Abstract
HIV-associated nephropathy (HIVAN) is characterized by collapsing FSGS. Because transgenic mice with podocyte-specific overexpression of the vascular endothelial growth factor 164 (VEGF164) isoform also develop collapsing FSGS, we sought to determine whether VEGF plays a role in HIVAN. Compared with controls, immunohistochemistry revealed that kidneys from HIV-1–transgenic mice (Tg26) and from patients with HIVAN had greater expression of both VEGF and its transcriptional regulator, hypoxia-inducible factor 2α (HIF-2α). Similarly, mRNA and protein levels of VEGF and HIF-2α were increased in HIV-infected podocytes in vitro, and this transcriptional upregulation was found to be stimulated by the HIV viral protein Nef in a Src kinase–and Stat3-dependent manner. HIV-1 also upregulated VEGFR2 and its co-receptor neuropilin-1 and suppressed the expression of semaphorin 3a in the podocyte. Exogenous VEGF stimulated proliferation and de-differentiation of podocytes, which are features of collapsing FSGS, and VEGFR2 neutralizing antibodies reversed these features in podocytes infected with HIV-1 or isolated from Tg26 mice. In conclusion, HIV-1 induces VEGF and VEGFR2 expression in podocytes, and this may be a critical step in the pathogenesis of HIVAN.
Vascular endothelial growth factor A (VEGF-A) belongs to a family of angiogenic growth factors. Its dysregulation plays a key role in disease states such as preeclampsia, diabetic nephropathy, and renal cell carcinoma.1–3 The biologic effects of VEGF correlate most closely to those of the VEGF165 isoform (VEGF164 in mice).4 VEGF's actions are mediated through two cell surface receptors, VEGFR1/Flt1 and VEGFR2/Flk1, and its co-receptors neuropilin 1/2 (Nrp).5
VEGF-A has been shown to play a critical role in both the establishment and maintenance of the glomerular filtration barrier.6 Transgenic mice with podocyte-specific overexpression of the VEGF164 isoform develop a collapsing glomerulopathy that resembles the classic renal lesion seen in HIV-associated nephropathy (HIVAN).6 Data have also provided evidence that VEGF may act as an autocrine factor for podocytes.7
The collapsing glomerular lesions that are classically seen in HIVAN are associated with podocyte proliferation and loss of podocyte differentiation markers, including WT1 and synaptopodin.8,9 In this study, we sought to determine whether VEGF is upregulated in podocytes from mice with HIVAN and whether blockade of VEGF receptors can ameliorate the podocyte proliferation and de-differentiation induced by HIV.
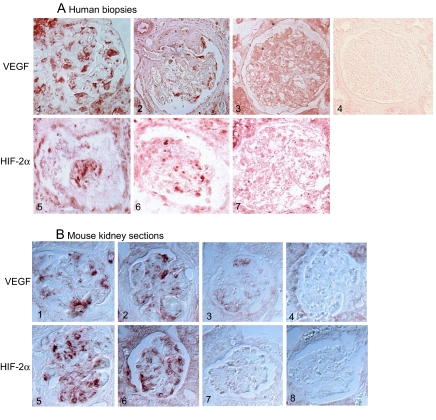
First, the expression of VEGF and hypoxia-inducible factor (HIF) was studied in vivo. By immunostaining, we found increased staining for both VEGF and its transcriptional regulator HIF-2α in kidneys of patients with HIVAN as well as in kidneys from Tg26 mice when compared with controls (Figure 1). We were not able to perform co-localization studies to determine whether the staining was podocyte specific because the markers of differentiation in podocytes are suppressed in Tg26 mice even in the absence of proliferation (Supplemental Figure 1).8 One limitation of our immunostaining is that VEGF staining with available antibodies does not distinguish between the proangiogenic (VEGF165) and antiangiogenic (VEGF165b) isoforms. VEGF165b binds VEGFR2 with the same affinity as VEGF165 but does not activate or stimulate downstream signaling pathways, thereby preventing VEGF165-mediated cell signaling.10 VEGF165b has been shown to be highly expressed in differentiated but not in de-differentiated podocytes.11
Figure 1.
