Abstract
The mortality rate for patients with acute renal failure (ARF) remains unacceptably high. Although dialysis removes waste products and corrects fluid imbalance, it does not perform the absorptive, metabolic, endocrine, and immunologic functions of normal renal tubule cells. The renal tubule assist device (RAD) is composed of a conventional hemofilter lined by monolayers of renal cells. For testing whether short-term (up to 72 h) treatment with the RAD would improve survival in patients with ARF compared with conventional continuous renal replacement therapy (CRRT), a Phase II, multicenter, randomized, controlled, open-label trial involving 58 patients who had ARF and required CRRT was performed. Forty patients received continuous venovenous hemofiltration + RAD, and 18 received CRRT alone. The primary efficacy end point was all-cause mortality at 28 d; additional end points included all-cause mortality at 90 and 180 d, time to recovery of renal function, time to intensive care unit and hospital discharge, and safety. At day 28, the mortality rate was 33% in the RAD group and 61% in the CRRT group. Kaplan-Meier analysis revealed that survival through day 180 was significantly improved in the RAD group, and Cox proportional hazards models suggested that the risk for death was approximately 50% of that observed in the CRRT-alone group. RAD therapy was also associated with more rapid recovery of kidney function, was well tolerated, and had the expected adverse event profile for critically ill patients with ARF.
Acute renal failure (ARF) arising from acute kidney injury (AKI) and acute tubular necrosis (ATN) secondary to nephrotoxic and/or ischemic renal tubule cell injury commonly results in a cascade of events culminating in multiorgan failure and death. Mortality rates from AKI requiring renal replacement therapy range from 50 to 70%.1,2 This high mortality rate has persisted over the past several decades despite greater understanding of the pathophysiology of the disorder and improvements in hemodialysis and hemofiltration therapy.
The pathophysiology of this disease is initiated with injury to the cellular elements of the kidney, predominantly the proximal tubule cells, leading to tubule cell necrosis and apoptosis and extensive microvascular abnormalities.3 This process evolves into intratubular obstruction, backleak of glomerular filtrate, and diminished peritubular capillary blood flow. When severe enough, renal failure evolves with diminished solute and water excretion and total organ failure. Current renal replacement therapies substitute for this small solute and volume clearance function of the kidney but do not replace the lost reclamation, metabolic, and endocrine functions of this solid organ. These synthetic functions reside in the cellular elements of the kidney. Furthermore, renal tubule cells may play an important immunoregulatory role in stressful clinical conditions. Accordingly, the addition of renal tubule cell therapy to continuous hemofiltration techniques may add more complete short-term renal replacement to allow the natural regenerative recovery of the damaged kidney to normal function and improve the multiorgan dysfunction resulting in poor outcomes in patients with AKI.4
An extracorporeal device has been fabricated with a standard hemofiltration cartridge containing approximately 0.5 to 1.0 × 108 nonautologous human renal tubule cells grown along the inner surface of the hollow fibers of the device.5,6 The nonbiodegradability and pore size of the hollow fibers allow the synthetic membranes to act as both a scaffold and an immunoprotective barrier for the cells. Preclinical studies of this renal tubule cell assist device (RAD) to provide renal cell therapy have demonstrated that these cells retain transport, metabolic, and endocrinologic activities.5 When the RAD is incorporated in series with a separate hemofiltration cartridge in an extracorporeal perfusion circuit, the two cartridge system replaces filtration, transport, metabolic, and endocrine functions in acutely uremic animals7 and ameliorates multiorgan dysfunction in Gram-negative septic shock in large-animal models.8,9 In an open-label Phase I/II human clinical trial at two clinical sites, the addition of human renal tubule cell therapy to continuous venovenous hemofiltration (CVVH) in 10 severely ill intensive care unit (ICU) patients with AKI and multiorgan failure demonstrated that the RAD was safely administered for up to 24 h. Importantly, acute physiologic improvements in several organ systems were temporally related to RAD and improved 30-d survival compared with predicted mortality from ICU scoring systems in these patients.10 We therefore evaluated whether addition of the RAD would reduce all-cause mortality at 28 d and later time points in ICU patients who had AKI and required continuous renal replacement therapy (CRRT) and whether the RAD has an acceptable safety profile during a treatment period up to 72 h.
