Abstract
Anti-myeloperoxidase (anti-MPO) antibodies have been implicated in the pathogenesis of small-vessel vasculitis, but the molecular mechanisms by which these antibodies contribute to disease are unknown. For determination of how anti-MPO antibodies affect inflammatory cell recruitment in small-vessel vasculitis, intravital microscopy was used to monitor leukocyte behavior in the accessible cremasteric microvessels under various experimental conditions. After local pretreatment of the cremaster muscle with cytokines (TNF-α, IL-1β, or keratinocyte-derived chemokine), administration of anti-MPO IgG to wild-type mice reduced leukocyte rolling in favor of augmented adhesion to and transmigration across the endothelium. This led to a decrease in the number of systemic circulating leukocytes and, similar to the early events in the development of vasculitic lesions, an increase in leukocyte recruitment to renal and pulmonary tissue. TNF-α led to the greatest recruitment of inflammatory cells, and IL-1β led to the least. When anti-CD18 was co-administered, anti-MPO IgG did not affect leukocyte rolling, adhesion, or transmigration; similarly, anti-MPO IgG did not produce these effects in Fc receptor γ chain−/− mice. This study provides direct in vivo evidence of enhanced leukocyte–endothelial cell interactions in the presence of anti-MPO IgG and highlights the critical roles of Fcγ receptors and β2 integrins in mediating these interactions. In addition, it suggests that neutrophils primed by cytokines in the presence of anti-MPO IgG can have systemic effects and target specific vascular beds.
Vasculitis is an inflammatory disease of blood vessel walls, typified by inappropriate recruitment of activated leukocytes with subsequent endothelial necrosis.1,2 Small-vessel vasculitis is associated with development of anti-neutrophil cytoplasmic autoantibodies (ANCA) directed against neutrophil intracellular enzymes, myeloperoxidase (MPO-ANCA), and proteinase 3 (PR3-ANCA).3–5 In response to cytokines such as TNF-α, these target antigens translocate to the neutrophil surface, thereby increasing their accessibility for binding by circulating autoantibodies.6,7 Direct evidence for ANCA pathogenicity was recently provided using a novel murine model.8 MPO-deficient mice immunized with murine MPO generated anti-MPO IgG, which was purified and transferred into naïve wild-type mice whose leukocytes expressed MPO. This triggered development of histologic changes typical of vasculitis within 6 d, including focal necrotizing glomerulonephritis with glomerular crescents and pulmonary capillaritis.8 Neutrophil depletion conferred protection against anti-MPO IgG–induced injury, providing evidence for neutrophil pathogenicity in disease induction.9
Further evidence for ANCA pathogenicity was presented in a rat model in which WKY rats immunized with human MPO generated anti-MPO ANCA that apparently cross-reacted with rat MPO.10 Intravital microscopy demonstrated that the chemokine Gro-α (CXCL1) enhanced leukocyte adhesion and transmigration in immunized rats and also when purified anti-MPO IgG was transferred from immunized to naïve animals.10 Because additional cytokines were not investigated, it is not known whether Gro-α is unique in its ability to enhance ANCA pathogenicity or whether other cytokines can act similarly.
Although those two studies demonstrated that ANCA is indeed pathogenic and proinflammatory, no studies have identified the actual molecular mechanisms that contribute to pathology in vivo. In this study, we hypothesized that Fc receptors and CD18 were essential for mediating ANCA-induced signaling and firm leukocyte–endothelial (L-E) cell interactions in vivo, because studies in vitro have identified a role for both.11–17 This was explored intravitally using a function blocking CD18 mAb and mice deficient for the Fc receptor γ chain (FcRγ chain−/−). This accessory chain is crucial for assembly and expression of activating Fc receptors (FcγRI and FcγRIII) that are abundantly expressed on the surface of murine leukocytes.18,19
This study is also novel in that we further addressed the relative contributions of different pathologically relevant cytokines in inducing ANCA-associated pathology in the cremaster microcirculation. The frequent association between infection and onset of vasculitis underscores the clinical relevance of studying the effects of cytokine priming; therefore, TNF-α, IL-1β, or keratinocyte-derived chemokine (KC; CXCL1), a murine functional homologue of human IL-8, was administered and L-E cell interactions in the presence of murine anti-MPO IgG were assessed and compared. More important, this mechanistic study has focused on L-E cell interactions at the earliest times after administration of murine IgG raised to murine MPO, obviating any species cross-reactivity to the antigen. The cremaster muscle is rarely affected by vasculitis, so we also studied renal and pulmonary capillaries. These are frequently affected in ANCA-associated vasculitis, leading to focal necrotizing and crescentic glomerulonephritis with renal failure and alveolar hemorrhage if left untreated.20,21 Injury to these tissues has previously been monitored after 6 d or between 6 and 7 wk in murine and rat vasculitis models, respectively, but it is not known whether any damage occurs upon immediate exposure to ANCA8,10; therefore, we also monitored the degree of injury and changes in leukocyte numbers in renal and pulmonary tissue in response to different cytokines, again at the earliest times after anti-MPO IgG.
RESULTS
Anti-MPO IgG Alone Does not Enhance Spontaneous L-E Cell Interactions in Wild-Type Mice
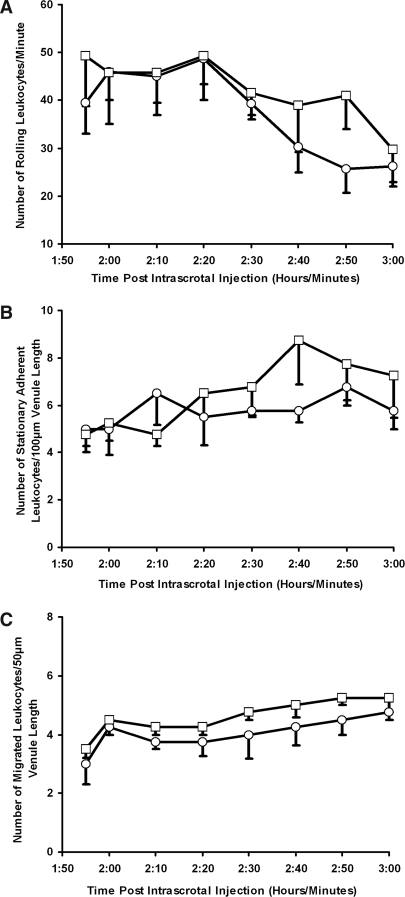
Control wild-type mice demonstrated spontaneous leukocyte rolling within the cremaster muscle microcirculation that reduced over time (Figure 1A). It is well established that this is because surgical preparation of the muscle acts as a mild inflammatory stimulus.22 Although a small increase in stationary adhesion and subsequent migration was observed with time after anti-MPO IgG, this was not significantly different when compared with control anti-BSA IgG (Figure 1, B and C).