(A) Immunohistochemical staining for VEGF-A and HIF-2α in human kidney biopsies. (1 through 3) VEGF staining: Representative pictures from biopsy specimens from HIV-positive patients with HIVAN show VEGF-A staining localized mostly to podocytes (1 and 2), and HIV-negative patients with minimal-change disease show only background staining (3). (4) Control: IgG staining shows diffuse background staining. (5 through 7) HIF-2α staining: Representative pictures from biopsy specimens from HIV-positive patients with HIVAN show strong HIF-2α expression localized mostly to the podocyte (5 and 6), and HIV-negative patients with minimal-change disease show little podocyte staining for HIF-2α (7). (B) Immunostaining for VEGF and HIF-2α in kidneys from Tg26 mice: (1 through 4) VEGF staining in kidney sections from Tg26 mouse (1 and 2), from littermate (3), or stained with control IgG (4). (5 through 8) HIF-2α staining in kidneys sections from Tg26 mouse (5 and 6), from littermate (7), or stained with control IgG (8). Bright light microscopy (Olympus BX60). Magnification, ×20.
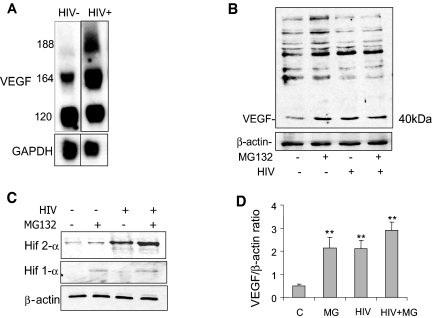
Next, we studied how HIV regulates VEGF expression in cultured podocytes. We found that the VEGF isoforms 164 and 188 were upregulated at the mRNA level in HIV-infected podocytes when compared with control cells, and protein expression of VEGF164 was increased (Figure 2, A and B). Treatment with MG132, a proteasomal inhibitor, augmented levels of VEGF in control podocytes, whereas treatment with MG132 in HIV-infected podocytes did not significantly increase levels of VEGF expression (Figure 2, B and D). We determined whether HIF levels are increased in HIV-infected podocytes, because HIF is known to activate transcription of VEGF and MG132 blocks degradation of HIF. Whereas treatment with MG132 slightly increased both HIF-1α and HIF-2α protein levels, HIV-infected podocytes had markedly increased expression of HIF-2α when compared with control podocytes (Figure 2C). Expression of HIF-1α did not change after HIV infection (Figure 2C). Our data suggest that HIV increases VEGF expression through stimulation of HIF-2α. HIF plays a key role in hypoxic conditions and serves as a transcription factor for genes that mediate adaptive responses to reduced oxygen availability, including VEGF and erythropoietin, commonly via changes that decrease HIF's degradation.12 Interestingly, our findings suggest that HIV markedly induces HIF-2α but not HIF-1α, indicating a hypoxia-independent pathway.
Figure 2.
(A) HIV-1 upregulates expression of the VEGF isoforms 164 and 188. mRNA levels for VEGF-A and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were analyzed in control vector-infected podocytes and HIV-infected podocytes by Northern blotting. (B) HIV upregulates expression of VEGF165 at the protein level, and inhibition of proteasomal degradation with MG132 treatment upregulates VEGF165. This is a representative blot of three independent experiments. (C) HIV upregulates expression of HIF-2α at the protein level, and inhibition of proteasomal degradation upregulates HIF-2α and HIF-1α levels in control cells. This is a representative blot of three independent experiments. (D) Densitometric analysis of blots in B are summarized, n = 3, **P < 0.01 versus control.
To identify which HIV-1–encoded gene was responsible for inducing expression of HIF-2α and VEGF, the genes for vpr, tat, and nef were introduced individually into conditionally immortalized podocytes. We found that only the nef gene was responsible for HIV-induced HIF-2α and VEGF expression in podocytes (Supplemental Figure 2). Because we have shown previously that Nef activates the Src/Stat3 pathway in podocytes13 and the Src/Stat3 pathway has been shown to mediate HIF-2α expression in other cell lines,14 we investigated whether Src/Stat3 mediates Nef-induced HIF-2α expression in podocytes. We found that both pp2 (a Src kinase inhibitor) as well as a dominant negative mutant for Stat3 inhibited Nef-induced HIF-2α expression, suggesting that the Src/Stat3 pathway indeed mediates the Nef-induced increase in HIF-2α expression in podocytes (Supplemental Figure 2E).