RESULTS
Patient Disposition, Demographics, and Baseline Assessments
Fifty-eight patients were randomly assigned to the study, 40 to receive the RAD and 18 to receive CRRT alone. One patient who was randomly assigned to the RAD group died after randomization but before the addition of the RAD to the CVVH circuit. A total of 25 of the 58 patients completed the study as planned, 31 patients died before day 180, and two patients withdrew prematurely from the study (one patient in each treatment group). A higher proportion of patients in the RAD group completed the study (21 [53%] of 40) compared with patients who received CRRT alone (four [22%] of 18).
As detailed in Table 1, with the exception of a trend toward a greater proportion of black patients in the RAD group, baseline demographics and clinical characteristics were similar. The majority of patients in both treatment groups were male (73 and 72% in the RAD and CRRT-alone groups, respectively). Mean age was 61 yr in the RAD group and 64 yr in the CRRT-alone group. The majority of patients in both treatment groups were white (58 and 89% in the RAD and CRRT-alone groups, respectively); the RAD group was composed of 38% black patients compared with 11% in the CRRT-alone group (P = 0.071). Mean sepsis-related organ failure assessment (SOFA) score at screening was 12.4 in the RAD group and 11.5 in the CRRT-alone group (range 5 to 19). Mean SOFA renal organ dysfunction subscores were 2.9 and 2.7 in the RAD and CRRT groups, respectively. Acute physiology and chronic health evaluation II (APACHE II)scores11 were slightly higher in the CRRT-alone group. Key clinical laboratory values were also similar between the two groups, including blood counts, electrolytes, blood urea nitrogen, creatinine, and albumin (data not shown). The acuity of illness upon study entry of the patients enrolled in the RAD and CRRT-alone groups is detailed in Table 2. By most parameters, the RAD group had a higher degree of disease severity and multiorgan failure than the CRRT-alone group (but no significant differences).
Table 1.
Patient characteristics
| Demographic Characteristic | RAD (n = 40; %) | CRRT Alone n = 18; %) |
|---|---|---|
| Age (yr, mean [SD]) | 60.9 (13.7) | 64.3 (12.3) |
| Male | 72.5 | 72.2 |
| White | 57.5 | 88.9 |
| Disease cause | ||
| infection/trauma | 37.5 | 33.3 |
| after cardiac surgery | 20.0 | 16.7 |
| after vascular surgery | 15.0 | 16.7 |
| chronic liver disease | 2.5 | 5.6 |
| other (multifactorial) | 25.0 | 27.8 |
Table 2.
Disease severity upon study entry (screening)a
| Disease Characteristic | RAD (n = 40) | CRRT Alone (n = 18) |
|---|---|---|
| SOFA score (mean [SD])b | 12.4 (3.05) | 11.5 (3.94) |
| APACHE II score (mean [SD])b | 24.5 (7.41) | 27.9 (7.86) |
| No. of organ failures (mean [SD]) | 4.0 (1.13) | 3.3 (1.19) |
| No. of organ failures (%)c | ||
| 1 | 0.0 | 5.6d |
| 2 | 7.5 | 16.7 |
| 3 | 25.0 | 38.9 |
| 4 | 42.5 | 16.7 |
| 5 | 25.0 | 22.2 |
| Respiratory failure (%) | 82.5 | 55.6 |
| Cardiac failure (%) | 67.5 | 66.7 |
| Liver failure (%) | 52.5 | 44.4 |
| Hematologic/coagulation failure (%) | 32.5 | 11.1 |
| CNS failure (%) | 67.5 | 55.6 |
| Sepsis (%) | 73.0 | 67.0 |
| Oliguria (%)e | 60.5 | 52.9 |
| Mechanical ventilation (%) | 90.0 | 50.0 |
CNS, central nervous system.
SOFA and APACHE II scores were assessed at baseline at study screening.
Organ failure = SOFA score ≥2.
One patient had sepsis and renal failure.
Oliguria defined as ≤480 ml in a 24-h period as reported on the case report form at screening; three patients were missing this screening measurement.
RAD Integrity and Performance
Median time to initiation of RAD therapy was 21.2 h (time of randomization to inclusion of the RAD in the CVVH circuit) with a range of 15.4 to 37.4 h. Median time on RAD therapy was 35.9 h with a range of 1.8 to 72.1 h. Malfunction of the RAD cartridge (leakage) was reported in only 1 (2.5%) of the 40 patients. None of the RAD cartridges was reported to have excessive cell release, membrane leakage, or clinically significant hemolysis.
