Abstract
Exercise is a treatment paradigm that can ameliorate cognitive dysfunction in Alzheimer disease (AD) and AD mouse models. Since exercise is also known to alter the peripheral immune response, one potential mechanism for the cognitive improvement following exercise may be by modulating the inflammatory repertoire in the central nervous system. We investigated the effects of voluntary exercise in the Tg2576 mouse model of AD at a time-point at which pathology has already developed. Inflammatory mRNA markers are increased in sedentary Tg2576 mice versus non-transgenic controls. We demonstrate that short-term voluntary wheel running improved spatial learning in aged transgenic mice as compared to sedentary Tg2576 controls. Inflammatory profiles of the Tg2576 and non-transgenic mice were different following exercise with the non-transgenic mice showing a broader response as compared to the Tg2576. Notably, exercising Tg2576 exhibited increases in a few markers including CXCL1 and CXCL12, two chemokines that may affect cognition.
Keywords: Exercise, Tg2576, Alzheimer disease, CXCL1, CXCL12, cognition, running, AD model, inflammation, neuroinflammation
INTRODUCTION
Alzheimer disease (AD) is the most common cause of dementia in the elderly population, characterized by progressive loss of cognitive abilities or activities of daily living. The neuropathological hallmarks of the disease are amyloid plaques and neurofibrillary tangles (NFT), which progressively accumulate in the brain. These neuropathologies are closely linked with chronic inflammation and neuronal dysfunction.
Increasing evidence suggests that the inflammatory response is integrally involved in the dementia component of the disease. The brains of clinical end-stage AD patients contain increased levels of activated microglia, cytokines and chemokines (Bajetto et al., 2001; Luterman et al., 2000). Also a study found a higher correlation between synapse loss and activated microglia than between Aβ and NFTs (Lue et al., 1996). There are some suggestions that inflammation may be involved early on during disease progression (Pasinetti, 2001; Zanjani et al., 2005). Our previous study showed a strong correlation between markers of the inflammatory response and the early stages of AD dementia (Parachikova et al., 2006) as seen through increased MHC II levels and decreased T-cell counts. This suggested a dysfunctional adaptive immune response during the early stages of AD dementia. In addition, we have recently shown that cognitive deficits coincide with altered CXCL12 chemokine levels in a mouse model of AD (Parachikova and Cotman, 2007). Notably, chemokines form part of the adaptive immune response, and antagonism of the CXCL12 receptor in wildtype mice mimics the cognitive impairments seen in AD mice.
Exercise appears to improve cognitive function, particularly in the aged population (Berkman et al., 1993; Blomquist and Danner, 1987; Colcombe and Kramer, 2003; Heyn et al., 2004; Hill et al., 1993; Rogers et al., 1990; Weuve et al., 2004). A large prospective study concluded that regular exercise in AD patients delays the onset of dementia and AD (Larson et al., 2006). This human data is supported by animal research demonstrating that exercise can facilitate learning in AD mouse models (O’Callaghan et al., 2007; Radak et al., 2006; Schweitzer et al., 2006; van Praag et al., 2005; Vaynman et al., 2004). Previous work from our laboratory explored the effects of exercise on cognition using the TgCRND8 mouse model of AD (Adlard et al., 2005). The study demonstrated that long term wheel running (five months) resulted in cognitive improvement and also significantly decreased amyloid load. In the current study, we have chosen a shorter time course of voluntary wheel running to identify if changes in cognition occur independently of the pathology and due to changes in the inflammatory system.
We used the Tg2576 mouse model of AD and characterized the profile of 84 inflammatory markers in the hippocampus of Tg2576 and non-transgenic mice following 3 weeks of voluntary wheel running and compared the inflammatory profile to cognitive functioning. The screen identified CXCL1 and CXCL12 as being significantly altered with running in the Tg2576 mice. These chemokines have been shown to induce neuroprotection (Watson and Fan, 2005) and mediate neuronal-glial communication (Bezzi et al., 2001) and could therefore participate in cognitive restoration.
MATERIALS AND METHODS
Mice
The study used aged (15–19 months old) Tg (HuApp695.K670-M671L) 2576 transgenic and age-matched C57Bl6/SJL non-transgenic mice obtained from Jackson Laboratories and a colony established. All animal housing and procedures were performed in accordance with the guidelines established by the University of California, Irvine.
Exercise Paradigm
Transgenic and non-transgenic mice were divided into either sedentary or running conditions for a period of 3 weeks (n=6/group). For voluntary wheel running, a metal running wheel (MiniMitter, Bend, OR) was placed in a 48 × 27 × 20 cm polyethylene cage. Running distance was recorded using Vital View software (MiniMitter, Bend, OR).
Behavioral Analysis
The radial arm Morris water maze (RAWM) (Hyde et al., 1998) is a task designed for the testing of spatial learning. This task is performed in a 5-arm radial arm maze placed inside a water tank. The goal of the task is for the animal to remember the location of a submerged platform based on external spatial cues. The 3 day protocol followed (Nichol et al., 2007). Days 1 and 2 consisted of 3 blocks of 5 trials each day for a total of 30 trials. After each block of 5 trials, animals rested for 30 minutes to allow memory consolidation. For each mouse, the platform location was kept the same for all trials for both days but we varied the goal arm for different mice in a cohort. While the platform location (goal arm) remained the same for each animal on both days, we varied the starting arm for each animal on all 30 trials on days 1 and 2. Animals were scored on mean latency to the target platform as well as on measures of reference and working memory (Hyde et al., 1998). The measure for reference memory was determined as the average latency to the target on trials 6 and 11 on day 1 and 1, 6, and 11 on day 2. These trials follow the brief period of rest, which allows for memory consolidation. The measure for working memory consisted of the remaining trials, i.e. on day 1, working memory was determined based on trials 1–5, 7–10, and 12–15 and on day 2, trials 2–5, 7–10, and 12–15.
