Summary
Purpose
Chronic renal failure is an acknowledged complication of hematopoietic stem cell transplantation (HSCT) in humans. It can be caused in part by radiation injury when HSCT is preceded by sufficient total body irradiation (TBI). Studies in laboratory animals show the benefit of angiotensin converting enzyme (ACE) inhibitors in mitigation and treatment of this complication. We thus conducted a randomized controlled trial testing whether the ACE inhibitor captopril was effective in mitigating chronic renal failure after HSCT.
Methods and materials
55 subjects undergoing TBI-HSCT were enrolled. Captopril, or identical placebo, was started at engraftment, and continued as tolerated until one year after HSCT.
Results
The baseline serum creatinine and calculated glomerular filtration rate (GFR) did not differ between groups. The one-year serum creatinine was lower, and the GFR higher, in the captopril compared to the placebo group ( p=0.07, for GFR). Patient survival was higher in the captopril compared to the placebo group, but this was also not statistically significant (p=0.09). In study subjects who were on study drug for more than two months, the one-year calculated GFRs were 92 ml/min and 80 ml/min, for the captopril and placebo groups, respectively (p=0.1). There was no adverse effect on hematological outcome.
Conclusion
We conclude that there is a trend in favor of captopril in mitigation of chronic renal failure after radiation-based HSCT.
Keywords: chronic renal failure, hematopoietic stem cell transplantation, radiation nephropathy, captopril
Introduction
Renal failure is a well-known and serious complication of hematopoietic stem cell transplantation (HSCT) (1). Acute renal failure, within the first thirty days after HSCT, occurs in up to 50% of patients and increases early mortality (2). Chronic renal failure is also common, and affects the health and well-being of people otherwise cured of the cancer for which the HSCT was done. If end-stage renal disease occurs, mortality rates are very high (3). Significant long-term loss of renal function may occur after HSCT without or with use of total body irradiation (TBI) for pre-transplant conditioning (4,5). The pathogenesis of that injury has been related to graft versus host disease (GVHD), calcineurin inhibitor toxicity, or other regimen toxicities. TBI-based HSCT has, however, been closely linked to the development of radiation nephropathy, which we and others have called bone marrow transplant (BMT) nephropathy (6). BMT nephropathy in its most severe form appears similar to hemolytic uremic syndrome (HUS), which has also been linked to TBI used for the conditioning regimen (7). Finally, partial kidney shielding at the time of TBI has reduced the occurrence rates of BMT nephropathy (8).
BMT nephropathy has been reproduced in laboratory animals using external beam TBI. In that model, chronic renal failure can be mitigated and treated by angiotensin converting enzyme (ACE) inhibitors (9). Others have shown the benefit of aldosterone blockade or angiotensin II blockade in an internal irradiation radioisotope model of radiation nephropathy (10). These data have overturned the former dogma that normal tissue radiation injury is untreatable. In additional experimental studies, we have shown that delaying the start of ACE inhibitors to three weeks after irradiation did not reduce their beneficial effect (11). This result suggested that ACE inhibitors might be used clinically after completion of radiation therapy, but before expression of injury, to mitigate the later development of renal failure. We therefore performed the present study, to attempt to mitigate radiation nephropathy occurring after TBI-based HSCT, using the ACE inhibitor captopril.
Methods
This is a prospective single-center masked randomized placebo-controlled trial to study the effect of the ACE-inhibitor captopril on the development of chronic renal failure and the BMT nephropathy syndrome after HSCT using preparative regimens that include TBI.
Captopril is a specific competitive inhibitor of angiotensin I converting enzyme, the enzyme responsible for the conversion of angiotensin I to angiotensin II. It is FDA-approved for treatment of hypertension, heart failure, and diabetic nephropathy.
Captopril is available in potencies of 6.25, 12.5 and 25 mg. This drug was encapsulated, and identical placebo capsules were made, but filled with gelatin (Panorama Pharmaceuticals, CA). Capsules of three different colors were made, indicating the three different captopril dose-strengths of 6.25, 12.5 and 25 mg. Identical placebo capsules were made for each dose. The starting dose was 6.25 mg b.i.d and the dose was increased to a maximum dose 25 mg t.i.d in adults, and 12.5 mg t.i.d. in children as described below. The dose of 25 mg t.i.d. was chosen because of its successful use in human diabetic nephropathy (12) and because of its correspondence, on the basis of body surface area, to doses used in experimental radiation nephropathy.
