Abstract
Death receptor 3 (DR3), a member of the TNF receptor (TNFR) superfamily, is induced in human renal tubular epithelial cells (TEC) in response to injury. This study examined the expression and actions of TL1A, the principal ligand for DR3. In histologically normal tissue from biopsy or nephrectomy specimens of renal allografts, TL1A mRNA and protein were expressed in vascular endothelial cells but not in TEC. In specimens of acute or antibody-mediated allograft rejection, vascular endothelial cells and infiltrating leukocytes expressed increased TL1A mRNA and protein, but TEC expressed TL1A protein without mRNA, consistent with uptake of exogenous ligand. Addition of TL1A to organ cultures of human or mouse kidney caused activation of NF-κB, expression of TNFR2, activation of caspase-3, and apoptosis in TEC. Inhibition of NF-κB activation increased TL1A-mediated caspase-3 activation and apoptosis of TEC, but it did not reduce the induction of TNFR2. In organ culture of DR3-deficient mouse kidneys, addition of TL1A induced TNFR2 but did not activate NF-κB and did not increase apoptosis of TEC. These data suggest that TL1A may contribute to renal inflammation and injury through DR3-mediated activation of NF-κB and caspase-3, respectively, but that an unidentified receptor may mediate the NF-κB–independent induction of TNFR2 in TEC.
Death receptors (DR), a subfamily of the TNF receptor (TNFR) superfamily, play an important role in cell proliferation, differentiation, and survival.1–3 DR are defined by the presence of an intracellular death domain (DD) that can bind adaptor proteins, which also contain a DD, initiating cellular responses.4 DR3 (also designated as TNFR superfamily member TNFRSF25) interacts with silencer of DD in unstimulated cells.5 Upon ligand binding to DR3, silencer of DD is displaced by TNFR-associated DD protein (TRADD). The DR–TRADD complex initially recruits TNFR-associated factor-2 and receptor-interacting protein, forming a signalosome that initiates both stress-activated protein kinase activation and NF-κB activation.4 At later times, TRADD can recruit Fas-associated DD protein, forming a distinct complex that can promote the autocatalytic activation of caspase 8, which, if unopposed by NF-κB–induced inhibitors, leads to caspase-3 activation and apoptotic cell death.
DR3 expression was initially reported to be restricted to lymphocytes,4,6,7 but we recently demonstrated inducible DR3 expression in both vascular endothelial cells (VEC) and tubular epithelial cells (TEC) in human renal allografts displaying either acute cellular rejection (ACR) or ischemic injury.5 The functions mediated by DR3 signaling in renal cells are unknown.
TL1A (also designated as TNFSF15) has been identified as the principal ligand for DR3.8 TL1A mRNA transcripts have been detected in various human tissues including kidney,8 and its expression is upregulated in T cells and macrophages in intestinal lamina propria of inflammatory bowel disease specimens in Crohn disease.9,10 In this study, we explored the hypothesis that TL1A may contribute to renal tubular injury. We examined TL1A expression in nephrectomy specimens and allograft biopsies interpreted as normal human kidney or undergoing ACR or antibody-mediated rejection. We also examined the function of TL1A in a kidney organ culture model using human tissue or tissue from wild-type (WT) and DR3 null (−/−) mice. Our findings suggest a complex role for TL1A and existence of an additional unidentified receptor for TL1A signaling.
RESULTS
TL1A mRNA and Protein Expression in Normal Human Kidney and Rejecting Allografts
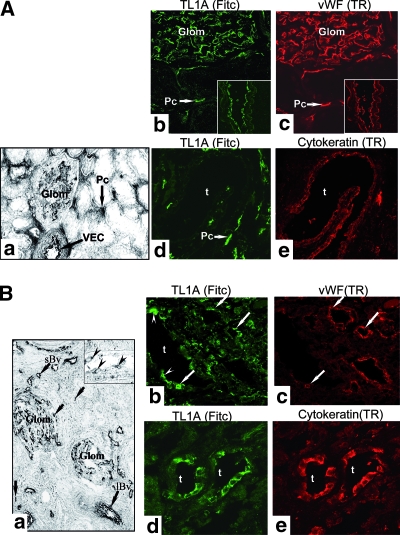
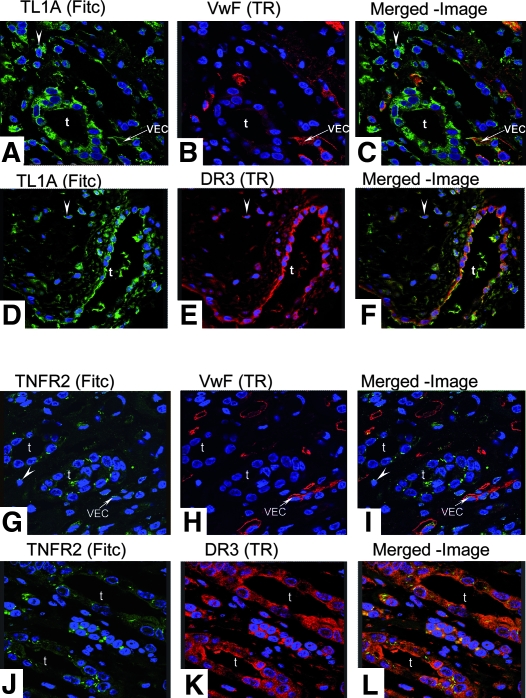