Figure 1.
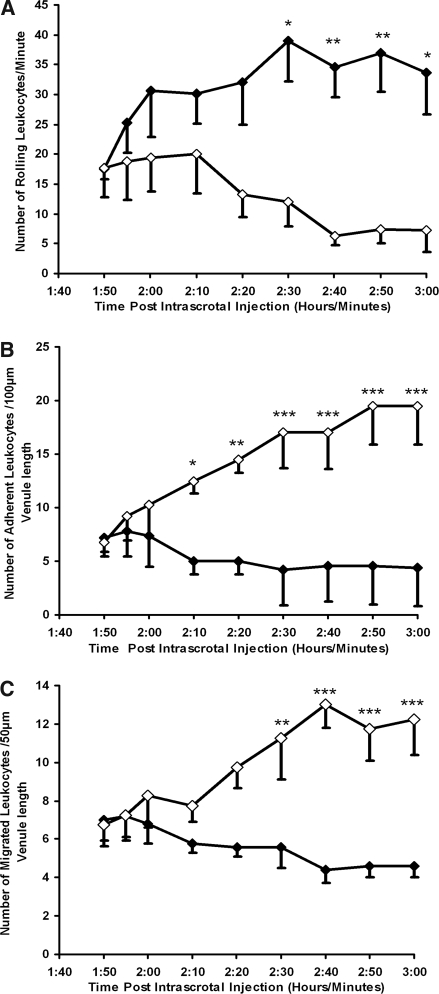
Anti-MPO IgG does not enhance spontaneous L-E cell interactions in wild-type mice. Anti-BSA IgG (○) or anti-MPO IgG (□) was administered intra-arterially (18 μg/g body wt) 2 h after intrascrotal injection of vehicle (saline containing 0.1% BSA). Any changes in leukocyte rolling (A), stationary adhesion (B), and migration (C) were measured between the groups during 60 min in postcapillary venules (20 to 50 μm). Data are means ± SEM (n = 5 for each treatment group).
Anti-MPO IgG Enhances L-E Cell Interactions in Wild-Type Mice in the Presence of Inflammatory Cytokines
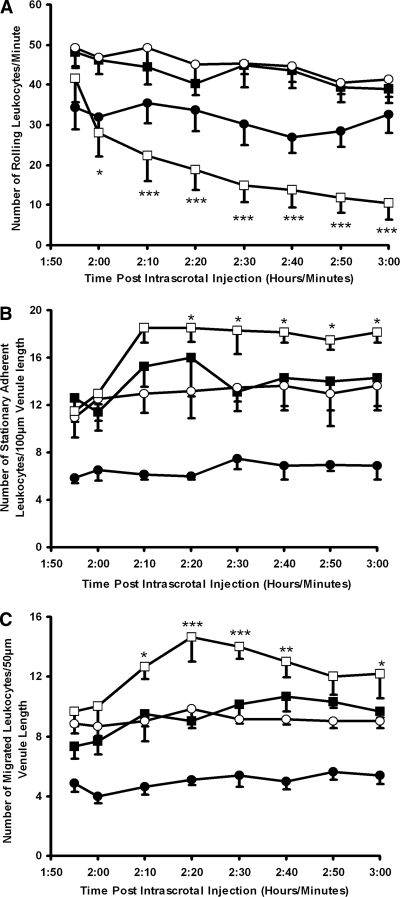
As expected, pretreatment with TNF-α increased leukocyte rolling, firm adhesion, and migration when compared with mice that received no cytokine (Figure 2). Anti-MPO IgG rapidly reduced TNF-α–induced leukocyte rolling (Figure 2A), which was sustained throughout the observation period (41.6 ± 6.0 before anti-MPO IgG infusion versus 10.5 ± 4.2 at 60 min after anti-MPO IgG) and was statistically significant (P < 0.001) when compared with control anti-BSA IgG. A rapid, concomitant, and sustained augmentation in leukocyte stationary adhesion (P < 0.05) and transmigration (P < 0.001) was also observed after anti-MPO IgG administration (Figure 2, B and C). Control anti-BSA IgG caused no such microcirculatory disturbances (Figure 2).
Figure 2.
Effects of anti-MPO IgG on L-E cell interactions in wild-type mice after intrascrotal TNF-α stimulation. Saline (▪), anti-BSA IgG (○), or anti-MPO IgG (□) was administered intra-arterially (18 μg/g body wt) 2 h after intrascrotal TNF-α (500 ng) injection. Changes in leukocyte rolling (A), stationary adhesion (B), and migration (C) were measured for 60 min in postcapillary venules (20 to 50 μm). The results for animals that received saline intra-arterially 2 h after intrascrotal injection of vehicle (saline containing 0.1% BSA) are also shown (•). Data are means ± SEM (n = 6 to 8 for each treatment group). *P < 0.05, **P < 0.01, ***P < 0.001 versus TNF-α–stimulated mice that received anti-BSA IgG.
Pretreatment with IL-1β also caused an increase in leukocyte rolling, firm adhesion, and migration compared with mice that received no cytokine (Figure 3, A, C, and E). Anti-MPO IgG reduced IL-1β–induced rolling (Figure 3A), although this failed to reach statistical significance when compared with control anti-BSA IgG; however, anti-MPO IgG induced a significant increase in leukocyte stationary adhesion (P < 0.01) and migration (P < 0.001) when compared with a control antibody, particularly at later time points (Figure 3, C and E).
Figure 3.
Effects of anti-MPO IgG on L-E cell interactions in wild-type mice after intrascrotal IL-1β or topical KC. Saline (▪), anti-BSA IgG (○), or anti-MPO IgG (□) was administered intra-arterially (18 μg/g body wt) 4 h after intrascrotal IL-1β (12.5 ng) injection. Changes in leukocyte rolling (A), stationary adhesion (C), and migration (E) were measured for 60 min in postcapillary venules (20 to 50 μm). The results for mice that received saline intra-arterially after intrascrotal injection of vehicle (saline containing 0.1% BSA) are also shown (•). Alternatively, KC (3.9 nM) or vehicle was continuously superfused over the cremaster muscle, and changes in rolling (B), stationary adhesion (D), and migration (F) were assessed. Data are means ± SEM (n = 5 for each treatment group). *P < 0.05, **P < 0.01, ***P < 0.001 versus cytokine-stimulated mice that received anti-BSA IgG.
