Abstract
In the fibrotic kidney, tubular epithelial cells express CCN2, formerly known as connective tissue growth factor. Because little is known about the transcriptional regulation of this profibrotic protein, this study investigated the mechanism underlying epithelial cell–selective upregulation of CCN2 in fibrosis. It was found that a previously unidentified cis-regulatory element located in the promoter of the murine CCN2 gene plays an essential role in basal and TGF-β1–induced gene transcription in tubular epithelial cells; this element acts in conjunction with the Smad-binding element and the basal control element-1. By protein mass fingerprint analysis and de novo sequencing, poly(ADP-ribose) polymerase-1 (PARP-1) was identified as a trans-acting protein factor that binds to this promoter region, which we termed the PARP-1–binding element. In vivo, knockdown of PARP-1 in proximal tubular epithelial cells significantly reduced CCN2 mRNA levels and attenuated interstitial fibrosis in the obstructed kidney. Thus, the PARP-1/PARP-1 binding element complex functions as a nonspecific, fundamental enhancer of both basal and induced CCN2 gene transcription in tubular epithelial cells. This regulatory complex may be a promising target for antifibrotic therapy.
CCN2 is a 38-kD cysteine-rich peptide that belongs to the CTGF, cyr 61/cef 10, nov family participating in a wide variety of biologic processes such as embryonic development and tissue repair.1 CCN2, formerly referred to as connective tissue growth factor, is a potent and ubiquitously expressed growth factor for fibroblasts, chondrocytes, and vascular endothelial cells. Previously, we showed that tubular epithelial cells also express CCN2, which played a pivotal role in renal fibrosis.2–4 A better understanding of the control of CCN2 expression in the tubular epithelial cells should facilitate the development of novel antifibrotic therapies targeting renal fibrosis. The expression of CCN2 has been studied in detail in fibroblasts, and TGF-β1 is a major stimulator of CCN2 gene expression in most mesenchymal cells.1,5 In skin fibroblasts, the TGF-β response element, known as the basal control element-1 (BCE-1) in the CCN2 promoter, is mainly responsible for the basal expression of CCN2.6,7 High constitutive CCN2 expression in scleroderma fibroblasts results from enhanced activation of BCE-1 and Sp1.7,8 In addition, a Smad-binding element (SBE) and an Ets1-binding site are necessary for TGF-β induction of CCN2 in skin fibroblasts.5,7,9 In addition, we and others also demonstrated that TGF-β is an important inducer of CCN2 in epithelial cells.2–5,10 Although several consensus gene regulatory motifs, including SBE, BCE-1, Ets1, and Sp1, have been identified in the murine CCN2 promoter, their functional relevance in renal tubular epithelial cells has not yet been demonstrated; therefore, in this study, we investigated the mechanism underlying CCN2 transcription in tubular epithelial cells.
RESULTS
A Novel Cis-Regulatory Element in the CCN2 Promoter
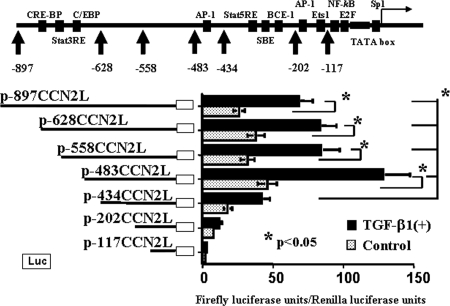
Bioinformatic analysis revealed a number of consensus motifs in the promoter sequence of the mouse CCN2 gene (representative ones are shown in Figure 1). Transient transfection experiments with firefly luciferase reporter minigenes bearing various CCN2 promoter fragments revealed that there were positive regulatory elements located within the −483- to −434-bp and −434- to −202-bp fragments (Figure 1). The latter fragment contains the SBE and BCE-1 sites that were shown to be responsive to TGF-β1, whereas the former fragment contains only a putative AP-1 binding site but enhances the basal and TGF-β1–induced CCN2 promoter activities approximately three-fold (Figure 1). Because the enhancement of reporter gene expression by the former −483- to −434-bp fragment was significant, further experiments were performed to identify additional cis-regulatory elements in this fragment. As shown in Figure 2, we identified cis-elements in the −483- to −434-bp fragment, other than the AP-1 binding site, that contributed significantly to the CCN2 promoter activity at the basal condition as well as in response to recombinant human TGF-β1 (rhTGF-β1), which depended on coexistence of both the SBE and BCE-1 sites. We next performed transient transfection analyses with additional luciferase reporter minigenes (Figure 3) so as to identify more precisely the location of the cis-elements within this promoter region. This analysis revealed that the −455- to −434-bp fragment (TGAGAAGTTTTTATGTCAGTAG) contained positive cis-regulatory elements. Bioinformatic reanalysis of this sequence suggested that it is not found within other promoters in the mouse genome but that it has some sequence homologies with known motifs, such as GRE_C, HFH1_01, MYB_Q6, SRY_01, and Oct1_03 sites (TFBIND; http://tfbind.ims.u-tokyo.ac.jp/), the former of which is bound by the zinc-finger protein site of glucocorticoid–glucocorticoid receptor complex. This sequence is highly conserved in the 5′ upstream genomic sequences of the rat and human CCN2 genes (TGAGAAATTTTTATATCAGTAG and TTCGAATTTTTTAGGAATTCCT, respectively; UCSC Genome Bioinformatics; http://genome.ucsc.edu/).
Figure 1.
Activity of murine CCN2 promoter fragments with or without rhTGF-β1 treatment in mProx24 cells. Known consensus motifs in the mouse CCN2 promoter and firefly luciferase reporter minigenes are shown. The genomic fragments corresponding to −483 to −434 bp and −434 to −202 bp within the CCN2 promoter contain positive cis-regulatory elements. The latter fragment that contains the SBE and BEC-1 enhances TGF-β1–induced CCN2 promoter activity rather than its basal activity (p-434CCN2L versus p-202CCN2L). In contrast, the former fragment enhances both basal and TGF-β1–induced CCN2 promoter activities (p-483CCN2L versus p-434CCN2L). The data were obtained from four independent experiments.
