Abstract
Several gene array studies have suggested that osteopontin (Opn) expression strongly correlates with albuminuria and glomerular disease. Urinary Opn concentration and kidney Opn immunoreactivity were found to be increased in patients with steroid-sensitive nephrotic syndrome. In addition, renal Opn mRNA was increased in the Ins2Akita mouse model of type 1 diabetic nephropathy, in the LPS-induced albuminuria model, and in glomeruli of puromycin aminonucleotide–induced nephrotic rats. Opn knockout mice did not develop albuminuria in response to LPS injection, and Opn knockout mice were protected from diabetes-induced albuminuria and mesangial expansion. In the glomerulus, Opn immunostaining was increased specifically in podocytes. Treatment of podocytes with recombinant Opn activated the NF-κB pathway, increased expression of urokinase plasminogen activator and matrix metalloproteinases 2 and 9, and increased podocyte motility. Taken together, these results indicate that Opn plays an important role in the development of albuminuria, possibly by modulating podocyte signaling and motility.
Osteopontin (Opn; or secreted phosphoprotein 1 [Spp1]) is a pleiotropic cytokine that is broadly expressed and upregulated during inflammation, cancer, and various other conditions.1–3 Secreted Opn can bind to αVβ3 integrin (vitronectin receptor) and can induce phosphoinositide-3-kinase/Akt-dependent NF-κB activation and urokinase plasminogen activator (uPA) secretion in cancer cells.1–3 Opn can also induce NF-κB activation through both IKK- and extracellular signal–regulated kinase–mediated pathways, which stimulates uPA-dependent matrix metalloproteinase 9 (MMP-9) activation. All of these could contribute to increased motility of cancer cells, invasion, tumor growth and metastasis.1–3 In addition, recent studies by Wei et al.4 showed that αVβ3 integrin (one of the Opn receptors) is a critical regulator of proteinuria.
Gene expression studies showed that higher Opn levels strongly correlate with more severe diabetic albuminuria and glomerulosclerosis in various diabetic nephropathy models.5 Gene profiling experiments performed on acute and chronic glomerulonephritis6,7 and transplant renal disease8 also found Opn mRNA to be highly increased and that it was one of the top genes that predicted renal damage.5,9 Similarly, gene array studies performed on isolated podocytes identified Opn as the gene with the highest increase in mRNA expression after sheer stress10; however, the reports are conflicting as to whether Opn plays a role in murine autoimmune glomerulonephritis.6,11
Here we show that Opn is regulated in mouse models of type 1 diabetic nephropathy-, LPS-, and puromycin aminonucleotide (PAN)-induced proteinuria and also in children with nephrotic syndrome. We found that Opn deletion (Spp1−/− mice) confers a protection against the development of albuminuria. We propose that podocytes are an important target of Opn, because we found that Opn treatment led to IκBα phosphorylation, upregulation of MMP-2 and MMP-9, and increased podocyte motility.
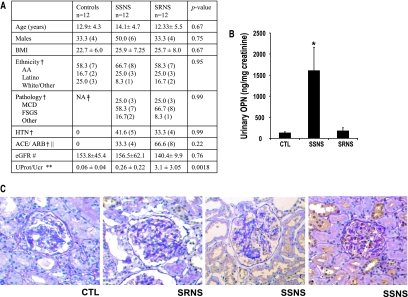
Because Opn is a secreted protein, we examined Opn levels in urine samples obtained from children with nephrotic syndrome. Thirty-six urine samples were obtained (at the time of the kidney biopsy), including 12 from control subjects without any evidence of renal disease and 24 patients with nephrotic syndrome. Patient characteristics are shown in Figure 1A. The indication for the renal biopsy was nephrotic syndrome with atypical presentation (age, presence of hypertension) or resistance to standard dosage of steroid treatment. FSGS was the most common cause of nephrotic syndrome in our cohort (58 to 66%), with a similar frequency between steroid-sensitive nephrotic syndrome (SSNS) and steroid-resistant nephrotic syndrome (SRNS). Urinary Opn levels were significantly increased in children with nephrotic syndrome to 824 ± 272 ng/mg creatinine compared with 135 ± 16 ng/mg creatinine in control subjects (P = 0.029). Urine Opn levels did not correlate with gender, proteinuria, renal histology, or GFR. Opn levels correlated with clinical response to steroids. Urine Opn levels (drawn after steroid treatment) were highly increased in children with SSNS (1611 ± 476 ng/mg creatinine), whereas they were not significantly different from control subjects in children who did not respond to the course of steroids (180 ± 61 ng/mg creatinine) (Figure 1B). Renal Opn expression was increased in kidney biopsies of patients with SSNS, indicating that the kidney is the likely source of increased Opn levels (Figure 1C).