Pretreatment with KC did not induce significant differences in leukocyte rolling in wild-type mice compared with mice that received no cytokine (Figure 3B). Increased adhesion and migration was observed but did not reach statistical significance (Figure 3, D and F). In contrast to the effects of TNF-α and IL-1β, there was no significant reduction in leukocyte rolling in KC-prestimulated mice after anti-MPO IgG (Figure 3B); however, anti-MPO IgG induced significant increases in leukocyte firm adhesion (P < 0.001) and migration (P < 0.001) compared with control anti-BSA IgG (Figure 3, D and F).
Anti-MPO IgG Depletes Leukocytes from the Systemic Circulation and Promotes Their Recruitment within Remote Organs
Circulating leukocyte numbers were significantly reduced after anti-MPO IgG infusion in TNF-α–prestimulated (3405 ± 400 before and 2063 ± 267 at 60 min after antibody; P < 0.001) and KC-prestimulated (2340 ± 149 before and 1970 ± 25 at 60 min after antibody; P < 0.05) mice. This effect was not observed in control mice that received anti-BSA IgG (data not shown). Anti-MPO IgG alone did not reduce circulating leukocyte numbers in the absence of TNF-α or in IL-1β (data not shown).
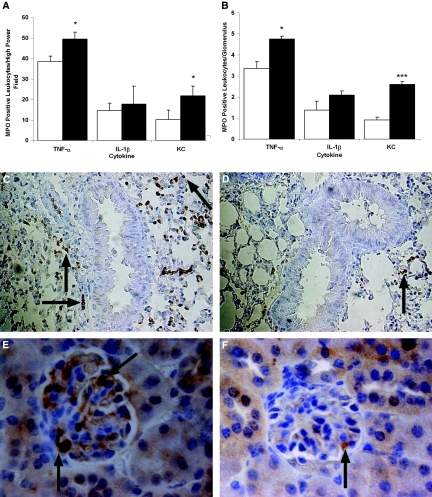
Quantitative analysis of immunostains demonstrated that anti-MPO IgG significantly increased MPO-positive inflammatory cells displaying multilobed morphology within pulmonary alveolar capillaries and renal glomeruli in TNF-α–pretreated (P < 0.05 pulmonary and renal) and KC-pretreated (P < 0.05 pulmonary and P < 0.001 for glomeruli) wild-type mice compared with mice that receiving control anti-BSA IgG (Figure 4, A and B). Anti-MPO IgG failed to promote significant differences in inflammatory cell recruitment to either vascular bed in IL-1β–pretreated mice (Figure 4, A–F).
Figure 4.
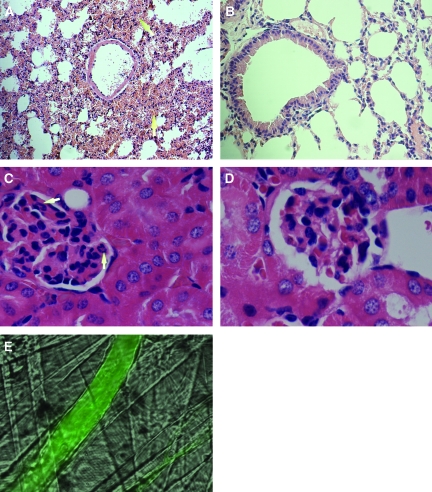
Anti-MPO IgG promotes leukocyte recruitment to remote organs in wild-type mice. MPO immunohistochemistry was performed on pulmonary (A) and renal (B) tissue harvested from TNF-α–, IL-1β–, or KC-pretreated wild-type mice 60 min after the infusion of anti-BSA IgG (□) or anti-MPO IgG (▪; 18 μg/g body wt). Quantitative results are means ± SEM (n = 5 for each treatment group). *P < 0.05, ***P < 0.001 versus cytokine-pretreated mice that received anti-BSA IgG. (C and D) Representative immunostains picturing pulmonary tissue after anti-MPO IgG (C) or anti-BSA IgG (D) infusion into TNF-α–stimulated mice. (E and F) Renal glomeruli after anti-MPO IgG (E) or anti-BSA IgG (F) infusion into TNF-α–prestimulated mice. MPO-positive cells appear brown and are highlighted by arrows. Magnifications: ×40 in C and D; ×100 in E and F.
Anti-MPO IgG Induces Hemorrhage in Specific Vascular Beds
Multiple sites of alveolar hemorrhage were observed in TNF-α–pretreated wild-type mice 60 min after anti-MPO IgG infusion (Figure 5A). Hemorrhage represented approximately 30% of total lung tissue examined. In comparison, well-preserved lung structure was observed after control anti-BSA IgG administration (Figure 5B), with hemorrhage not exceeding 8%. Anti-MPO IgG infusion was accompanied by areas of fibrin deposition and edema (data not shown). Erythrocyte extravasation was also identified within pulmonary tissue after anti-MPO IgG infusion in all KC-treated mice (approximately 15%) but in only one of six mice pretreated with IL-1β (approximately 8% of total lung examined).
Figure 5.
Anti-MPO IgG induces hemorrhage in specific vascular beds in wild-type mice. (A) Analysis of hematoxylin- and eosin-stained pulmonary tissue sections identified sites of alveolar hemorrhage (arrows) in wild-type mice prestimulated with TNF-α 60 min after anti-MPO IgG infusion (18 μg/g body weight). (B) Well-preserved lung structures after anti-BSA IgG administration. (C and D) No significant structural abnormalities in the glomeruli after the administration of anti-MPO IgG (C; recruited neutrophils indicated by arrows) or anti-BSA IgG (D). Changes in vascular integrity in cremaster muscle microvessels were assessed by measuring the leakage of FITC-conjugated BSA from the microvessels into the surrounding tissue. (E) FITC-conjugated BSA is retained within the venules. Magnifications: ×40 in A and B; ×100 in C and D.
Examination of renal tissue revealed no abnormalities in renal glomerular structure in TNF-α–, IL-1β–, or KC-prestimulated mice that received anti-MPO IgG or control anti-BSA IgG (Figure 5, C and D; data not shown for IL-1β or KC). Locally, no FITC-BSA macromolecular leakage was observed in the entire cremaster microcirculatory bed in TNF-α–, IL-1β–, or KC-prestimulated mice that received anti-MPO IgG. This was evidenced by the retention of albumin within all vessels (Figure 5E). Furthermore, no leakage was identified 120 or 180 min after anti-MPO IgG (data not shown).
