Abstract
Conjugation or deconjugation of ubiquitin (Ub) or ubiquitin-like proteins (UBLs) to or from cellular proteins is a multifaceted and universal means of regulating cellular physiology, controlling the lifetime, localization, and activity of many critical proteins. Deconjugation of Ub or UBL from proteins is performed by a class of proteases called isopeptidases. Herein is described a readily quantifiable novel isopeptidase assay platform consisting of Ub or UBL fused to the reporter enzyme phospholipase A2 (PLA2). Isopeptidase activity releases PLA2, which cleaves its substrate, generating a signal that is linear with deubiquitylase (DUB) concentration and is able to discriminate DUB, deSUMOylase, deNEDDylase, and deISGylase activities. The power and sensitivity of the UBL-PLA2 assay are demonstrated by its ability to differentiate the contrasting deISGylase and DUB activities of two coronavirus proteases: severe acute respiratory syndrome papain-like protease (SARS-CoV PLpro) and NL63 CoV papain-like protease 2 (PLP2). Furthermore, direct comparisons with the current Ub-7-amino-4-methylcoumarin (Ub-AMC) assay demonstrated that the Ub-PLA2 assay is an effective tool for characterizing modulators of isopeptidase activity. This observation was expanded by profiling the inhibitory activity of the nonselective isopeptidase inhibitor NSC 632839 against DUBs and deSUMOylases. Taken together, these studies illustrate the utility of the reporter-based approach to measuring isopeptidase activity.
Keywords: ubiquitin, deubiquitylase, deSUMOylase, deISGylase, deNEDDylase
The content of most proteins in the cell is governed by the ubiquitin–proteasomal pathway (Hershko and Ciechanover 1998). Ubiquitin (Ub) and ubiquitin-like proteins (UBLs) such as SUMO, NEDD8, and ISG15 regulate proteins by additional mechanisms, for example, intracellular compartmentation, signal transduction, and the regulation of some E3 ligases (Welchman et al. 2005). Degradation of a targeted protein by the ubiquitin system typically involves the concerted action of two to three enzymes (for review, see Hershko and Ciechanover 1998). Typically, polyubiquitylated polypeptides are delivered to the proteasome complex, which hydrolyzes the polypeptide into short oligopeptides and releases free ubiquitin, which is recycled. The process is reversible; ubiquitin, as well as other UBLs, can be deconjugated by proteases called ubiquitin or UBL isopeptidases. The term isopeptidase herein will refer to proteases that specifically cleave ubiquitin or UBLs after the terminal carboxyl group of ubiquitin (Gly76) or UBL (Amerik and Hochstrasser 2004). Deubiquitylases (DUBs), isopeptidases that cleave at the carboxyl terminus of ubiquitin, have been divided into five distinct groups, the largest being the ubiquitin-specific proteases (USP/UBP) (Amerik and Hochstrasser 2004; Nijman et al. 2005).
The approval of the proteasome inhibitor bortezomib (velcade) for multiple myeloma validated the ubiquitin proteasome pathway for cancer treatment (Bross et al. 2004). Unfortunately, toxicities associated with proteasome inhibition limit the clinical application of bortezomib and other proteasome inhibitors. In contrast, therapeutic strategies that target specific aspects of the ubiquitin–proteasome pathway are predicted to be better tolerated. Genomics has identified at least 530 human genes that putatively encode enzymes involved in the conjugation and deconjugation of ubiquitin; of these, at least 79 are thought to encode functional DUBs, some of which have multiple isoforms (Wong et al. 2003; Nijman et al. 2005). Considerable progress has been made in the study of ubiquitin conjugation; however, the study of DUBs is still in its nascent stages. The importance of aberrant DUB activity in carcinogenesis has been recently reviewed (Nicholson et al. 2007).
In addition to the DUBs, multiple families of UBL-specific isopeptidases exist, such as SENP1 and SENP2, which cleave SUMO, and DEN1 (SENP8), which cleaves NEDD8 (Gan-Erdene et al. 2003; Cheng et al. 2006). UBP43, the mouse homolog of the human isopeptidase USP18, was originally reported to be a deISGylase with no DUB activity, and UBP43 −/− mice were characterized by premature death, brain cell injury, and elevated levels of ISG15 conjugates in the brain (Malakhov et al. 2002; Ritchie et al. 2002). However, the relevance of the deISGylase activity of UBP43 was questioned following the observation that the phenotype of UBP43 −/− mice was not rescued by the additional knockout of ISG15 (Knobeloch et al. 2005). The coronavirus severe acute respiratory syndrome papain-like protease (SARS-CoV PLpro) was reported to be a DUB with some deISGylase activity (Harcourt et al. 2004; Barretto et al. 2005; Lindner et al. 2005). Using the fluorogenic reporters Ub-AMC (Ub-7-amino-4-methylcoumarin) and ISG15-AMC, it was very recently reported that SARS Co-V PLpro functioned preferentially as a deISGylase (Lindner et al. 2007). Another report demonstrated that a second coronavirus, NL63, encoded an isopeptidase, papain-like protease 2 (PLP2), with DUB activity (Chen et al. 2007). Interestingly, the presence or absence of deISGylating activity was not reported (Chen et al. 2007). The medical relevance of NL63 is illustrated by studies demonstrating that NL63 is associated with both upper and lower respiratory tract infections (Fouchier et al. 2004; van der Hoek et al. 2004, 2005).
The first DUBs were cloned over a decade ago (Wilkinson et al. 1989; Tobias and Varshavsky 1991), but to date the only commercially available options of monitoring isopeptidase activity in a high-throughput screening (HTS) manner are the UBL–AMC conjugates or the Lanthascreen DUB substrate (Dang et al. 1998; Gan-Erdene et al. 2003; Lindner et al. 2005; Horton et al. 2007). Given the potential drawbacks associated with other assays for measuring isopeptidase activity, we sought to develop a superior reporter-based assay. Previously, it was reported that a number of DUBs are able to cleave Ub conjugates linked by an ε- or α-peptide linkage with similar efficiency (Larsen et al. 1998; Lin et al. 2001). Furthermore, selected enzymes require a free amino terminus for catalytic activity. Initially, we described a prototype of an isopeptidase assay in a study of the activity of the catalytic core of the yeast SUMO protease, ULP1 (Arnold et al. 2006). Data presented herein describe the generation of a reporter system consisting of Ub or UBL fused to the amino terminus of an alternative reporter, phospholipase A2 (PLA2), by an α-peptide bond. This assay is able to determine the contrasting substrate specificities of the coronavirus proteases PLpro and PLP2 and characterize a novel modulator of isopeptidase activity, demonstrating its broad utility.