Figure 1.
Increased urinary Opn levels in children with nephrotic syndrome. (A) Demographics of the research participants. Continuous variables are means ± SD, with P values calculated by one-way ANOVA. AA, African American; †P value calculated by Fisher exact test. ‡Not obtained. ‖Treatment with angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers at the time of urine collection. #Estimated GFR (ml/min per 1.73 m2). **Urinary protein/creatinine ratio; P value calculated by Kruskal-Wallis test. (B) Urine Opn levels were measured with human Opn ELISA kits (R&D Systems) and normalized to urinary creatinine values. *Categorical variables presented as percentage with P values calculated by Fisher exact test. (C) Representative immunostainings with anti-Opn antibody of kidney sections obtained from the research participants (counterstained with periodic acid-Schiff), CTL is a time 0 biopsy of a living transplant allograft.
These observations suggest that urinary Opn levels might be a useful biomarker of steroid sensitivity in children with nephrotic syndrome. SRNS of children is associated with a bad prognosis, because this disease is often resistant to other secondary forms of treatment and leads to ESRD.12 The renal histology is often not a reliable predictor of treatment response. A biomarker that predicts outcome would be useful to prevent treatment with steroids that are associated with significant risk and adverse effect. Our study suggested that urine Opn levels might be a useful marker. Our study used a cross-sectional study design, and urine was collected from children after they were treated with prednisone; therefore, additional larger and prospective studies are needed to determine whether Opn can prospectively predict steroid sensitivity.
In addition to patients, we examined the expression and regulation of Opn in animal models of albuminuria. Twenty-four hours after LPS injection, 6-wk-old male C57/B6J mice developed steroid-sensitive and transient proteinuria (Figure 2A). This rapid and abundant albuminuria was not associated with significant glomerular changes under light microscopy (Supplemental Figure 1); however, quantitative real-time-PCR analysis performed on whole-kidney lysates showed a significant increase in Opn mRNA level (Figure 2B).
Figure 2.
Increased Opn expression in animal models of nephrotic syndrome. (A, C, and E) Urine albumin creatinine ratios of control (CTL); (A) LPS-treated animals (LPS), (C) Sixteen-week-old Ins2Akita diabetic mice (Akita), (E) PAN-treated rats (PAN). (B, D, and F) Relative Opn mRNA level by QRT-PCR in whole kidneys or glomeruli (G) of control C57/B6J mice (CTL). LPS-treated (B) or diabetic (D) mice and PAN-treated (F and G) rats (n = 6 to 8 per group). *Statistically significant difference, P < 0.05. Kidney sections were stained with anti-Opn antibody and developed with peroxidase-conjugated secondary antibody (without counterstaining). (H through M) Control animals (CTL; H and K), wild-type (I), or Opn knockout (J) LPS-treated mice. Wild-type (L) or Opn knockout (M) 16-wk-old male Akita mice. (N and O) Kidneys obtained from LPS-treated mice were stained with anti-Opn antibody (green; N) and with anti-synaptopodin antibody (red; O). (P) Overlay of synaptopodin and Opn staining.
Single (intraperitoneal) injection of PAN to Sprague-Dawley rats caused severe nephrotic syndrome (Figure 2E) as early as 4 d after the injection. We found increased Opn mRNA levels in PAN-treated rats. Although it did not reach statistical significance (Figure 2F) in whole-kidney homogenate, the increase was significant and much greater (approximately 70-fold) in glomerular extracts (Figure 2G).
The Opn mRNA level was also significantly increased in kidneys of 16-wk-old male Akita mice (Figure 2D), which is a model of diabetic nephropathy. This model developed significantly increased albuminuria and mesangial expansion as well (Figure 2C and Supplemental Figure 1).