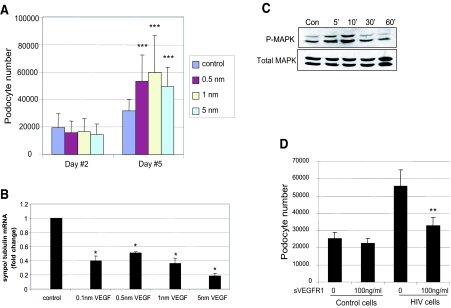
In vitro HIV-1–infected podocytes and podocytes from HIV-1–transgenic mice exhibit podocyte proliferation and loss of differentiation markers.9,15,16 In vitro studies support a clear role for Nef in podocyte proliferation and de-differentiation9; therefore, we determined whether VEGF mediates HIV/Nef-induced podocyte proliferation. We found that treatment with exogenous VEGF significantly increased podocyte number (Figure 3A). VEGF administration did not increase podocyte apoptosis or necrosis (data not shown). Levels of mRNA of the podocyte-specific marker synaptopodin were significantly decreased in VEGF-stimulated podocytes (Figure 3B). VEGF also induced p44/42 mitogen-activated protein kinase (MAPK) phosphorylation (Figure 3C). Treatment of HIV-infected cells with recombinant sVEGFR1, which traps endogenous VEGF and inhibits its binding with cell surface receptors, significantly reduced podocyte proliferation induced by HIV (Figure 3D). These data suggest that VEGF mediates the effects of HIV-1 infection on podocyte proliferation and de-differentiation. Consistent with our studies, Ding et al.17 showed that podocyte-specific deletion of the Von Hippel-Lindau gene (Vhlh) leads to stabilization of HIF-α subunits, causing podocyte proliferation and the development of the clinical features of rapidly progressive glomerulonephritis.
Figure 3.
(A) Effect of VEGF on podocyte proliferation. Podocytes were exposed to no VEGF or VEGF at indicated concentration. Cell number was determined at days 2 and 5. Bars represent mean cell number ± SE of three samples. ***P < 0.005 versus cells without VEGF treatment. (B) VEGF causes reduction of synaptopodin expression. Podocytes were incubated with or without VEGF for 2 d, and RNA was isolated. The synaptopodin/tubulin ratio was determined by real-time PCR. The fold of increase as compared with control podocytes is expressed. Means ± SEM of three independent experiments are shown; *P < 0.05 versus control. (C) VEGF stimulates MAPK phosphorylation. Podocytes were incubated with 1 nm of VEGF or no VEGF for 5, 10, 30, and 60 min. Total and phosphorylated MAPK was determined by Western blot using anti-MAPK and anti–p-MAPK. The representative blot of three independent experiments is shown. (D) Effect of recombinant sVEGFR1 on HIV-induced podocyte proliferation. Control and HIV-infected podocytes were treated with recombinant sVEGFR1 or control vehicle daily for 3 d, and then cell number was determined. **P < 0.01; n = 4 versus vehicle-treated cells.
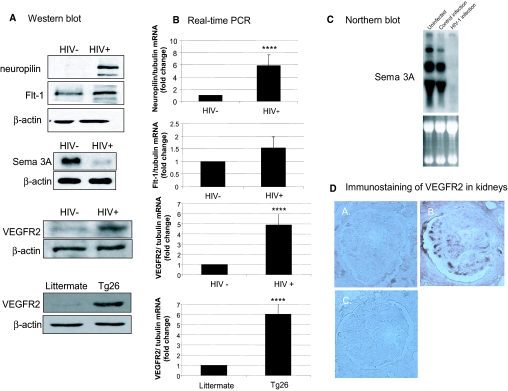
We also examined whether HIV infection regulates VEGF receptor expression in podocytes. We found that both protein and RNA expression of Flt1, Nrp-1, and VEGFR2 was increased and expression of Semaphorin 3A (Sema 3A) was downregulated in HIV-infected podocytes (Figure 4A through C). VEGFR2 expression was also increased in podocytes from Tg26 mice (Figure 4A) and in HIVAN kidneys (Figure 4D). Human podocytes have been shown to express VEGFR1, VEGFR3, Nrp-1, and Nrp-2, and recent evidence suggests that VEGFR2 may be inducible in differentiated murine podocytes.7,18 Our data suggest that HIV may induce VEGFR2 expression in both human and murine podocytes.
Figure 4.