Because captopril may cause leukopenia, the study drug was not started until marrow engraftment had occurred. This timing was justified on our experimental data that showed that a delayed start of captopril could successfully mitigate radiation nephropathy (13).
Patient Selection
Eligible candidates were patients over the age of 6 years receiving marrow or peripheral stem cells from autologous, HLA-identical sibling or unrelated donors at the Children’s Hospital of Wisconsin or Froedtert Hospital, Medical College of Wisconsin Hematopoietic Cell Transplant Programs. Only patients receiving myeloablative TBI as part of the preparative regimen were included.
Eligibility criteria included a plasma creatinine of less than 1.8 mg% in adults and less than 1.2 mg% in children under 16 years of age. These criteria were chosen to avoid including patients with substantial renal disease before bone marrow transplantation. The baseline plasma creatinine for analysis was the value that was obtained at the time of the start of the study drug.
Ineligibility criteria included a history of intolerance to ACE inhibitors, or existing ongoing treatment with an ACE inhibitor. Because of the teratogenic potential of ACE inhibitors, women of child bearing potential and not practicing adequate contraception were not eligible.
Registration/Randomization
Patients were randomized to receive Captopril or placebo. Randomization was stratified by age [pediatric (< 16 yrs. vs. ≥ 16 yrs.) versus adult] and marrow stem cell source (autologous, HLA-identical sibling, donor other than HLA-identical sibling). Randomization was carried out at the International Bone Marrow Transplant Registry (IBMTR) Statistical Center, Medical College of Wisconsin, using a random number table.
Treatment Plan
Patients started captopril or placebo depending on randomization. The start of captopril or placebo was on the day of engraftment, when the blood absolute neutrophil count was ≥ 1000/mm3. The starting dose was 6.25 mg p.o. b.i.d, which was increased to 25 mg p.o. t.i.d as tolerated. In children, the study drug was crushed, or given as a slurry, as required. In children weighing more than 40 kg, the protocol for adults was used. Captopril was continued for the first post-transplant year. Study drug was dispensed by the Froedtert Hospital central pharmacy.
HSCT, including preparative regimens, TBI, graft versus host disease (GVHD) prophylaxis, and supportive care was performed according to the protocols and standard institutional guidelines of the Adult and Pediatric BMT programs of the Medical College of Wisconsin. Chemo-irradiation conditioning was done as described in Lawton et al (8). The standard TBI dose was 14 Gy, in nine equal fractions over three days, using a dose rate of 8 to 20 cGy/minute and with at least four hours between fractions. Kidney shielding was 15 % in the PA and the AP direction, yielding a total kidney dose of 9.8 Gy. GVHD prophylaxis used cyclosporin in almost all cases. Two received tacrolimus, one in each arm. Three had no calcineurin inhibitor use, two in the captopril arm, and one in the placebo arm.
If a patient in the control or captopril arm became hypertensive (three consecutive readings greater than 140/80 mmHg in adults, and greater than 120/80 in children) after HSCT, anti-hypertensive drugs could be used that were not ACE inhibitors or angiotensin II blockers. Study drug could be stopped, according to the preference of the treating physician.
If a patient became hypotensive (systolic blood pressure ≤ 100 mmHg) during follow-up, the study drug was withheld temporarily, and then restarted as tolerated.
For azotemia (defined as a doubling of the baseline plasma creatinine or plasma creatinine ≥ 2.5 mg/dl), the study drug was withheld temporarily at the discretion of the treating physician. Study drug could be stopped, according to the preference of the treating physician.
On Study Evaluation
Blood pressure and serum creatinine were obtained twice weekly during hospitalization and weekly after discharge until day 100 or as long as the patient was followed weekly in the outpatient department.
Blood pressure, plasma creatinine (in mg/dl), and haptoglobin, were obtained at days 100, 180, 365 post-transplant.