Results
Ub-PLA2: A novel reporter
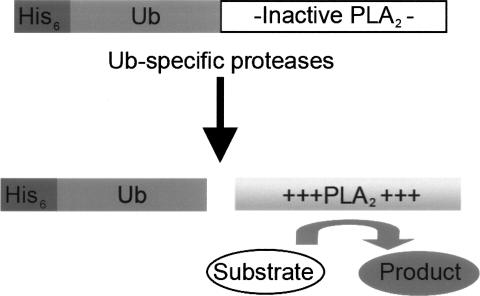
The Ub-PLA2 assay is based on the fact that PLA2 requires a free amino terminus to be catalytically active. PLA2 cleaves the 2-acyl linkage of 3-sn-phosphoglycerides in a Ca2+-dependent reaction and requires a free amino terminus for catalytic activity (Fig. 1; Dijkstra et al. 1981). Initially, a recombinant reporter construct consisting of Ub fused to the amino terminus of mouse soluble PLA2 group X was expressed and purified. In addition, the core catalytic domain of the isopeptidase USP2a/2b (hereafter referred to as USP2) was overexpressed as a fusion protein in Escherichia coli.
Figure 1.
Ub-PLA2, a novel isopeptidase reporter construct. Specific cleavage by the isopeptidase at the carboxy-terminal glycine of the Ub releases catalytically active PLA2, which is then able to turn over its substrate, liberating a fluorescent product.
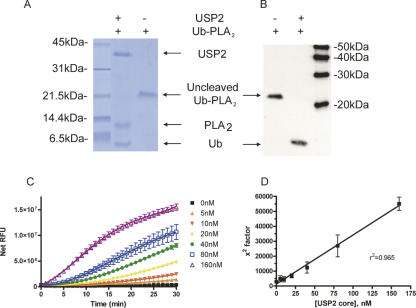
Proof of concept for the coupled assay was provided by the observation that USP2 was able to cleave Ub-PLA2 completely following a 30-min incubation at room temperature (Fig. 2A,B). Furthermore, the cleavage of Ub-PLA2 was dose dependent as detected by the liberation of the fluorophore 7-nitrobenz-2-oxa-1,3-diazole (NBD) from 2-(6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine (NBD C6-HPC) (Fig. 2C; Supplemental Fig. S1). The initial lag in the cleavage of the PLA2 substrate NBD C6-HPC is partially due to the coupled nature of the USP2, Ub-PLA2 assay. To determine if the fluorescent readout of the assay accurately reflected USP2 activity, the fluorescence intensity was fitted to a fourth-order polynomial equation (Fig. 2D). When the x 2 factor was plotted against USP2 concentration, a very strong linear relationship was revealed, confirming the dose dependency of the assay (see Discussion). An additional DUB, USP7, was expressed and purified from Sf9 insect cells, and a similar linear relationship was observed when 0–160 nM USP7 was incubated with 30 nM Ub-PLA2 and 20 μM NBD C6-HPC (Supplemental Fig. S2). Thus, the DUB, Ub-PLA2 assay can be used to monitor dose-dependent DUB activity.
Figure 2.
USP2 core cleaves Ub-PLA2 resulting in a dose-dependent increase in NBD fluorescence. Complete cleavage of Ub-PLA2 is observed at room temperature within 30 min. (A) Gel analysis of USP2 cleavage of Ub-PLA2. Coomassie blue stained gel of 500 ng of Ub-PLA2 ± 500 ng of USP2. (B) Anti-Ub Western blot analysis of 62.5 ng of Ub-PLA2 ± 62.5 ng of USP2. As expected for proteins lacking the Ub amino-terminal fusion, neither USP2 nor free PLA2 is visible on the anti-Ub Western blot. MagicMark size markers are indicated. (C) Varying concentrations of USP2 were incubated with 30 nM Ub-PLA2 and 20 μM NBD C6-HPC. Data are mean ± SD of triplicate determinations. (D) The data were fit to a fourth-order polynomial equation, and linear regression analysis revealed a tight correlation between the derived x 2 values and 6His USP2 core concentration. Data shown are mean ± SD of three determinations.
The UBL-PLA2 assay can be applied to a spectrum of isopeptidases
To gauge the utility of the assay and expand on our observation that DUBs effectively and specifically cleave Ub from PLA2, we tested the ability of other isopeptidases to cleave alternative UBL domains from PLA2. Recombinant fusion proteins of SUMO3-PLA2, ISG15-PLA2, and NEDD8-PLA2 were expressed and purified from E. coli. Additional isopeptidases were expressed and purified from bacteria and insect cells.
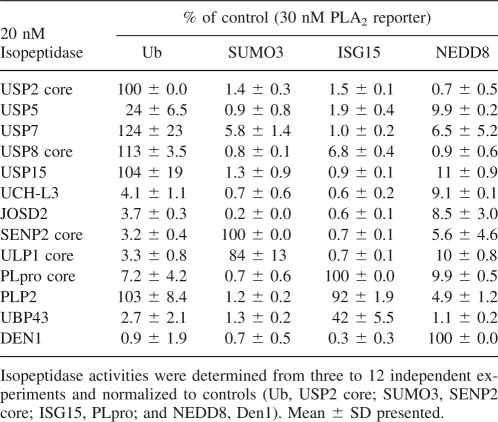
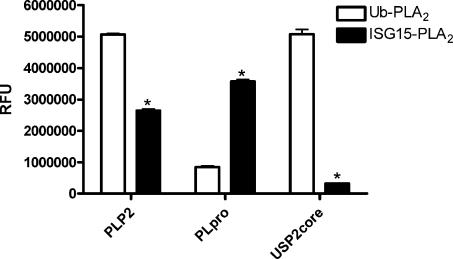
We examined the relative isopeptidase activities of 13 isopeptidases against four UBL-PLA2 reporter constructs (Table 1). As expected, USP2, USP5, USP7, USP8, and USP15 were primarily deubiquitinating enzymes. Consistent with published reports, USP5 cleavage of Ub-PLA2 was significantly enhanced by incubating in the presence of 25–100 nM mono Ub (Supplemental Fig. S3; Dang et al. 1998; Reyes-Turcu et al. 2006). Similarly, the mammalian and yeast deSUMOylases SENP2 core and ULP1 core were confirmed to be deSUMOylases, and the deNEDDylating enzyme DEN1 to be a deNEDDylase. SARS-CoV PLpro core and UBP43 were both preferentially deISGylases. As reported previously, PLpro also had some DUB activity; however, when equimolar amounts of Ub-PLA2 and ISG15-PLA2 were tested concurrently, PLpro generated a much stronger signal with the ISG15-PLA2 than with the Ub-PLA2 reporter (Barretto et al. 2005; Lindner et al. 2005, 2007). Interestingly, the selectivity data suggested that 20 nM NL63 PLP2 was able to cleave 30 nM Ub-PLA2 or 30 nM ISG15-PLA2 with similar efficiency (Table 1). We were unable to determine Km values of PLP2 for Ub and ISG15-PLA2 due to a lack of saturable kinetics (data not shown). However, when compared at a lower concentration (1 nM) with 30 nM of UBL-PLA2, a twofold higher signal was observed when PLP2 was incubated with Ub-PLA2 relative to ISG15-PLA2 (Fig. 3). In contrast, the fluorescent readout for 1 nM PLpro + ISG15-PLA2 was 3.8-fold greater relative to Ub-PLA2 (Fig. 3). As expected, the relative fluorescence unit (RFU) value was enhanced 17-fold for USP2 and Ub-PLA2 (vs. ISG15-PLA2).