Immunostaining studies showed increased podocyte-specific Opn immunostaining in the glomeruli of LPS-treated mice and in 16-wk-old male Akita mice compared with control mice (Figure 2, H through P). The specificity of the antibody was tested on kidney sections obtained from Opn knockout mice (Figure 2, J and M).
Our next question was whether Opn plays a role in the development of albuminuria. To answer this question, we took advantage of the Opn knockout mice. Opn null (B6.Cg-Spp1tmBlh/J or Spp1−/−) mice are viable without any renal abnormalities.13 Six-week-old male Opn knockout mice were administered an injection of 20 μg of LPS (intraperitoneally). Wild-type mice developed significant albuminuria 24 h after the injection; however, albuminuria did not appear in Opn knockout mice (Table 1).
Table 1.
Phenotype analysis of LPS-treated wild-type (C57/B6J) Opn knockout (Spp1−/−) micea
| Group | n | Age (wk) | Body Weight (g) | Albumin/Creatinine (μg/mg) |
|---|---|---|---|---|
| C57/B6 | 9 | 6 | 20.9 ± 2.0 | 71.6 ± 23.0 |
| C57/B6 LPS | 10 | 6 | 20.4 ± 1.0 | 292.4 ± 37.0b |
| Spp1−/− | 8 | 6 | 19.9 ± 1.0 | 63.4 ± 23.0 |
| Spp1−/− LPS | 10 | 6 | 21.8 ± 1.0 | 91.8 ± 35.0 |
Body weight and albuminuria were measured at the time animals were killed, 24 h after LPS injection. Albuminuria was measured by murine albumin-specific ELISA kit and creatinine with colorimetric reaction and expressed as μg albumin/mg creatinine.
Statistically significant difference, P < 0.05 versus C57/B6 control groups.
We also tested whether Opn plays a role in the development of diabetic albuminuria and mesangial expansion. We generated combined mutant mice that both were Opn null and had the Ins2Akita mutation (both strains were in C57/B6J background). Body weight and blood glucose levels were not different in male wild-type Ins2Akita and in the Opn null Ins2Akita groups (Tables 1 and 2). Wild-type Ins2Akita mice had increased albuminuria at 16 wk of age.14,15 Albuminuria was significantly lower in the Spp1−/− Ins2Akita mice compared with nondiabetic controls. Mesangial expansion, a hallmark of diabetic glomerulopathy, was also significantly decreased in Ins2Akita mice with Opn deletion (Supplemental Figure 2). In summary, Opn knockout mice showed protection from LPS- and diabetes-induced albuminuria.
Table 2.
Phenotype analysis of wild-type, Ins2Akita, and Spp1−/− Ins2Akita micea
| Group | n | Age (wk) | Body Weight (g) | Blood Glucose (mg/dl) | Albumin/Creatinine (μg/mg) |
|---|---|---|---|---|---|
| C57/B6 | 10 | 16 | 31.0 ± 1.0 | 204.0 ± 14.0 | 9.0 ± 4.0 |
| Akita | 10 | 16 | 24.0 ± 2.0 | 559.0 ± 40.0b | 438.0 ± 98.0b,c |
| Spp1−/− | 8 | 16 | 31.0 ± 1.0 | 246.0 ± 20.0 | 17.0 ± 6.0 |
| Spp1−/− Akita | 9 | 16 | 26.0 ± 2.5 | 575.0 ± 25.0b | 82.0 ± 8.0b |
Body weight, blood glucose, and albuminuria were measured at the time animals were killed, at 16 wk of age. Albuminuria was measured by murine albumin-specific ELISA kit and creatinine with colorimetric reaction and expressed as μg albumin/mg creatinine.
Statistically significant difference, P < 0.05 versus C57/B6 control groups.
Statistically significant difference, P < 0.05 Akita versus Spp1−/− Akita mice.
Further cell culture experiments examining the role and regulation of Opn in cultured podocytes showed that LPS and hyperglycemia induced upregulation of Opn mRNA in podocytes in vitro. This response is reminiscent of the observed in vivo increase of Opn expression in podocytes in diabetic or LPS-treated mice (Supplemental Figure 3). Activation of the protein kinase C and the extracellular signal–regulated kinase pathways likely via the release of intracellular reactive oxygen species seems to play an important role in regulating Opn mRNA levels in cultured podocytes (Supplemental Figure 4).