On Day 3, we performed reversal training. The platform for each animal was placed in a new location and for 5 trials, mice were scored on the number of times they entered the arm where the platform was located on the previous 2 days. The average of the first 2 trials on Day 3 was used as a representative of a probe trial. At the end of testing on Day 3, we performed tasks for swim speed and visible platform as control measures. Statistical analysis of the data employed a Student T-test (unpaired, unequal variance) with P≤0.05 being significant.
Amyloid Beta Load analysis
Thioflavin S (Sigma, St. Louis, MO) was used to stain hippocampal sections for β-pleated sheet structures. 3 comparable coronal sections from 3 different animals each/group were incubated with 0.5% thioflavin S in 50% ethanol for 10 min. Sections were then washed twice with 50% ethanol for 5 min each and once with water for 5 min. Staining was visualized under a confocal microscope (Bio-Rad Radiance 2100 confocal system). All thioflavin S positive plaques were counted by two independent observers within the hippocampal region. Statistical analysis employed a Student T-test.
Western blot analyses for total APP levels
Brains (n=6) were homogenized in T-PER buffer (Pierce, Rockford, IL) in the presence of a complete protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis, IN) and centrifuged at 100,000 × g for 1 h at 4°C. Supernatants were collected as the detergent-soluble fraction. Protein quantification was performed using the bicinchoninic acid (BCA) assay (BioRad, Hercules, CA). 10 micrograms of total protein was resolved by SDS-PAGE and electrophoretically transferred onto nitrocellulose membrane. Membranes were blocked in 5% milk, washed, then incubated with the monoclonal 6E10 primary antibody (1:1000; overnight at 4ºC). On the following day, the membrane was washed and incubated with HRP-conjugated goat anti-mouse secondary (1:1000; 1hr; BioRad, Hercules, CA). Membranes were visualized using enhanced chemiluminescence (Pierce, Rockford, IL). Quantification of total APP protein levels was performed using Scion Image Software (NIH).
Aβ40 and Aβ42 ELISAs
Detergent-insoluble fractions were used to detect Aβ40 and Aβ42 using quantitative sandwich enzyme immunoassay technique (ELISA). The FA fractions were diluted 1:20 in neutralization buffer (1 M Tris base and 0.5 M Na2HPO) prior to loading. MaxiSorp immunoplates (Nunc, Naperville, IL) were coated with the monoclonal Aβ20.1 antibody at a concentration of 25 μg/ml in coating buffer (0.1 M NaCO3 buffer, pH 9.6) and blocked using 3% BSA. Standards of both Aβ40 and Aβ42 were prepared in antigen capture buffer (20 mM NaH2PO4, 2 mM EDTA, 0.4 M NaCl, 0.5 g of 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, and 1% BSA, pH 7.0) and loaded onto the ELISA plates in duplicate. Cortex samples were also loaded in duplicate and incubated overnight at 4°C. The plates were subsequently washed and probed with either HRP-conjugated anti-Aβ35–40 (MM32–13.1.1) or anti-Aβ35–42 (MM40–21.3.4) overnight at 4°C. 3,3′,5,5′-Tetramethylbenzidine was used as the chromagen, and the reaction was stopped by 30% O-phosphoric acid and read at 450 nm on a Molecular Dynamics (Sunnyvale, CA) plate reader. Results were normalized to protein concentrations. Data analysis used Student T-test statistics.
mRNA expression level analysis of inflammatory markers
RNA was isolated from the frozen hippocampus by the guanidium thiocyanate method (Chomczynski and Sacchi, 1987) with TRIzol Reagent (LifeTechologies, Carlsbad, CA). Total RNA samples were purified using RNeasy quick spin columns (Qiagen, Valencia CA) and quantified using a UV spectrophotometer. RNA quality was assessed by Bioanalyzer on DNA 500 chips (Agilent Technologies, Santa Clara, CA) ensuring high RNA quality (28S/18S ratio of > 1.5).
RT2Profiler™ PCR Array (Superarray)
The gene expression level of 84 inflammatory markers, 3 house-keeping genes and 2 negative controls were determined simultaneously using Superarray technology. This method is a quantitative real time polymerase chain reaction (qRT-PCR) based approach performed in a 96-well plate format. RNA samples (n=6) were pooled onto individual Superarrays with two samples per array for a total of 3 arrays per condition. The RNA is first converted into first strand cDNA using the ReactionReady™ First Strand cDNA Synthesis Kit following manufacturer’s guidelines. Next, the cDNA is added to the provided RT2 PCR Master Mix containing SYBR Green. The mixture is then aliquoted into each well of the 96-well plate containing pre-dispensed gene-specific primer sets, and the PCR reaction is performed. Statistical analysis was based of the cycle threshold (CT) values using Student T test (unpaired) statistics and fold change (ΔΔCT method).