Table 1.
Relative isopeptidase activities
Figure 3.
The contrasting DUB and deISGylase activities of PLpro and PLP2. The cleavage activity of 1 nM protease in the presence of 30 nM Ub-PLA2 or 30 nM ISG15-PLA2 was monitored at 45 min. Representative experiment is shown. Data are mean ± SD of three wells. (*) P < 0.001 vs. Ub-PLA2 (students two tailed t-test).
In contrast to the other DUBs, 20 nM UCH-L3 was unable to cleave Ub-PLA2 or any of the other UBL-PLA2 reporter constructs. The UCH-L3 enzyme was highly active as demonstrated by its ability to cleave the alternative reporter, Ub-AMC (Supplemental Fig. S4). Our data are consistent with the observation that ubiquitin C-terminal hydrolases such as UCH-L3 preferentially cleave Ub from small adducts (exemplified by Ub-AMC) (Larsen et al. 1998). In addition, 20 nM JOSD2, a member of the Machado–Joseph disease domain subfamily of DUBs, was unable to cleave 30 nM of any of the UBL-PLA2 reporter fusion proteins as detected by PLA2 activity. However, higher concentrations of JOSD2 cleaved Ub-PLA2 (Supplemental Fig. S5A). Comparable results were seen with Ub-AMC, suggesting that JOSD2 is a relatively weak DUB or exhibits substrate specificity (Supplemental Fig. S5B). Taken together, these data demonstrate that the UBL-PLA2 assay format has utility as a tool to dissect the specificities of purified isopeptidases.
The Ub-PLA2 reporter compares favorably with other reporter systems
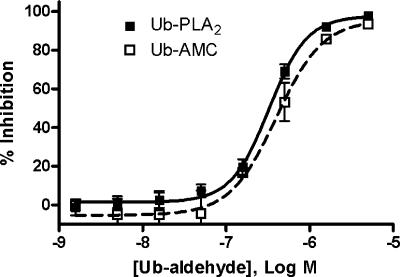
We wished to determine the relative activity of the UBL-PLA2 system in comparison to other isopeptidase reporter assays. When equimolar concentrations of Ub-PLA2 and Ub-AMC were directly compared, low (12.5–50 nM) concentrations of Ub-PLA2 were found to produce significantly higher signal-to-background (S/B) ratios than the same concentrations of Ub-AMC (Supplemental Fig. S6). One application of isopeptidase reporters is the discovery and characterization of novel isopeptidase inhibitors. Prior to screening for inhibitors, we determined the assay robustness and reproducibility in a HTS format, designated by the Z′ value (Zhang et al. 1999). The USP2, Ub-PLA2 assay exceeded the Z′ value required for a usable HTS assay (0.5) with a Z′ value of 0.72 ± 0.04 (Supplemental Table 1; Supplemental Fig. S7). To test whether the Ub-PLA2 reporter system was capable of identifying an inhibitor of USP2, the EC50 value of the well-characterized DUB inhibitor, ubiquitin aldehyde (Ub-ald), against USP2 was determined using 27.2 nM Ub-PLA2/20 μM NBD C6-HPC or 316 nM Ub-AMC (Fig. 4). Comparable EC50 values were obtained for Ub-ald mediated inhibition of USP2 as measured by Ub-PLA2/NBD C6-HPC (363 ± 50 nM) or Ub-AMC (473 ± 103 nM). The Lanthascreen DUB substrate represents an alternative reporter system for measuring DUB activity. Direct comparison revealed that for the USPs tested, the Ub-PLA2 assay was significantly more sensitive (Supplemental Fig. S8). Additional applications of the UBL-PLA2 assay platform include measuring the isopeptidase activity of crude cell lysates and monitoring partial fractionation schemes from HeLa cells or Sf9 cells (Supplemental Fig. S9A,B). The observed isopeptidase activities were inhibited by nonspecific sulfhydryl alkylating agent N-Ethylmaleimide (NEM), confirming that the assay measured cysteine protease activity (Supplemental Fig. S9C) (data not shown). These data confirm that UBL-PLA2 is a sensitive reporter system that has utility to measure activity of crude cell lysates in addition to purified proteins.
Figure 4.
EC50 values from Ub-PLA2 and Ub-AMC assay are comparable. USP2 core was incubated with varying concentrations of Ub-ald for 30 min before addition of Ub-PLA2/NBD C6-HPC or Ub-AMC. Representative experiment is shown. Data are mean ± SD of triplicate wells. Ub-ald does not inhibit free PLA2 (data not shown).