Outcome Assessment
The BMT nephropathy syndrome is defined as azotemia (doubling of base-line serum creatinine or a >50% decrease in the glomerular filtration rate (GFR) ), hypertension and anemia after HSCT in the absence of any other identifiable cause of kidney malfunction and in the absence of nephrotoxic drugs (14). The GFR (in ml/min) in adults were calculated by the Modification of Diet in Renal Disease (MDRD) formula and the GFR in children was done by nuclear medicine testing. The MDRD formula was calculated as in Stevens et al. (15). The MDRD values and the nuclear medicine values are normalized to body surface area of 1.73 m2.
Stop-points included: the development of the BMT nephropathy syndrome as defined or hypertension or azotemia, according the assessment of the treating physician. HUS, which overlaps with BMT nephropathy, was also a stop point. Patients reaching a stop-point had the study drug withdrawn but were followed thereafter.
Sample Size
The study was designed to detect a 20% reduction (25% to 5%) in the probability of post-transplant nephropathy in the Captopril group as compared to the control group. Forty patients per arm were needed to have 80% power to detect this difference in incidence based on a 5% level using the log rank test. Due to problems with accrual we observed only 27 patients on the Captopril arm and 28 patients on the placebo arm. With this number of patients, we had 67% power to detect a 20% difference in the post-transplant nephropathy rate.
Non-study cohort
Adult subjects who did not participate in this study, but who underwent myeloablative TBI-based HSCT during the time of this study were identified, specifically for the development, or not, of the BMT nephropathy syndrome and HUS. Their non-participation was because of refusal or in-eligibility due to enrollment in other clinical studies. Neither median age nor type of transplant differed between this group and the adult study cohort.
Statistical analysis
Continuous data are shown as the median with the interquartile range. Group differences were tested by Mann-Whitney tests with exact p-values computed by a Monte Carlo routine based on 10,000 replicates. The cumulative incidence function was used to summarize the incidence of post-transplant nephropathy or HUS with death without nephropathy as a competing risk (16). Intergroup differences were assessed by Fisher exact test. Survival was summarized using the Kaplan-Meier method. Comparisons for time to event data was made by the log rank test.
Institutional Review Board Approval was obtained and maintained throughout the study.
Results
Of a total of 183 eligible subjects, 55 were enrolled in this study from July 1998 to January 2006; 52 were adults, 3 were children. Five additional adults were enrolled but did not obtain study drug at the pharmacy (Figure 1). The total TBI dose was 14 Gy in all but six; of those, one had 14.1 Gy, one had 13.5, one had 13.2, and three had 12 Gy.
Figure 1.
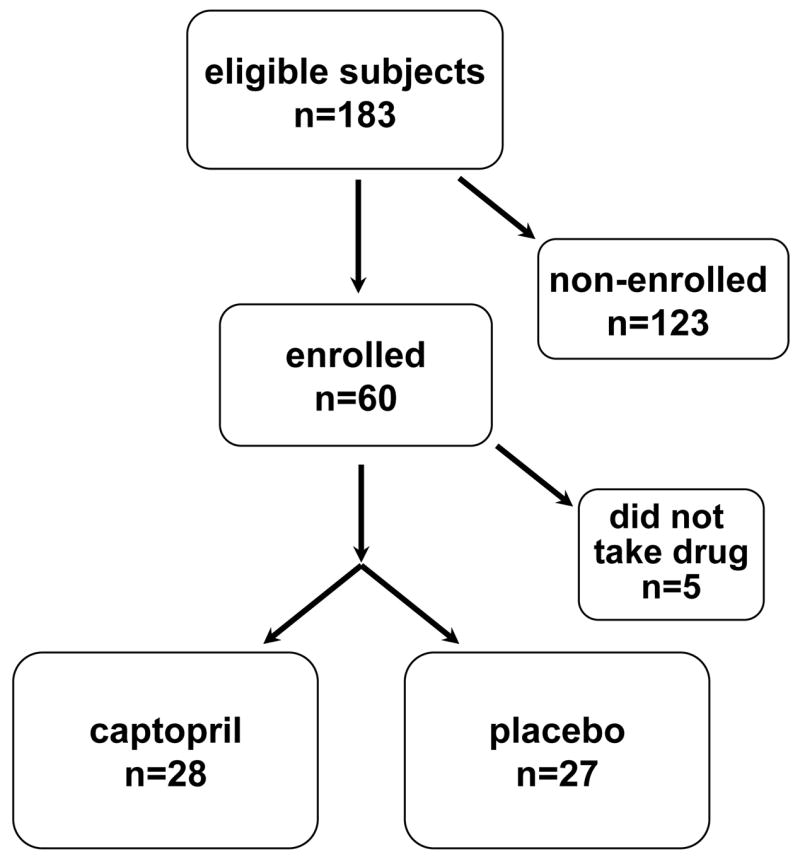
Diagram of patient recruitment and accrual to this study
Recruitment to this study was 30%. Adult recruitment rates were over 50% for the first two years of the study, and then decreased steadily. Over this same time, the number of TBI-based myeloablative BMT in adults at our center decreased from 35 per year to 8 per year, and these two trends caused steadily decreasing enrollment numbers. Overall enrollment of adults was 35% of those eligible for the study. Overall enrollment of children was 6%.