Quantitative RT-PCR
Validation of CXCL1 and CXCL12 mRNA expression levels in Tg2576 runners and sedentary animals was performed using real time quantitative PCR performed on the individual samples (n=6/condition). CXCL1 and CXCL12 primers were designed against the same regions as the primer sets used in the Superarray (personal communication with Superarray representative). Specifically, we used the CXCL1 primers 5′TGTTGTGCGAAAAGAAGTGC3′ and 5′ACACGTGCGTGTTGACCATA3′ and the CXCL12 primers 5′GCAGACTGTGTTGGGTGAGA3′ and 5′CATCTATCCTCCCCACGAGA3′. Real time PCR was performed on 100ng of total RNA using the One-Step iQ SYBR Green supermix (Bio-Rad, Hercules, CA) on a Bio-Rad thermal-cycler. Threshold cycle (Ct) values were calculated with MyiQ software (Bio-Rad, Hercules, CA), and the quantitative fold changes in CXCL1 and CXCL12 mRNA were determined as relative to 18S mRNA levels. Data analysis used Student T-test statistics.
CXCL1 and CXCL12 protein analysis
CXCL1 and CXCL12 protein levels were determined using quantitative sandwich ELISA. Mouse CXCL12 and CXCL1 Immunoassays using pre-coated plates were obtained from R&D Systems (Minneapolis, MN) and Immuno-Biological Laboratories (Minneapolis, MN) respectively. Briefly, standards and cortex samples were added in duplicate to the pre-coated plate and incubated at room temperature for 2 hours. Plates were subsequently washed to remove unbound substances and next an enzyme-linked polyclonal antibody specific for mouse CXCL1 or CXCL12 was added to the wells. Plates were then washed again followed by the addition of a substrate solution and color develops in proportion to the amount of chemokine bound. The plates were read at 450 nm on a Molecular Dynamics (Sunnyvale, CA) plate reader. Results were normalized to protein concentrations. Data analysis used Student T-test statistics.
RESULTS
Aged Tg2576 mice exposed to 3 weeks of voluntary wheel running exhibit improved memory as compared to their sedentary counterparts
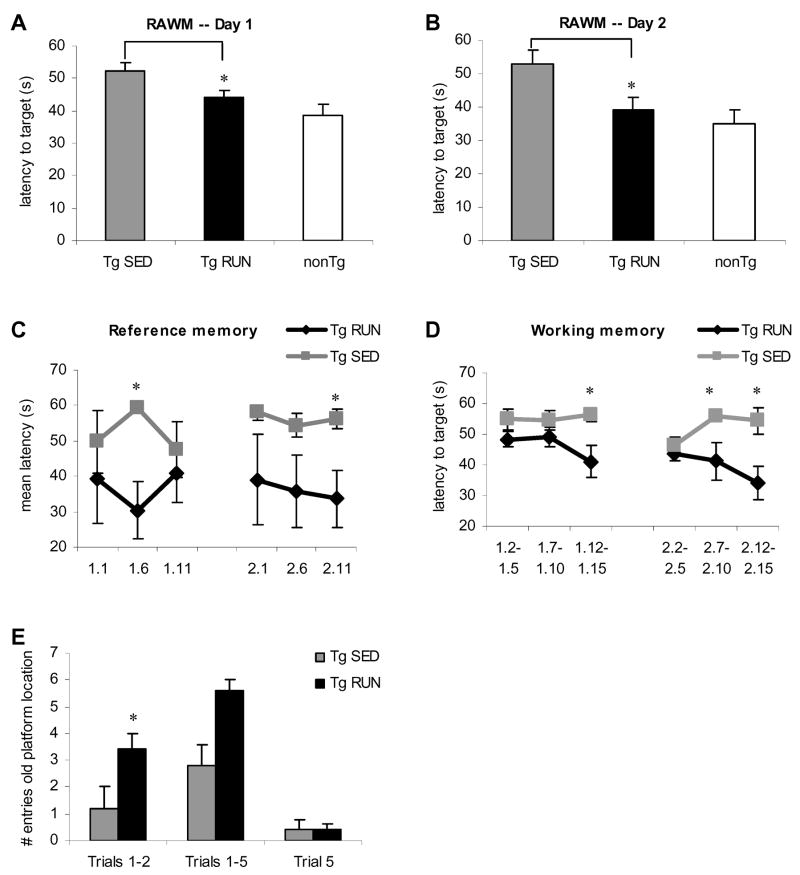
In order to determine the effects of exercise on learning and memory in aged Tg2576 mice, we exposed mice to voluntary wheel running for a period of three weeks. Short term voluntary exercise could ameliorate the learning and memory deficits found in aged Tg2576 mice in accordance with our previous finding (Nichol et al., 2007). We used the radial arm water maze (RAWM) behavior paradigm for 3 days consisting of 15 trials per day with days 1 and 2 being for working and reference memory and day 3 examining reversal training. We found that Tg2576 animals exposed to 3 weeks of voluntary wheel running performed significantly better on the RAWM than sedentary transgenic mice on both days 1 and 2 of testing (day 1 P=0.04, day 2 P=0.02) (Figure 1A, B), restoring cognitive function to non-transgenic levels (runners and sedentary combined; Figure 1A, B). Cognitive improvements were specific to the Tg2576 mice as we found no significant differences between running and sedentary non-transgenic mice (data not shown). Importantly, this suggests that short term exercise paradigms can fully restore behavioral deficits induced by AD pathology in the Tg2576 model of AD.