CD18 Integrin Blockade Prevents Conversion of Leukocyte Rolling to Firm Adhesion in TNF-α–Prestimulated Wild-Type Mice after Infusion of Anti-MPO IgG
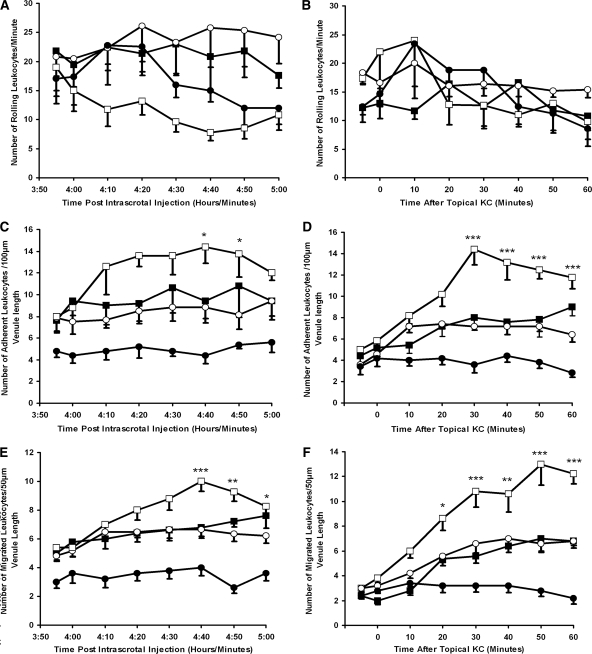
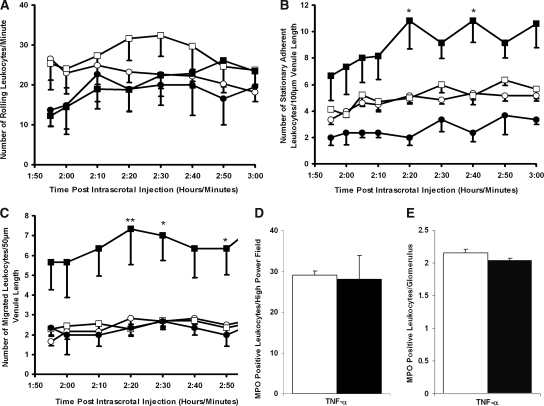
In TNF-α–prestimulated wild-type mice that were administered the CD18 mAb (GAME-46), anti-MPO IgG was unable to induce a decrease in leukocyte rolling or significantly increase stationary adhesion and migration when compared with mice that were administered isotype control antibody (rat IgG1; Figure 6). Leukocyte depletion from the systemic circulation was also prevented in mice that received GAME-46 (2035 ± 156 before and 2028 ± 310 at 60 min after anti-MPO IgG). By comparison, mice that received the isotype control antibody before anti-MPO IgG demonstrated a significant (P < 0.05) reduction (2095 ± 304 before and 1240 ± 134 at 60 min after anti-MPO IgG) in systemic leukocyte numbers. Differential cell counts performed on whole-blood smears demonstrated that neutrophil levels changed from 22.5 ± 4.24% of circulating white blood cells before to 11.2 ± 3.14% after infusion of isotype control and anti-MPO IgG. In mice given GAME-46 and anti-MPO IgG, neutrophil numbers remained constant throughout the experiment (18.3 ± 1.4 and 17.9 ± 1.7%).
Figure 6.
CD18 integrin blockade inhibits the conversion of leukocyte rolling to firm adhesion in TNF-α–prestimulated wild-type mice. A CD18 mAb (♦) or the isotype control (⋄) was infused intra-arterially (30 μg/mouse) 5 min before anti-MPO IgG (18 μg/g body wt). (A through C) Changes in leukocyte rolling (A), stationary adhesion (B), and migration (C) were measured for 60 min in postcapillary venules (20 to 50 μm). Data are means ± SEM (n = 5 for each treatment group). *P < 0.05, **P < 0.01, ***P < 0.001 versus control mice.
Lack of FcRγ Chain Prevents Conversion of Leukocyte Rolling to Firm Adhesion in TNF-α–Prestimulated Wild-Type Mice after Infusion of Anti-MPO IgG
To characterize further the mechanism of anti-MPO IgG–mediated leukocyte activation in vivo, we used FcRγ chain−/− mice. In knockout mice that were prestimulated with TNF-α, anti-MPO IgG was unable to decrease in leukocyte rolling or significantly increase stationary adhesion and migration (Figure 7, A through C); however, an elevated dosage of TNF-α (750 ng) confirmed that it was possible to increase significantly leukocyte firm adhesion (P < 0.05) and migration (P < 0.05) above that induced by TNF-α 500 ng (Figure 7, B and C), indicating that there was no failure in adhesive or migratory potential of leukocytes in FcRγ chain−/− mice. Anti-MPO IgG administration in TNF-α–prestimulated (500 ng) FcRγ chain−/− mice also failed to induce significant depletion of leukocytes from the systemic circulation (2004 ± 306 before and 1867 ± 257 at 60 min after anti-MPO IgG). Furthermore, quantitative MPO immunohistochemistry demonstrated no significant difference between MPO-positive cell number within pulmonary and renal tissue of FcRγ chain−/− mice 60 min after administration of anti-MPO IgG or control anti-BSA IgG (Figure 7, D and E). There was no evidence of fluid accumulation, fibrin deposition, or erythrocyte extravasation into alveolar spaces in knockout mice above background levels (8%; data not shown).
Figure 7.
Effects of Anti-MPO IgG in FcRγ chain−/− mice after intrascrotal TNF-α stimulation. Anti-BSA IgG (○) or anti-MPO IgG (□) was administered intra-arterially (18 μg/g body wt) 2 h after intrascrotal TNF-α (500 ng) injection. The results for mice that received saline intra-arterially 2 h after intrascrotal injection of an elevated concentration of TNF-α (750 ng; ▪) or vehicle (saline containing 0.1% BSA) are also shown (•). (A through C) Changes in leukocyte rolling (A), stationary adhesion (B), and migration (C) were measured for 60 min in postcapillary venules (20 to 50 μm). (D and E) Quantitative MPO immunohistochemistry was performed on pulmonary (D) and renal (E) tissue harvested from TNF-α–pretreated FcRγ chain−/− mice 60 min after the infusion of anti-BSA IgG (□) or anti-MPO IgG (▪; 18 μg/g body wt). Data are means ± SEM (n = 5 for each treatment group). *P < 0.05, **P < 0.01 versus pretreated mice that received intra-arterial anti-BSA IgG (500 ng).
DISCUSSION
ANCA pathogenicity was previously demonstrated in two experimental models that also highlighted a dependence on neutrophils for injury to occur8–10; however, the molecular mechanisms by which inflammatory cell recruitment is achieved has not been addressed previously. Our detailed study extends the original findings considerably by demonstrating that murine anti-MPO IgG can also augment leukocyte adhesion and transmigration in vivo and illustrates the rapidity of these interactions, because they are evident within minutes of antibody exposure. This study also provides the first in vivo mechanistic data highlighting critical roles for Fcγ receptors and β2 integrins in mediating L-E cell interactions. We also provide novel evidence that a number of cytokines are capable of cooperating with ANCA in mediating inflammatory events; however, a marked hierarchy existed regarding the strength of responses supported by different cytokines with TNF-α being the most potent. Remarkably, overt injury was not detected within the local cremaster microcirculation, although it was apparent within distant pulmonary tissue. The presence of injury so soon after anti-MPO IgG infusion within a remote site and the direct effects on circulating leukocyte numbers has not been demonstrated previously and is an interesting finding; however, because 8% of pulmonary tissue demonstrated evidence of injury after infusion of anti-BSA IgG, we cannot exclude a nonspecific component after anti-MPO IgG, but a nearly four-fold enhancement of injury (30%) suggests a largely specific response.