We evaluated the effect of exogenous recombinant Opn in vitro on cultured murine podocytes. After Opn treatment, we observed an increase in IκBα phosphorylation, peaking at approximately 40 min (Figure 3A and Supplemental Figure 5) and a decrease in total IκBα levels. Preincubation of the cells with Bay11-7082 and pyrrolidine dithiocarbamate (PDTC) inhibited IκBα phosphorylation, indicating NF-κB activation. Similarly, Opn treatment increased the Bay11- and PDTC-sensitive NF-κB p50 DNA binding (Figure 3B). These observations suggest that Opn treatment leads to the activation of the NF-κB pathway in cultured podocytes.
Figure 3.
Effect of Opn treatment on cultured murine podocytes. (A) Western blot analysis of cultured murine podocytes treated with recombinant murine Opn (50 ng/ml) and harvested after the indicated time points. Membranes were probed as indicated with phospho-IκBα antibodies, total IκBα, and β-actin serving as loading controls. When indicated, cells were preincubated with 5 μM Bay11-7082 (Bay) or 100 μM PDTC. The data are a representative of three independent experiments. (B) Transfactor NF-κB p50 DNA binding assay of control and Opn-treated cells in the presence or absence of indicated inhibitors. (C) Cultured podocytes were treated with 50 ng/ml mouse recombinant Opn for 1, 4, 6 or 24 h. uPA and MMP-2 and -9 mRNA levels were determined by QRT-PCR. (D) Cells were incubated with Bay of PDTC followed by treatment with recombinant Opn (for 6 h). Significance was determined by one-way ANOVA. Scrape wound assay was performed on podocytes in the presence and absence of recombinant Opn and various inhibitors of Bay (5 μM) or PDTC (100 μM). The number of migrated cells was counted (by DAPI staining) 16 h after Opn incubation. (E) Representative images of control (sham), Opn, Bay, or PDTC plus Opn-treated podocytes are shown. (F) Quantification of three independent experiments. *Statistically significant difference (P < 0.05) compared with control. (G) The proposed model of Opn action in podocytes mediating albuminuria.
Increased Opn expression in cancer cells regulates basement membrane turnover via the activation of NF-κB, subsequently increasing expression of key extracellular matrix enzymes, uPA, MMP-2 and -9. Recent observations indicated that MMP is involved in glomerular disease development.16 We found a time-dependent increase in MMP-2 and -9 and uPA mRNA levels after Opn treatment (Figure 3C). To test whether the increased MMP transcript levels are the consequence of the NF-κB activation, we incubated cells with inhibitors of the NF-κB pathway including Bay11-7082 and PDTC. We found that whereas MMP-2 transcription was sensitive to the inhibitors of the NF-κB pathway, MMP-9 was not sensitive to Bay11-7082 or PDTC (Figure 3D). We performed wound-healing assays to examine the functional effect of Opn on podocytes, because podocyte motility has been strongly linked to the development of proteinuria.4,17 We found that Opn-treated podocytes migrated faster than control untreated cells, and they covered the wounded area faster than control cells (Figure 3E). The NF-κB pathway seems to be an important regulator of podocyte migration, because inhibitors of this pathway (Bay11-7082, PDTC) blocked Opn-induced podocyte migration (Figure 3, E and F). In summary, we found that Opn-treated podocytes have increased motility and increased expression of matrix-degrading enzymes. This is likely to be the consequence of Opn-induced enhanced NF-κB signaling.
What could the relationship between Opn levels and SSNS be? Although we attempted to study different animal models of albuminuria, we note that the PAN- and LPS-induced albuminuria models are sensitive to steroid treatment,18 indicating a correlation with our human data. The differential expression of Opn in SSNS could also suggest that the pathomechanism of SSNS might be different from SRNS, with Opn and downstream NF-κB perhaps being involved in SSNS. Glucocorticoids are known to interfere with the NF-κB pathway, via inhibiting the DNA binding of NF-κB p65 subunit.19 This opens the possibility that glucocorticoids might be effective in SSNS via interfering with the NF-κB pathway (downstream of Opn) in podocytes. The presence of glucocorticoid receptors have been demonstrated in cultured podocytes, a finding consistent with our hypothesis.18,20 We also found that dexamethasone inhibits Opn-induced podocyte motility (data not shown).