Figure 1.
Behavioral analysis of exercising and transgenic Tg2576 mice relative to non-transgenic controls (n=6). Mean latencies to the target platform for Day 1 (A) and Day 2 (B) of testing. On both days, transgenic runners (Tg RUN) take a significantly shorter amount of time to get to the target platform than transgenic sedentary mice (Tg SED). Transgenic runners perform at the level of non-transgenic (nonTg) mice. Analysis of reference memory and working memory are depicted in (C) and (D) respectively. On both measures of memory, Tg RUN perform significantly better than Tg SED. (E) Day 3 probe trial data of Tg SED and Tg RUN animals. Tg RUN animals reenter the arm where the platform had been located on the previous two days significantly more than Tg SED on trials 1 and 2. However by trial 5, animals have learned that the platform location has been changed from the previous two days.
Performance on the RAWM can be dissociated into working and reference memory (Hyde et al., 1998). We found significant differences between running and sedentary Tg2576 mice on both of these memory measures. For reference memory, we found that APP runners performed significantly better than APP sedentary mice on both day 1 (P=0.02) and day 2 (P=0.04) (Figure 1 C). On measures of working memory, transgenic runners performed significantly better than sedentary APP on both days (P=0.03, P=0.04, P=0.02) (Figure 1 D).
On Day 3, reversal training, we found that running Tg2576 mice returned to the arm where the platform had been located on the previous two days (old platform arm) more often than Tg2576 sedentary animals indicating improved memory of platform location (Figure 1E). On trials 1–2, Tg2576 runners entered the old platform arm 3 times more often than Tg2576 sedentary animals (P=0.03). When compared on all 5 trials, we found a trend towards greater entry into the old platform arm for APP runners compared to APP sedentary (P=0.08) while on trial 5, both groups performed equally. Hence, while the first couple of trials represent a probe trial, both groups of animals learn the task (novel platform location) by trial 5. To control for motivation and physical differences, we assessed swim speed and tested latency to visible platform and found no significant differences between sedentary and running APP on either test (data not shown).
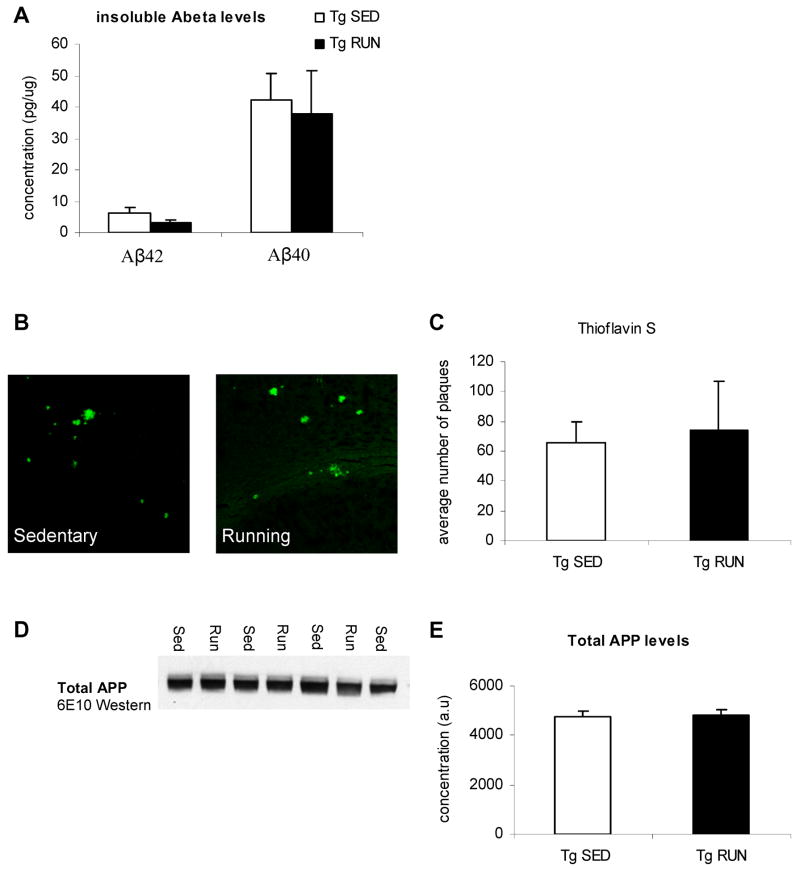
Amyloid beta levels remain unaltered following 3 weeks of voluntary wheel running
Behavioral analysis had shown that 3 weeks of voluntary exercise could restore cognition to age-matched non-transgenic levels. Cognitive deficits in the Tg2576 mice are thought to be due to overproduction of APP resulting in elevated soluble, oligomeric and insoluble Aβ levels (Hsiao et al., 1996; Westerman et al., 2002) mirroring human AD (McLean et al., 1999; Wang et al., 1999). We have previously shown that long term exercise could reduce Aβ load and thus restore these cognitive deficits (Adlard et al., 2005). However, we chose a much shorter exercise period here, and were looking at already established pathology in aged animals. Quantitative analysis of Aβ40 and Aβ42 by sensitive sandwich ELISA showed that insoluble levels showed no significant difference between transgenic animals exposed to a running wheel as compared to their sedentary transgenic counterparts (Figure 2A). Thioflavin S staining for β-sheet structures showed the presence of plaques in both sedentary transgenic animals as well as transgenic mice exposed to voluntary running (Figure 2B). Analysis of thioflavin S staining revealed no difference between sedentary and running transgenic animals (Figure 2C). Importantly, this suggested that cognitive deficits were restored back to non-transgenic levels independently from effects on insoluble Aβ, unlike longer, preventative, exercise paradigms (Adlard et al., 2005). In concurrence, steady state levels of APP were unaffected by short term exercise (Fig 2D, E). In conclusion, our data suggests that 3 weeks of voluntary wheel running in the Tg2576 mouse model of AD does not alter amyloid beta levels.