(A) Effects of HIV-1 infection on protein expression of neuropilin-1, Flt1, VEGFR2, and Sema 3A in podocytes. Neuropilin-1, Flt1, VEGFR2, and Sema 3A expression was determined by Western blot in HIV-infected and control-infected podocytes. VEGFR2 expression was also examined in podocytes isolated from Tg26 mice and their littermates. These are representative blots of three independent experiments. (B) The neuropilin-1/tubulin, Flt1/tubulin, and VEGFR2/tubulin ratios were determined by real-time PCR. The fold of increase as compared with control podocytes is expressed. Means ± SEM of three independent experiments are shown. ****P < 0.001 versus control. (C) Northern blot analysis of Sema 3A expression was performed using RNA from podocytes infected with HIV-1 versus the control construct and uninfected podocytes. A representative blot of three independent experiments is shown demonstrating dramatic reduction in the expression of Sema 3A in response to the HIV-1 transgene as compared with the control infection (top). The ethidium bromide–stained RNA gel before transfer is shown to demonstrate that equivalent amounts of RNA were used for the analysis (bottom). (D) Immunostaining of VEGFR2 in kidney biopsies from patients with HIVAN (B) compared with patients with minimal-change disease (A) and IgG control (C).
Next, we determined whether VEGFR2 mediates the effects of HIV-1 on podocytes using both in vitro HIV-infected podocytes and podocytes isolated from Tg26 mice. We found that treatment with neutralizing antibodies against VEGFR2 caused less proliferation and increased synaptopodin expression both in HIV-infected and Tg26 podocytes when compared with podocytes treated with anti-flag antibody (Supplemental Figure 3) or rat IgG2a (same IgG class of anti-VEGFR2 antibody; data not shown). These data support a role for VEGFR2 in HIV-induced podocyte proliferation and de-differentiation.
The precise role of VEGFR2 and Nrp-1 in podocyte biology and pathology remains unclear. Recent studies show that tyrosine kinase inhibitors that block the actions of the VEGF receptors can decrease podocyte proliferation.7 Although Nrp-1 had previously been considered a nonsignaling VEGF co-receptor, VEGF has been shown to signal in an autocrine manner through Nrp-1 in the absence of Flk1 in breast carcinoma cells that are tyrosine kinase receptor negative.19 HIV also induces upregulation of both VEGFR2 and its co-receptor Nrp-1, which may facilitate and enhance VEGF165's interaction with VEGFR2.20 Interestingly, HIV infection also reduces podocyte expression of Sema 3A, which exerts its effects through its receptors Nrp-1 and plexinA.21 Sema 3A has been recently shown to regulate expression and interactions among several slit-diaphragm proteins.22
Our study demonstrates that Nef-Src/Stat3-HIF-VEGF is a novel pathway for podocyte proliferation and de-differentiation in HIVAN. In vivo, podocyte-specific expression of Nef in transgenic mice induces an abnormal podocyte phenotype characterized by increased Ki67, loss of synaptopodin, and increased phospho-Stat3.15 These mice, however, do not have significant glomerulosclerosis (GS) or podocyte proliferation. One possible explanation is that these transgenic mice are on the C57/b6 background, which is known to be resistant to GS.23 In contrast, recent studies have shown that podocyte-specific expression of Nef in transgenic mice on the FVB/N genetic background develop significant GS.24 In these mice, podocytes exhibit a loss of differentiation markers in the absence of proliferation. Thus, the exact combination of factors that lead to podocyte proliferation in vivo remains unclear.
Our study also suggests the presence of a unique autocrine loop for VEGF in podocytes. Autocrine loops have been previously described in both endothelial and hematopoietic stem cells25,26; however, in both of these systems, treatment with VEGFR antagonists that act extracellularly fail to reverse the effects of VEGF, whereas those that are able to penetrate the intracellular compartment mimic the deletion of VEGF.25,26 In addition, exogenous treatment with VEGF is unable to compensate for the loss of endogenous VEGF in endothelial cells in which VEGF has been specifically deleted, suggesting the presence of an internal VEGF-dependent autocrine loop.25 In contrast, we found that treatment with either soluble VEGFR or VEGFR2 neutralizing antibodies can reverse the podocyte proliferation and de-differentiation induced by HIV infection. Thus, it seems that the VEGF autocrine loop functions differently in podocytes.