Ten patients were treated for the full 72 h. Seven patients were discontinued before 72 h because of clinical improvement (21 to 68 h), whereas four patients were discontinued early (5 to 34 h) because of worsening clinical conditions. Two patients who were randomly assigned to RAD were not treated because of death before RAD integration into the blood circuit and incorrect insertion of the RAD into the circuit, respectively. Two patients had treatment terminated early because of vascular access problems (44 and 53 h). One RAD was disconnected prematurely because of leakage at one of the tubing connectors (57 h). The remaining 14 patients were terminated early in the treatment regimen because of clotting (3 to 57 h). In the majority of cases, clotting that resulted in early termination was due to initiation of clot within the hemofiltration cartridge with extension into the pre-RAD bloodline.
Efficacy
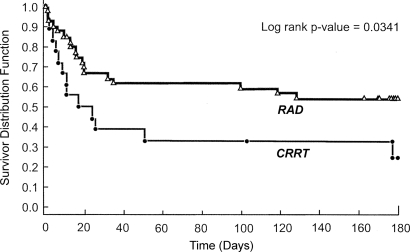
A summary of all-cause mortality at days 28, 90, and 180 is displayed in Table 3. By day 28, 33 of the 58 patients were alive, 24 patients had died, and one (RAD group) had withdrawn consent. In the RAD group, 13 (33%) of the 39 patients had died by day 28 compared with 11 (61%) of the 18 patients in the CRRT-alone group (P = 0.082). The absolute reduction in mortality observed in the RAD group was sustained at days 90 and 180. As determined by Kaplan-Meier methods, survival through 180 d was significantly improved in the RAD group as compared with the CRRT-alone group (P = 0.034; Figure 1). The hazard ratio (HR) for death in the RAD group compared with the CRRT-alone group adjusted for disease cause was 0.481 (95% confidence interval [CI] 0.23 to 0.99), indicating that the relative risk for death in the RAD group was approximately 50% of that observed in the CRRT-alone group.
Table 3.
Summary of all-cause mortality (intention-to-treat population)
| Time Point | RAD (n = 40; n[%])
|
CRRT Alone (n = 18; n [%])
|
Pb | ||||
|---|---|---|---|---|---|---|---|
| Died | Alive | Unknowna | Died | Alive | Unknowna | ||
| 28 d | 13 (33.3) | 26 (66.7) | 1 | 11 (61.1) | 7 (38.9) | 0 | 0.0821 |
| 90 d | 15 (38.5) | 24 (61.5) | 1 | 12 (66.7) | 6 (33.3) | 0 | 0.0855 |
| 180 d | 18 (50.0) | 18 (50.0) | 4 | 13 (76.5) | 4 (23.5) | 1 | 0.0817 |
One patient in the RAD group withdrew consent before day 28, and three patients were alive but had their last study visit outside of the protocol-specified window of 180 ± 7 days. When these three patients are included at day 180, survival in the RAD group was 54% (21 of 39 patients). In the CRRT-alone group, one patient was lost to follow-up after day 90.
P values from exact Pearson χ 2 test; analysis excludes patients with unknown status.
Figure 1.
Kaplan-Meier estimates of survival between patients in the RAD and conventional CRRT groups.
Renal Recovery
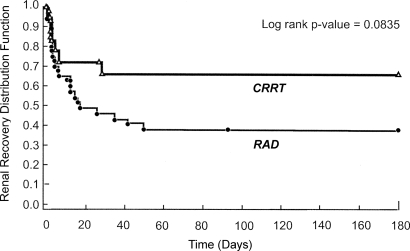
By day 28, 21 (53%) of the 40 patients in the RAD group had recovered renal function, 10 (25%) had died before recovery, eight (20%) remained on renal support, and one (3%) had withdrawn consent. In the CRRT-alone group, a lower proportion of patients had recovered renal function (five [28%] of 18) by day 28, a higher proportion had died before recovery (nine [50%] of 18), and a similar proportion remained on renal support (four [22%] of 18) compared with the RAD group. The Kaplan-Meier plot of time to renal recovery in the two groups is displayed in Figure 2. At day 180, only one (3%) patient in the RAD group and one (6%) patient in the CRRT-alone group were still on long-term dialysis.