Figure 2.
Amyloid beta level analysis in exercising and sedentary Tg2576 mice. (A) ELISA data for insoluble Aβ40 and Aβ42 levels. No differences were found in either species of Aβ. (B) Thioflavin S staining in the hippocampus of sedentary and running Tg2576 mice. (C) Thioflavin S quantification. No differences were found in Thioflavin S levels in the hippocampus. (D, E) Western blot for total APP levels and total APP quantification. No differences were found in total APP levels.
Gene expression changes of inflammatory markers
As we had shown that we could restore working and reference memory with short term voluntary exercise independently from any changes in insoluble Aβ, we sought to identify secondary changes that could account for the cognitive improvement, which was specific to the APP overexpressing mice. As we had previously shown that inflammatory markers correlate strongly with cognitive decline in clinically mild to moderate AD patients, and that exercise is known to also modulate inflammation, we focused on inflammatory changes. We therefore conducted a widespread screen of the mRNA expression level of inflammatory markers in the hippocampus of sedentary and exercised Tg2576 and age-matched non-transgenic control mice.
APP overexpression modulates the inflammatory response in the hippocampus
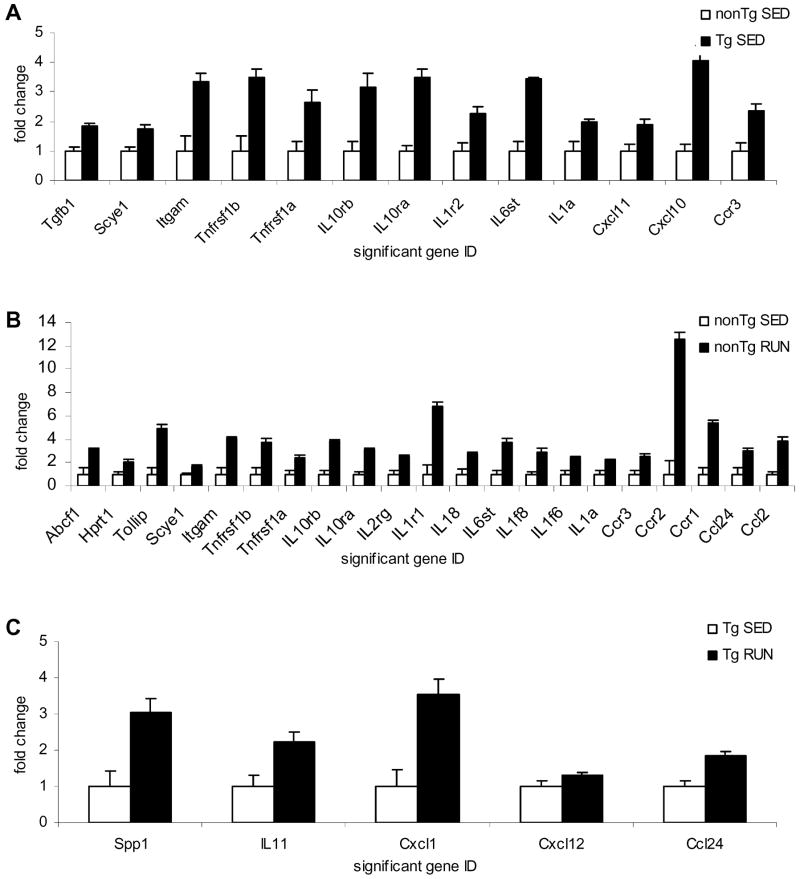
Sedentary APP mice exhibited increased gene expression levels for numerous inflammatory markers as compared to age-matched sedentary non-transgenic mice including the pro-inflammatory IL1α and the IL1 receptor (IL1r2) (Figure 3A). The largest change in gene expression between transgenic and non-transgenic mice was in the chemokine CXCL10 (IP10). In addition to CXCL10, transgenic mice exhibited increased mRNA levels for CXCL11 and the CCR3 chemokine receptor. A number of cytokine receptors including the receptors for IL1 (IL1r2), IL10 (IL10rβ) and TNF (Tnfrsf1a) were also found to be increased in the brains of the transgenic mice as compared to non-transgenic mice. These findings agree with the previous literature that there is an inflammatory response associated with Aβ plaques in AD and transgenic animals.
Figure 3.