In conclusion, our data show that HIV infection stimulates VEGF production in podocytes through the Nef-Src/Stat3–HIF-2α pathway. Excess of VEGF production, along with upregulation of its receptors (VEGFR2 and Nrp-1) and downregulation of its protective ligand (Sema 3A), contributes to podocyte proliferation and loss of synaptopodin in HIVAN. Our data provide new insight into the underlying molecular mechanisms of podocyte disease of HIVAN. Future studies will determine whether administration of anti-VEGFR2 drugs could have beneficial effects in Tg26 mice, an animal model of HIVAN.
CONCISE METHODS
Immunohistochemistry and Immunofluorescence
Human biopsy specimens from patients with HIVAN and minimal-change disease were obtained courtesy of Dr. Vivette d'Agati (Columbia University) from routine biopsies performed after institutional review board approval. Paraffin-embedded and paraformaldehyde-fixed kidneys were obtained from Tg26 mice and their littermates. Five specimens from each group were tested with each antibody after they were dewaxed and rehydrated. Antigen retrieval was performed on Tg26 and littermate specimens after deparaffinizing and rehydrating the sections. The slides were immersed in citrate buffer (10 mM citric acid and 0.05% Tween 20 [pH 6.0]), heated to boiling point for 5 min, cooled to room temperature for 20 min, washed in PBS two times, and then followed by standard staining protocol. All specimens were incubated with primary antibody (anti-VEGF [Invitrogen, Carlsbad, CA], HIF-2α antibody [Novus Biologicals, Littleton, CO], anti-human VEGFR2 antibody [R&D Systems, Minneapolis, MN], rabbit IgG [Sigma-Aldrich, St. Louis, MO], or mouse IgG [Santa Cruz Biotechnology, Santa Cruz, CA]). This was followed by incubation with biotinylated secondary antibody and streptavidin/biotin–horseradish peroxidase complex prepared using Vectastain ABC Elite reagents (Vector Laboratories, Burlingame, CA).
Immunofluorescence staining was performed for VEGF, HIF-2α, and synaptopodin in mouse kidney sections from Tg26 mice and their littermates. Paraffin-embedded sections were de-paraffinized as described. Sections were incubated with primary antibodies (anti-VEGF [Invitrogen]; HIF-2α antibody [Novus Biologicals]; anti-synaptopodin [gift from Dr. Peter Mundel, Mount Sinai School of Medicine, New York, NY]) for 1 h at room temperature and then with FITC-labeled second antibodies with different wavelengths for co-localization study. Sections were examined using fluorescence microscopy.
Conditionally Immortalized Murine Podocytes with HIV-1–Expressing or Control Vector
Conditionally immortalized murine podocytes were isolated as described previously.27 Production of HIV-1 constructs has been described previously.9 The podocytes used in these experiments are from an established line of pNL4-3:d1443 and pHR-CMV-IRES2-GFP-ΔB–infected podocytes. In all experiments, cells were grown at 37°C on type 1 collagen–coated dishes for 10 d to inactivate temperature-sensitive T antigen and allow for differentiation.
Northern Blot Analysis
Total RNA was extracted using Trizol (Life Technologies BRL, Grand Island, NY). VEGF, Sema 3A, and glyceraldehyde-3-phosphate dehydrogenase probes were generated by reverse transcriptase–PCR from RNA isolated from normal mouse glomeruli. The cDNA probes were radiolabeled with [32P-α]dCTP and hybridized with Hybrisol (Intergen, Purchase, NY).
Western Blot
pNL4-3:d1443 and pHR-CMV-IRES2-GFP-ΔB–infected podocytes were lysed and subjected to Western blot analysis using the following antibodies: Neuropilin-1 (Abcam, Cambridge, MA), Flk1 (Santa Cruz Biotechnology), VEGFR2 (R&D Systems), Sema 3A (Abcam), and β-actin (Sigma-Aldrich). pNL4-3:d1443 and pHR-CMV-IRES2-GFP-ΔB podocytes were grown to confluence and stimulated with Mg132 (10 μM; Calbiochem, La Jolla, CA) or dimethyl-sulfoxide (Sigma-Aldrich) at a concentration of 1:1000 overnight. Cell lysates and nuclear extracts were subjected to Western Blot analysis with anti-VEGF antibody (R&D Systems) and HIF-1α and HIF-2α antibodies (Novus Biologicals).