Having previously observed upregulation of DR3 on TEC in renal transplants with ACR or ischemic injury,5 we investigated expression of TL1A mRNA and protein in nephrectomy specimens and human allograft biopsies. In histologically normal kidney, TL1A mRNA and protein were constitutively expressed by glomerular and peritubular capillary VEC (Figure 1A, a and c) but not by TEC (Figure 1A, d through e). ACR specimens showed increased TL1A mRNA expression in VEC and in infiltrating mononuclear cells (MNC), but TL1A mRNA was still not observed in TEC (Figure 1B, a). Nevertheless, TL1A protein was detected in both TEC and VEC (Figure 1B, b through e). The expression of TL1A protein but not mRNA in TEC suggests that tubular epithelium acquires TL1A protein secreted by other cells, such as endothelium and MNC, possibly via binding to DR3, which is upregulated in TEC during ACR.5 In specimens with antibody-mediated rejection, TL1A was found in TEC, MNC infiltrate, and VEC, where it co-stains with anti–von Willebrand factor (anti-vWF; Figure 2, A through C). TL1A co-stained with DR3 predominantly in TEC and in MNC infiltrate (arrowheads; Figure 2, D through F). These specimens also showed punctuate staining for TNFR2 in TEC, some VEC, and MNC infiltrate, where it co-localized with DR3-expressing cells (Figure 2, G through L). In other words, similar changes in TL1A, DR3, and TNFR2 expression are observed in ACR and antibody-mediated rejection.
Figure 1.
TL1A and DR3 mRNA and protein expression in human kidney biopsies. (A) In normal kidney, TL1A mRNA is demonstrated constitutively in VEC of glomerular and peritubular capillaries (a). Co-staining for TL1A and vWF is evident in EC of glomeruli and peritubular capillaries and larger vessels (inset) but not in TEC (b through e). (B) Sections of ACR show increased TL1A mRNA in EC of glomeruli, in large (lBv) and small vessels (sBv) and in MNC infiltrates (arrows; a). Inset shows TL1A mRNA in VEC (arrowheads). Co-staining for TL1A and vWF is evident in VEC (arrows) and in TEC (arrowheads; b and c). TL1A is strongly expressed by TEC (d and e). Similar patterns of staining were seen in five different samples of normal kidney or of ACR. Glom, glomeruli; t, tubular cells; Pc, peritubular capillaries. Magnifications: ×116 in A, a; ×63 in A, b through e, and B, d through e; ×55 in B, a; ×235 in B, inset a; ×40 in B, b and c.
Figure 2.
(A through C) Sections of antibody-mediated rejection show TL1A in TEC, MNC infiltrates (arrowheads), and VEC as confirmed by anti-vWF. (D through F) Co-localization for TL1A and DR3 was evident in TEC and MNC infiltrates (arrowheads). (G through L) TNFR2 was demonstrated in VEC with anti-vWF and in TEC, where it co-localizes with DR3. t, tubular cells. Magnification, ×63.
Despite the presence of detectable TL1A protein in biopsies of kidney allografts with rejection, ELISA failed to detect evidence of TL1A protein in urine of patients with rejection (data not shown). We interpret this as being consistent with efficient uptake of TL1A by TEC, preventing release into the urine. We cannot detect TL1A secretion by cultured human TEC, also measured by ELISA, whereas human umbilical vein epithelial cells (HUVEC) do secrete TL1A basally and at higher levels after treatment with TNF (Supplementary Figure 1). These data are consistent with our findings that renal VEC but not TEC express mRNA for TL1A.
TL1A Effects on TEC in Human Renal Organ Culture
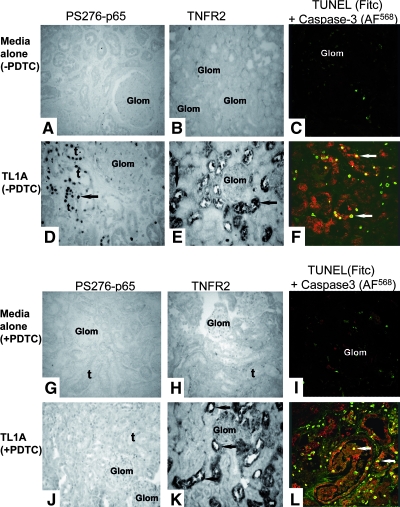
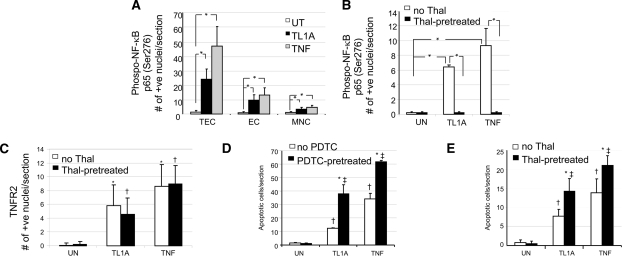
We next analyzed responses of human kidney organ cultures to TL1A, using TNF as a positive control.11 Unstimulated cultures showed no activation of NF-κB, detected with antibody to P-S276-p65 (Figure 3A), or expression of TNFR2 (Figure 3B) and contained only a few isolated active caspase-3–negative/terminal deoxynucleotidyl transferase–mediated digoxigenin-11-dUTP nick-end labeling (TUNEL)-positive nuclei within the interstitium (Figure 3C). In contrast, TL1A-treatment induced abundant NF-κB activation in TEC (Figure 3D; quantified in Figure 4, A and B) and to a lesser extent in VEC and resident MNC (P < 0.01; Figure 4A). TL1A also induced marked expression of TNFR2 in TEC (Figure 3E) and an increased TEC death (Figure 3F). Similar changes were detected in TNF-treated cultures (quantified in Figure 4C).