To investigate the signaling mechanisms through which anti-MPO IgG augmented leukocyte interactions, we focused on the role of Fcγ receptors because in vitro experiments suggested that ANCA induces leukocyte activation through FcγR ligation.15,16 Enzymatic removal of the Fc portion of ANCA or function blocking antibodies against human FcγRIIa and FcγRIIIb inhibit the ANCA-induced respiratory burst response.17,23,24 We used FcRγ chain−/− mice lacking the γ-chain subunit essential for Fc receptor expression and neutrophil function and presented novel data demonstrating that knockout mice were resistant to effects of anti-MPO IgG.18,19,25 This was evidenced by a failure to increase numbers of rolling leukocytes that became firmly adherent and transmigrating. In Addition, histologic examination of pulmonary tissue revealed no increase in inflammatory cell recruitment. This study is also the first to demonstrate the importance of β2 integrin in mediating leukocyte responses to ANCA in vivo, and this again supports findings in vitro.11,13,26
As well as increasing endothelial adhesion molecule expression, cytokines may prime circulating leukocytes, allowing L-E cell interactions to be enhanced in response to an otherwise nonpathogenic concentration of anti-MPO IgG.22,27,28 TNF-α but not IL-1β is known to prime neutrophils for responsiveness to ANCA IgG, which may be one reason for more potent effects of anti-MPO IgG in pulmonary and cremaster microvasculature after TNF-α administration compared with IL-1β. Priming effects of TNF-α include increasing target antigen expression on the neutrophil surface, but, in the case of MPO, evidence for this is limited and other priming mechanisms may be operational.7,29–32 It is interesting that KC permitted local conversion of leukocyte rolling to firm adhesion and migration after anti-MPO IgG, although injurious effects in pulmonary tissue were less marked than after TNF-α administration. This suggests that combinations of TNF-α and anti-MPO IgG induce greater proinflammatory leukocyte functions, which may be relevant to human disease, in which increased levels of TNF-α expression are evident within target organs.33
Although we observed high levels of recruited leukocytes in pulmonary tissue, we acknowledge that we did not fully discriminate between leukocyte infiltration (secondary to transmigration, as occurred in cremaster microcirculation) and margination within the capillary lumen. Indeed, previous work form our group highlighted the potential pathogenic significance of intravascular trapping of leukocytes within glomeruli in ANCA-associated disease.34 We anticipate that either process would be injurious in concert with ANCA-driven leukocyte activation because multiple areas of alveolar capillary damage, hemorrhage, and fluid accumulation, as well as respiratory distress, were observed in this study. Pulmonary injury suggests that significant proportions of adherent leukocytes detached from cremasteric endothelium and rejoined the systemic circulation, where they promoted injury to remote organs possibly by undergoing degranulation and a respiratory burst.35–37 It is possible that these cells are neutrophils, because a large proportion of leukocytes engaging in adhesive interactions with cytokine-stimulated vascular endothelium in vivo are of this lineage (97%).38 Furthermore, differential counts of blood smears demonstrated reduced neutrophil numbers.
The rapidity of glomerular and pulmonary leukocyte recruitment in this study is of particular interest. In the original description of this model, injury was assessed 6 d after transfer of anti-MPO IgG, suggesting a significant lag phase before onset of organ damage8; however, in a subsequent report, microscopic hematuria and glomerular neutrophil infiltration were documented 1 d after IgG transfer, which would be more compatible with the time course of our own experiments.39 Concurrent administration of proinflammatory cytokines may well have ha an impact on the speed of onset of tissue injury in our experiments. Consistent with this hypothesis, a relative increase in glomerular neutrophil recruitment at 24 h in mice given anti-MPO IgG and LPS compared with those that received anti-MPO IgG alone has been observed.39 In those experiments, LPS administration increased serum TNF-α concentrations to approximately 5 ng/ml, and although we did not measure TNF-α in our studies, it is possible that the systemic concentrations were higher still and this influenced the time to the onset of injury.
In our study, there seems to be a difference in susceptibility of different vascular beds to ANCA-neutrophil–mediated tissue damage. It is unclear why specific vascular beds are preferentially targeted in ANCA pathology, and sites other than the kidney and lungs that were not monitored may also be affected. This may reflect differences in endothelial cell morphology and phenotype, which is defined by the functional demands of that particular microenvironment.40,41 The observation that injury occurred more readily in pulmonary than glomerular microcirculation may also reflect the higher volume of cardiac output to the lungs (100% rather than 20 to 25%) and/or that the pulmonary microcirculation is a lower pressure system than the glomerulus. This may be relevant from a mechanical aspect because we previously showed that ANCA induced changes in neutrophil cytoskeleton and reduced their deformability, potentially enhancing neutrophil trapping.42
In conclusion, this study demonstrates that circulating anti-MPO IgG acts synergistically with inflammatory stimuli to potentiate cytokine-induced leukocyte firm adhesion and transmigration within murine cremasteric venules and also promotes changes within leukocytes that drive injury at susceptible distant sites. Injury occurred more readily within lungs than either the cremaster muscle, which was anatomically closest to the cytokine stimulus, or in glomerular capillaries known to be targeted as disease progresses. Neither local nor distant inflammatory changes were observed in mice that received a CD18 antibody before anti-MPO IgG. Furthermore, FcRγ chain−/− mice were also protected from the effects of anti-MPO IgG infusion. These results suggest a key role for both of these molecules in contributing to the mechanisms underlying anti-MPO IgG–induced tissue damage, thereby pointing to new strategies to target the underlying pathology in small-vessel vasculitis.