Figure 2.
Kaplan-Meier estimates of time to renal recovery in the RAD and CRRT-alone groups.
Patient Subgroup Analysis
Subgroup analyses, including severity of illness (SOFA and APACHE scores), number of organ failures (Table 4), and presence of sepsis at study entry were evaluated. Consistently higher survival rates at 28 d were observed in the RAD group compared with the CRRT-alone group, regardless of the number of organ failures. Of note, patients with five or more organ failures had a 60% mortality rate in the RAD group compared with a 100% rate in the CRRT-alone group. The incidence of sepsis in the two groups was high, at 73 and 67% in RAD and CRRT-alone groups, respectively. RAD therapy decreased the mortality rate in patients with sepsis from 67% in the CRRT-alone group to 34%. In this regard, the RAD was associated with a reduction in the risk for death compared with CRRT alone when analyzed by age, race, and baseline SOFA or APACHE II score (Table 5). Cox proportional hazards models were fit separately for patients with high and low values of the baseline illness severity scores APACHE II and SOFA. These models were unadjusted. For APACHE II ≥26 (n = 34), an HR of RAD to CVVH alone of 0.47 (95% CI 0.20 to 1.10; P = 0.08) was seen versus 0.58 (95% CI 0.14 to 2.33; P = 0.44) for APACHE II <26 (n = 24). Similarly, for SOFA ≥12 (n = 35), an HR of 0.39 (95% CI 0.16 to 0.90; P = 0.03) was observed versus 0.45 (95% CI 0.11 to 1.80; P = 0.26) in the SOFA <12 group (n = 23). Thus, it seems that treatment effects may be more pronounced with greater illness severity at baseline, although given the small sample sizes, the CI are overlapping.
Table 4.
All-cause mortality by day 28 by number of organ failures
| No. of Organ Failures | RAD (Deaths/Total Patients) | CRRT Alone (Deaths/Total Patients) |
|---|---|---|
| 1 | 0/0 | 0/1 |
| 2 | 0/3 | 1/3 (33.3%) |
| 3 | 2/10 (20.0%) | 4/7 (57.1%) |
| 4 | 6/17 (35.3%) | 2/3 (66.7%) |
| 5+ | 5/9 (55.6%)a | 4/4 (100%) |
Excludes one patient with unknown status.
Table 5.
Cox HR: Risk for death by day 180 by patient subgroup (intention-to-treat population)
| Patient Subgroup | HR (95% CI) | P |
|---|---|---|
| Age (yr) | ||
| ≥65 (n = 26) | 0.51 (0.20 to 1.28) | 0.15 |
| <65 (n = 32) | 0.59 (0.18 to 1.91) | 0.38 |
| Race | ||
| white (n = 39) | 0.49 (0.21 to 1.17) | 0.11 |
| nonwhite (n = 19) | 0.13 (0.02 to 0.78) | 0.03 |
| Baseline SOFA | ||
| ≥12 (n = 35) | 0.47 (0.20 to 1.10) | 0.08 |
| <12 (n = 23) | 0.58 (0.14 to 2.33) | 0.45 |
| Baseline APACHE II | ||
| ≥26 (n = 34) | 0.39 (0.16 to 0.90) | 0.03 |
| <26 (n = 24) | 0.45 (0.11 to 1.80) | 0.26 |
Safety
Table 6 summarizes all severe adverse events (SAE) reported in two or more patients in either treatment group. SAE were reported in 68% of patients (27 of 40) receiving the RAD and in 89% of patients (16 of 18) receiving CRRT alone. The reported SAE were typical of a seriously ill patient population in the ICU with ARF and receiving dialysis therapy. Only two of the SAE (hypoglycemia in one patient in the RAD group and supraventricular tachycardia in one patient in the CRRT-alone group) were considered to be treatment related by the investigator.
Table 6.