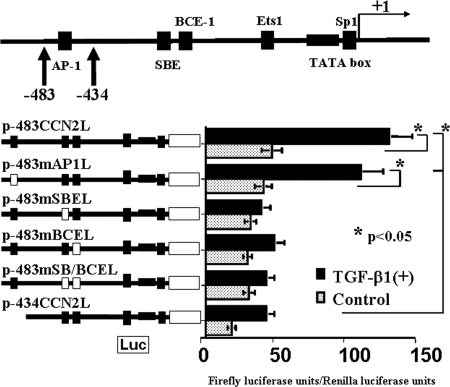
Figure 2.
Analysis of positive cis-regulatory elements at −483 to −434 bp within the CCN2 promoter. A putative AP-1 site is identified in this region, and there are known positive regulatory elements such as SBE, BCE-1, Ets1, and SP-1 sites within a region corresponding to −434 to 1 bp within the CCN2 promoter. The plasmids p-483mAP1L, p-483mSBEL, p-483mBCEL, and p-483mSB/BCEL contained mutated consensus motifs in their genomic sequences as shown in Table 1. Mutation of the AP-1 site was without effects so that other active elements would seem to be present within the region corresponding to −483 to −434 bp (p-483CCN2L versus p-483mAP1L). All of the regulatory elements (i.e., ones within a region corresponding to −483 to −434 bp and the SBE and BCE-1) were necessary for the full activity of the CCN2 promoter in response to rhTGF-β1 in mProx24 cells (p-483CCN2L versus p-483mSBEL, p-483mBCEL, p-483mSB/BCEL, and p-434CCN2L). The data were obtained from four independent experiments.
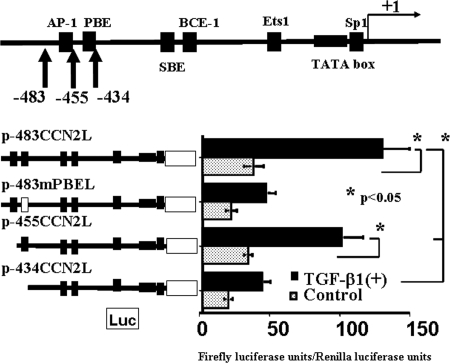
Figure 3.
Analysis of positive cis-regulatory elements within the −455- to −434-bp region of the CCN2 promoter. Deletion of the −483- to −455-bp region in the CCN2 promoter from p-483CCN2L was without effects (p-483CCN2L versus p-455CCN2L). In contrast, mutation or deletion of sequences corresponding to −455 to −434 bp within the CCN2 promoter significantly reduced both basal and TGF-β1–induced CCN2 promoter activities in mProx24 cells (p-455CCN2L versus p-483mPBEL and p-434CCN2L), suggesting that this region contains positive cis-regulatory elements. The plasmid p-483mPBEL contained a mutated consensus motif in the genomic sequence as shown in Table 1. The data were obtained from four independent experiments.
Electrophoretic Mobility Shift Assay
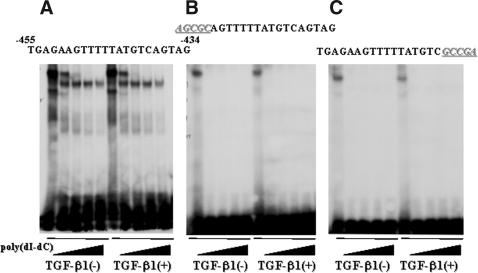
We next performed an electrophoretic mobility shift assay (EMSA) to determine whether proteins from tubular epithelial cell nuclear extracts specifically bind the −455- to −434-bp fragment. As shown in Figure 4, we observed a single protein–DNA complex that was not observed when we used oligodeoxynucleotide (ODN) probes containing mutations in either the 5′ or 3′ site, suggesting that this protein complex was specific for this sequence motif. This result also revealed that the binding site likely spanned −455 to −434 bp in the CCN2 promoter, yielding a 22-bp-long cis-element. In addition, nuclear factors binding to that site seemed to be expressed constitutively in tubular epithelial cells, because treatment of the cells with rhTGF-β1 before preparation of the nuclear extracts did not affect formation of the protein–DNA complex observed in this EMSA (Figure 4). This finding seems compatible with the observation that the −455- to −434-bp fragment enhances basal as well as TGF-β1–induced CCN2 gene transcription, suggesting that this is a nonspecific, fundamental enhancer active in tubular epithelial cells (Figures 1 through 3).
Figure 4.
Nuclear trans-acting factors bind to the −455- to −434-bp CCN2 promoter fragment. (A) Proteins within nuclear extracts prepared from mProx24 cells incubated in the presence or absence of rhTGF-β1 bound to the −455- to −434-bp CCN2 promoter fragment to form a single shifted band in the EMSA. (B and C) The shifted band was abolished when either the 5′- or 3′-most five bases of the sequence were mutated. These results indicated that this trans-factor (complex) was constitutively expressed and that it recognized the whole sequence of this fragment. The data were obtained from four independent experiments in each case.