Figure 3.
TL1A responses in human kidney organ cultures. Sections were co-immunostained for P-S276-p65 or TNFR2 or active caspase-3 and TUNEL assay for evidence of cell death. Unstimulated cultures were negative for P-S276-p65 (A) and TNFR2 (B) and for active caspase-3/TUNEL (C). In contrast, TL1A-treated cultures without PDTC showed a strong signal for P-S276-p65 (D) and TNFR2 (E; arrows) and active caspase-3–positive/TUNEL-negative nuclei (arrows) in TEC (F). Unstimulated cultures pretreated with PDTC were negative for P-S276-p65 (G), TNFR2 (H), and active caspase-3–positive/TUNEL (I). PDTC-pretreated cultures exposed to TL1A show a diminished expression for P-S276-p65 (J) but a strong signal for TNFR2 (K; arrows) and an even increased active caspase-3/TUNEL-positive nuclei in TEC (L; arrows) compared with cultures not pretreated. Images are from one of five separate experiments with similar results. Magnification, ×235.
Figure 4.
Quantitative analysis of nuclear P-S276-p65 expression, TNFR2, and TUNEL in human kidney organ cultures. (A) Addition of TL1A or TNF significantly increased nuclear P-S276-p65 expression in TEC, EC, and MNC, with staining predominantly in TEC. *P < 0.01. (B) Thalidomide pretreatment abolished the rise in nuclear P-S276-p65 expression induced by either TL1A or TNF. *P < 0.01. (C) However, thalidomide did not block the significant increase in the TL1A- or TNF-induced expression of TNFR2 in TEC. *P < 0.01 TL1A or TNF versus untreated; †P < 0.01 TL1A or TNF versus untreated all with Thal. (D and E) TUNEL-positive TEC with apoptotic morphology were counted in 10 fields of view in six (D) or two (E) different experiments. A significant increase in apoptosis was observed in TL1A- or TNF-treated cultures compared with controls, which increased further in cultures treated with PDTC or thalidomide as compared with cultures without PDTC or thalidomide (in all 10 fields were analyzed in 6 or 2 [E] experiments). In panel D, *P < 0.01 PDTC pre-treatment versus no PDTC; †P < 0.01 TL1A or TNF versus untreated; and ‡P < 0.01 TL1A or TNF versus untreated all with Thal. In panel E, *P < 0.01 Thal pre-treatment versus no Thal; †P < 0.01 TL1A or TNF versus untreated; and ‡P < 0.01 TL1A or TNF versus untreated all with Thal. ANOVA P < 0.001; post hoc Tukey's Honestly Significantly Different; P < 0.01.
For assessment of whether increased TNFR2 expression was dependent on NF-κB activation, kidney organ cultures were pretreated either with pyrrolidine dithiocarbamate (PDTC)12,13 or thalidomide,14 inhibitors of NF-κB activation, before TL1A or TNF treatment. Unstimulated cultures pretreated with PDTC or thalidomide showed no staining for P-S276-p65 or for TNFR2 and minimal TUNEL-positive nuclei (Figure 4); however, although pretreatment with PDTC or thalidomide effectively inhibited TL1A-mediated activation of NF-κB (Figure 4, A and B), it surprisingly did not significantly alter TNFR2 induction on TEC (Figures 3 and 4C). PDTC or thalidomide also increased the extent of TL1A-induced cell death (Figures 3 and 4, D and E), increasing the apoptotic-positive tubular cells from <100 to 300 (P < 0.01). The effects of PDTC or thalidomide on TL1A paralleled those seen in cultures treated with TNF (i.e., inhibition of NF-κB activation) and increased apoptosis of TEC but had minimal effect on TNFR2 induction (Figure 4, B through E, and data not shown). We were unable to investigate TL1A responses further using cultured human TEC because these cells, unlike TEC in situ, failed to respond to exogenous TL1A assessed by immunoblotting for IκB-α degradation (Supplementary Figure 2a) or caspase-3 cleavage (Supplementary Figure 2b).