Patient demographics were largely similar, with the exception of an over-representation of underlying acute myeloid leukemia in the captopril group. These differences were not statistically significant (table 1). The distribution of types of HSCT (haplo-identical, non-related, related match, related partial match, syngeneic, or autologous) did not differ between the two groups.
Table 1.
Underlying diseases in study subjects (ALL is acute lymphocytic leukemia, AML is acute myelocytic leukemia, CML is chronic myeloid leukemia, CLL is chronic lymphoid leukemia, MDS is myelodysplastic syndrome)
| Disease | captopril | placebo |
|---|---|---|
| ALL | 4 | 4 |
| AML | 12 | 4 |
| CML | 5 | 7 |
| CLL | 1 | 2 |
| Lymphoma | 3 | 6 |
| MDS | 3 | 3 |
| Plasma cell leukemia | 1 |
The baseline serum creatinine and calculated GFR values did not differ between the captopril and placebo groups (table 2). The median TBI dose was 14 Gy in both the captopril and placebo arms. The geometric mean doses, 1393 cGy, and 1379 cGy, respectively, did not differ (p=0.3)
Table 2.
Evolution of kidney function in study subjects. The data are shown as medians with interquartile ranges in parentheses.
| Group | Starting serum creatinine | Starting GFR | One year serum creatinine | One year GFR | One year GFR, 60 day+ group |
|---|---|---|---|---|---|
| Captopril | 0.80 (0.7–1.0) | 100.5 (82.1–119.0) | 0.95 (0.8–1.4) | 85.8 (56.8–110.9) | 91.9 (69.3–110.9) |
| Placebo | 0.85 (0.7–1.2) | 93.8 (71.2–113.3) | 1.10 (0.9–1.8) | 76.7 (32.8–93.6) | 80.0 (47.6–93.9) |
| P value | 0.35 | 0.30 | 0.2 | 0.07 | 0.1 |
The average time on study drug was 1.8 months for those on captopril and 2.4 months for those on placebo (p=0.5). Five subjects in each group completed one year on study drug. Reasons for stopping study drug are shown on table 3. Hypertension or azotemia forced the stop of study drug in five subjects on placebo, but none on captopril. These differences were not statistically significant.
Table 3.
Reasons for stopping study drug in study subjects
| Captopril | Reason | Placebo |
|---|---|---|
| 5 | completed one year on study drug | 5 |
| 5 | death | 6 |
| 3 | non-compliant | 6 |
| 3 | hypotension | 2 |
| 2 | dizzy | |
| 2 | neutropenia | |
| 2 | disease relapse | 2 |
| hypertension | 2 | |
| azotemia | 2 | |
| BMT nephropathy | 1 | |
| cough | 1 | |
| 1 | dyspnea | |
| 1 | hematuria | |
| 1 | hyperkalemia | |
| 1 | infection | |
| 1 | mental status change | |
| 1 | palpitation |
In one-year survivors the serum creatinine tended to be lower (0.95 vs. 1.10, p=0.2) and the calculated GFR values were higher (85.8 vs. 76.7, p=0.07) in the captopril group as compared to the placebo group (table 2).
Because the median time of subjects on study drug was two months, an additional analysis was performed for those subjects on study drug for two months or more. For these subjects (n=19), there was better-preserved kidney function in the captopril group compared to the placebo group as shown by the median GFR at one year (table 2).