Superarray analysis of inflammatory markers in transgenic and non-transgenic mice. (A) Significantly altered markers in sedentary transgenic mice (Tg SED) compared to sedentary non-transgenic mice (nonTg SED). (B) Significantly altered markers in non-transgenic runners (nonTg RUN) compared to non-transgenic sedentary mice (nonTg SED). (C) Significantly altered markers in transgenic runners (Tg RUN) compared to transgenic sedentary mice (Tg SED).
Exercise modulates the inflammatory response in the hippocampus in non-transgenic mice
We also compared the inflammatory mRNA profile of non-transgenic animals following the voluntary exercise regime. Voluntary exercise increased mRNA levels of a number of chemokines and chemokine receptors (Figure 3B). This is in accordance with a recent study showing microglia and T cell recruitment into the hippocampus following environmental enrichment (Ziv et al., 2006), as chemokines are known to be chemoattractant molecules. The largest change in gene expression between sedentary and exercising non-transgenic mice was in the CCR2 chemokine receptor, a key microglia chemoattractant molecule, but large increases were also seen in a number of cytokines and cytokine receptors such as IL6st, IL1, and the IL10 receptor. Interestingly, while this voluntary exercise paradigm seems to be very broadly pro-cytokine/chemokine, there were no differences in cognitive function between sedentary and exercised animals. This may be because the non-transgenic animals are already performing at peak cognitive function, and cannot improve further, or because this exercise paradigm does not improve cognition in non-transgenic animals.
Exercise alters the inflammatory response in the hippocampus of Tg2576 mice
The 3 week voluntary exercise paradigm restored cognitive deficits back to non-transgenic levels in the Tg2576 mice, without affecting Thioflavine S and Aβ. We have shown that APP overexpression leads to a pro-inflammatory response in these sedentary animals, which could contribute to the cognitive deficits reported in these animals (Figure 3A). We looked to a further altered inflammatory response to explain the restoration of cognitive deficits in these animals with voluntary exercise. Interestingly, APP transgenic mice exposed to 3 weeks of voluntary wheel running did not return any of the increased inflammatory markers due to APP overexpression back to baseline (Figure 3A vs. Figure 3C), but instead additionally increased mRNA levels of 5 other chemokines (Figure 3C). The largest change at the mRNA level in running compared to sedentary transgenic mice was observed for the chemokine CXCL1. In addition to CXCL1, exercise increased mRNA levels of IL11, Spp1, and CXCL12. CXCL1 was previously shown to induce neuroprotection from Aβ in the hippocampus whereas the CXCL12 molecule is a key neuron-glia/neuron-neuron communication marker. We hypothesized that the increase in these 5 chemokines may be protective and may contribute to the cognitive amelioration. This is supported by our recent findings that one of the chemokines, CXCL12, is a modulator of cognitive function and is downregulated in AD and in Tg2576 mice at ages when cognitive decline begins (Parachikova and Cotman, 2007).
Validation of the CXCL12 and CXCL1 Superarray data
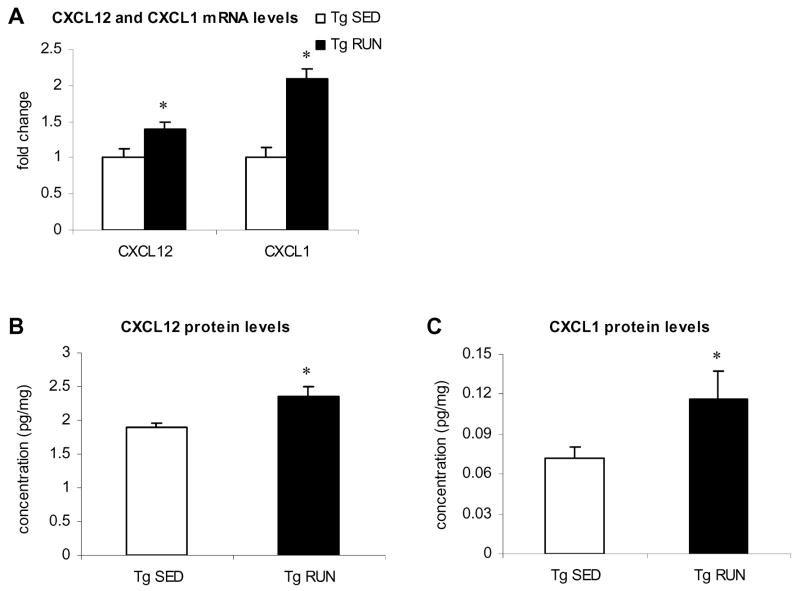
We chose to focus on CXCL1 and CXCL12 since these chemokines have been shown to be neuroprotective and improve neuron/glia communication in the brain, respectively, and could thus contribute to learning and memory. The changes in CXCL12 and CXCL1 were further validated using quantitative real time PCR on individual samples from transgenic sedentary and transgenic runners (n=6/condition). The data obtained validated the Superarray findings (Figure 4A). Specifically, we found a significant 1.4 fold increased in CXCL12 and a significant 2.1 fold increased in CXCL1 mRNA levels in running as compared to sedentary transgenic animals. These changes are comparable to the changes found in these two chemokines using Superarray technology. We next studied the protein levels of the chemokines CXCL12 and CXCL1 and demonstrate that 3 weeks of voluntary wheel running in Tg2576 mice resulted in increased protein levels for both CXCL12 (1.3 fold, Figure 4B) and CXCL1 (1.6 fold, Figure 4C). In conclusion, 3 weeks of voluntary wheel running in Tg2576 mice compared to sedentary Tg2576 controls induces increased mRNA and protein level for the chemokines CXCL12 and CXCL1.