A Trans-Binding Factor, Poly(ADP-Ribose) Polymerase-1
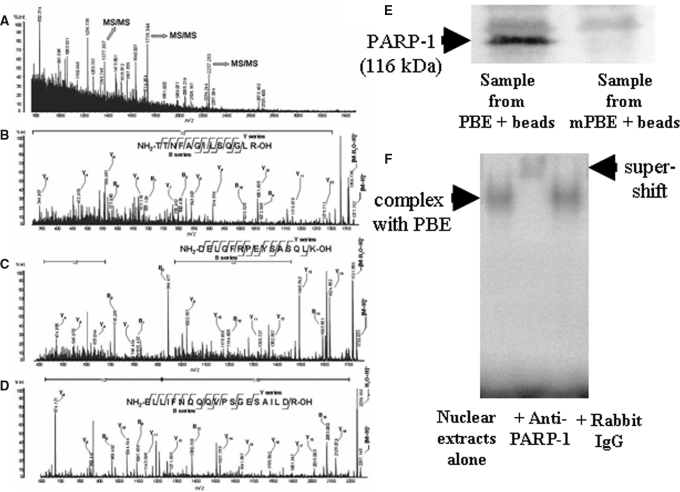
Purified target proteins formed a single band of an apparent molecular weight of 116 kD after analysis by 10% SDS-PAGE (data not shown). Protein identification by protein mass fingerprint (PMF) analysis provided mass information on the in-gel digested peptides of the target protein, as shown in Figure 5A. The spectra did not correspond to that of any known proteins in a protein database search; therefore, we performed de novo sequencing on three major ions in the PMF spectra using Matrix-assisted laser-desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS; Figure 5, B through D). The predicted amino acid sequences of the three ions were identical to murine poly(ADP-ribose) polymerase-1 (PARP-1), and this protein had been contained in the list of the proteins suggested by the PMF analysis. In additional experiments, the target protein purified using the biotin-streptavidin DNA affinity technique was electrophoresed through a 10% SDS-PAGE gel, transferred to a nitrocellulose membrane, and identified as the 116-kD PARP-1 protein by immunoblotting with an anti–PARP-1 antibody (Figure 5E). A supershift assay performed with the anti–PARP-1 antibody also confirmed that the trans-acting factor bound to this −455- to −434-bp CCN2 promoter fragment is the PARP-1 protein (Figure 5F).
Figure 5.
Representative MALDI-TOF MS spectra in PMF analysis (A) and de novo sequence analyses (B through D). MS/MS spectra of each parent peptide ion m/z 1377.807, 1739.944, and 2257.283 in A are B, C, and D, respectively. Bn and Yn show generic description of potential ions that are formed by fragmentation of a parent peptide. The Bn and Yn ions are fragments that retain the charge at the amino end (N-terminus) and carboxy end (C-terminus) of the peptide molecules, respectively. Location and N-terminus–or C-terminus–derived direction of the fragment ions that reflect the peptide bonds in the database search are shown with amino acid sequence of the parent peptides. (E) Nuclear protein purified using the biotin-streptavidin DNA affinity technique with the identified putative DNA binding sequence (PBE) was targeted for immunoblotting, which revealed that the trans-factor protein was a 116 kD, PARP-1 protein. mPBE, mutated PBE. (F) Anti–PARP-1 antibody caused a supershift of the shifted band of the −455- to −434-bp CCN2 promoter fragment (PBE) bound to the nuclear protein extracts in the EMSA analysis, but application of rabbit IgG was without effects, indicating that PARP-1 protein interacted with the PBE. The blot is representative of four independent experiments.
Roles of PARP-1 In Vitro
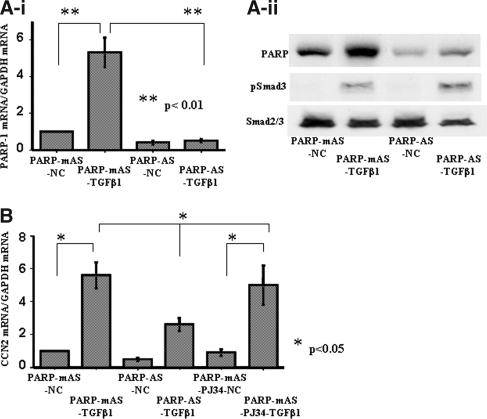
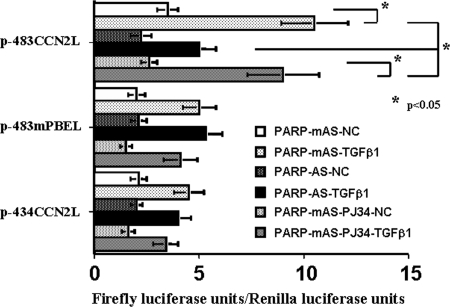
To determine whether PARP-1 is of importance for CCN2 gene transcription in tubular epithelial cells, we transfected PARP-1 antisense ODN into mProx24 cells by electroporation to knockdown PARP-1 mRNA and then treated the transfected cells with rhTGF-β1. This procedure significantly reduced the levels of PARP-1 mRNA and protein in the mProx24 cells (Figure 6A, i and ii). In contrast, neither expression of Smad2/3 nor phosphorylation of Smad3 was affected by the PARP-1 antisense ODN (Figure 6Aii), suggesting that the effect of the PARP-1 antisense was mediated by a direct effect on PARP-1 expression and not by effects on other genes that contribute to the regulation of CCN2 transcription. We also found that TGF-β1 significantly increased PARP-1 expression in the tubular epithelial cells. Knockdown of PARP-1 mRNA but not pretreatment with a PARP inhibitor, PJ34, significantly reduced CCN2 mRNA levels in mProx24 cells stimulated with rhTGF-β1 (Figure 6B). In transient transfection experiments using luciferase reporter constructs, knockdown of PARP-1 mRNA also significantly blocked the CCN2 promoter activities of p-483CCN2L under rhTGF-β1 treatment, whereas polyribosylation blockade by PJ34 did not affect them (Figure 7). We further observed that the activities of luciferase reporter minigenes lacking the −455- to −434-bp fragment (p-483mPBEL and p-434CCN2L) were unaffected by knockdown of PARP-1 mRNA in mProx24 cells under control conditions or after treatment with rhTGF-β1, suggesting that this element, which we termed the PARP-1–binding element (PBE), mediated the enhancer action of PARP-1 (Figure 7).
Figure 6.
(A) Effects of PARP-1 antisense ODN in vitro. Transfection of PARP-1 antisense ODN by electroporation significantly reduced PARP-1 at the mRNA (i) and protein (ii) levels in control mProx24 cells and cells treated with rhTGF-β1. Because PARP-1 antisense ODN did not affect the expression of Smad2/3 or the phosphorylation of Smad3 (ii), the ODN was unlikely to cause nonspecific knockdown of other mRNA. (B) PARP-1 antisense ODN significantly reduced CCN2 mRNA levels in mProx24 cells treated with rhTGF-β1, whereas treatment with a polyribosylation inhibitor PJ34 (3 μM) was without effects. NC, negative control without rhTGF-β1 treatment; AS, antisense ODN; mAS, mutated antisense ODN. The data were obtained from four independent experiments.