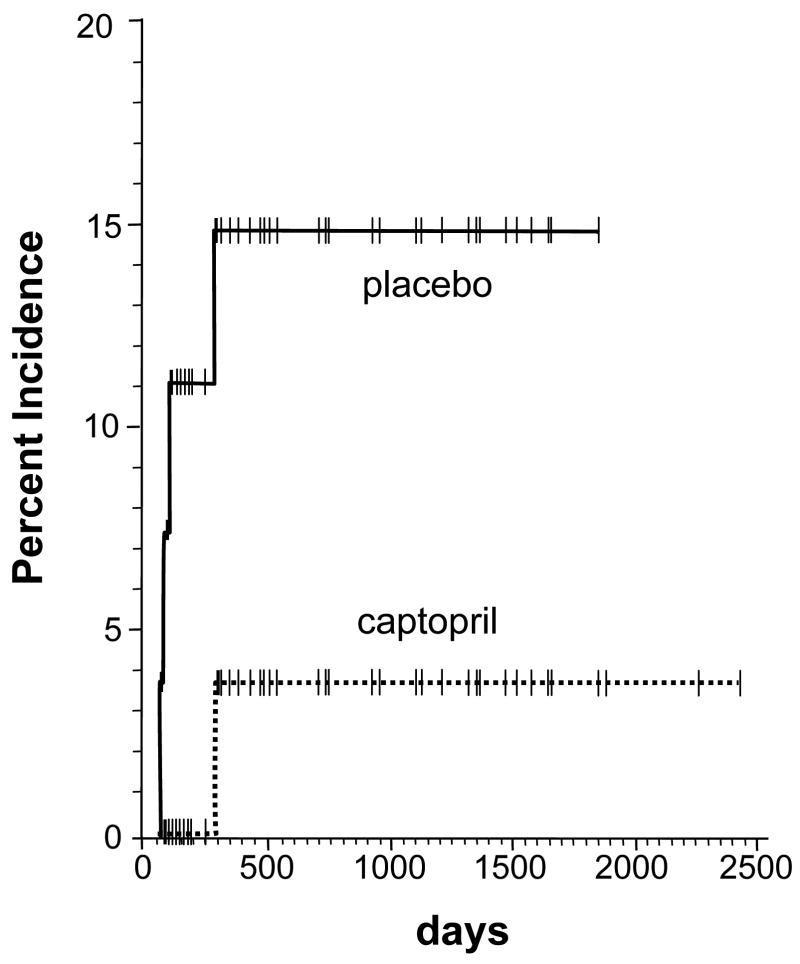
One subject in the group on captopril developed clinical BMT nephropathy, as did one in the placebo group. An additional three subjects in the placebo group developed HUS. As shown on figure 2, the cumulative incidence of nephropathy or HUS showed that there was a trend in favor of captopril, a 3.7 % incidence compared to placebo, a 15 % incidence; but this did not attain statistical significance (p=0.1). In addition, the one-year actuarial patient survival for subjects in the captopril arm, 67 %, was better than that of the placebo arm, 48 %, but this did not attain statistical significance (p=0.09).
Figure 2.
The cumulative incidence of BMT nephropathy and HUS according to use of captopril or placebo. The occurrence of BMT nephropathy and HUS was within the first year after the BMT, as has been the case for BMT nephropathy in previous studies (6,12). The placebo group had a higher rate than the captopril group, but this did not attain statistical significance (p=0.1)
There was no significant association of the use of calcineurin inhibitor, as cyclosporin or tacrolimus, or occurrence of graft versus host disease to the occurrence of BMT nephropathy or HUS (p > 0.6 for both, Fisher’s exact test).
A parallel analysis was performed of 61 adult subjects undergoing myeloablative BMT during the time of the study, at a TBI dose of 14 Gy, but who did not participate in the study (the non-study cohort). Of these, five developed the BMT nephropathy syndrome, of whom one progressed to chronic renal failure and long-term dialysis. An additional subject in this group also developed chronic renal failure requiring long term chronic dialysis, but did not have clear features of BMT nephropathy or HUS. There was one case of HUS, in addition to the five BMT nephropathy cases. Survival free of BMT nephropathy or HUS in the non-study cohort was not different from that of the study cohort on placebo (p=0.8).