Characterization of novel isopeptidase inhibitors using the UBL-PLA2 reporter
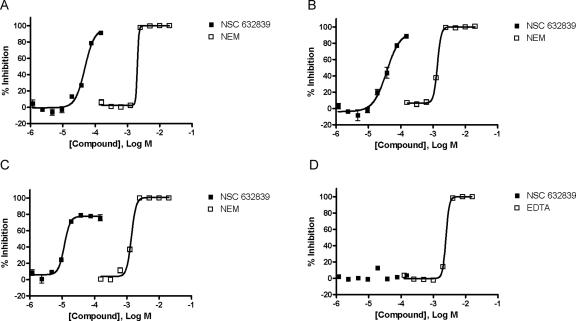
Aleo and colleagues reported that NSC 632839 (F6) inhibited ubiquitin isopeptidases as illustrated by its ability to inhibit z-LRGG-AMC cleavage by crude lysates in the mid-micromolar range (Aleo et al. 2006). To further characterize NSC 632839 against purified enzymes, we determined its inhibitory potential against purified USP2, USP7, and SENP2 and demonstrated that NSC 632839 was not only a DUB inhibitor, but also a deSUMOylase inhibitor (Fig. 5A–C). Specifically, NSC 632839 inhibited USP2, USP7, and SENP2 with EC50 values of 45 ± 4 μM, 37 ± 1 μM, and 9.8 ± 1.8 μM, respectively. Importantly, NSC 632839 did not inhibit the reporter enzyme PLA2 over the concentration range tested (1.2–150 μM), indicating that the reported inhibition was selective for isopeptidases (Fig. 5D). As expected, NEM inhibited USP2, USP7, and SENP2 with EC50 values of 1.9 ± 0.2 mM, 1.4 ± 0.07 mM, and 1.3 ± 0.2 mM, respectively (Fig. 5A–C). NEM does not inhibit PLA2 over the concentration range tested (0.2–25 mM) (data not shown). Ethylenediaminetetraacetic acid (EDTA) inhibited the Ca2+-dependent PLA2 enzyme (in the presence of 2 mM CaCl2) with an EC50 value of 2.5 ± 0.006 mM (Fig. 5D).
Figure 5.
Characterization of NSC 632839. The inhibitory activity of NSC 632839 was determined against USP2 (A), USP7 (B), SENP2 (C), and free PLA2 (D). Representative graphs are shown. Data are mean ± SD of triplicate determinations.
Discussion
The generation of the UBL-PLA2 reporter system was based on previously reported observations that a number of DUBs are able to cleave Ub conjugates linked by an ε- or α-peptide linkage with similar efficiency (Larsen et al. 1998; Lin et al. 2001). We took advantage of the observation that mature PLA2 requires a free amino terminus for catalytic activity by creating a fusion protein of an UBL linked to PLA2 by an α-peptide bond (Fig. 1; Dijkstra et al. 1981). The data show that the isopeptidase assay configured in this format has a very broad utility and scope, in terms of both ubiquitin family members and efficacy in diverse systems, including cell lysates and subsequent fractionations.
Initial experiments revealed that USP2 and USP7 cleaved Ub-PLA2, which liberated NBD in a dose-dependent manner as reflected by the r 2 values of 0.965 and 0.953, respectively, when the x 2 factor of a polynomial fit of the curves was plotted against DUB concentration (Fig. 2D; Supplemental Fig. S2B). Thus, the Ub-PLA2 fusion protein linked by an α-peptide bond is appropriate for monitoring purified DUB activity. The shape of the progress curves such as Figure 2C is due to several factors. First, there will be a lag in fluorescence appearance due to the coupled nature of the assay, and this is further complicated by the fact that the substrate for PLA2 is largely micellar, requiring bulk transport of monomeric substrate before the PLA2 can act. Thus, the use of a polynomial fit is purely phenomenological. The apparent relationship between the x 2 factor and enzyme amount may arise from the fact that [E] and [S] are similar in these assays. This means that the concentration of the ES complex leading to product formation will be determined by the fact that [ES] = [E] × [S], and since [E] ∼ [S], then [ES] ∼ [E]2.
Subsequently, we profiled the relative activity of seven DUBs against Ub-PLA2 (Table 1). These data revealed that the Ub-PLA2 assay is an effective tool for profiling the activity of the largest class of DUBs, the USPs. There are likely >50 functional USPs; four of five USPs tested had robust activity against Ub-PLA2 with negligible activity against the other UBL-PLA2 conjugates. USP5 activity was enhanced in the presence of exogenous Ub (Supplemental Fig. S3), consistent with its known specificity for polyubiquitin (Dang et al. 1998; Reyes-Turcu et al. 2006). In contrast, the Ub-PLA2 assay does not appear to be appropriate for measuring the activity of UCH enzymes such as UCH-L3. The Ub-PLA2 assay also had low activity against a member of the Machado–Joseph disease domain proteases. However, it should be noted that at higher concentrations of JOSD2, dose-dependent DUB activity was observed; a similar observation was made for JOSD2 with Ub-AMC (Supplemental Fig. S5). Interestingly, the relatively poor in vitro cleavage of Ub conjugates by JOSD2 is consistent with a previous report of another member of the Machado–Joseph domain, Ataxin 3 (Mao et al. 2005).
We chose to expand these initial observations by expressing and purifying three additional UBL-PLA2 reporters, SUMO3-PLA2, NEDD8-PLA2, and ISG15-PLA2. The specificity and relative activity of five other isopeptidases were determined against the four UBL-PLA2 reporter constructs (Table 1). As expected, SENP2 and ULP1 behaved as deSUMOylating enzymes and DEN1 as a deNEDDylase. Consistent with previous in vitro data, UBP43 was preferentially a deISGylase (Malakhov et al. 2002). In addition, in our hands PLpro is a deISGylase (Table 1; Fig. 3). Moreover, our data are in agreement with the very recent report that SARS Co-V PLpro functioned preferentially as a deISGylase (Lindner et al. 2007). In contrast, 20 nM of the related NL63 coronavirus protease (PLP2) appeared to cleave both Ub and ISG15 conjugates equally well when tested against 30 nM Ub-PLA2 or 30 nM ISG15-PLA2. Follow-up experiments demonstrated that lower concentrations of PLP2 (1 nM) were able to generate a significantly greater signal when incubated with Ub-PLA2 relative to ISG15-PLA2 suggesting that PLP2 has a preference for Ub relative to ISG15 (Fig. 3). Confirmatory kinetic experiments were uninformative due to a lack of substrate saturation (data not shown).