Most common SAE (intention-to-treat population)a
| MedDRA Preferred Term | RAD (n = 40; n [%]) | CRRT Alone (n = 18; n [%]) |
|---|---|---|
| Cardiac arrest | 8 (20.0) | 3 (16.7) |
| Sepsis | 3 (7.5) | 4 (22.2) |
| Multiorgan failure | 3 (7.5) | 2 (11.1) |
| Respiratory failure | 2 (5.0) | 2 (11.1) |
| Deep vein thrombosis | 2 (5.0) | 1 (5.6) |
| ARDS | 1 (2.5) | 2 (11.1) |
| Anemia | 2 (5.0) | 0 (0.0) |
| Cardiorespiratory arrest | 2 (5.0) | 0 (0.0) |
| Colon cancer | 2 (5.0) | 0 (0.0) |
| Hematocrit decreased | 2 (5.0) | 0 (0.0) |
| Hypoxia | 2 (5.0) | 0 (0.0) |
| Hepatic failure | 0 (0.0) | 2 (11.1) |
| Hypotension | 0 (0.0) | 2 (11.1) |
Presents all SAE reported in two or more of patients in either treatment group. ARDS, acute respiratory distress syndrome; MedDRA, Medical Dictionary for Regulatory Activities Terminology.
DISCUSSION
In this randomized, multicenter trial, the addition of renal tubule cell therapy to CVVH treatment trended to reduce all-cause mortality at 28 d in ICU patients with AKI. This primary end point of 28-d mortality, however, did not reach statistical significance (P = 0.08). The cumulative survival benefit was observed over the 180 d of follow-up as a secondary end point, with a 50% reduction in mortality risk that was statistically significant (P = 0.038). This treatment effect was observed despite a heterogeneous spectrum of patients. A consistent survival benefit with cell therapy was observed in various subgroup analyses, including age, race, baseline disease severity (SOFA and APACHE II), number of organ failures, and the presence of sepsis. A higher percentage of patients from the RAD group demonstrated renal recovery at 28 d compared with the CRRT control group. Thus, the primary effect of RAD therapy was to improve overall patient survival and earlier renal recovery, which was associated with long-term survival benefit. Of note, a recent epidemiologic study has reported a lower in-hospital mortality rate in black patients with ARF and AKI than in white patients.12 The RAD group had a higher number of black patients compared with the conventional CRRT group. This difference may have had an influence in the results.
The most common cause of early RAD treatment termination was clotting of the hemofilter cartridge with extension into the pre-RAD bloodlines and into the RAD cartridge itself. The RAD also demonstrated integrity, with minor cell loss and functionality in critically ill patients for up to 72 h of therapy.
Treatment with the RAD was well tolerated, and the adverse event profile was consistent with that expected for a seriously ill population with AKI. The most common clinically meaningful adverse events observed during RAD treatment were hypotension, thrombocytopenia, and hypoglycemia. Hypotension most frequently occurred during the first few minutes of RAD treatment and was generally responsive to standard therapy. The development of thrombocytopenia in patients exposed to an extracorporeal circuit and heparin therapy is not uncommon, and, although a relationship to RAD treatment cannot be excluded at this time, no significant clinical sequelae related to thrombocytopenia have been reported. Hypoglycemia has been observed after insertion of the RAD into the extracorporeal circuit, which was attributed to nonspecific adsorption of insulin to the cartridge membrane from the culture medium, which was then released when the RAD was perfused.10 This issue has been addressed by instituting a flushing procedure before shipment, as well as instituting guidelines in the protocol for supplemental intravenous glucose administration and careful glucose monitoring during the initial 24 h of RAD therapy.
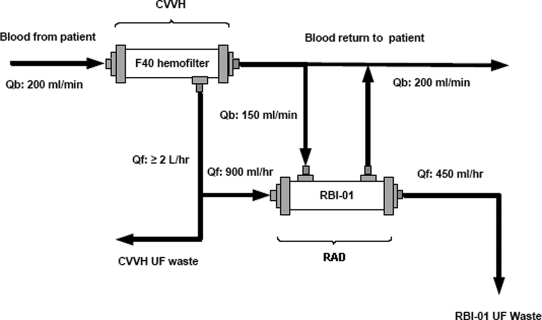
The degree of impact of this therapeutic approach in this study is compelling. The mortality rate of these patients has consistently been in the range of 50 to 70% during the past four decades despite improvements in ICU care and advances in synthetic materials and extracorporeal circuits.1,2 Hemodialysis and hemofiltration interventions have made an impact on preventing death from hyperkalemia, acidemia, uremia, and volume overload, but these patients continue to progress into a vicious spiral of events, including systemic inflammatory response syndrome, sepsis, cardiovascular collapse, ischemic damage to solid organs, and multiorgan failure and death.13,14 Dialysis dosage has not been consistently shown to have an impact on the mortality outcome of these types of patients. In fact, a lower small solute clearance would be expected from use of the RAD, which re-processes effluent already cleared by CVVH and returns half of this volume to the circulation (Figure 3). No differences in serum blood urea nitrogen or creatinine values were observed during the first 3 d after randomization (data not shown). The mechanism by which renal tubule cell therapy reverses this spiraling cascade of clinical events is unknown, although it is most likely multifactorial. The critical nature of renal tubule cells in the development of AKI is clearly recognized. Although the pathologic findings in this disorder are patchy necrosis and apoptosis of proximal renal epithelial cells, the overall ability of renal epithelium during this disorder to promote paracrine and endocrine effects to alter immunologic and distant organ performance is limited not only because of cell injury but also because of severe microvascular damage and reductions in tubular blood flow.3
Figure 3.