HIV-1 Constructs
The production of HIV-1 constructs has been described previously.28 The vpr, tat, and nef constructs as well as a control green fluorescence protein–containing construct and a Stat3 dominant negative mutant (a gift from Dr. C.M. Horvath, The Rockefeller University, New York, NY) were individually introduced into wild-type conditionally immortalized podocytes maintained at 33°C via nucleofection (Amaxa Biosystems, Cologne, Germany), and the cells were transferred to 37°C for 3 d.29 The transfection efficiency was evaluated by fluorescence microscopy confirming 70 to 80% efficiency.
Real-Time PCR
Real-time PCR was performed with a Roche LightCycler using a QuantiTect One Step RTPCR SYBR green kit (Qiagen, Valencia, CA) using validated primer sets for VEGF, endothelial PAS domain protein, and VEGFR2/kinase insert domain receptor (Qiagen). Melting-curve analysis was performed to check for a single amplicon and verified by size using gel electrophoresis. Specific primers for synaptopodin (5′-GCC AGG GAC CAG CCA GAT A-3′ and 3′-AGG AGC CCA GGC CTT CTC T-5′) and tubulin (5′-TGC CTT TGT GCA CTG GTA TG-3′ and 3′-CTG GAG CAG TTT GAC GAC AC-5′) were designed (MWG-Biotech, High Point, NC). LightCycler analysis software was used for determining crossing points using the second derivative method. Data were analyzed by the 2−ΔΔ CT method and presented as fold increase normalized to the housekeeping gene tubulin.30
Cell Proliferation and Determination of Cell Signaling Activation and Differentiation
Conditionally immortalized podocytes were split and plated into 24-well collagen-coated plates at a density of 20,000 cells/well after differentiation. The cells were serum-starved overnight and exposed to medium with or without recombinant human VEGF165 (R&D Systems) in concentrations of 0.1, 0.5, 1, or 5 nm. HIV-infected podocytes and their control cells were also exposed to recombinant sVEGFR1 (R&D Systems; 100 ng/ml) daily for 3 d to block the effects of VEGF. Cells were trypsinized and counted on days 2 and 5. RNA was also isolated from samples and used for real-time PCR using a synaptopodin-specific primer.
Differentiated podocytes were stimulated for 0, 10, 20, 30, and 60 min with 1 nm of VEGF. Cell lysates were analyzed using Western blot analysis using anti–phospho-MAPK1 and 2 and anti-MAPK1 and 2 (Cell Signaling Laboratory, Danvers, MA).
Determination of Podocyte Apoptosis/Necrosis
Differentiated podocytes were exposed to medium with or without VEGF in concentrations ranging from 0.1 to 10 nm, and apoptosis was assessed by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling staining (Promega, Madison, WI). Apoptotic cells with nuclei staining dark brown were counted by light microscopy by two independent investigators. Apoptosis was expressed as the ratio of apoptotic cells to the total number of cells in the field of view. FACS was used to quantify apoptotic and necrotic podocytes after annexin V-FITC and propidium iodide labeling (BD Bioscience, San Jose, CA).
VEGFR2 Inhibition
Transgenic podocytes and in vitro HIV-1–infected podocytes were differentiated at 37°C for 7 d and then treated with either neutralizing antibody against VEGFR2/Flk1 (1 μg/ml; cat. no. MAB4431, rat IgG2a class; R&D Systems) or an equivalent amount of a nonrelevant antibody (anti-flag mAb of mouse IgG1; cat. no. F3165; Sigma) or a rat IgG2a isotype control.
The transgenic and HIV-infected podocytes were also split and plated into 24-well dishes at a density of 20,000 cells/well and exposed to medium with anti-VEGFR2/Flk1 (1 μg/ml) or anti-flag antibody. Cells were trypsinized and counted on day 3.
Statistical Analysis
All experiments were performed at least three times. In cell proliferation and real-time PCR results, data are expressed as means ± SEM. Statistically significant differences between the means were determined by unpaired t test where appropriate. Significance was defined as P ≤ 0.05.
DISCLOSURES
None.
Acknowledgments
This work was supported by National Institutes of Health grants DK065495 and DK078897 (to J.H.), DK056492 (to P.K.), DK038761 (to R.I.), DK079781 (to T.L.), and DK076523 (to S.K.).