Figure 4.
CXCL1 and CXCL12 levels in transgenic runners and transgenic sedentary mice. (A) Quantitative RT-PCR confirmation of increased CXCL1 and CXCL12 levels in Tg RUN compared to Tg SED. CXCL12 (B) and CXCL1 (C) protein levels as measured via ELISA. For both chemokines, Tg RUN show significantly increased protein levels compared to Tg SED.
DISCUSSION
The present study provides evidence that exercise is a modulator of the inflammatory response in the CNS of aged C57B6 and Tg2576 mice. Tg2576 mice exposed to voluntary wheel running exhibit an altered inflammatory response as compared to non-transgenic runners and improved cognition. We propose that exercise exerts its beneficial effects on cognition in the aged Tg2576 runners in part by altering the expression level of inflammatory molecules; including increasing levels of the chemokines CXCL1 (Groα) and CXCL12 (SDF1). In support of this, exercise has been repeatedly shown to exert positive effects on cognitive function in humans (Colcombe and Kramer, 2003; Heyn et al., 2004; Kramer et al., 1999; Weuve et al., 2004; Young, 1979) and enhance learning/memory in mice (Fordyce and Wehner, 1993; Nichol et al., 2007; O’Callaghan et al., 2007; Schweitzer et al., 2006; van Praag et al., 2005; Vaynman et al., 2004). Studies in the periphery have documented leukocytosis and changes in circulating cytokines including TNFα, IL1, IL6, IL10 with physical exercise (Bruunsgaard, 2005; Jonsdottir et al., 2000; Lancaster et al., 2004; Ostrowski et al., 1998a; Ostrowski et al., 1998b; Pedersen et al., 1997; Rohde et al., 1997). The effects of exercise on the inflammatory response in the brain however remain poorly defined. Here we show that in non-transgenic mice exercise increases a wide variety of chemokines and chemokine receptors in the CNS complementing these studies in the periphery.
APP overexpression also resulted in the elevation of a number of inflammatory markers including the pro-inflammatory IL1α and the IL1 receptor. These findings confirm and extend previous literature (Abbas et al., 2002; Benzing et al., 1999; Frautschy et al., 1998; Mehlhorn et al., 2000; Sly et al., 2001) also showing increased pro-inflammatory markers due to Aβ. It is important to point out that resting levels of cytokines and chemokines are higher in the Tg2576 than the non-transgenic controls, which explains why fewer markers were further increased with exercise in the transgenic mice compared to exercising non-transgenic mice. Voluntary wheel running in the transgenic mice did not affect the inflammatory markers that were altered as result of APP overexpression. Instead, we found that running resulted in the increased mRNA expression of only a few molecules, predominantly chemokines. Notably, as voluntary exercise restored cognition back to non-transgenic levels in the absence of changes in Aβ levels, it may be that these increased chemokines can contribute to cognitive functioning.
In particular, two chemokines CXCL1 and CXCL12 were increased in the Tg2576 mice following three weeks of voluntary wheel running. CXCL1 (Groα) is a ligand of the CXCR2 receptor. CXCR2 as well as CXCL1 have been shown to be found in amyloid plaques in the brains of AD patients (Xia et al., 1997), while an in vitro study demonstrated that CXCR2 ligands protected hippocampal neurons from Aβ-induced cell death (Watson and Fan, 2005). Hence we propose that, in part, the cognitive amelioration in aged Tg2576 mice following running may be due to CXCL1 mediated neuronal dysfunction and degeneration. CXCL12 (SDF-1) is the sole ligand for the CXCR4 receptor. In addition to its neuroinflammatory function, CXCL12/CXCR4 signaling has been proposed to regulate neuronal excitability and synaptic transmission. CXCL12 enhances glutamate release from astrocytes and regulates neuronal excitability (Bezzi et al., 2001), signal propagation within glial networks (Innocenti et al., 2000) and synaptic transmission (Kang et al., 1998). We have previously speculated that increased CXCL12 might improve neuron-glia/neuron-neuron communication thereby improving and enhancing learning and memory. Recently, we have been able to demonstrate a direct link between CXCL12/CXCR4 signaling and learning and memory. We found that systemic administration of AMD3100, a specific CXCR4 antagonist, results in impaired learning and memory in young non-transgenic mice (Parachikova and Cotman, 2007).
In conclusion, the findings of the current study suggest that cognitive amelioration in Tg2576 mice due to exercise, may be in part mediated via changes in the inflammatory response.