Schematic of the extracorporeal perfusion circuit for renal cell therapy. Flow rates approximate those used clinically. The hemofilter perfusion PUMP system used the BBraun's (Bethlehem, PA) Diapact System; the RAD perfusion system used an Alaris (San Diego, CA) intravenous pump for the pre-RAD ultrafiltrate line and a Minntech (Minneapolis, MN) blood pump for the post-RAD blood line. Qb, blood flow; Qf, rate of fluid filtration.
Evaluation of a potential mechanism of action of renal tubule cell therapy has been directed toward an immunoregulatory role of tubule cells in stressful clinical conditions. Preclinical8,9 and initial clinical data10,15–17 have demonstrated an alteration of this form of cell therapy on the systemic inflammatory cascade, which is activated by AKI. Further evaluation in ongoing clinical studies of the RAD will further test this possibility because of the small number of patients in this study, especially in the control group. Of importance, a subsequent 53-patient Phase IIb bridging study was initiated to evaluate a commercial scale-up manufacturing process, use of citrate as a regional anticoagulation process, and incorporating a blinded sham non–cell-containing cartridge into the study design. This follow-up clinical study was discontinued after an interim analysis projected that completing the study would not meet its efficacy goal. Preliminary analysis of the clinical data suggested that the multiple changes incorporated into this more recent Phase IIb clinical study influenced the negative results. At least two changes are being further evaluated and followed up with additional preclinical studies. As these data develop to a conclusion, we plan to publish the results of these further analyses.
Despite the critical nature and life-threatening illnesses of the patients enrolled in this clinical study, the addition of renal tubule cell therapy to CVVH resulted in a substantive clinical impact on survival compared with a conventional CRRT group. RAD treatment for up to 72 h promoted a statistically significant survival advantage over 180 d of follow-up in ICU patients with AKI and demonstrated an acceptable safety profile. Ultimately, a pivotal Phase III randomized, multicenter trial is required to evaluate further this new therapeutic approach for this disorder with an unacceptably high mortality rate.
CONCISE METHODS
Patients
From March 2004 through December 2005, this prospective, randomized, controlled, open-label, clinical trial was conducted at 12 medical centers in the United States. The study was carried out under a corporate-sponsored (RenaMed Biologics, Inc., Lincoln, RI) Investigational New Drug application in accordance with the Declaration of Helsinki and good clinical practice. The institutional review board at each medical center approved the protocol, along with the Cleveland Clinic institutional review board as a coordinating review group. Written informed consent was obtained from all participants or their authorized representatives. A centralized telephone randomization system was established with 24-h availability.
Enrollment Criteria
Adult male and female (nonpregnant) patients who were aged 18 to 80 yr and required CRRT for the treatment of ARF secondary to ATN in an ICU setting were enrolled in the study. ATN was defined as acute renal failure occurring in a setting of acute ischemic or nephrotoxic injury and oliguria (<20 ml/h) for >24 h or an increase in serum creatinine concentration ≥2 mg/dl (≥1.5 mg/dl in women) during a period of ≤4 d. Patients were required to have received CRRT for a minimum of 4 h but not longer than 48 h before randomization. Eligible patients were also required to have at least one nonrenal organ failure (modified SOFA score ≥2) or presence of sepsis as defined by Bone et al.18,19 Exclusion criteria were irreversible brain damage, presence of organ transplant, preexisting chronic renal insufficiency (baseline serum creatinine ≥3.0 mg/dl for men or ≥2.5 mg/dl for women), chronic immunosuppression, Xigris therapy at time of randomization, nonpregnancy status, and do-not-resuscitate status. Before randomization, each patient was reviewed by the RenaMed Biologics, Inc., Medical Monitor to confirm study eligibility.
