francisco, CA; May 6, 2007.
Published online ahead of print. Publication date available at www.cjasn.org.
The data reported in this study were supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
References
- 1.Meier-Kriesche HU, Schold JD, Kaplan B: Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant 4: 1289–1295, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B: Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 4: 378–383, 2004 [DOI] [PubMed] [Google Scholar]
- 3.US Renal Data System: USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006, pp 147–158
- 4.Chang SH, Russ GR, Chadban SJ, Campbell SB, McDonald SP: Trends in kidney transplantation in Australia and New Zealand, 1993–2004. Transplantation 84: 611–618, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Ferraris JR, Tambutti ML, Cardoni RL, Prigoshin N: Conversion from cyclosporine A to tacrolimus in pediatric kidney transplant recipients with chronic rejection: Changes in the immune responses. Transplantation 77: 532–537, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Kaufman DB, Shapiro R, Lucey MR, Cherikh WS, T Bustami R, Dyke DB: Immunosuppression: Practice and trends. Am J Transplant 4[Suppl 9]: 38–53, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Meier-Kriesche HU, Chu AH, David KM, Chi-Burris K, Steffen BJ: Switching immunosuppression medications after renal transplantation: A common practice. Nephrol Dial Transplant 21: 2256–2262, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Morales JM, Andres A, Rengel M, Rodicio JL: Influence of cyclosporin, tacrolimus and rapamycin on renal function and arterial hypertension after renal transplantation. Nephrol Dial Transplant 16[Suppl 1]: 121–124, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Jindal RM, Hjelmesaeth J: Impact and management of posttransplant diabetes mellitus. Transplantation 70: SS58–SS63, 2000 [PubMed] [Google Scholar]
- 11.Agha IA, Lopez-Rocafort L, Wang C, Miller B, Hardinger K, Flavin K, Lowell J, Jendrisak M, Shenoy S, Schnitzler M, Storch G, Brennan CD: A prospective evaluation of BK virus infection in renal transplant patients [Abstract]. Am J Transplant 2: 260, 2002. 12096789 [Google Scholar]
- 12.Binet I, Nickeleit V, Hirsch HH, Prince O, Dalquen P, Gudat F, Mihatsch MJ, Thiel G: Polyomavirus disease under new immunosuppressive drugs: A cause of renal graft dysfunction and graft loss. Transplantation 67: 918–922, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Almond PS, Matas A, Gillingham K, Dunn DL, Payne WD, Gores P, Gruessner R, Najarian JS: Risk factors for chronic rejection in renal allograft recipients. Transplantation 55: 752–756, discussion 756–757, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Pirsch JD, Ploeg RJ, Gange S, D’Alessandro AM, Knechtle SJ, Sollinger HW, Kalayoglu M, Belzer FO: Determinants of graft survival after renal transplantation. Transplantation 61: 1581–1586, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Burroughs TE, Lentine KL, Takemoto SK, Swindle J, Machnicki G, Hardinger K, Brennan DC, Irish WD, Schnitzler MA: Influence of early posttransplantation prednisone and calcineurin inhibitor dosages on the incidence of new-onset diabetes. Clin J Am Soc Nephrol 2: 517–523, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM: Identifying persons with diabetes using Medicare claims data. Am J Med Qual 14: 270–277, 1999 [DOI] [PubMed] [Google Scholar]
- 17.McBean AM, Li S, Gilbertson DT, Collins AJ: Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: Whites, blacks, Hispanics, and Asians. Diabetes Care 27: 2317–2324, 2004 [DOI] [PubMed] [Google Scholar]
- 18.O’Connor PJ, Rush WA, Pronk NP, Cherney LM: Identifying diabetes mellitus or heart disease among health maintenance organization members: Sensitivity, specificity, predictive value, and cost of survey and database methods. Am J Manag Care 4: 335–342, 1998 [PubMed] [Google Scholar]
- 19.Rector TS, Wickstrom SL, Shah M, Thomas Greeenlee N, Rheault P, Rogowski J, Freedman V, Adams J, Escarce JJ: Specificity and sensitivity of claims-based algorithms for identifying members of Medicare+Choice health plans that have chronic medical conditions. Health Serv Res 39: 1839–1857, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosio FG, Pesavento TE, Osei K, Henry ML, Ferguson RM: Post-transplant diabetes mellitus: Increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int 59: 732–737, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Revanur VK, Jardine AG, Kingsmore DB, Jaques BC, Hamilton DH, Jindal RM: Influence of diabetes mellitus on patient and graft survival in recipients of kidney transplantation. Clin Transplant 15: 89–94, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Boudreaux JP, McHugh L, Canafax DM, Ascher N, Sutherland DE, Payne W, Simmons RL, Najarian JS, Fryd DS: The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation 44: 376–381, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Friedman EA, Shyh TP, Beyer MM, Manis T, Butt KM: Posttransplant diabetes in kidney transplant recipients. Am J Nephrol 5: 196–202, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Miles AM, Sumrani N, Horowitz R, Homel P, Maursky V, Markell MS, Distant DA, Hong JH, Sommer BG, Friedman EA: Diabetes mellitus after renal transplantation: As deleterious as non-transplant-associated diabetes? Transplantation 65: 380–384, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Roth D, Milgrom M, Esquenazi V, Fuller L, Burke G, Miller J: Posttransplant hyperglycemia: Increased incidence in cyclosporine-treated renal allograft recipients. Transplantation 47: 278–281, 1989 [PubMed] [Google Scholar]
- 26.Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, Hernandez D, Kasiske BL, Kiberd B, Krentz A, Legendre C, Marchetti P, Markell M, van der Woude FJ, Wheeler DC: New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 75: SS3–SS24, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 26[Suppl 1]: S5–S20, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM: Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int 62: 1440–1446, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Ducloux D, Kazory A, Chalopin JM: Posttransplant diabetes mellitus and atherosclerotic events in renal transplant recipients: A prospective study. Transplantation 79: 438–443, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Gill JS, Abichandani R, Kausz AT, Pereira BJ: Mortality after kidney transplant failure: The impact of non-immunologic factors. Kidney Int 62: 1875–1883, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Knoll G, Muirhead N, Trpeski L, Zhu N, Badovinac K: Patient survival following renal transplant failure in Canada. Am J Transplant 5: 1719–1724, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Meier-Kriesche HU, Kaplan B: Death after graft loss: A novel endpoint for renal transplantation. Transplant Proc 33: 3405–3406, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Kaplan B, Meier-Kriesche HU: Death after graft loss: An important late study endpoint in kidney transplantation. Am J Transplant 2: 970–974, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK: Long-term survival in renal transplant recipients with graft function. Kidney Int 57: 307–313, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Johnston O, Ghadder S, Rose C, Gill JS: Refining the Tacrolimus associated risk of post-transplant diabetes mellitus [Abstract]. Am J Transplant 7: 186. 2007 [Google Scholar]
- 36.Johnston O, Rose C, Gill JS: Sirolimus: an important risk factor for post transplant diabetes mellitus [Abstract]. Am J Transplant 7: 186. 2007 [Google Scholar]
- 37.Reisaeter AV, Hartmann A: Risk factors and incidence of posttransplant diabetes mellitus. Transplant Proc 33: 8S–18S, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Sumrani NB, Delaney V, Ding ZK, Davis R, Daskalakis P, Friedman EA, Butt KM, Hong JH: Diabetes mellitus after renal transplantation in the cyclosporine era: An analysis of risk factors. Transplantation 51: 343–347, 1991 [DOI] [PubMed] [Google Scholar]