CONCISE METHODS
Recombinant murine TNF-α and IL-1β were purchased from R&D Systems (Abingdon, UK). KC was purchased from Peprotech (Rocky Hill, NJ). All were dissolved in PBS containing 0.1% BSA (Sigma, Gillingham, UK) and stored in aliquots at −20°C. No azide/low endotoxin anti-mouse CD18 (GAME-46) and the isotype control (rat IgG1) were obtained from BD Biosciences (Oxford, UK). W-CAP Tec buffer was purchased from Surgipath (Peterborough, UK). Polyclonal rabbit anti-human MPO and the REAL EnVision detection system were purchased from Dako (Cambridge, UK). Concanavalin A–Sepharose and the Hi Trap protein-G column were purchased from Amersham Biosciences (Roosendaal, Netherlands). Diff-Quik rapid staining set was purchased from Dade Behring (Marburg, Germany). Alkaline phosphatase–conjugated goat antibody was purchased from Jackson ImmunoResearch (Cambridgeshire, UK), and the Mono S cation exchange column was obtained from BioRad (Veenendaal, Netherlands). McCoy5A medium and all other reagents were purchased from Sigma.
Mice
Male C57BL/6 mice weighing 20 to 30 g were used in all wild-type animal experiments and were purchased from Harlan (Oxon, UK). Mpo−/− and FcRγ chain−/− mice were backcrossed into the C57BL/6 background and bred in-house.24,43 Genotype was confirmed by PCR and agarose gel electrophoresis using DNA isolated from mouse tail clippings. All procedures performed in the United Kingdom were in accordance with the Home Office Animals (Scientific Procedures) Act of 1986 (Project License Nos. 40/2212 and 40/2749).
MPO Purification
MPO was purified from a murine cell line (WEHI-3) as described previously.8 In short, WEHI-3 cells were cultured at 37°C in HEPES-buffered McCoy5A medium supplemented with 10% FCS and 2% penicillin/streptomycin. Cells were harvested at a density of 1.5 × 106/ml and lysed by dounce homogenization in a buffer containing 6.7 mM sodium acetate, 3 mM MgCl2, 3 mM NaCl, 0.5 mM PMSF, and 1% CTAB (pH 6.0). After stirring overnight at room temperature, any insoluble material was removed by centrifugation (20 min, 20,000 × g at 4°C). The sample was loaded into dialysis tubing with a 12- to 14-kD molecular weight cutoff point, immersed in buffer (100 mM sodium acetate and 100 mM NaCl [pH 6.3]), and dialyzed overnight at 4°C. The sample was bound to concanavalin A–Sepharose (prepared according to the manufacturer's instructions) by mixing for 8 h at 4°C, washed to remove impurities (5 min, 1200 × g at 4°C), and then eluted with a buffer containing 100 mM sodium acetate, 100 mM NaCl, and 750 mM methyl a-d-mannopyranoside (pH 6.3). The eluted sample was immersed in dialysis buffer containing 50 mM sodium acetate and 100 mM NaCl (pH 8.5) overnight at 4°C. The sample was then loaded into a Mono S cation exchange column, eluted using 1 M NaCl, and dialyzed overnight against PBS (4°C). Fractions containing MPO were identified using spectrofluorometry and also by their intense green coloration.
Antibody generation
Mpo−/− mice were immunized with purified murine MPO as described previously.8,39 Briefly, 10 μg of purified MPO mixed with complete Freund's adjuvant was administered intraperitoneally on day 0 with boosters (10 μg of MPO mixed with incomplete Freund's adjuvant, intraperitoneally) given on days 14 and 21. Control antibodies were generated after immunization with BSA. Antibody development was assessed by ELISA using a small aliquot of serum isolated from tail-vein blood. Microtiter plates were coated overnight with 0.5 μg/ml purified murine MPO (4°C) and blocked with BSA. Serial dilutions (1:100 starting dilution) of mouse serum were prepared, pipetted in duplicate into appropriate microtiter wells, and incubated at room temperature for 60 min. Wells were further incubated (60 min, room temperature) with alkaline phosphatase–conjugated goat anti-mouse IgG. 4-Nitrophenyl phosphate was used as the substrate, and wells were analyzed spectrophotometrically at 405 nm. Terminal bleeds were performed on day 36 with the serum extracted and stored at −20°C. The final antibody titer was also determined by ELISA as detailed previously. Anti-MPO IgG (or control antibody) was used as purified IgG after ammonium sulfate precipitation and isolation on protein G affinity columns.8,39 All antibodies were used intact and without preparation of F(ab′)2 fragments because Fc engagement is required for ANCA activation, so intact anti-BSA antibodies were a more rigorous control.15,17
Intravital Microscopy
Effects of anti-MPO IgG on leukocyte rolling, stationary adhesion, and migration were measured in vivo by direct observation of the murine cremaster muscle microcirculation using intravital microscopy (IVM).44,45 Briefly, mice (five to eight per experimental group) were anesthetized with ketamine hydrochloride (100 mg/kg Vetalar; Amersham Biosciences [Buckinghamshire, UK] and Upjohn Ltd., [Northamptonshire, UK]) and xylazine hydrochloride (10 mg/kg; Millpledge Pharmaceuticals, Nottinghamshire, UK). The trachea and carotid artery were cannulated to facilitate respiration and to allow the administration of maintenance anesthesia and antibodies, respectively. Blood samples (10 μl) were also withdrawn via the carotid artery at 10-min intervals and analyzed for total systemic leukocyte numbers using a hemocytometer. In selected experiments, differential cell counts were also performed on whole-blood smears stained with Diff-Quik rapid staining set. The testis was exposed through a small scrotal incision, and the cremaster muscle was exteriorized, cleared of connective tissue, and pinned across a glass coverslip on a specialized microscope stage. The cremaster muscle was continuously superfused with bicarbonate buffered saline (131.7 mM NaCl, 4.69 mM KCl, 2.7 mM CaCl2, 2.1 mM MgCl2, and 14.44 mM NaHCO3 [pH 7.4]), equilibrated with 5% CO2 in N2, and maintained at 37°C.
Before intra-arterial anti-BSA IgG or anti-MPO IgG administration (18 μg/7 μl for each gram of body weight), mice were pretreated with an intrascrotal injection of TNF-α (500 to 750 ng in 200 μl) or IL-1β (12.5 ng in 200 μl) for 2 and 4 h, respectively. This provided local microvascular endothelial activation or direct leukocyte stimulation before anti-MPO IgG. Control mice received vehicle (saline containing 0.1% BSA in 200 μl, intrascrotally). Alternatively, KC was dissolved in the superfusion buffer and continuously applied at a final concentration of 3.9 nM. In all experiments, L-E cell interactions were recorded 20 min after the exposure of the cremaster muscle for IVM. Optimal concentrations of TNF-α, IL-1β, and KC were determined in preliminary experiments. In selected TNF-α experiments, the CD18 antibody (GAME-46; 30 μg/mouse) or an equal concentration of IgG1 isotype control was infused intra-arterially 5 min before administration of anti-MPO IgG.38,46 L-E cell interactions in single unbranched postcapillary venules (20 to 50 μm in diameter) were observed using an upright microscope (Olympus BX61W1, Middlesex, UK) fitted with a water immersion objective (×40/0.80W). Digital images were captured using a CoolSNAP camera (Roper Scientific, Buckinghamshire, UK), and results were analyzed off-line during video playback analysis.