Discussion
This study supports the idea that captopril may mitigate chronic renal failure after radiation-based HSCT. Declining enrollment may have caused a type 2 error; i.e., that captopril is truly effective despite the not-quite-significant p value of the GFR or survival endpoints.
The relative percent reduction in the occurrence of the BMT nephropathy syndrome, in the captopril group compared to the placebo group, was over 50% (figure 2). Although this reduction did not achieve statistical significance, it corresponds to the range of clinical benefits seen with use of ACE inhibitors for treatment of kidney diseases. For example, the risk reduction of renal failure by use of captopril in diabetic nephropathy was almost 50% in the study of Lewis et al (12).
Both the captopril and placebo groups had a substantial fall in their GFR. Indeed, four of the eleven subjects in the placebo group evaluated at one year had GFR < 60 ml/minute, which is in the range for stage 3 chronic kidney disease (15). In this range of GFR and below, pathophysiological changes occur, such as declining ability to concentrate the urine, reduced synthesis of calcitriol, and greater tendency towards hypertension. There is also increased general and cardiovascular mortality risk in this range of reduced kidney function (17). Even modest protection against this fall in GFR may thus be beneficial.
The precise cause of the long-term loss of kidney function in these cases is not known because there were no kidney biopsies done. The use of TBI in all cases and the presence of five cases of BMT nephropathy and or HUS in the entire cohort supports the notion that TBI played a role. There appeared to be no significant association with the use of calcineurin inhibitors or graft versus host disease.
Use of HSCT worldwide is now common, being over 40,000 per year. It is likely that there are many subjects who have undergone HSCT and now have reduced renal function as a normal tissue sequel of the HSCT. Occurrence of chronic renal failure after HSCT may be related to myeloablative chemo-irradiation conditioning, but can also occur with non-myeloablative transplants (4). In the most severe cases, that would result in end-stage renal disease and the need for chronic dialysis. Outcomes of chronic dialysis after HSCT are poor (3). End-stage renal disease, the requirement for dialysis or kidney transplant to maintain life, has a sixteen-fold higher occurrence after BMT compared to the age-matched general population (18). HSCT is thus very clearly associated with severe chronic renal failure. Long-term surveillance of all subjects who undergo curative HSCT is imperative, to identify and treat kidney disease, whatever its causes.
As a percentage of eligible subjects, enrollment in this study was satisfactory. In the United States, for adult patients, a mere 2 to 7% of cancer patients are enrolled in National Cancer Institute sponsored trials (19,20), while our enrollment for adults was 30%. There was declining enrollment with time, however. This resulted from a decline in use of myeloablative HSCT at our center, and may have also resulted from competing priorities in the care of HSCT patients. The enrollment of children to this study was well below the expected 50% rate, which is more typical for cancer trials in children.
The non-study cohort provided a parallel and internal control group. The ongoing occurrence of BMT nephropathy or HUS in this group suggests that our BMT practice did not differ between the study cohort and the non-study cohort, and that there was no unaccounted variation in renal disease that caused the favorable trend for the captopril compared to the placebo group.
Less than half of study subjects completed one year on study drug. Attending physicians caring for these patients had a low threshold for stopping study drug. The reasons for stopping study drug, such as neutropenia or hypotension, could not be specifically linked to the study drug, because of numerous other possible causes for these events in HSCT patients. Long-term patient survival in the captopril cohort was actually better than that of the placebo cohort, confirming that use of captopril did not have an adverse effect on overall outcomes in these patients. This better survival may suggest additional benefit of captopril in mitigating normal tissue injury after HSCT.
The analysis by intent-to-treat included subjects in the captopril and placebo arms regardless of the length of time that they were taking the study drug. In laboratory animals, captopril used from three weeks to ten weeks after irradiation results in long-term mitigation and attenuation of radiation nephropathy (11,13). Use of captopril for less than six weeks has a lesser benefit. Thus, in a post-hoc analysis, the evolution of GFR was tested in subjects on the study drug for 60 days or more. This time period corresponded to the median time on study drug for the whole group. In these 60-day subjects in the captopril and placebo groups, the one-year median GFR was 92 ml/min and 80 ml/min, respectively. This difference was not statistically significant, but was in favor of captopril.