Recently, Catic and colleagues profiled the DUB, deSUMOylase, and deISGylase activity of 22 DUBs (three of which overlapped with this study) using suicide inhibitors (Catic et al. 2007). Interestingly, both approaches found that USP2, USP5, and USP7 were DUBs; however, Catic and colleagues reported that USP2 and USP5 also bound to ISG15-vinyl sulfone, suggesting that they had deISGylating activity as well as DUB activity. In contrast, we did not detect any significant enzymatic deISGylating activity for USP2 or USP5 (Table 1). Previously, the same group described the ability of USP5 to bind ISG15-vinyl sulfone, but to our knowledge the enzymatic cleavage of ISG15 conjugates by USP5 has not been demonstrated (Hemelaar et al. 2004). No other reports of USP2 deISGylating activity have been published. This conflict highlights the importance of profiling isopeptidase activities by measuring proteolytic cleavage activity in addition to substrate binding.
A number of DUB assays have been reported, including the commercially available Ub-AMC and Lanthascreen DUB substrate. In contrast to Ub-AMC and Lanthascreen DUB substrate, in the Ub-PLA2 assay, the DUB cleaves a 14-kDa protein from the C terminus of Ub, likely representing a more physiological substrate for DUBs such as USP7 (Layfield et al. 1999). In addition, although Ub-AMC has been used to screen for inhibitors, its excitation wavelength is in the UV range, which is known to result in false positive rates as high as 20% in HTS (Liu et al. 2003; Tirat et al. 2005). Importantly, comparable EC50 values were obtained for the inhibition of USP2 by the specific DUB inhibitor Ub-ald as detected by Ub-PLA2/NBD C6-HPC or Ub-AMC, illustrating that the Ub-PLA2 assay is appropriate for screening for inhibitors of isopeptidases (Fig. 4). Further, the utility of the Ub-PLA2 assay as a HTS screening platform for modulators of isopeptidase activity is illustrated by the assay exhibiting Z′ values ranging from 0.69 to 0.92 (Supplemental Table 1; Supplemental Fig. S7). As in the case of all coupled enzyme assays, there is the possibility that a compound will directly modulate the activity of PLA2; these compounds, however, can be rapidly eliminated by a counterscreen against free PLA2 (Fig. 5D). Taken together, these data demonstrate that with respect to the most numerous class of DUBs, the USPs, the Ub-PLA2 assay is ideal for determining relative enzyme activity. In contrast, Ub-AMC and the Lanthascreen DUB substrate seem better suited to the UCH family of DUBs.
Further evidence of the utility of the UBL-PLA2 assay was provided by the characterization of a previously reported DUB inhibitor (Aleo et al. 2006). In the initial report, NSC 632839 was not tested against purified proteins; rather, it was shown to inhibit cleavage of an Ub mimetic (z-LRGG-AMC) by transformed fibroblast lysates. In this study, we have expanded on the observations of Aleo and colleagues by demonstrating that NSC 632839 inhibits purified USP2- and USP7-mediated cleavage of Ub-PLA2 (Fig. 5A,B). In addition, NSC 632839 inhibits the deSUMOylase SENP2, suggesting that this compound is a relatively nonselective isopeptidase inhibitor (Fig. 5C). Moreover, the isopeptidase inhibitory activity of NSC 632839 was confirmed by the observation that it did not inhibit free PLA2 over the concentration range tested (Fig. 5D). Thus, the UBL-PLA2 assay is ideal for HTS applications such as screening for USP inhibitors.
In summary, data presented within have demonstrated that the UBL-PLA2 reporter assay is a robust and sensitive assay. Further, as exemplified by PLpro and PLP2, the same platform can be used to discriminate DUB, deSUMOylase, deISGylase, and deNEDDylase activities. As such, the UBL-PLA2 reporter system is beginning to provide new mechanistic insights in the field of UBL isopeptidases. Furthermore, additional reporter fusions consisting of alternative reporter enzymes that require a free amino terminus for activity fused to UBLs are currently in development (data not shown). Isopeptidases are clearly an emerging disease target, and we propose that the UBL-PLA2 reporter system will be an extremely useful tool to identify novel inhibitors and activators of target USPs (Nicholson et al. 2007). We estimate that >500,000 datapoints have been generated against ∼40 therapeutically important isopeptidases using the current assay in 96- to 1536-well-plate formats. Importantly, when screened in a 1536-well-plate format, the assay exceeded a throughput of 400,000 data points per day with a Z′ value of 0.73 ± 0.27 (PubChem AID 927; H. Veith and D. Auld, pers. comm.). These results show that the assay is robust, and data describing the results of this and other screening campaigns will be the subject of future publications.
Materials and Methods
Materials
Unless stated otherwise all reagents were obtained from Sigma Chemical Company and were at a minimum of reagent grade or better. Plasmids encoding UBP43 and JOSD2 were purchased from Open Biosystems. MagicMark size markers, NBD C6-HPC, and Lanthascreen DUB substrate were procured from Invitrogen. ECL reagents, ubiquitin aldehyde, and USP15 were procured from BIOMOL.
Expression and purification of UBL-PLA2
The mature mouse group X PLA2 (amino acids 29–151) was cloned and expressed in BL21 (DE3) bacteria (Novagen) using standard molecular biology techniques (see Supplemental Table 2 for primer information). 6His-Ub-PLA2 was expressed and purified as described (Gasparian et al. 2003; Leach and Michael 2005). Subsequently, PLA2 was also subcloned into the bacterial expression vectors pET24d-6His-SUMO3, pET24d-6His-NEDD8, and pET24d-6His-ISG15 to generate recombinant 6His-SUMO3-PLA2, 6His-NEDD8-PLA2, and 6His-ISG15-PLA2, respectively. Expression and purification were as described above. Free catalytically active PLA2 was generated by incubating SUMO3-PLA2 with SENP2 overnight at room temperature to ensure complete cleavage prior to affinity subtraction with Ni-NTA beads.
Expression and purification of isopeptidases
The cloning, expression, and purification from BL21 (DE3) bacteria of the catalytic core fragments of USP2 core (amino acids 259–605), USP8 core (amino acids 710–1110), SENP2 (amino acids 366–590), SARS-CoV PLpro (amino acids 1541–1855), and full-length JOSD2 were performed using standard molecular biology techniques (see Supplemental Table 2 for primer information). Codon-optimized PLP2 cDNA (amino acids 1565–1894) was prepared synthetically by DNA2.0 (www.dna20.com/index.php?pageID-78) before expressing and purifying from BL21 (DE3) bacteria. Full-length USP7 and UBP43 were cloned, expressed, and purified from Sf9 cells using standard molecular biology techniques (see Supplemental Table 2 for primer information). The isopeptidases USP5, UCH-L3, and ULP1 core were expressed and purified as described (Larsen et al. 1996; Malakhov et al. 2004; Russell and Wilkinson 2005; Marblestone et al. 2006).