Role of DR3 in TL1A Responses
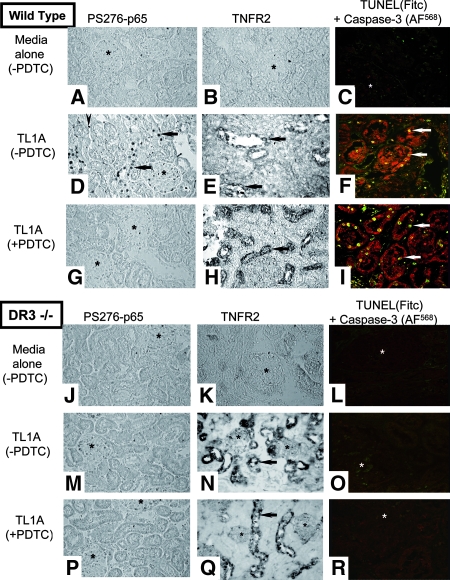
To investigate the importance of DR3 in the responses of TL1A in the kidney, we made use of the DR3-null mouse.15 WT mouse kidney organ cultures incubated in medium alone expressed no P-S276-p65, TNFR2, or active caspase-3/TUNEL (Figure 5, A through C). In contrast, TL1A-treated cultures showed strong expression of nuclear P-S276-p65 (Figure 5D) and also marked TNFR2 expression in TEC (Figure 5E) and increased active caspase-3/TUNEL-positive nuclei predominantly in TEC (Figure 5F). With PDTC pretreatment, TL1A-treated cultures showed only rare nuclear P-S276-p65 staining (Figure 5G), but TNFR2 (Figure 5H) and active caspase-3/TUNEL-positive nuclei (Figure 5I) in TEC were still increased.
Figure 5.
TL1A responses in WT and DR3−/− mouse kidney organ cultures. Unstimulated cultures from WT mice (without PDTC) are negative for P-S276-p65 (A), TNFR2 (B), and active caspase-3/TUNEL (C). In contrast, TL1A-treated cultures (D through F) show a strong signal for P-S276-p65 in TEC (arrows) and in MNC infiltrate (arrowhead) and a strong signal for TNFR2 (E) and active caspase-3/TUNEL (F) in TEC (arrows). TL1A-treated cultures (pretreated with PDTC) show a diminished expression for P-S276-p65 (G) but a strong signal for TNFR2 (H) and active caspase-3/TUNEL (I) in TEC (arrows). In DR3−/− mice (without PDTC), unstimulated cultures are negative for P-S276-p65, TNFR2, and active caspase-3/TUNEL (J through L). TL1A-treated cultures are negative for P-S276-p65 (M) but positive for TNFR2 (N) with a similar pattern of staining as WT animals and are negative for active caspase-3/TUNEL (O). TL1A-treated cultures (pretreated with PDTC; P through R) show similar pattern of staining as cultures without PDTC. Glomeruli (*). Magnification, ×253.
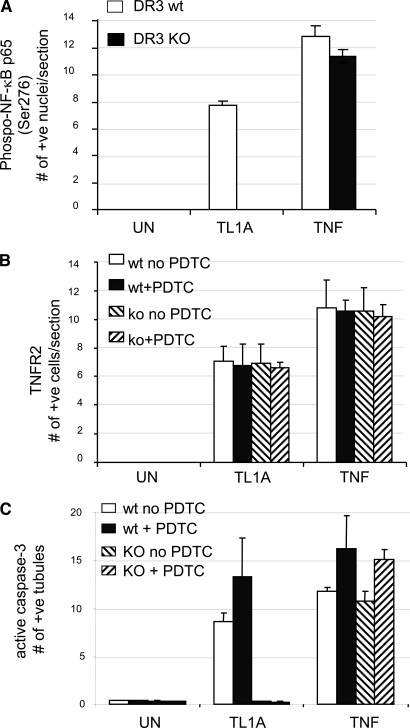
DR3 −/− mouse kidney organ cultures (Figure 5, J through L) showed the same pattern of basal staining as the WT tissue; however, TL1A did not elicit nuclear P-S276-p65 staining (Figure 5M) or activation of caspase-3/TUNEL (Figure 5O), whereas treatment with TNF did (data not shown). Most striking, TNFR2 upregulation was still strongly induced by TL1A in TEC (Figure 5N). With PDTC pretreatment, TL1A-treated cultures remained negative for P-S276-p65 (Figure 5P) and active caspase-3/TUNEL (Figure 5R) yet still became strongly positive for TNFR2 (Figure 5Q). Quantitative data for all three parameters obtained from 10 sections chosen at random from three WT and three DR3 −/− mice for each condition are shown in Figure 6. These results suggest that the induction of TNFR2 by TL1A in TEC is independent of NF-κB and independent of DR3, the only known receptor for TL1A.
Figure 6.
Quantification for expression of P-S276-p65 (A), TNFR2 (B), and active caspase-3 (C); in mouse organ cultures of WT, TL1A-treated cultures show an increased expression of all three parameters (P < 0.05), whereas DR3−/− (knockout [KO]) cultures show no rise in P-S276-p65 or active caspase-3, but TNFR2 expression is not diminished. By contrast, TNF-stimulated expression of all three parameters is not diminished in DR3 KO cultures. Note that PDTC pretreatment increased the amount of active caspase-3 in TL1A- or TNF-treated WT cultures similar to human organs. Moreover, in DR3 KO cultures, PDTC likewise still increased further the number of caspase-3–positive cells induced by TNF.