Treatment Assignment
Patients were randomly assigned (2:1) to receive CVVH plus RAD or CRRT consisting of CVVH, continuous venovenous hemodialysis, or continuous venovenous hemodiafiltration (control). Patients were categorized at randomization into one of five classifications on the basis of the likely cause of AKI: (1) postvascular surgery, (2) postcardiac surgery, (3) chronic liver disease, (4) infection or trauma, or (5) other. Randomization was accomplished from a central source and was stratified by ATN classification and by site so that treatment balance was maintained within strata and within site, using an institution-balancing algorithm.20 Because the number of patients per site and per stratum was small, true randomization concealment may not have been achieved and needs to be considered as a limitation in this trial. Conventional CRRT in the control group was determined by the clinical team according to patient need and conventional CRRT protocols used at each specific clinical site.
All hemofilters contained noncellulose biocompatible membranes. A new hemofilter was placed into the circuit at the time of randomization and replaced with a new cartridge according to institutional policy. For patients who were randomly assigned to control therapy (CVVH, continuous venovenous hemodialysis, or continuous venovenous hemodiafiltration), the effluent rate was ≥2 L/h (i.e., replacement fluid plus dialysate). The CVVH + RAD group received hemofiltration at a rate ≥2 L/h with a pre-RAD ultrafiltrate (UF) flow rate of 900 ± 50 ml/h and a post-RAD UF rate of 50 ± 5% of the pre-RAD UF rate. The recommended CRRT blood flow rate for both treatment groups was ≥200 ml/min. The extracorporeal circuit for CVVH + RAD is schematized in Figure 3. Because of the time required to prepare and ship the RAD to a clinical site from the manufacturing and storage site (Walkersville, MD, or Lincoln, RI), there was an inherent delay between randomization and initiation of RAD therapy (8 to 28 h). The manufacture and storage of RAD have been detailed in previous publications.10 Only one RAD was used for each patient. Reasons for early termination from RAD treatment were documented. Supportive care required for the treatment of these critically ill patients (e.g., antibiotics, fluid balance, vasopressors, ventilatory support) was provided according to institutional policy and recommendations of the treating physician(s).
Patient Evaluation
The primary objective of this study was to evaluate the impact of the RAD on all-cause mortality at day 28. Additional efficacy end points included 90- and 180-d all-cause mortality, recovery of renal function (time on dialysis), time to ICU discharge, and time to hospital discharge.
Safety assessments included monitoring for adverse events, vital signs, and laboratory evaluations. In addition, RAD integrity and cell loss were assessed. An independent data monitoring committee, composed of three clinicians and a statistician, conducted unblinded safety and effectiveness assessments at periodic intervals during the course of the trial.
Statistical Analyses
The primary efficacy end point was all-cause mortality 28 d after randomization (intent-to-treat population). Statistical differences between the two groups were evaluated at 28, 90, and 180 d after randomization with the exact Pearson χ2 test. Kaplan-Meier methods were also used to assess differences in survival between groups. The log-rank test statistic was computed to assess the overall differences through 180 d. Cox proportional hazards models were used to identify risk factors. HR and corresponding 95% CI were computed. The Cox model provided estimate of risk compared with control for each Kaplan-Meier (180-d survival, time to renal recovery) as well as by patient subgroup: Age greater or less than 65 yr, race, and SOFA and APACHE II scores at study entry during screening period. Also, the variables included in the Cox model were age, race, baseline APACHE II score, and baseline SOFA score.