Figure 7.
PARP-1 antisense ODN significantly reduced the basal and TGF-β1–induced CCN2 promoter activities of p-483CCN2L containing an intact PBE site (the −455- to −434-bp CCN2 promoter fragment), but PJ34 was without effects. In contrast, promoter activities of p-483mPBEL and p-434CCN2L, both of which lacked an intact PBE site and reactions to rhTGF-β1 treatment, were not affected by either PARP-1 antisense ODN or PJ34. The data were obtained from four independent experiments.
Roles of PARP-1 In Vivo
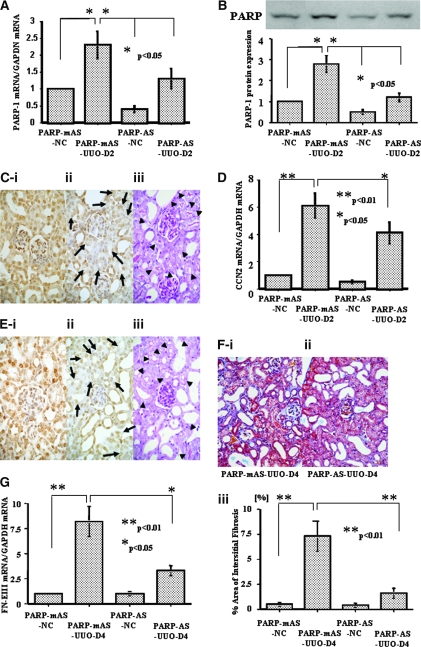
Unilateral ureter obstruction (UUO) significantly increased PARP-1 expression in the obstructed kidney (Figure 8, A and B). Intravenous injection of PARP-1 antisense ODN reduced PARP-1 mRNA and protein levels in the kidney with or without ureter obstruction (Figure 8, A and B) and significantly downregulated PARP-1 protein levels mainly in the proximal tubular epithelial cells in those kidneys after 48 h (Figure 8C). CCN2 gene expression has been reported to be increased in the obstructed kidney, which was localized to tubular epithelial cells and interstitial cells.11 Compared with the unobstructed kidney, CCN2 mRNA levels were significantly increased in the obstructed kidney at day 2; however, injection of PARP-1 antisense ODN blocked the increases in CCN2 mRNA levels (Figure 8D) and those protein levels mainly in proximal tubular epithelial cells (Figure 8E). Finally, knockdown of PARP-1 expression by PARP-1 antisense ODN injection significantly attenuated interstitial fibrosis in the obstructed kidney by day 4 (Figure 8, F and G). These results suggest that PARP-1 is an important protein factor controlling basal and induced CCN2 gene expression in tubular epithelial cells in vivo.
Figure 8.
Effects of PARP-1 antisense ODN in vivo. Intravenous administration of PARP-1 antisense ODN (1 mg/kg wt) reduced PARP-1 at the mRNA (A) and protein (B) levels in the obstructed (UUO) and unobstructed (NC) kidneys. (C) PARP-1 protein expression was localized exclusively to cell nucleus and perinuclear cytoplasm in all of the cell components such as glomerular, tubular, and interstitial cells in the obstructed kidney treated with PARP-1 mutated antisense ODN (i) (DAB). PARP-1 antisense ODN injected intravenously significantly reduced PARP-1 protein signals in tubular epithelium (arrows; ii) (DAB), which were suggested to be mainly proximal tubules because they had a brush border demonstrated by a periodic acid-Schiff (PAS) stain in the consecutive section (arrowheads; iii). (D) CCN2 mRNA levels were increased in the obstructed kidney at day 2, compared with the unobstructed kidney, and PARP-1 mRNA knockdown by antisense ODN in the tubular epithelial cells significantly reduced such CCN2 mRNA levels. (E) CCN2 protein was localized to cell nucleus and cytoplasm in the tubular epithelial cells in the obstructed kidney treated with mutated antisense ODN at day 2 (i) (DAB). PARP-1 mRNA knockdown in the proximal tubular epithelial cells significantly reduced such CCN2 protein signals in tubular epithelium (arrows; ii) (DAB), which were also suggested to be mainly proximal tubules because they had a brush border demonstrated by a PAS stain in the consecutive section (arrowheads; iii). (F) Interstitial fibrosis in the day 4 obstructed kidneys was significantly reduced in the mice treated with PARP-1 antisense ODN (ii) as compared with those treated with mutated antisense ODN (i; Masson's trichrome). (iii) The percentage of fibrous, interstitial area in each group was quantified as described in the Concise Methods section. (G) FN-EIIIA mRNA levels were significantly reduced in the day 4 obstructed kidneys in the mice treated with PARP-1 antisense ODN compared with those treated with mutated antisense ODN (P < 0.05). The data of A, B, D, F, and G were obtained from five independent experiments. Magnification, ×200.
DISCUSSION
In this study, we found that PARP-1 bound to the PBE within the murine CCN2 promoter and thereby enhanced both basal and TGF-β1–induced CCN2 gene transcription in proximal tubular epithelial cells in concert with Smads and the BCE-1 binding protein and that PARP-1 was involved in renal interstitial fibrosis. PARP-1 is an abundantly expressed 116-kD eukaryotic nuclear protein comprising three main domains: The N-terminal DNA-binding domain containing two zinc fingers, the automodification domain, and the C-terminal catalytic domain.12–14 Upon binding to DNA mainly through the second zinc-finger domain, PARP-1 catalyzes the cleavage of NAD+ into nicotinamide and ADP-ribose and then uses the latter to synthesize branched nucleic acid–like polymers poly(ADP-ribose) covalently attached to a variety of nuclear proteins, which significantly affects their function.12 Poly(ADP-ribosyl)ation has been implicated in chromatin remodeling, DNA repair in the presence of low levels of DNA damage and cell death in the presence of extensive DNA damage, and transcriptional regulation, which are critical for many physiologic and pathophysiologic outcomes.14 In addition to histones and PARP-1 itself, a number of transcription factors and signaling molecules such as NF-κB, AP-2, Oct-1, YY1, and p53 have been shown to become poly(ADP-ribosyl)ated by PARP-1.15–19 The effect of PARP-1 on the function of these proteins is carried out not only by covalent poly(ADP-ribosyl)ation but also by noncovalent protein–protein interactions.