IVM Data Analysis
Leukocyte rolling was determined by counting numbers of cells rolling along a 100-μm segment of vessel within 60 s. A leukocyte was considered stationary or firmly adherent when it remained stationary for 30 s.47 Migrated leukocytes were quantified by counting those that had transmigrated within 50 μm of either side of the venule.28 For assessment of changes in macromolecular leakage of labeled albumin, FITC-BSA was injected via the carotid cannula. This conjugated albumin molecule is normally retained within the vasculature, which appears green against a nongreen interstitial background; however, under conditions leading to a disturbance in vascular integrity, FITC-BSA leaks out and appears as a green flare surrounding the microvessel.44 Vessel length and diameter were measured using digital microscopy software (Slidebook; Intelligent Imaging Innovations, Goettingen, Germany).
Histology
At the end of each intravital experiment, both lungs and kidneys were harvested, fixed in 10% formal saline, embedded in paraffin blocks, and sectioned (2 μm thick). For basic histology, sections were stained with hematoxylin and eosin according to standard protocols, and neutrophils were identified by their multilobed nuclei. For immunohistochemistry, the histologic specimens were dewaxed and rehydrated by treating with W-CAP Tec buffer (pH 8.0) for 40 min at 98°C. Endogenous peroxidases were blocked with 1% hydrogen peroxide in methanol for 10 min at room temperature before incubation (60 min) with the primary antibody, a polyclonal rabbit anti-human MPO antibody (cross-reactive with murine MPO). The primary antibody was diluted 1:5000 with universal antibody diluent. This was followed by incubation with Dako REAL EnVision detection reagent consisting of a peroxidase-conjugated polymer that carries goat antibodies to rabbit Ig. The reaction was visualized by Dako REAL DAB + Chromogen and counterstained with Mayers hemalum. The secondary antibody detection system is believed to have low cross-reactivity for murine IgG, but even if the injected murine anti-MPO antibody that was bound to leukocytes was detected, this would be of no consequence because the aim was to enumerate numbers of MPO-positive cells. Tissue sections were analyzed blind by a histopathologist and quantified by counting the number of MPO-positive leukocytes in 10 high-power fields (in three to five pulmonary sections) or the number in 50 glomeruli (in three to five renal sections) and calculating the average. The images were captured using Lucia G version 4 (Nikon, Surrey, UK).
Statistical Analyses
The number of rolling, stationary adherent, and migrated leukocytes was analyzed using two-way ANOVA followed by Bonferroni post hoc tests to compare the values at individual time points. The effects of anti-MPO IgG and anti-BSA IgG on leukocyte recruitment within the lung and also within the kidney were compared using a t test. All statistical tests were performed using GraphPad Prism (version 4; GraphPad, San Diego, CA). P ≤ 0.05 was considered significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by British Heart Foundation project grant PG/04/075/17280. P.H. is supported by the Netherlands Organisation for Scientific Research (NWO VIDI 917.66.341).
S.L.N. designed experiments, recorded the data, analyzed data, and wrote the article; N.K. designed experiments, interpreted data, and wrote the article; G.B.N. contributed theoretical knowledge; P.H. contributed vital new reagents; D.K. analyzed histology data; and C.O.S.S. designed experiments, interpreted data, and wrote the article.
We thank Dr. Dennis Huugen for immunizing the MPO knockout mice and Dr. Graham Caine for processing the tissue samples for histologic analysis. We also thank Dr. Peter Hewins for reading the manuscript.
This work has been partially published as four abstracts after presentation at several conferences (J Am Soc Nephrol 18: 1A, 2007; Clin Exp Rheum 4: S-101, 2007; Microcirculation 14: 635–665, 2007; Microcirculation 13: 511–534, 2006).
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Hewins P, Savage COS: Anti-neutrophil cytoplasm antibody associated vasculitis. Int J Biochem Cell Biol 35: 277–282, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Savage COS: The evolving pathogenesis of systemic vasculitis. Clin Med 2: 458–464, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki K, Okazaki T: Contribution of myeloperoxidase in vasculitis development. Jpn J Infect Dis 57: S2–S3, 2004 [PubMed] [Google Scholar]
- 4.Suzuki K, Muso E, Nauseef WM: Contribution of peroxidases in host-defense, diseases and cellular functions. Jpn J Infect Dis 57: S1–S2, 2004 [PubMed] [Google Scholar]
- 5.Morgan MD, Harper L, Williams J, Savage COS: Anti-neutrophil cytoplasm–associated glomerulonephritis. J Am Soc Nephrol 17: 1224–1234, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Harper L, Savage COS: Pathogenesis of ANCA-associated systemic vasculitis. J Pathol 190: 349–359, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Hoshino A, Nagao T, Ito-Ihara T, Ishida-Okawara A, Uno K, Muso E, Nagi-Miura N, Ohno N, Tokunaka K, Naoe S, Hashimoto H, Yasuhara M, Yamamoto K, Suzuki K: Trafficking of QD-conjugated MPO-ANCA in murine systemic vasculitis and glomerulonephritis model mice. Microbiol Immunol 51: 551–566, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Charles JJ: Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110: 955–963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao H, Heeringa P, Liu Z, Huugen D, Hu P, Maeda N, Falk RJ, Jennette JC: The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol 167: 39–45, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little M, A, Smyth CL, Yadav R, Ambrose L, Cook HT, Nourshargh S, Pusey C, D: Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood 106: 2050–2058, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Radford DJ, Savage COS, Nash GB: Treatment of rolling neutrophils with antineutrophil cytoplasmic antibodies causes conversion to firm integrin-mediated adhesion. Arthritis Rheum 43: 1337–1345, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Radford D, Luu N, Hewins P, Nash G, Savage COS: Antineutrophil cytoplasmic antibodies stabilize adhesion and promote migration of flowing neutrophils on endothelial cells. Arthritis Rheum 44: 2851–2861, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Calderwood JW, Williams JM, Morgan MD, Nash GB, Savage COS: ANCA induces beta-2 integrin and CXC chemokine-dependent neutrophil-endothelial cell interactions that mimic those of highly cytokine-activated endothelium. J Leukoc Biol 77: 33–43, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Reumaux D, Kuijpers TW, Hordijk PL, Duthilleul P, Roos D: Involvement of Fc gamma receptors and beta-2 integrins in neutrophil activation by anti-proteinase-3 or anti-myeloperoxidase antibodies. Clin Exp Immunol 134: 344–350, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JM, Ben-Smith A, Hewins P, Dove SK, Hughes P, McEwan R, Wakelam