Immunoblotting
Following transfer to nitrocellulose, the blot was placed in boiling water for 5 min before blocking, probing sequentially with anti-Ub (Sigma Chemical Company) and anti-rabbit-HRP (Jackson ImmunoResearch Laboratories), and visualizing with ECL reagents.
UBL-PLA2 assay
Unless stated otherwise, recombinant isopeptidase was mixed with UBL-PLA2 and NBD C6-HPC to final concentrations of 20 nM, 30 nM, and 20 μM, respectively, in a total volume of 100 μL in a well in a black-walled 96-well-plate (Greiner Bio-One). All dilutions were performed in PLA2 assay buffer (20 mM Tris-HCl, pH 8.0, 2 mM CaCl2, and 2 mM β-mercaptoethanol). Unless stated otherwise, the increase in fluorescence intensity over time was determined on a Perkin-Elmer Envision fluorescence plate reader with excitation and emission filters of 475 nm and 555 nm, respectively, at room temperature. Net RFUs were determined by subtracting the blank RFU value (20 μM NBD C6-HPC in PLA2 assay buffer) from each data point.
USP2 core, Ub-PLA2 dose dependency experiments were performed by mixing 0–160 nM USP2 core with 30 nM Ub-PLA2 and 20 μM NBD C6-HPC in a total volume of 100 μL as above. The data were fit to a fourth-order polynomial equation such that y = a + bx + cx 2 + dx 3 + ex 4 using GraphPad Prism 4.0 (GraphPad Software). The linearity of signal (relative to isopeptidase concentration) was determined by performing linear regression of x 2 factor (c) versus isopeptidase concentration.
Relative isopeptidase activity against various UBL-PLA2 fusions was determined by adding the test isopeptidase to a final concentration of 20 nM in combination with 20 μM NBD C6-HPC and 30 nM of the individual UBL-PLA2 reporter constructs (Ub-PLA2, SUMO3-PLA2, NEDD8-PLA2, or ISG15-PLA2). Net RFU was determined as described above and the signal (isopeptidase + reporter) to background (reporter) ratio was determined within the linear range of the assay (30–45 min) before expressing the S/B as a percentage of the control isopeptidases: USP2 (Ub-PLA2), SENP2 (SUMO3-PLA2), PLpro (ISG15-PLA2) and DEN1 (NEDD8-PLA2). Follow-up experiments were performed by adding the test isopeptidase to a final concentration of 1 nM in combination with 20 μM NBD C6-HPC and 30 nM of Ub-PLA2 or ISG15-PLA2. Data (RFU) from the 45-min time point were used for analysis. The UBL-PLA2 assay reagents are available from LifeSensors, Inc. (www.lifesensors.com).
Ub-aldehyde inhibition
In a 96-well-plate, 31.25 nM USP2 core was incubated with a concentration range of Ub-aldehyde for 30 min before supplementation with an equal volume of 54.4 nM Ub-PLA2/40 μM NBD C6-HPC or 632 nM Ub-AMC (BIOMOL or Boston Biochem). The increase in fluorescence was monitored over time using an Ascent Fluoroskan using the following wavelengths: Ex460; Em538 (Ub-PLA2) or Ex355; Em460 (Ub-AMC). The RFU values within the initial linear range were normalized such that reporter alone = 100% inhibition and USP2 core + reporter = 0% inhibition and were plotted using GraphPad Prism 4.0. EC50 values were determined by fitting the data to a sigmoidal dose response (variable slope) equation.
Characterization of NSC 632839
In a 96-well-plate, 40 nM USP2, 40 nM USP7, or 20 nM SENP2 was preincubated with a concentration range of NSC 632839 (NCI/NIH developmental therapeutics program) or control for 30 min before supplementation with an equal volume of 60 nM Ub-PLA2/40 μM NBD C6-HPC (USP2 or 7) or 20 nM SUMO3-PLA2/40 μM NBD C6-HPC (SENP2). Relative activity of the enzymes was determined by measuring the RFU values at single time points within the initial linear range (USP, 50 min; USP7, 50 min; and SENP2, 30 min). The RFU values within the initial linear range were normalized such that isopeptidase + vehicle = 0% inhibition and isopeptidase + NEM = 100% inhibition. The EC50 values were determined as above. The inhibitory activity of the test compound against the reporter enzyme PLA2 was performed as described above except there was no preincubation step and the data were normalized such that free PLA2 + vehicle = 0% inhibition and free PLA2 + EDTA = 100% inhibition. PLA2 activity was determined 8 min after the addition of the reagents.
Electronic supplemental material
Supplemental materials consist of tables of the Z′ values and PCR primers. Figures illustrate dose-dependent USP2 and USP7 activity, enhancement of USP5 activity in the presence of mono-Ub, dose-dependent UCH-L3 activity (measured by Ub-AMC), dose-dependent JOSD2 activity (measured by Ub-PLA2 or Ub-AMC), a comparison of Ub-PLA2 and Ub-AMC or the Lanthascreen substrate, USP2 Z′ data distribution, and the utility of the UBL-PLA2 assay for monitoring the fractionation of whole-cell lysates.
Acknowledgments
We thank Drs. Simon Wing, Susan Weiss, Edward Yeh, Zhen-Qiang Pan, Roland Hilgarth, and Li Liu for providing research materials and Dr. James Strickler for helpful discussions. NSC 632839 was obtained from the NCI/NIH developmental therapeutics program. Part of this work was funded by grants 1R43DK07139-01 from the NIDDK/NIH to D.E.S. and 1R43CA115205-01 from the NCI/NIH to M.R.M.
Footnotes
Supplemental material: see www.proteinscience.org
Reprint requests to: Benjamin Nicholson, Progenra, Inc., 271A Great Valley Parkway, Malvern, PA 19355, USA; e-mail: nicholson@progenra.com; fax: (610) 647-4705.