Acknowledgments
We would like to thank Vadim Fedulov for the expert assistance and Dr. Kim Green for the critical review of the manuscript. This work was supported by AG000538 and AG16573.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas N, et al. Up-regulation of the inflammatory cytokines IFN-gamma and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APP(SWE) transgenic mice. J Neuroimmunol. 2002;126:50–7. doi: 10.1016/s0165-5728(02)00050-4. [DOI] [PubMed] [Google Scholar]
- Adlard PA, et al. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–21. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajetto A, et al. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001;22:147–84. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- Benzing WC, et al. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–9. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Berkman LF, et al. High, usual and impaired functioning in community-dwelling older men and women: findings from the MacArthur Foundation Research Network on Successful Aging. J Clin Epidemiol. 1993;46:1129–40. doi: 10.1016/0895-4356(93)90112-e. [DOI] [PubMed] [Google Scholar]
- Bezzi P, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–10. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Blomquist KB, Danner F. Effects of physical conditioning on information-processing efficiency. Percept Mot Skills. 1987;65:175–86. doi: 10.2466/pms.1987.65.1.175. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. 2005 doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Wehner JM. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain Res. 1993;619:111–9. doi: 10.1016/0006-8993(93)91602-o. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, et al. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–17. [PMC free article] [PubMed] [Google Scholar]
- Heyn P, et al. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Hill RD, et al. The impact of long-term exercise training on psychological function in older adults. J Gerontol. 1993;48:P12–7. doi: 10.1093/geronj/48.1.p12. [DOI] [PubMed] [Google Scholar]
- Hsiao K, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Hyde LA, et al. Water version of the radial-arm maze: learning in three inbred strains of mice. Brain Res. 1998;785:236–44. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Innocenti B, et al. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci. 2000;20:1800–8. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir IH, et al. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol. 2000;528 Pt 1:157–63. doi: 10.1111/j.1469-7793.2000.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, et al. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–92. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kramer AF, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–9. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Lancaster GI, et al. Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc Immunol Rev. 2004;10:91–106. [PubMed] [Google Scholar]
- Larson EB, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Lue LF, et al. Inflammation, A beta deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. J Neuropathol Exp Neurol. 1996;55:1083–8. [PubMed] [Google Scholar]
- Luterman JD, et al. Cytokine gene expression as a function of the clinical progression of Alzheimer disease dementia. Arch Neurol. 2000;57:1153–60. doi: 10.1001/archneur.57.8.1153. [DOI] [PubMed] [Google Scholar]
- McLean CA, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–6. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mehlhorn G, et al. Induction of cytokines in glial cells surrounding cortical beta-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int J Dev Neurosci. 2000;18:423–31. doi: 10.1016/s0736-5748(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Nichol KE, et al. Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behav Brain Res. 2007;184:124–32. doi: 10.1016/j.bbr.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan RM, et al. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176:362–6. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, et al. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol. 1998a;513(Pt 3):889–94. doi: 10.1111/j.1469-7793.1998.889ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K, et al. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998b;508(Pt 3):949–53. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parachikova A, Cotman CW. Reduced CXCL12/CXCR4 results in impaired learning and is downregulated in a mouse model of Alzheimer disease. Neurobiol Dis. 2007;28:143–53. doi: 10.1016/j.nbd.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinetti GM. Cyclooxygenase and Alzheimer’s disease: implications for preventive initiatives to slow the progression of clinical dementia. Arch Gerontol Geriatr. 2001;33:13–28. doi: 10.1016/s0167-4943(01)00091-7. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, et al. Exercise-induced immunomodulation--possible roles of neuroendocrine and metabolic factors. Int J Sports Med. 1997;18(Suppl 1):S2–7. doi: 10.1055/s-2007-972695. [DOI] [PubMed] [Google Scholar]
- Radak Z, et al. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int. 2006;49:387–92. doi: 10.1016/j.neuint.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Rogers RL, et al. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc. 1990;38:123–8. doi: 10.1111/j.1532-5415.1990.tb03472.x. [DOI] [PubMed] [Google Scholar]
- Rohde T, et al. Prolonged submaximal eccentric exercise is associated with increased levels of plasma IL-6. Am J Physiol. 1997;273:E85–91. doi: 10.1152/ajpendo.1997.273.1.E85. [DOI] [PubMed] [Google Scholar]
- Schweitzer NB, et al. Exercise-induced changes in cardiac gene expression and its relation to spatial maze performance. Neurochem Int. 2006;48:9–16. doi: 10.1016/j.neuint.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Sly LM, et al. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain Res Bull. 2001;56:581–8. doi: 10.1016/s0361-9230(01)00730-4. [DOI] [PubMed] [Google Scholar]
- van Praag H, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, et al. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer’s disease from normal and pathologic aging. Exp Neurol. 1999;158:328–37. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- Watson K, Fan GH. Macrophage inflammatory protein 2 inhibits beta-amyloid peptide (1–42)-mediated hippocampal neuronal apoptosis through activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Mol Pharmacol. 2005;67:757–65. doi: 10.1124/mol.104.004812. [DOI] [PubMed] [Google Scholar]
- Westerman MA, et al. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22:1858–67. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, et al. Physical activity, including walking, and cognitive function in older women. Jama. 2004;292:1454–61. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Xia M, et al. Interleukin-8 receptor B immunoreactivity in brain and neuritic plaques of Alzheimer’s disease. Am J Pathol. 1997;150:1267–74. [PMC free article] [PubMed] [Google Scholar]
- Young RJ. The effect of regular exercise on cognitive functioning and personality. Br J Sports Med. 1979;13:110–7. doi: 10.1136/bjsm.13.3.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanjani H, et al. Complement Activation in Very Early Alzheimer Disease. Alzheimer Dis Assoc Disord. 2005;19:55–66. doi: 10.1097/01.wad.0000165506.60370.94. [DOI] [PubMed] [Google Scholar]
- Ziv Y, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:26875. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]