There is no consensus in the literature regarding whether the modulation of NF-κB–mediated transcription by PARP-1 depends on the catalytic activity of the enzyme that modifies histones and other nuclear proteins to alter gene transcription, where PARP inhibitors suppress such an action, or, alternatively, on its physical presence as a transcriptional co-activator.13,20,21 In other cases, PARP-1 catalytic activity is unnecessary for its transcriptional co-regulator function in combination with either HTLV Tax or B-Myb22,23 and in the transcriptional DNA/protein complex formation that enhances Reg gene transcription.24 In this study, knockdown of PARP-1 mRNA by antisense ODN treatment but not treatment of tubular epithelial cells with a potent PARP inhibitor, PJ34, suppressed basal and TGF-β1–induced CCN2 gene transcription in tubular epithelial cells. These findings suggest that enzymatic polyribosylation of either target proteins or PARP-1 itself is not involved in CCN2 gene transcription in tubular epithelial cells. In addition, knockdown of PARP-1 mRNA specifically reduced the activities of the luciferase reporter minigenes bearing an intact PBE site, whereas pretreatment with PJ34 had no effects on the activities of luciferase reporter minigenes regardless of whether they contained an intact PBE site. The dosage of PJ34 (3 μM) used in this study seemed adequate because that dosage was effective in murine glomerular epithelial cells25 and inhibited the increase in poly(ADP-ribosyl)ation of proteins in mProx24 cells in response to high glucose to an extent similar to that observed in the presence of 10 μM (data not shown). The observation that mice deficient in PARP-1 seem normal suggests functional redundancy of this protein.26 This means that poly(ADP-ribose) polymerase activities likely remained even during PARP-1 mRNA knockdown, but CCN2 gene transcription was significantly suppressed; therefore, these results suggest that PARP-1 binds to the PBE in the CCN2 promoter and enhances basal and TGF-β1–induced CCN2 gene transcription not via its catalytic activity but mediated by its physical presence. In this study, we also demonstrated that both the SBE and BCE-1, in concert with the PBE, were necessary for full CCN2 promoter activity in tubular epithelial cells. This suggests that PARP-1 bound to the PBE may interact with Smads bound to the SBE and an unknown factor bound to the BCE-1 to yield its full enhancing activity.
The PBE was found to comprise nearly 22 bp (Figure 4), which is relatively longer than other consensus motifs (Cxcl1, Reg, and cTnT) previously identified as PARP-1–binding sites.9,27,28 As such, we cannot rule out the possibility that the PBE may contain more than one binding site. The various PARP-1 –binding sites differ considerably in sequence, suggesting that PARP-1 may bind to these sites as part of an enhancer/promoter binding complex containing other DNA binding factors and co-activators.29 Because a portion of the PBE is highly homologous to the consensus GRE (GGTACAnnnTGTTCT),3 PARP-1 alone or in combination with other co-factors may bind to this motif via its zinc fingers to regulate the CCN2 promoter activity in a manner similar to the way glucocorticoid–glucocorticoid receptor complex binds to the GRE. Alternatively, PARP-1 was recently reported to bind to the promoter region of the inducible nitric oxide synthase gene as a trans-activation factor in a sequence-specific manner in murine mesangial cells.30 That 9-bp consensus motif, located at −859 to −850 bp within the inducible nitric oxide synthase promoter, consists solely of A and T residues. In addition, PARP was reported to bind to a 9-bp pJα sequence found in human centromeric α-satellite DNA and a 28-bp tandemly repeated AT28 sequence found within a neocentromere DNA at metaphase.31 Both of these sequences, like other centromeric sequences, are also highly AT rich. Because the PBE has a high A and T content, PARP-1 likely interacts with AT-rich sequences possibly via its cysteine residues within the zinc-finger domains.30
In conclusion, we have demonstrated that the PARP-1/PBE complex functions as a nonspecific, fundamental enhancer and is necessary for full transcription of the CCN2 gene in murine renal tubular epithelial cells under basal and TGF-β1–induced conditions in vitro and in vivo. We previously demonstrated that specific CCN2 mRNA knockdown in tubular epithelial cells significantly attenuated renal fibrosis.4 These results provide a new insight into the pathologic processes in fibrotic kidney diseases and suggest new targets against which to develop new, selective antifibrotic strategies against a potent profibrotic growth factor, CCN2.
CONCISE METHODS
Cell Culture
Cultured mouse proximal tubular epithelial cells, mProx24, derived from C57BL/6 mice, were maintained in DMEM containing 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin. For experimental purposes, the mProx24 cells were seeded in six-well plates (1 × 105 cells/well) and incubated overnight in growth medium, after which the medium was replaced with modified K-1 medium (50:50 Ham's F-12/DMEM with 5 μg/ml transferrin and 5 μg/ml insulin), and the cells were incubated for 48 h before subsequent experiments. To examine the mechanism of rhTGF-β1–induced CCN2 transcription in these cells, a PARP inhibitor, PJ34 (3 μM; Calbiochem, San Diego, CA), was added to the cultures 30 min before stimulation with rhTGF-β1 (3.0 ng/ml). After an incubation of another 3 h, the cells were harvested for RNA extraction.