Abbreviations: DTT, dithiothreitol; DUB, deubiquitylase; EDTA, Ethylenediaminetetraacetic acid; FRET, fluorescence resonance energy transfer; HTS, high-throughput screening; IPTG, isopropyl β-D-1-thiogalactopyranoside; MJD, Machado–Joseph domain; NBD C6-HPC, 2-(6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine; MOI, multiplicity of infection; NEM, N-Ethylmaleimide; OTU, ovarian tumor related; PCR, polymerase chain reaction; PLA2, phospholipase A2; PLP2, papain-like protease 2; RFU, relative fluorescence unit; SARS-CoV PLpro, severe acute respiratory syndrome coronavirus papain-like protease; S/B, signal: background; Ub, ubiquitin; Ub-ald, ubiquitin aldehyde; Ub-AMC, Ub-7-amino-4-methylcoumarin; UBL, ubiquitin-like; UCH, ubiquitin C-terminal hydrolase; USP/UBP, ubiquitin-specific protease.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.083450408.
Reference
- Aleo, E., Henderson, C.J., Fontanini, A., Solazzo, B., Brancolini, C. Identification of new compounds that trigger apoptosome-independent caspase activation and apoptosis. Cancer Res. 2006;66:9235–9244. doi: 10.1158/0008-5472.CAN-06-0702. [DOI] [PubMed] [Google Scholar]
- Amerik, A.Y., Hochstrasser, M. Mechanism and function of deubiquitinating enzymes. Biochim. Biophys. Acta. 2004;1695:189–207. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Arnold, J.J., Bernal, A., Uche, U., Sterner, D.E., Butt, T.R., Cameron, C.E., Mattern, M.R. Small ubiquitin-like modifying protein isopeptidase assay based on poliovirus RNA polymerase activity. Anal. Biochem. 2006;350:214–221. doi: 10.1016/j.ab.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretto, N., Jukneliene, D., Ratia, K., Chen, Z., Mesecar, A.D., Baker, S.C. The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J. Virol. 2005;79:15189–15198. doi: 10.1128/JVI.79.24.15189-15198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bross, P.F., Kane, R., Farrell, A.T., Abraham, S., Benson, K., Brower, M.E., Bradley, S., Gobburu, J.V., Goheer, A., Lee, S.L., et al. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin. Cancer Res. 2004;10:3954–3964. doi: 10.1158/1078-0432.CCR-03-0781. [DOI] [PubMed] [Google Scholar]
- Catic, A., Fiebiger, E., Korbel, G.A., Blom, D., Galardy, P.J., Ploegh, H.L. Screen for ISG15-cross-reactive deubiquitinases. PLoS ONE. 2007;2:e679. doi: 10.1371/journal.pone.0000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z., Wang, Y., Ratia, K., Mesecar, A.D., Wilkinson, K.D., Baker, S.C. Proteolytic processing and deubiquitinating activity of papain-like proteases of human coronavirus NL63. J. Virol. 2007;81:6007–6018. doi: 10.1128/JVI.02747-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, J., Bawa, T., Lee, P., Gong, L., Yeh, E.T. Role of desumoylation in the development of prostate cancer. Neoplasia. 2006;8:667–676. doi: 10.1593/neo.06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, L.C., Melandri, F.D., Stein, R.L. Kinetic and mechanistic studies on the hydrolysis of ubiquitin C-terminal 7-amido-4-methylcoumarin by deubiquitinating enzymes. Biochemistry. 1998;37:1868–1879. doi: 10.1021/bi9723360. [DOI] [PubMed] [Google Scholar]
- Dijkstra, B.W., Drenth, J., Kalk, K.H. Active site and catalytic mechanism of phospholipase A2. Nature. 1981;289:604–606. doi: 10.1038/289604a0. [DOI] [PubMed] [Google Scholar]
- Fouchier, R.A., Hartwig, N.G., Bestebroer, T.M., Niemeyer, B., de Jong, J.C., Simon, J.H., Osterhaus, A.D. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl. Acad. Sci. 2004;101:6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan-Erdene, T., Nagamalleswari, K., Yin, L., Wu, K., Pan, Z.Q., Wilkinson, K.D. Identification and characterization of DEN1, a deneddylase of the ULP family. J. Biol. Chem. 2003;278:28892–28900. doi: 10.1074/jbc.M302890200. [DOI] [PubMed] [Google Scholar]
- Gasparian, M.E., Ostapchenko, V.G., Schulga, A.A., Dolgikh, D.A., Kirpichnikov, M.P. Expression, purification, and characterization of human enteropeptidase catalytic subunit in Escherichia coli . Protein Expr. Purif. 2003;31:133–139. doi: 10.1016/s1046-5928(03)00159-1. [DOI] [PubMed] [Google Scholar]
- Harcourt, B.H., Jukneliene, D., Kanjanahaluethai, A., Bechill, J., Severson, K.M., Smith, C.M., Rota, P.A., Baker, S.C. Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain-like protease activity. J. Virol. 2004;78:13600–13612. doi: 10.1128/JVI.78.24.13600-13612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemelaar, J., Borodovsky, A., Kessler, B.M., Reverter, D., Cook, J., Kolli, N., Gan-Erdene, T., Wilkinson, K.D., Gill, G., Lima, C.D., et al. Specific and covalent targeting of conjugating and deconjugating enzymes of ubiquitin-like proteins. Mol. Cell. Biol. 2004;24:84–95. doi: 10.1128/MCB.24.1.84-95.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko, A., Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Horton, R.A., Strachan, E.A., Vogel, K.W., Riddle, S.M. A substrate for deubiquitinating enzymes based on time-resolved fluorescence resonance energy transfer between terbium and yellow fluorescent protein. Anal. Biochem. 2007;360:138–143. doi: 10.1016/j.ab.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Knobeloch, K.P., Utermohlen, O., Kisser, A., Prinz, M., Horak, I. Re-examination of the role of ubiquitin-like modifier ISG15 in the phenotype of UBP43-deficient mice. Mol. Cell. Biol. 2005;25:11030–11034. doi: 10.1128/MCB.25.24.11030-11034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, C.N., Price, J.S., Wilkinson, K.D. Substrate binding and catalysis by ubiquitin C-terminal hydrolases: Identification of two active site residues. Biochemistry. 1996;35:6735–6744. doi: 10.1021/bi960099f. [DOI] [PubMed] [Google Scholar]
- Larsen, C.N., Krantz, B.A., Wilkinson, K.D. Substrate specificity of deubiquitinating enzymes: Ubiquitin C-terminal hydrolases. Biochemistry. 1998;37:3358–3368. doi: 10.1021/bi972274d. [DOI] [PubMed] [Google Scholar]