DISCUSSION
The regulated expression of TNFRSF and their ligands in the kidney may modulate the response to renal injury. We previously reported that both DR35 and TNFR216 are upregulated in renal TEC in ACR and in renal ischemic injury. Like TNFR1, DR3 mediates inflammatory and cell death responses through activation of NF-κB and caspase-3, whereas TNFR2, which is not a DR, promotes cell survival and proliferation, responses correlated with phosphorylation of Etk.11 Here, we investigated the responses to the DR3 ligand TL1A8 using a kidney organ culture model previously developed by our laboratory.11 We report several new findings: (1) TL1A is expressed by VEC of glomeruli and microvessels in normal human kidney and synthesis, and expression of TL1A by VEC is elevated in both ACR and antibody-mediated rejection. (2) TEC are negative for both TL1A mRNA and protein in normal kidney but express high levels of TL1A protein—but not mRNA—in ACR and antibody-mediated rejection, leading us to infer that TL1A produced by VEC and/or infiltrating MNC is taken up by TEC in ACR, where high levels of DR3 are induced.5 In support of this hypothesis, we detected TL1A in the supernatant of cultured HUVEC but were unable to detect TL1A by ELISA in the supernatant of untreated or TNF-treated primary cultures of TEC or by immunoblotting in lysates of cultured TEC (unpublished observations). Furthermore, we were unable to detect TL1A by ELISA in serial samples of urine from patients who underwent renal transplantation, either during periods of normal renal function or renal transplant rejection, consistent with uptake rather than secretion by TEC. (3). In human and WT mouse kidney organ culture models, TL1A upregulates TNFR2, activates NF-κB and caspase-3, and induces apoptotic cell death in TEC. (4) The induction of TNFR2 by TL1A is independent of NF-κB because it occurs in the presence of PDTC or thalidomide, agents that block NF-κB activation and enhance TL1A-mediated apoptosis. (5) In TL1A-treated cultures from DR3 −/− mice, there is no NF-κB or caspase-3 activation, showing that these functions are mediated by DR3, whereas TNFR2 upregulation is undiminished in TEC, demonstrating that TL1A-mediated TNFR2 induction does not occur via DR3 but rather through an as-yet-unidentified receptor.
Our experiments suggest that a number of NF-κB–dependent genes may be induced in TEC via DR3. We have not analyzed our specimens for specific examples, but these are likely to include both proinflammatory genes and antiapoptotic genes. It is interesting that one gene in the former category may be DR3 itself, which is induced on TEC in response to TL1A and whose induction is blocked by PDTC or thalidomide (R.S.A.-L., unpublished data). There are consensus recognition sequences with 85 to 95% homology for NF-κB/cRel binding both upstream (four sites) and downstream (three sites) of the genomic human DR3 sequence, consistent with the possibility that the DR3 promoter is regulated by NF-κB. Evidence for the induction of antiapoptotic genes is strongly suggested by our observations that treatment with PDTC or thalidomide markedly sensitized TEC to TL1A-induced caspase 3 activation and apoptotic cell death. Previous studies on the signaling mechanisms of death receptors, including TNFR1, DR3, and Fas, revealed that members of the NF-κB and caspase family are key determinants of life and death decision-making, with cell fate ultimately determined by a balance between positive and negative regulators.2,4,6 Hence, the effect of PDTC or thalidomide may relate to inhibition of NF-κB–induced antiapoptotic factors such as c-FLIP17,18 and activation of proapoptotic effectors such as caspases,8 which are essential for the cell-death machinery.
Although we found reproducible DR3-dependent and -independent effects of TL1A in renal tissue in organ culture, we found little response to TL1A of isolated cultured cells, including human TEC lines (DPK-KTEC-H and HK2), HUVEC, and human embryonic kidney cells (HEK293). It is unclear why in situ responses differ from those of isolated cells, but we found similar differences in responses to TNF.11 Cell culture may profoundly alter gene expression and behaviors.19,20 We believe that organ culture, which preserves in situ cell geometries and although less subject to experimental manipulations, offers a valid and important corrective for experiments with isolated cells and may better reflect what happens in vivo.
We previously reported that TNF can mediate TNFR2 induction in the human kidney organ culture system.11 In experiments performed as controls for this report, TNF-mediated induction of TNFR2 also seemed to be independent of NF-κB in that it was also not inhibited by PDTC or thalidomide. Thus, our PDTC/thalidomide experiments also suggested that there are distinct pathways regulating DR3 and TNFR2 expression on TEC despite the concordant induction in injured kidney. Specifically, whereas TL1A induction of DR3 is NF-κB dependent, TNFR2 is regulated through NF-κB–independent pathways.
Once induced, DR3, like TNFR1, also seems to mediate renal injury in a caspase-dependent manner8 as indicated by our data here that increased active caspase-3 expression enhances apoptotic cell death in TEC in TL1A-treated cultures. In contrast, the induction of TNFR2 seems to play a role in tubular repair.11 Specifically, by use of TNFR subtype-selective muteins, we demonstrated that TNFR2 induces proliferative nuclear cell antigen (a marker for cell proliferation) on TEC. This finding suggests that TNFR2 may provide an important protective signal for TEC proliferation and regeneration in renal injury. More recent observations have confirmed the prediction that renal ischemia/reperfusion injury is ameliorated in TNFR1 −/− mice but exacerbated in TNFR2 −/− mice (W.M., unpublished observations).