Real-Time Reverse Transcription–PCR
Total RNA was extracted from the lysates of cultured cells and the homogenates of kidneys with TRIzol (Life Technologies BRL, Grand Island, NY) according to the manufacturer's instructions. All RNA samples were pretreated with the RNase-free DNase I (Qiagen, Basel, Switzerland). Real-time quantitative one-step reverse transcription–PCR (RT-PCR) assay was performed to quantify mRNA levels using QuantiTect SYBR Green RT-PCR (Qiagen) and an ABI PRISM 7700 sequence detection system (Applied Biosystems, Tokyo, Japan). The primers used for real-time RT-PCR were as follows: CCN2 primer, forward 5′-GTGGAATATTGCCGGTGCA-3′, reverse 5′-CCATTGAAGCATCTTGGTTCG-3′; PARP-1 primer, forward 5′-GGAAAGGGATCTACTTTGCCG-3′, reverse 5′-TCGGGTCTCCCTGAGATGTG-3′; fibronectin EIIIA isoform (FN-EIIIA) primer, forward 5′-ATCCGGGAGCTTTTCCCTG-3′, reverse 5′-TGCAAGGCAACCACACTGAC-3′; and glyceraldehyde-3-phosphate dehydrogenase primer, forward 5′-TGCAGTGGCAAAGTGGAGATT-3′, reverse 5′-TTGAATTTGCCGTGAGTGGA-3′. All of these ODN were designed by using Primer Express software (Perkin Elmer, Foster City, CA). Preliminary RT-PCR experiments in which these primer sets were used yielded appropriately sized, single products.
Luciferase Reporter Constructs and Transient Transfection Assay
A series of firefly luciferase reporter minigenes were constructed bearing various 5′ fragments of murine CCN2 gene. The plasmids p-897CCN2L, p-628CCN2L, p-558CCN2L, p-483CCN2L, p-434CCN2L, p-202CCN2L, and p-117CCN2L contained genomic DNA −897, −628, −558, −483, −455, −434, −202 and −117 bp upstream of the transcription start site, respectively. All of these genomic DNA fragments were obtained from liver DNA of C57BL/6J mice by PCR amplification. In separate experiments, a series of mutated p-483CCN2L (e.g. p-483mAP1L, p-483mSBEL, p-483mBCEL, p-483mSB/BCEL, and p-483mPBEL) were generated by PCR-based mutagenesis. The plasmids p-483mAP1L, p-483mSBEL, p-483mBCEL, p-483mSB/BCEL, and p-483mPBEL contained mutated consensus motifs in their genomic sequences as shown in Figures 2 and 3 and Table 1. In these plasmids, genomic fragments were placed 5′ of the firefly luciferase cDNA in pGL3basic (Promega, Madison, WI). The accuracy of all constructed plasmids was verified by sequencing.
Table 1.
Mutations generated in consensus motifs in the luciferase reporter constructs
| Construct (Motif) | Native Motif Sequence | Mutated Sequence |
|---|---|---|
| p-483mAP1L | −464 −456 | −464 −456 |
| (AP-1) | −TTTGAGTCT— | −TTCAAGGAT— |
| p-483mSBEL | −250 −243 | −250 −243 |
| (SBE) | —CAGACGGA— | —CATGTGTA— |
| p-483mBCEL | −232 −220 | −232 −220 |
| (BCE-1) | —GTGTCAAGGGGTC— | —GTGTAGCTTGATC— |
| p-483mSB/BCEL | (as above) | (as above) |
| (SBE and BCE-1) | ||
| p-483mPBEL | −455 −434 | −455 −434 |
| (PBE) | —TGAGAAGTTTTTATGTCAGTAG— | —TGAGCAGTCGTTATGGACGTAG— |
Transient transfection experiments were performed using TransFast and Dual Luciferase System (Promega) as described previously.3 In these experiments, the medium was changed at 12 h after transfection, and the cells were incubated with or without rhTGF-β1 (3.0 ng/ml) in the modified K-1 medium for another 36 h.
EMSA
The mProx24 cells were seeded in Petri dishes and incubated overnight in growth medium, after which the medium was replaced with modified K-1 medium. After 48 h, 3 ng/ml rhTGF-β1 was added to the dishes and the cells were incubated for 3 h. Then, nuclear proteins were prepared using NE-PER nuclear extraction reagent (Pierce Chemical Co., Rockford, IL). Protein concentration was determined by the Bradford method, and aliquots were frozen at −80°C until use. Eight micrograms of nuclear proteins was incubated with 100 pg of 32P-labeled probes consisting of oligonucleotides shown in Figure 4 in buffer containing 10 mM HEPES (pH 7.8), 2 mM MgCl2, 50 mM KCl, 1 mM dithiothreitol, 0.1 mM EDTA, and 20% glycerol in the presence of poly(dI-dC) (Pharmacia Biotech, Piscataway, NJ) for 20 min at room temperature. When antibodies were included, they were added to the buffer with nuclear proteins and incubated on ice for 15 min before the addition of probe. Rabbit anti–PARP-1 antibody and rabbit IgG were purchased from Alexis Biochemicals (San Diego, CA) and Sigma (St. Louis, MO), respectively. The mixture was loaded on a 4% polyacrylamide 0.5× TBE gel. The gels were dried under vacuum, after which standard autoradiography was performed.
Purification of Trans-Factor Protein(s) from Nuclear Extracts
Aliquots of nuclear extract protein (100 μg) were diluted in binding buffer (20 mM HEPES at pH 7.9, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1 mM PMSF, 12.5 μg/ml N-acetyl-Leu-Leu-norleucinal, 2 μg/ml leupeptin, and 0.25 mg/ml poly[dI-dC] as final concentration) and were subjected to precipitation using biotinylated PBE (the −455- to −434-bp CCN2 promoter fragment) ODN or biotinylated mutated-PBE ODN followed by Dynabeads M-280 Streptavidin (Invitrogen, Carlsbad, CA). After 6 h of incubation at 4°C and magnetic separation, the beads were washed four times in wash buffer (20 mM HEPES at pH 7.9, 150 mM NaCl, 1 mM EDTA, and 1% Triton X-100 supplemented with protease inhibitors) and were then boiled in SDS-PAGE sample buffer to elute proteins for subsequent electrophoresis.