- Layfield, R., Franklin, K., Landon, M., Walker, G., Wang, P., Ramage, R., Brown, A., Love, S., Urquhart, K., Muir, T., et al. Chemically synthesized ubiquitin extension proteins detect distinct catalytic capacities of deubiquitinating enzymes. Anal. Biochem. 1999;274:40–49. doi: 10.1006/abio.1999.4234. [DOI] [PubMed] [Google Scholar]
- Leach, C.A., Michael, W.M. Ubiquitin/SUMO modification of PCNA promotes replication fork progression in Xenopus laevis egg extracts. J. Cell Biol. 2005;171:947–954. doi: 10.1083/jcb.200508100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, H., Yin, L., Reid, J., Wilkinson, K.D., Wing, S.S. Divergent N-terminal sequences of a deubiquitinating enzyme modulate substrate specificity. J. Biol. Chem. 2001;276:20357–20363. doi: 10.1074/jbc.M008761200. [DOI] [PubMed] [Google Scholar]
- Lindner, H.A., Fotouhi-Ardakani, N., Lytvyn, V., Lachance, P., Sulea, T., Menard, R. The papain-like protease from the severe acute respiratory syndrome coronavirus is a deubiquitinating enzyme. J. Virol. 2005;79:15199–15208. doi: 10.1128/JVI.79.24.15199-15208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner, H.A., Lytvyn, V., Qi, H., Lachance, P., Ziomek, E., Menard, R. Selectivity in ISG15 and ubiquitin recognition by the SARS coronavirus papain-like protease. Arch. Biochem. Biophys. 2007;466:8–14. doi: 10.1016/j.abb.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Lashuel, H.A., Choi, S., Xing, X., Case, A., Ni, J., Yeh, L.A., Cuny, G.D., Stein, R.L., Lansbury P.T., Jr Discovery of inhibitors that elucidate the role of UCH-L1 activity in the H1299 lung cancer cell line. Chem. Biol. 2003;10:837–846. doi: 10.1016/j.chembiol.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Malakhov, M.P., Malakhova, O.A., Kim, K.I., Ritchie, K.J., Zhang, D.E. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J. Biol. Chem. 2002;277:9976–9981. doi: 10.1074/jbc.M109078200. [DOI] [PubMed] [Google Scholar]
- Malakhov, M.P., Mattern, M.R., Malakhova, O.A., Drinker, M., Weeks, S.D., Butt, T.R. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J. Struct. Funct. Genomics. 2004;5:75–86. doi: 10.1023/B:JSFG.0000029237.70316.52. [DOI] [PubMed] [Google Scholar]
- Mao, Y., Senic-Matuglia, F., Di Fiore, P.P., Polo, S., Hodsdon, M.E., De Camilli, P. Deubiquitinating function of ataxin-3: Insights from the solution structure of the Josephin domain. Proc. Natl. Acad. Sci. 2005;102:12700–12705. doi: 10.1073/pnas.0506344102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marblestone, J.G., Edavettal, S.C., Lim, Y., Lim, P., Zuo, X., Butt, T.R. Comparison of SUMO fusion technology with traditional gene fusion systems: Enhanced expression and solubility with SUMO. Protein Sci. 2006;15:182–189. doi: 10.1110/ps.051812706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, B., Marblestone, J.G., Butt, T.R., Mattern, M.R. Deubiquitinating enzymes as novel anticancer targets. Future Oncol. 2007;3:191–199. doi: 10.2217/14796694.3.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijman, S.M., Luna-Vargas, M.P., Velds, A., Brummelkamp, T.R., Dirac, A.M., Sixma, T.K., Bernards, R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Reyes-Turcu, F.E., Horton, J.R., Mullally, J.E., Heroux, A., Cheng, X., Wilkinson, K.D. The ubiquitin binding domain ZnF UBP recognizes the C-terminal diglycine motif of unanchored ubiquitin. Cell. 2006;124:1197–1208. doi: 10.1016/j.cell.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Ritchie, K.J., Malakhov, M.P., Hetherington, C.J., Zhou, L., Little, M.T., Malakhova, O.A., Sipe, J.C., Orkin, S.H., Zhang, D.E. Dysregulation of protein modification by ISG15 results in brain cell injury. Genes & Dev. 2002;16:2207–2212. doi: 10.1101/gad.1010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, N.S., Wilkinson, K.D. Deubiquitinating enzyme purification, assay inhibitors, and characterization. Methods Mol. Biol. 2005;301:207–219. doi: 10.1385/1-59259-895-1:207. [DOI] [PubMed] [Google Scholar]
- Tirat, A., Schilb, A., Riou, V., Leder, L., Gerhartz, B., Zimmermann, J., Worpenberg, S., Eidhoff, U., Freuler, F., Stettler, T., et al. Synthesis and characterization of fluorescent ubiquitin derivatives as highly sensitive substrates for the deubiquitinating enzymes UCH-L3 and USP-2. Anal. Biochem. 2005;343:244–255. doi: 10.1016/j.ab.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Tobias, J.W., Varshavsky, A. Cloning and functional analysis of the ubiquitin-specific protease gene UBP1 of Saccharomyces cerevisiae . J. Biol. Chem. 1991;266:12021–12028. [PubMed] [Google Scholar]
- van der Hoek, L., Pyrc, K., Jebbink, M.F., Vermeulen-Oost, W., Berkhout, R.J., Wolthers, K.C., Wertheim-van Dillen, P.M., Kaandorp, J., Spaargaren, J., Berkhout, B. Identification of a new human coronavirus. Nat. Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek, L., Sure, K., Ihorst, G., Stang, A., Pyrc, K., Jebbink, M.F., Petersen, G., Forster, J., Berkhout, B., Uberla, K. Croup is associated with the novel coronavirus NL63. PLoS Med. 2005;2:e240. doi: 10.1371/journal.pmed.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welchman, R.L., Gordon, C., Mayer, R.J. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat. Rev. Mol. Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- Wilkinson, K.D., Lee, K.M., Deshpande, S., Duerksen-Hughes, P., Boss, J.M., Pohl, J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- Wong, B.R., Parlati, F., Qu, K., Demo, S., Pray, T., Huang, J.D., Payan, P.G., Bennett, M.K. Drug discovery in the ubiquitin regulatory pathway. Drug Discov. Today. 2003;8:746–754. doi: 10.1016/s1359-6446(03)02780-6. [DOI] [PubMed] [Google Scholar]
- Zhang, J.H., Chung, T.D., Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]