Finally, our results offer the possibility of using Opn inhibitors as a novel therapeutic target of albuminuria. Peptides that target RGD molecules such as Opn have already been developed by various pharmaceutical laboratories as a potential therapy for cancer and metastasis.21
CONCISE METHODS
Animals
Mice with homozygous deletion of Opn (B6.Cg-Spp1tmBlh/J, stock no. 004936), Ins2Akita mice on C57/B6J background, and wild-type C57/B6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). Genotyping PCR was performed on genomic DNA according to published protocol (http://www.jax.org).
Puromycin (150 mg/kg body wt; Sigma-Aldrich Co., St. Louis, MO) was injected intraperitoneally into male Sprague-Dawley rats (Charles River Co., Wilmington, MA) that weighed 100 g (n = 6). Rats developed severe proteinuria on day 4, and they were killed on day 8 after the injection. Glomeruli were isolated by differential sieving. All procedures were approved by the Albert Einstein College of Medicine Animal Care and Use Committee.
Phenotypic Analysis
Blood glucose was measured by OneTouch Ultra2 Glucometer (Johnson & Johnson, New Brunswick, NJ). Albuminuria was determined using a mouse albumin-specific ELISA kit (Exocell Laboratories, Philadelphia, PA), and urine creatinine was determined using Creatinine Companion (Exocell), following the manufacturer's protocol.
Immunohistochemistry
Immunohistochemistry was performed either on OCT-embedded frozen or formalin-fixed paraffin-embedded kidney sections as described previously (15) using anti-Opn antibody obtained from University Hybridoma Bank (Iowa), and some was also a gift from Dr. David D. Dendhardt (University of New Jersey)22; antisynaptopodin antibody was obtained from Sigma-Aldrich.
QRT-PCR
QRT-PCR results were normalized to ubiquitin C and HPGRT gene expression using the ΔΔCT value method. Supplemental Table 1 contains all primer sequences and further details.
Cell Wound-Healing Assay
Cell wound-healing assay was performed as described previously.23
Western Blotting
Western blotting was performed as detailed in the supplemental information. The following primary antibodies were used in this study: Total-IκBα was purchased from Cell Signaling (Danvers, MA), and phospho-IκBα (Ser32) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA).
Patient Characteristics
Pediatric patients with nephrotic syndrome were recruited at the Montefiore Medical Center (Bronx, NY). The institutional review board for clinical trials involving human subjects approved the study protocol. Fresh midstream urine samples were collected at the clinic or at the time of the kidney biopsy. Control kidney biopsy samples were collected from living transplant allografts right after removal from the donor (time 0).
NF-κB p50 Nuclear Binding
NFκB p50 nuclear binding assay was performed using TransFactor Colorimetric kit (Clontech BD, Mountain View, CA) according to the manufacturer's protocol.
Human Osteopontin Quantikine ELISA Kit
Human Osteopontin Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) was used for quantification of urine Opn levels. The assay was performed according to the manufacturer's protocol.
DISCLOSURES
None.
Acknowledgments
This work was supported by grants from the Juvenile Diabetes Foundation, Gottschalk Award of the American Society of Nephrology, and the National Institutes of Health. Cell culture and histology service was provided by the O'Brian Kidney Center Imaging Core Facility.
Part of this work was presented at the annual meeting of the American Society of Nephrology; November 14 through 19, 2006; San Diego, CA.