The kidney is not the only organ in which TL1A may contribute to human disease. TL1A/DR3 interactions have been implicated in the pathogenesis of inflammatory bowel disease, in which both TL1A and DR3 mRNA are upregulated in the inflamed intestinal mucosa of murine ileitis.21 Interaction between TL1A on antigen-presenting cells and DR3 on lymphocytes may exacerbate injury through induction of memory T cell proliferation and IFN-γ secretion.10,21 This has provided a rationale for blockade of the TL1A/DR3 pathway as a potential therapy for Crohn's disease.22 Our results suggest that blockade of TL1A and blockade of DR3 may not have the same effects.
In summary, TL1A seems to play several roles in renal tubular injury that may be antagonistic to each other and involve distinct receptors. Further elucidation of the pathways underlying these countervailing responses may lead to new therapies that tip the balance from injury to repair.
CONCISE METHODS
Analysis of Tissue from Normal Kidney and Renal Allografts
Experiments using human tissue were performed with informed consent and approval of the local ethical committee and tissue bank. Non-pathologic renal tissue was obtained from allograft biopsies taken immediately after reperfusion of nine renal transplants (time 0 biopsy) or from the uninvolved pole of kidney excised because of renal tumors and from nine renal allograft biopsies with ACR or antibody-mediated rejection. Tissue was either encapsulated in CRYO-M-BED embedding compound (Bright Instrument Co. Ltd., Huntingdon, Cambridgeshire, UK) and snap-frozen in liquid nitrogen or briefly fixed by immersion in 4% formaldehyde (BDH Merck Ltd., Poole, Dorset, UK) pH 7.5 for 1.5 h at 4°C and paraffin wax-embedded for immunohistochemistry and hematoxylin and eosin staining.
Mouse Kidney
All protocols involving animals were approved by the UK Home Office and the Cambridge University Local Ethical Committee. C57Bl/6 DR3 −/− mice were crossed once into a CD1 background, and the F1 heterozygote progeny were crossed to yield WT and DR3−/− littermates. Animals were killed, and the kidneys were harvested and processed as described in the previous section.
Kidney Organ Cultures
Duplicate 1-mm3 fragments of histologically normal kidney and fresh kidney tissue from WT or DR3 −/− mice were placed in flat-bottomed 96-well tissue culture plates and immediately immersed in medium M199 containing 10% heat-inactivated FCS and 2 mM l-glutamine. Tissue was incubated either in medium alone or with 0.2 μg/ml human recombinant TL1A (R&D Systems, Oxford, UK) or 10 ng/ml human recombinant TNF-α (R&D Systems) for 3 h at 37°C. Additional, duplicate samples were either treated with 50 μg/ml (or 300 μM) PDTC13 (Merck Biosciences Ltd., Beeston, Nottingham, Nottinghamshire, UK) or 100 μM thalidomide14 (Sigma-Aldrich, Gillingham, Dorset, UK) for 30 min before TL1A or TNF treatment. Half of the harvested tissue was cryoprotected and snap-frozen, and half was processed for paraffin wax embedding and hematoxylin and eosin staining.
In Situ Hybridization
In situ hybridization was carried out as described previously.5,11 Cryosections were incubated overnight at 37°C with hybridization solution containing single-stranded antisense DNA oligonucleotide probes 5′end labeled with digoxigenin specific for TL1A (4.1 μg/ml; catgaggagctgggttggctcagggttgctgtctgttaccttggtgatgaccacagtgat; MWG Biotech, Milton Keynes, UK) and then incubated with alkaline-phosphatase–conjugated-sheep anti-digoxigenin antibody (Roche Diagnostics Ltd., Burgess Hill, West Sussex, UK) for 2 h at room temperature and visualized with alkaline-phosphatase substrate (5-brom-4-chloro-3-indoxyly phosphate/nitro blue tetrazolium chloride solution; Sigma-Aldrich). Negative controls included incubation of sections with a sense probe to either DR3 or TL1A.
TL1A or TNFR2 and DR3 Staining of Normal and Rejecting Human Kidney
For detection of TL1A, TNFR2 and DR3 cryosections were permeabilized in methanol at −20°C for 5 min and rinsed in MilliQ water followed by 0.1 M Tris buffer (pH 7.4) + 0.01% Tween-20 (TBS) before incubation in blocking buffer (10% FCS in TBS). Excess fluid was removed, and sections were incubated overnight at 4°C with 1:50 dilution of mouse anti-hTL1A (IgG2a; provided by Human Genome Sciences, Rockville, MD) or mouse anti-hTNFR2 (R&D Systems) and 1:500 dilution of rabbit anti-vWF (Dakocytomation, Ely, Cambridgeshire, UK) or rabbit anti-cytokeratin or rabbit anti-DR3. After rinses in TBS, sections were incubated further in horse anti-mouse FITC and goat anti-rabbit Texas Red (Vector Laboratories, Orton Southgate, Peterborough, UK) at 1:100 dilution for 1 h at room temperature. Sections were rinsed in MilliQ water, mounted in Vectashield Mounting Medium (Vector Laboratories), imaged using Leica laser scanning confocal microscope (Leica Microsystem Ltd., Knowlhill, Milton Keynes, UK), and processed as described previously.