Portions of this work were presented in abstract form at the annual meeting of the American Society of Nephrology; November 14 through 19, 2006; San Diego, CA.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA: Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Takahashi A, Sasaki H, Kim SJ, Tobisu K, Kakizoe T, Tsukamoto T, Kumamoto Y, Sugimura T, Terada M: Markedly increased amounts of messenger RNAs for vascular endothelial growth factor and placenta growth factor in renal cell carcinoma associated with angiogenesis. Cancer Res 54: 4233–4237, 1994 [PubMed] [Google Scholar]
- 3.Khamaisi M, Schrijvers BF, De Vriese AS, Raz I, Flyvbjerg A: The emerging role of VEGF in diabetic kidney disease. Nephrol Dial Transplant 18: 1427–1430, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N: Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem 267: 26031–26037, 1992 [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP, LeCouter J: The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster RR, Hole R, Anderson K, Satchell SC, Coward RJ, Mathieson PW, Gillatt DA, Saleem MA, Bates DO, Harper SJ: Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes. Am J Physiol Renal Physiol 284: F1263–1273, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Barisoni L, Kriz W, Mundel P, D'Agati V: The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Sunamoto M, Husain M, He JC, Schwartz EJ, Klotman PE: Critical role for Nef in HIV-1-induced podocyte dedifferentiation. Kidney Int 64: 1695–1701, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, Foster R, Digby-Bell J, Shields JD, Whittles CE, Mushens RE, Gillatt DA, Ziche M, Harper SJ, Bates DO: VEGF165b, an inhibitory vascular endothelial growth factor splice variant: Mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res 64: 7822–7835, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Cui TG, Foster RR, Saleem M, Mathieson PW, Gillatt DA, Bates DO, Harper SJ: Differentiated human podocytes endogenously express an inhibitory isoform of vascular endothelial growth factor (VEGF165b) mRNA and protein. Am J Physiol Renal Physiol 286: F767–F773, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ: Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001 [DOI] [PubMed] [Google Scholar]
- 13.He JC, Husain M, Sunamoto M, D'Agati VD, Klotman ME, Iyengar R, Klotman PE: Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J Clin Invest 114: 643–651, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, Cheng JQ, Jove R, Yu H: Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene 24: 5552–5560, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Husain M, D'Agati VD, He JC, Klotman ME, Klotman PE: HIV-1 Nef induces dedifferentiation of podocytes in vivo: a characteristic feature of HIVAN. AIDS 19: 1975–1980, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Kajiyama W, Kopp JB, Marinos NJ, Klotman PE, Dickie P: Glomerulosclerosis and viral gene expression in HIV-transgenic mice: Role of nef. Kidney Int 58: 1148–1159, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Ding M, Cui S, Li C, Jothy S, Haase V, Steer BM, Marsden PA, Pippin J, Shankland S, Rastaldi MP, Cohen CD, Kretzler M, Quaggin SE: Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med 12: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Guan F, Villegas G, Teichman J, Mundel P, Tufro A: Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol Renal Physiol 291: F422–F428, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM: Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res 61: 5736–5740, 2001 [PubMed] [Google Scholar]
- 20.Fuh G, Garcia KC, de Vos AM: The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J Biol Chem 275: 26690–26695, 2000 [DOI] [PubMed] [Google Scholar]
- 21.He Z, Tessier-Lavigne M: Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90: 739–751, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Guan F, Villegas G, Teichman J, Mundel P, Tufro A: Autocrine class 3 semaphorin system regulates slit diaphragm proteins and podocyte survival. Kidney Int 69: 1564–1569, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Gharavi AG, Ahmad T, Wong RD, Hooshyar R, Vaughn J, Oller S, Frankel RZ, Bruggeman LA, D'Agati VD, Klotman PE, Lifton RP: Mapping a locus for susceptibility to HIV-1-associated nephropathy to mouse chromosome 3. Proc Natl Acad Sci U S A 101: 2488–2493, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo Y, Matsusaka T, Zhong J, Ma J, Ma LJ, Hanna Z, Jolicoeur P, Fogo AB, Ichikawa I: HIV-1 genes vpr and nef synergistically damage podocytes, leading to glomerulosclerosis. J Am Soc Nephrol 17: 2832–2843, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML: Autocrine VEGF signaling is required for vascular homeostasis. Cell 130: 691–703, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N: VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417: 954–958, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Husain M, Gusella GL, Klotman ME, Gelman IH, Ross MD, Schwartz EJ, Cara A, Klotman PE: HIV-1 Nef induces proliferation and anchorage-independent growth in podocytes. J Am Soc Nephrol 13: 1806–1815, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Chuang PY, Yu Q, Fang W, Uribarri J, He JC: Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int 72: 965–976, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]