Enrollment of at least 90 patients with 60 randomly assigned to CVVH + RAD and 30 assigned to conventional CRRT treatment was initially planned. This Phase II “screening” trial was based on a three-category decision guideline: Whether the estimated improvement in 28-d survival for the RAD + CVVH arm versus the CRRT-alone arm was <10%, between 10 and 23.3%, or >23.3%. A 10 to 23.3% effect would suggest P < 0.2 and would be reason to go forward with additional confirmatory trials. Because of slow patient enrollment and the corporate need to assess the program, an interim analysis was conducted after 58 patients had been enrolled (40 patients assigned to CVVH + RAD and 18 patients to CRRT alone). Enrollment was suspended pending review of the results from the interim analysis by the independent data monitoring committee. After the interim analysis, a corporate decision was made to discontinue enrollment in this study and proceed to the design of a confirmatory Phase II study.
DISCLOSURES
H.D.H. is a shareholder of Nephrion (formerly RenaMed Biologics, Inc.), a biotechnology spinout company of the University of Michigan.
Acknowledgments
Abstracts regarding the results of this study were previously published (J Am Soc Nephrol 16: 46A, 2005 and J Am Soc Nephrol 17: 49A, 2006).
We are grateful for the contributions of staff of RenaMed Biologics, Inc., in particular Bradley Maroni, MD, Michael F. DeBruin, MD, Karen M. Brennan, BSN, MBA, and J. Ricardo Da Silva, RN, BSN.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Toward the Promise of Renal Replacement Therapy,” on pages 839–840.
REFERENCES
- 1.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM, for the Program to Improve Care in Acute Renal Disease (PICARD): Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int 66: 1613–1621, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Ympa YP, Sakr Y, Reinhart K, Vincent JL: Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med 118: 827–832, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Sutton TA, Fisher CJ, Molitoris BA: Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 539–549, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Humes HD: Bioartificial kidney for full renal replacement therapy. Semin Nephrol 20: 71–82, 2000 [PubMed] [Google Scholar]
- 5.Humes HD, MacKay SM, Funke AJ, Buffington DA: Tissue engineering of a bioartificial renal tubule assist device: In vitro transport and metabolic characteristics. Kidney Int 55: 2502–2514, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Humes HD, Fissell WH, Weitzel WF, Buffington DA, Westover AJ, MacKay SM, Gutierrez JM: Metabolic replacement of renal function in uremic animals with a bioartificial kidney containing human cells. Am J Kidney Dis 39: 1078–1087, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Humes HD, Buffington DA, MacKay SM, Funke AJ, Weitzel WF: Replacement of renal function in uremic animals with a tissue-engineered kidney. Nat Biotechnol 17: 451–455, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Fissell WH, Lou L, Abrishami S, Buffington DA, Humes HD: Bioartificial kidney ameliorates gram-negative bacteria-induced septic shock in uremic animals. J Am Soc Nephrol 14: 454–461, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Humes HD, Buffington DA, Lou L, Abrishami S, Wang M, Xia J, Fissell WH: Cell therapy with a tissue-engineered kidney protects against the multi-organ consequences of septic shock. Crit Care Med 31: 2421–2428, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Humes HD, Weitzel WF, Bartlett RH, Swaniker FC, Paganini EP, Luderer JR, Sobota J: Initial clinical results of the bioartificial kidney containing human cells in ICU patients with acute renal failure. Kidney Int 66: 1578–1588, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: A severity of disease classification system. Crit Care Med 13: 818–829, 1985 [PubMed] [Google Scholar]
- 12.Waikar SS, Curhan GC, Ayanian JZ, Chertow GM: Race and mortality after acute renal failure. J Am Soc Nephrol 18: 2740–2748, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly KJ: Acute renal failure: Much more than a kidney disease. Semin Nephrol 26: 105–113, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M: Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int 63: 600–606, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Humes HD, Weitzel WF, Bartlett RH, Swaniker FC, Paganini EP: Renal cell therapy is associated with dynamic and individualized responses in patients with acute renal failure. Blood Purif 21: 64–71, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K, Spratt D, Shyr Y, Ikizler TA, for the PICARD study group: Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int 65: 1357–1365, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Himmelfarb J, Le P, Klenzak J, Freedman S, McMenamin ME, Ikizler TA, for the PICARD group: Impaired monocyte cytokine production in critically ill patients with acute renal failure. Kidney Int 66: 2354–2360, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Bone RC: Sepsis, the sepsis syndrome, multi-organ failure: A plea for comparable definitions. Ann Intern Med 114: 332–333, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 22: 707–710, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Zelen M: The randomization and stratification of patients to clinical trials. J Chronic Dis 27: 365–375, 1974 [DOI] [PubMed] [Google Scholar]