P-S276-p65 or TNFR2 Immunostaining of Human or Mouse Kidney Organ Cultures
Formaldehyde-fixed wax sections of human or mouse kidney organ cultures from WT and DR3−/− mice were incubated with 50 μg/ml Proteinase-K (Roche Diagnostics) for 8 min at room temperature and endogenous peroxidase–quenched with 3% hydrogen peroxide in absolute methanol for 20 min at room temperature. After blocking with blocking buffer for 20 min, sections were incubated at 4°C overnight with 1:100 dilution of rabbit anti–P-S276-p65 (Cell Signaling Technology, Danvers, MA) or 1:50 dilution of mouse anti-hTNFR2 or goat anti-mouse TNFR2 (R&D Systems) and then with either anti-rabbit or anti-mouse horseradish peroxidase or anti-goat horseradish peroxidase (Vector Laboratories) and visualized using diaminobenzidine (Sigma-Aldrich). For controls, the primary antibody was replaced by either nonimmune serum or isotype-specific antisera. The number of P-S276-p65 on TEC, VEC and MNC, and TNFR2 on TEC was determined by counting 10 representative fields at a magnification of ×235 by an observer unaware of the treatment group.
TUNEL and Immunostaining for Active Caspase-3
Apoptotic cells were detected on sections of human or mouse kidney organ cultures by TUNEL assay as described previously11,23 followed by immunostaining for cleaved caspase-3 Asp175 antibody (Cell Signaling). Briefly, sections were incubated with FITC-conjugated TUNEL label mixture (Roche Diagnostics Ltd., Manheim, Germany) followed by rabbit anti-cleaved caspase-3 Asp175 antibody (Cell Signaling). Antigen-binding sites were detected using anti-rabbit AlexFluor–conjugated secondary antibody. Approximately 1000 TEC from six different samples of human kidney organ culture from each of the three treatments were counted at a high power (×235) to determine the percentage of TUNEL-positive cells with apoptotic morphology. TUNEL-positive cells within glomeruli were not counted.
Detection of Soluble TL1A by ELISA
Cultured human TEC or urine or serum of transplant recipients was screened for detectable levels of soluble TL1A. HUVEC was used as positive control.12 Cultured human kidney TEC (Dominion Pharmakine, Derio Bizkaia, Spain) or HUVEC (Lonza, Walkerville, MD) were grown in a six-well culture plate until confluent. The cells were either left in culture alone (untreated) or in medium containing 10 ng/ml TNF (R&D Systems) for 24 h. The culture medium was then collected, and cells were tested for presence of soluble TL1A using an ELISA kit (PeproTech, Business Park, Rocky Hill, NJ) according to the manufacturer's instructions. In addition, urine and serum from renal transplant recipients collected either at time of transplantation (times zero biopsy) or at day 1 to 10 after a rejection episode were examined for the presence of secreted TL1A. Briefly, a 96-well ELISA plate was blocked with 3% BSA in PBS (250 μl/well) for 2 h at room temperature. The rTL1A standard protein, two-fold serially diluted (starting at 4 ng/ml to 0 in diluent) in 1% BSA in PBS, along with samples of interest were incubated in wells (100 μl/well) for 2 h at room temperature or 4°C overnight. Plates were washed three times with wash buffer (PBS + 0.1% BSA + 0.05% Tween 20). A biotinylated anti-TL1A polyclonal antibody, 0.2 μg/ml (100 μl/well), was incubated to wells for 2 h at room temperature. After washing three times with wash buffer, 1:2000 dilution (100 μl/well) of peroxidase-conjugated streptavidin (Vector Laboratories) was added to wells followed by 1 h incubation at room temperature. Plates were washed three times with wash buffer, and ABTS liquid substrate solution (100 μl/well) was added to wells for color development. Plates were read in an ELISA plate reader (Molecular Device, Sunnyvale CA) at 450 nm.
Detection of IκB-α Degradation or Cleaved Caspase-3 in Human TEC
TEC were grown to confluence in six-well plates and then treated with or without TNF (1 or 10 ng/ml) or TL1A (100 or 1000 ng/ml) for 30 min for detection of IκB-α degradation or for cleaved caspase-3 activation for 24 h. Cells were then washed with ice-cold PBS twice and lysed in 25 mM Tris base, 135 mM NaCl, 2.6 mM KCl, 1% Nonidet P-40, protein inhibitor mixture, and 1 mM PMSF for 30 min. Samples were centrifuged, and the supernatants were collected and boiled in sample buffer (75 mM Tris/HCl, 10% sucrose, 0.2 mg/ml bromphenol blue, and 2% SDS) for 3 min before analysis by immunoblotting as described previously.24 Rabbit polyclonal anti–IkB-α antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit polyclonal anti-cleaved caspase-3 (Asp175; Cell Signaling Technology) were used at a dilution of 1:1000 and detected by enhanced chemiluminescence using ECL according to the manufacturer's instructions.
Statistical Analyses
The significance of differences between experimental values was assessed using an unpaired t test or by ANOVA followed by Tukey's Honestly Significantly Different.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by Kidney Research UK, Genzyme Renal Innovations Program, British Heart Foundation, National Institute for Health Research Cambridge Biomedical Research Centre, and the US National Institutes of Health.