MJ, Savage COS: Activation of the Gi heterotrimeric G protein by ANCA IgG F(ab′)2 fragments is necessary but not sufficient to stimulate the recruitment of those downstream mediators used by intact ANCA IgG. J Am Soc Nephrol 14: 661–669, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Hewins P, Williams JM, Wakelam MJ, Savage COS: Activation of Syk in neutrophils by antineutrophil cytoplasm antibodies occurs via Fc gamma receptors and CD18. J Am Soc Nephrol 15: 796–808, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Ben-Smith A, Dove SK, Martin A, Wakelam MJ, Savage COS: Antineutrophil cytoplasm autoantibodies from patients with systemic vasculitis activate neutrophils through distinct signaling cascades: Comparison with conventional Fc gamma receptor ligation. Blood 98: 1448–1455, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV: Fc gamma RIV: A novel FcR with distinct IgG subclass specificity. Immunity 23: 41–51, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Nimmerjahn F, Ravetch JV: FcY receptors: Old friends and new family members. Immunity 24: 19–28, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kamesh L, Harper L, Savage COS: ANCA-positive vasculitis. J Am Soc Nephrol 13: 1953–1960, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Heeringa P, Huugen D, Tervaert JW: Anti-neutrophil cytoplasmic autoantibodies and leukocyte-endothelial interactions: A sticky connection? Trends Immunol 26: 561–564, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Ley K, Tedder TF: Leukocyte interactions with vascular endothelium: New insights into selectin-mediated attachment and rolling. J Immunol 155: 525–528, 1995 [PubMed] [Google Scholar]
- 23.Reumaux D, Vossebeld P, Roos D, Verhoeven A: Effect of tumor necrosis factor-α induced integrin activation on Fc gamma receptor II mediated signal transduction: Relevance for activation of neutrophils by anti-proteinase 3 or anti-myeloperoxidase antibodies. Blood 86: 3189–3195, 1995 [PubMed] [Google Scholar]
- 24.Porges A, Redecha P, Kimberly W, Csernok E, Gross W, Kimberly R: Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fc gamma RIIa. J Immunol 153: 1271–1280, 1994 [PubMed] [Google Scholar]
- 25.Park SY, Ueda S, Ohno H, Hamano Y, Tanaka M, Shiratori T, Yamazaki T, Arase H, Arase N, Karasawa A, Sato S, Ledermann B, Kondo Y, Okumura K, Ra C, Saito T: Resistance of Fc receptor–deficient mice to fatal glomerulonephritis. J Clin invest 102: 1229–1238, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takagi J, Petre BM, Walz T, Springer TA: Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110: 599–611, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Gotsch U, Jager U, Dominis M, Vestweber D: Expression of P-selectin on endothelial cells is upregulated by LPS and TNF-alpha in vivo. Cell Adhes Commun 2: 7–14, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Muller WA: Leukocyte-endothelial cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol 24: 326–333, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Falk RJ, Terrell RS, Charles LA, Jennette JC: Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A 87: 4115–4119, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day CJ, Hewins P, Savage COS: New developments in the pathogenesis of ANCA-associated vasculitis. Clin Exp Rheumatol 21: S35–S48, 2003 [PubMed] [Google Scholar]
- 31.Bosch X, Guilabert A, Font J: Antineutrophil cytoplasmic antibodies. Lancet 368: 404–418, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Hewins P, Morgan MD, Holden N, Neil D, Williams JM, Savage COS, Harper L: IL-18 is upregulated in the kidney and primes neutrophil responsiveness in ANCA-associated vasculitis. Kidney Int 69: 605–615, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Noronha I, Kruger C, Andrassy K, Ritz E, Waldherr R: In situ production of TNF-α, IL-1β and IL-2R in ANCA positive glomerulonephritis. Kidney Int 43: 682–692 [DOI] [PubMed]
- 34.Cockwell P, Brooks CJ, Adu D, Savage COS: Interleukin-8: A pathogenetic role in antineutrophil cytoplasmic autoantibody associated glomerulonephritis. Kidney Int 55: 852–863, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Kunkel EJ, Dunne JL, Ley K: Leukocyte arrest during cytokine-dependent inflammation in vivo. J Immunol 164: 3301–3308, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Smith CW: Possible steps involved in the transition to stationary adhesion of rolling neutrophils: A brief review. Microcirculation 7: 385–394, 2000 [PubMed] [Google Scholar]
- 37.Bathe M, Shirai A, Doerschuk CM, Kamm RD: Neutrophil transit times through pulmonary capillaries: The effects of capillary geometry and fMLP-stimulation. Biophys J 83: 1917–1933, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunkel EJ, Ley K: Distinct phenotype of E-selectin-deficient mice: E-selectin is required for slow leukocyte rolling in vivo. Circ Res 79: 1196–1204, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Huugen D, Xiao H, van Esch A, Falk RJ, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, Jennette JC, Heeringa P: Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: Role of tumor necrosis factor-alpha. Am J Pathol 167: 47–58, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aird WC, Edelberg JM, Weiler-Guettler H, Simmons WW, Smith TW, Rosenberg RD: Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J Cell Biol 138: 1117–1124, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edelberg JM, Aird WC, Wu W, Rayburn H, Mamuya WS, Mercola M, Rosenberg RD: PDGF mediates cardiac microvascular communication. J Clin Invest 102: 837–843, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tse WY, Nash GB, Hewins P, Savage COS, Adu D: ANCA-induced neutrophil F-actin polymerization: Implications for microvascular inflammation. Kidney Int 67: 130–139, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Aratani Y, Koyama H, Nyui S-i, Suzuki K, Kura F, Maeda N: Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun 67: 1828–1836, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalia N, Pockley GA, Wood RFM, Brown NJ: Effects of FK409 on intestinal ischemia-reperfusion injury and ischemia-induced changes in the rat mucosal villus microcirculation. Transplantation 72: 1875–1880, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Hicks AE, Nolan SL, Ridger VC, Hellewell PG, Norman KE: Recombinant P-selectin glycoprotein ligand-1 directly inhibits leukocyte rolling by all 3 selectins in vivo: Complete inhibition of rolling is not required for anti-inflammatory effect. Blood 101: 3249–3256, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Ridger VC, Wagner BE, Wallace WA, Hellewell PG: Differential effects of CD18, CD29 and CD49 integrin subunit inhibition on neutrophil migration in pulmonary inflammation. J Immunol 166: 3484–3490, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Dangerfield JP, Wang S, Nourshargh S: Blockade of alpha 6 integrin inhibits IL-1 beta but not TNF-alpha-induced neutrophil transmigration in vivo. J Leukoc Biol 77: 159–165, 2005 [DOI] [PubMed] [Google Scholar]