PMF Analysis and De Novo Sequence Analysis
Purified protein samples were separated by 10% SDS-PAGE and stained with Coomassie Brilliant Blue G250, and the visualized band of interest was excised. The protein sample in the gel was processed and analyzed with MALDI-TOF MS using an AXIMA-CFR mass spectrometer (Shimadzu, Kyoto, Japan).32
For focusing the protein candidates from PMF analysis, de novo peptide sequencing were carried out on three peptides of m/z 1377.807, 1739.944, and 2257.283 in the raw MS/MS spectrum with AXIMA-QIT mass spectrometer (Shimadzu). The measurement conditions were based on the procedure reported previously by Suzuki et al.33,34 The PMF and de novo sequence data were used to search the Matrix Science protein database using the program MASCOT (http://www.matrixscience.com/search_form_select.html).
PARP-1 Antisense ODN Transfection by Electroporation
The mProx24 cells (2 × 106) were washed in serum-free medium and resuspended in 100 μl of the supplemented Nucleofector solution (L-type; Amaxa Biosystems, Gaithersburg, MD) containing 4 μg of ODN. The ODN used in this study were as follows: PARP-1 antisense ODN 5′-GCCTCCGCCATCCTTCTC-3′ and mutated-antisense ODN 5′-GACTCCACCAGCATGCTC-3′. The sample was transferred into a cuvette and electroporated in the Nucleofector device (Amaxa) under the preoptimized program T-30 for mProx24 cells. Immediately after addition of 500 μl of growth medium, the whole sample was transferred into the six-well plate prefilled with 2 ml of growth medium per well and cultured for 4 h. Then, the medium was replaced with serum-free, modified K-1 medium, and after another 20 h of incubation, 3.0 ng/ml rhTGF-β1 was added to the well with or without pretreatment with PJ34 (3 μM), and the cells were harvested for mRNA extraction after 3 h. In another experiment, cells transfected with ODN by electroporation were used for transient transfection experiments with luciferase reporter minigenes described previously. PARP-1 mRNA knockdown was confirmed at mRNA levels by real-time RT-PCR as described previously and at protein levels by Western blotting using anti–PARP-1 antibody (1:1000) as described previously.35 Smad2/3 proteins were also evaluated by Western blotting using anti-Smad2/3 and anti-phosphorylated Smad3 antibodies (1:500; Cell Signaling Technology, Danver, MA) to test whether PARP-1 antisense ODN nonspecifically affected other biologic properties influencing CCN2 gene transcription.
Involvement of PARP-1 in the CCN2 Promoter Activity in the Kidney In Vivo
To reveal an involvement of PARP-1 in the CCN2 promoter activity in tubular epithelial cells in vivo, we conducted an in vivo experiment in which PARP-1 was blocked in mice with UUO. Manipulation for ureter ligation in detail was reported previously.36 We also reported that when a given antisense ODN was injected intravenously into rodents, it was absorbed into the proximal tubular epithelium, where it was retained for nearly 48 h, during which time it could block transcription of a target gene.3,4,35 Therefore, for groups of male, 5- to 6-wk-old C57BL/6J mice (n = 5, respectively) were purchased from Charles River Laboratories Japan (Yokohama, Japan), and two groups were administered an intravenous injection of the PARP-1 antisense ODN and the others of mutated-antisense ODN (as the negative control) at a concentration of 1.0 mg/kg twice at 48 and 6 h before ureter ligation. At 48 and 96 h after ureter ligation, mice were killed, and obstructed and unobstructed kidney tissues were sampled for RNA extraction, protein extraction, and paraformaldehyde fixation for paraffin blocks. PARP-1 mRNA knockdown was confirmed at mRNA levels by real-time RT-PCR and at protein levels by immunoblotting as described previously. Immunoreactive protein bands captured on x-ray film were analyzed by computerized densitometry using Mac SCOPE software (version 2.5; Mitani Corp., Fukui, Japan). Sections (4 μm) cut from paraffin blocks were processed for Masson's trichrome staining, and consecutive sections were used for periodic acid Schiff staining and immunohistochemistry using anti–PARP-1 and anti-CCN2 as described previously.3 Interstitial fibrosis was quantitatively determined with Mac SCOPE in 10 high-power (×200) cortical fields, and the interstitial fibrosis indices were expressed as the mean percentage area in blue per one cortical field in Masson's trichrome–stained sections.35 Animal care and treatment were conducted in accordance with the institutional guidelines.
Statistical Analyses
Values are presented as means ± SEM. Statistical differences between groups were evaluated using a Bonferroni/Dunnett test; P ≤ 0.05 was considered to be statistically significant.
DISCLOSURES
None.
Acknowledgments
This study was partially supported by a research grant from Daiichi Asubio Pharma Co., Ltd.