Many thanks to Helen Bye for help with animal husbandry and genotyping. Mrs. Bye was funded by a grant from the Wellcome Trust (064232 to A.T.).
Published online ahead of print. Publication date available at www.jasn.org.
R.S.A.-L. and J.W. contributed equally to this work
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Marsters SA, Sheridan JP, Donahue CJ, Pitti RM, Gray CL, Goddard AD, Bauer KD, Ashkenazi A: Apo-3, a new member of the tumor necrosis factor receptor family, contains a death domain and activates apoptosis and NF-kappa B. Curr Biol 6: 1669–1676, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A: Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer 2: 420–430, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Bhardwaj A, Aggarwal BB: Receptor-mediated choreography of life and death. J Clin Immunol 23: 317–332, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chinnaiyan AM, O'Rourke K, Yu GL, Lyons RH, Garg M, Duan DR, Xing L, Gentz R, Ni J, Dixit VM: Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science 274: 990–992, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Al-Lamki RS, Wang J, Thiru S, Pritchard NR, Bradley JA, Pober JS, Bradley JR: Expression of silencer of death domains and death-receptor-3 in normal human kidney and in rejecting renal transplants. Am J Pathol 163: 401–411, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitson J, Raven T, Jiang YP, Goeddel DV, Giles KM, Pun KT, Grinham CJ, Brown R, Farrow SN: A death-domain-containing receptor that mediates apoptosis. Nature 384: 372–375, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Bodmer JL, Burns K, Schneider P, Hofmann K, Steiner V, Thome M, Bornand T, Hahne M, Schroter M, Becker K, Wilson A, French LE, Browning JL, MacDonald HR, Tschopp J: TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas(Apo-1/CD95). Immunity 6: 79–88, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, Cho YH, Ullrich S, Kanakaraj P, Carrell J, Boyd E, Olsen HS, Hu G, Pukac L, Liu D, Ni J, Kim S, Gentz R, Feng P, Moore PA, Ruben SM, Wei P: TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity 16: 479–492, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Bamias G, Martin C III, Marini M, Hoang S, Mishina M, Ross WG, Sachedina MA, Friel CM, Mize J, Bickston SJ, Pizarro TT, Wei P, Cominelli F: Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol 171: 4868–4874, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Prehn JL, Mehdizadeh S, Landers CJ, Luo X, Cha SC, Wei P, Targan SR: Potential role for TL1A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin Immunol 112: 66–77, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Al-Lamki RS, Wang J, Vandenabeele P, Bradley JA, Thiru S, Luo D, Min W, Pober JS, Bradley JR: TNFR1-and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J 19: 1637–1645, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Zhang L: Identification of naturally secreted soluble form of TL1A, a TNF-like cytokine. J Immunol Methods 298: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Liu SF, Ye X, Malik AB: Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents In vivo expression of proinflammatory genes. Circulation 100: 1330–1337, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Aerbajinai W, Zhu J, Gao Z, Chin K, Rodgers GP: Thalidomide induces gamma-globin gene expression through increased reactive oxygen species-mediated p38 MAPK signaling and histone H4 acetylation in adult erythropoiesis. Blood 110: 2864–2971, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang EC, Thern A, Denzel A, Kitson J, Farrow SN, Owen MJ: DR3 regulates negative selection during thymocyte development. Mol Cell Biol 21: 3451–3461, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Lamki RS, Wang J, Skepper JN, Thiru S, Pober JS, Bradley JR: Expression of tumor necrosis factor receptors in normal kidney and rejecting renal transplants. Lab Invest 81: 1503–1515, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Marconi A, Atzei P, Panza C, Fila C, Tiberio R, Truzzi F, Wachter T, Leverkus M, Pincelli C: FLICE/caspase-8 activation triggers anoikis induced by beta1-integrin blockade in human keratinocytes. J Cell Sci 117: 5815–5823, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M: The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell 124: 601–613, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Dai C, Wu C, Liu Y: PINCH-1 promotes tubular epithelial-to-mesenchymal transition by interacting with integrin-linked kinase. J Am Soc Nephrol 18: 2534–2543, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Yang J, Luo JH, Dedhar S, Liu Y: Tubular epithelial cell dedifferentiation is driven by the helix-loop-helix transcriptional inhibitor Id1. J Am Soc Nephrol 18: 449–460, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Bamias G, Mishina M, Nyce M, Ross WG, Kollias G, Rivera-Nieves J, Pizarro TT, Cominelli F: Role of TL1A and its receptor DR3 in two models of chronic murine ileitis. Proc Natl Acad Sci U S A 103: 8441–8446, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young HA, Tovey MG: TL1A: A mediator of gut inflammation. Proc Natl Acad Sci U S A 103: 8303–8304, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavrieli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119: 493–501, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Al-Lamki RS, Zhang H, Kirkiles-Smith N, Gaeta ML, Thiru S, Pober JS, Bradley JR: Histamine antagonizes tumor necrosis factor (TNF) signaling by stimulating TNF receptor shedding from the cell surface and Golgi storage pool. J Biol Chem 278: 21751–21760, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.