We thank Dr. Peter Mundel (Mount Sinai School of Medicine) for providing the murine podocyte cell line and Dr. Thomas Hostetter for critical reading of the manuscript.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Rangaswami H, Bulbule A, Kundu GC: Osteopontin: Role in cell signaling and cancer progression. Trends Cell Biol 16: 79–87, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Rangaswami H, Bulbule A, Kundu GC: JNK1 differentially regulates osteopontin-induced nuclear factor-inducing kinase/MEKK1-dependent activating protein-1-mediated promatrix metalloproteinase-9 activation. J Biol Chem 280: 19381–19392, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Rangaswami H, Bulbule A, Kundu GC: Nuclear factor-inducing kinase plays a crucial role in osteopontin-induced MAPK/IkappaBalpha kinase-dependent nuclear factor kappaB-mediated promatrix metalloproteinase-9 activation. J Biol Chem 279: 38921–38935, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J: Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Susztak K, Böttinger E, Novetsky A, Liang D, Zhu Y, Ciccone E, Wu D, Dunn S, McCue P, Sharma K: Molecular profiling of diabetic mouse kidney reveals novel genes linked to glomerular disease. Diabetes 53: 784–794, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Yu XQ, Nikolic-Paterson DJ, Mu W, Giachelli CM, Atkins RC, Johnson RJ, Lan HY: A functional role for osteopontin in experimental crescentic glomerulonephritis in the rat. Proc Assoc Am Physicians 110: 50–64, 1998 [PubMed] [Google Scholar]
- 7.Lan HY, Yu XQ, Yang N, Nikolic-Paterson DJ, Mu W, Pichler R, Johnson RJ, Atkins RC: De novo glomerular osteopontin expression in rat crescentic glomerulonephritis. Kidney Int 53: 136–145, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Alchi B, Nishi S, Kondo D, Kaneko Y, Matsuki A, Imai N, Ueno M, Iguchi S, Sakatsume M, Narita I, Yamamoto T, Gejyo F: Osteopontin expression in acute renal allograft rejection. Kidney Int 67: 886–896, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Hudkins KL, Giachelli CM, Eitner F, Couser WG, Johnson RJ, Alpers CE: Osteopontin expression in human crescentic glomerulonephritis. Kidney Int 57: 105–116, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Endlich N, Sunohara M, Nietfeld W, Wolski EW, Schiwek D, Kränzlin B, Gretz N, Kriz W, Eickhoff H, Endlich K: Analysis of differential gene expression in stretched podocytes: Osteopontin enhances adaptation of podocytes to mechanical stress. FASEB J 16: 1850–1852, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Bonvini JM, Schatzmann U, Beck-Schimmer B, Sun LK, Rittling SR, Denhardt DT, Le Hir M, Wüthrich RP: Lack of in vivo function of osteopontin in experimental anti-GBM nephritis. J Am Soc Nephrol 11: 1647–1655, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Bellew CA, Silverstein DM, Aviles DH, Boineau FG, Vehaskari VM: High incidence of initial and late steroid resistance in childhood nephrotic syndrome. Kidney Int 68: 1275–1281, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL: Altered wound healing in mice lacking a functional osteopontin gene (spp1). J Clin Invest 101: 1468–1478, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Susztak K, Raff AC, Schiffer M, Bottinger EP: Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 15.Szabo C, Biser A, Benko R, Bottinger E, Susztak K: Poly(ADP-ribose) polymerase inhibitors ameliorate nephropathy of type 2 diabetic Leprdb/db mice. Diabetes 55: 3004–3012, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Zeisberg M, Khurana M, Rao VH, Cosgrove D, Rougier JP, Werner MC, Shield CF 3rd, Werb Z, Kalluri R: Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med 3: e100, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P: Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8: 485–491, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ: Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: Role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol 16: 2615–2625, 2005 [DOI] [PubMed] [Google Scholar]
- 19.De Bosscher K, Vanden Berghe W, Vermeulen L, Plaisance S, Boone E, Haegeman G: Glucocorticoids repress NF-kappaB-driven genes by disturbing the interaction of p65 with the basal transcription machinery, irrespective of coactivator levels in the cell. Proc Natl Acad Sci U S A 97: 3919–3924, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW: Direct effects of dexamethasone on human podocytes. Kidney Int 70: 1038–1045, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Shen SI, Kotamraj PR, Bhattacharya S, Li X, Jasti BR: Synthesis and characterization of RGD-fatty acid amphiphilic micelles as targeted delivery carriers for anticancer agents. J Drug Target 15: 51–58, 2007 [DOI] [PubMed] [Google Scholar]
- 22.D'Alonzo RC, Kowalski AJ, Denhardt DT, Nickols GA, Partridge NC: Regulation of collagenase-3 and osteocalcin gene expression by collagen and osteopontin in differentiating MC3T3–E1 cells. J Biol Chem 277: 24788–24798, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP: Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 23: 1155–1165, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]