We thank M. Otobe and M. Funabashi for technical assistance.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Takigawa M: CTGF/Hcs24 as a multifunctional growth factor for fibroblasts, chondrocytes and vascular endothelial cells. Drug News Perspect 16: 11–21, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Inoue T, Okada H, Kobayashi T, Watanabe Y, Kanno Y, Kopp JB, Nishida T, Takigawa M, Ueno M, Nakamura T, Suzuki H: Hepatocyte growth factor counteracts transforming growth factor-β1, through attenuation of connective tissue growth factor induction, and prevents renal fibrogenesis in 5/6 nephrectomized mice. FASEB J 17: 268–270, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Okada H, Kikuta T, Inoue T, Kanno Y, Ban S, Sugaya T, Takigawa M, Suzuki H: Dexamethasone induces connective tissue growth factor expression in renal tubular epithelial cells in a mouse strain-specific manner. Am J Pathol 168: 737–747, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, Sugaya T, Kopp JB, Suzuki H: Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol 16: 133–143, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Blom IE, Goldschmeding R, Leask A: Gene regulation of connective growth factor: New targets for antifibrotic therapy? Matrix Biol 21: 473–482, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Grotendorst GR, Okochi H, Hayashi N: A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ 7: 469–480, 1996 [PubMed] [Google Scholar]
- 7.Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A: CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem 276: 10594–10601, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Holmes A, Abraham DJ, Chen Y, Denton C, Shi-wen X, Black CM, Leask A: Constitutive connective tissue growth factor expression in scleroderma fibroblasts is dependent on Sp1. J Biol Chem 278: 41728–41733, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Nakerakanti SS, Kapanadze B, Yamasaki M, Markiewicz M, Trojanowska M: Fli1 and Ets1 have distinct roles in connective tissue growth factor/CCN2 gene regulation and induction of the profibrotic gene program. J Biol Chem 281: 25259–25269, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Inoue T, Okada H, Kobayashi T, Watanabe Y, Kikuta T, Kanno Y, Takigawa M, Suzuki H: TGF-β1 and HGF coordinately facilitate collagen turnover in subepithelial mesenchyme. Biochem Biophys Res Commun 297: 255–260, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Yokoi H, Mukoyama M, Nagae T, Mori K, Suganami T, Sawai K, Yoshioka T, Koshikawa M, Nishida T, Takigawa M, Sugawara A, Nakao K: Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc Nephrol 15: 1430–1440, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Smith S: The world according to PARP. Trends Biochem Sci 26: 174–179, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Jagtap P, Szabo C: Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov 4: 421–440, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Kim MY, Zhang T, Kraus WL: Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev 19: 1951–1967, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Oliver FJ, Menissier de Murcia J, Nacci C, Decker P, Andriantsitohaina R, Muller S, de la Rubia G, Stoclet JC, de Murcia G: Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly(ADP-ribose) polymerase-1 deficient mice. EMBO J 18: 4446–4454, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kannan P, Yu Y, Wankhade S, Tainsky MA: PolyADP-ribose polymerase is a coactivator for AP-2-mediated transcriptional activation. Nucleic Acid Res 27: 866–874, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nie J, Sakamoto S, Song D, Qu Z, Ota K, Taniguchi T: Interaction of Oct-1 and automodification domain of poly(ADP-ribose) synthase. FEBS Lett 424: 27–32, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Oei SL, Shi Y: Transcription factor Yin Yang 1 stimulates poly(ADP-ribosyl)ation and DNA repair. Biochem Biophys Res Commun 284: 450–454, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Tong WM, Hande MP, Landsdorp PM, Wang ZQ: DNA strand break-sensing molecule poly(ADP-ribose) polymerase cooperates with P53 in telomere function, chromosome stability and tumor suppression. Mol Cell Biol 21: 4046–4054, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kameoka M, Ota K, Tetsuka T, Tanaka Y, Itaya A, Okamoto T, Yoshihara K: Evidence for regulation of NF-kappaB by poly(ADP-ribose) polymerase. Biochem J 346: 641–649, 2000 [PMC free article] [PubMed] [Google Scholar]
- 21.Hassa PO, Buerki C, Lombardi C, Imhof R, Hottiger MO: Transcriptional coactivation of nuclear factor-κB-dependent gene expression by p300 is regulated by poly(ADP)-ribose polymerase-1. J Biol Chem 278: 45145–45153, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Anderson MG, Scoggin KE, Simbulan-Rosenthal CM, Steadman JA: Identification of poly(ADP-ribose) polymerase as a transcriptional coactivator of the human T-cell leukemia virus type 1 Tax protein. J Virol 74: 2169–2177, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cervellera MN, Sala A: Poly(ADP-ribose) polymerase is a B-MYB coactivator. J Biol Chem 275: 10692–10696, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Akiyama T, Takasawa S, Nata K, Kobayashi S, Abe M, Shervani NJ, Ikeda T, Nakagawa K, Unno M, Matsuno S, Okamoto H: Activation of Reg gene, a gene for insulin-producing β-cell regeneration: Poly(ADP-ribose) polymerase binds Reg promoter and regulates the transcription by autopoly(ADP-ribosyl)ation. Proc Natl Acad Sci U S A 98: 48–53, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabo C, Biser A, Benko R, Boettinger E, Susztak K: Poly(ADP-ribose) polymerase inhibitors ameliorate nephropathy of type 2 diabetic Leprdb/db mice. Diabetes 55: 3004–3012, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Burkart V, Wang ZQ, Radons J, Heller B, Herceg Z, Stingl L, Wagner EF, Kolb H: Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic β-cell destruction and diabetes development induced by streptozotocin. Nat Med 5: 314–319, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Nirodi C, NagDas S, Gygi SP, Olson G, Aebersold R, Richmond A: A role for poly(ADP-ribose) polymerase in the transcriptional regulation of the melanoma growth stimulatory activity (CXCL1) gene expression. J Biol Chem 276: 9366–9374, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler AJ, Ordahl CP: Poly(ADP-ribose)polymerase binds with transcription enhancer factor 1 to MCAT1 elements to regulate muscle-specific transcription. Mol Cell Biol 19: 296–306, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraus WL, Lis JT: PARP goes transcription. Cell 113: 677–683, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Yu Z, Kuncewicz T, Dubinsky WP, Kone BC: Nitric oxide-dependent negative feedback of PARP-1 trans-activation of the inducible nitric-oxide synthase gene. J Biol Chem 281: 9101–9109, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Earle E, Saxena A, MacDonald A, Hudson DF, Shaffer LG, Saffery R, Cancilla MR, Cutts SM, Howman E, Choo KH: Poly(ADP-ribose) polymerase at active centromeres and neocentromeres at metaphase. Hum Mol Genet 9: 187–194, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Hirosawa N, Yano K, Suzuki Y, Sakamoto Y: Endocrine disrupting effects of di-(2-ethylhexyl)phthalate on female rats and proteome analyses of their pituitaries. Proteomics 6: 958–971, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki Y, Suzuki M, Nakahara Y, Ito Y, Ito E, Goto N, Miseki K, Iida J, Suzuki A: Structural characterization of glycopeptides by N-terminal protein ladder sequencing. Anal Chem 78: 2239–2243, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Zhong H, Zhang Y, Wen Z, Li L: Protein sequencing by mass analysis of polypeptide ladders after controlled protein hydrolysis. Nat Biotechnol 22: 1291–1296, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Okada H, Inoue T, Kikuta T, Watabe T, Kanno Y, Ban S, Sugaya T, Horiuchi M, Suzuki H: A possible anti-inflammatory role of angiotensin II type 2 receptor in immune-mediated glomerulonephritis during type 1 receptor blockade. Am J Pathol 169: 1577–1589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG: Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]