We conclude that captopril has a favorable trend towards protection against chronic renal failure in subjects undergoing radiation-based HSCT.
Figure 3.
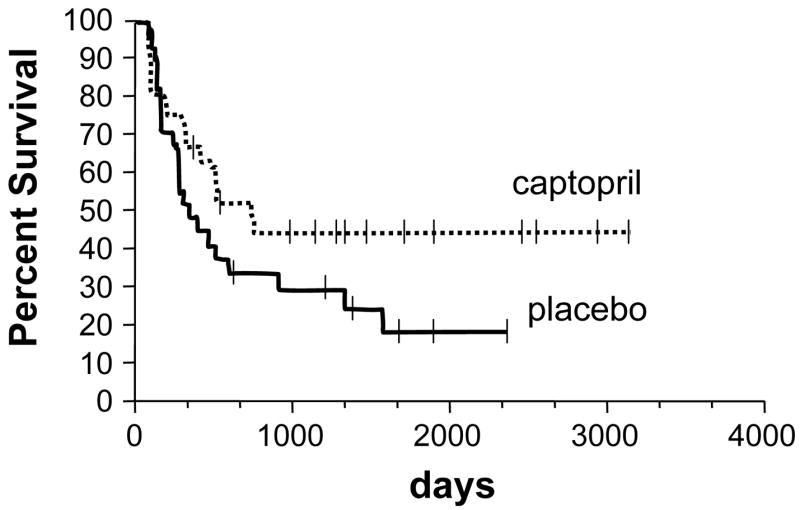
The actuarial patient survival according to use of captopril or placebo. There was better patient survival in the subjects of the captopril group, with a possible increasing survival advantage over time. This survival difference did however not attain statistical significance (p=0.09)
Acknowledgments
This study was supported by NIH CA24652 (JEM, EPC) and by a grant from the American Cancer Society, ROG 00-350-01 (EPC). The assistance of Kristin Hansen R.Ph., Deb Pastorek, RN, Senad Novic, and Vicki Johnson, RN, is gratefully acknowledged. These data were presented in abstract form at the Radiation Research Society meeting, Philadelphia, PA, 2006
Footnotes
Conflicts of interest
No conflicts of interest exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen EP. Renal failure after bone marrow transplantation. Lancet. 2001;357:6–7. doi: 10.1016/S0140-6736(00)03561-3. [DOI] [PubMed] [Google Scholar]
- 2.Zager RA. Acute renal failure in the setting of bone marrow transplantation. Kidney Int. 1994;46:1443–58. doi: 10.1038/ki.1994.417. [DOI] [PubMed] [Google Scholar]
- 3.Cohen EP, Piering WF, Kabler-Babbitt C, Moulder JE. End-stage renal disease after bone marrow transplantation: poor survival compared to other causes of ESRD. Nephron. 1998;79:408–412. doi: 10.1159/000045085. [DOI] [PubMed] [Google Scholar]
- 4.Weiss AS, Sandmeier BM, Storer B, et al. Chronic kidney disease following non-myeloablative hematopoietic cell transplantation. Am J Transplant. 2006;6:89–94. doi: 10.1111/j.1600-6143.2005.01131.x. [DOI] [PubMed] [Google Scholar]
- 5.Deconinck E, Kribs M, Rebibou JM, et al. Cytomegalovirus infection and chronic graft versus host disease are significant predictors of renal failure after allogeneic hematopoietic stem cell transplantation. Haematologia. 2005;90:569–570. [PubMed] [Google Scholar]
- 6.Cohen EP. Radiation nephropathy after bone marrow transplantation. Kidney Int. 2000;58:903–918. doi: 10.1046/j.1523-1755.2000.00241.x. [DOI] [PubMed] [Google Scholar]
- 7.Chappell ME, Keeling DM, Prentice HG, et al. Haemolytic uraemic syndrome after bone marrow transplantation: an adverse effect of total body irradiation? Bone Marrow Transplant. 1988;3:339–347. [PubMed] [Google Scholar]
- 8.Lawton CA, Cohen EP, Murray KJ, et al. Long-term results of selective renal shielding in patients undergoing total body irradiation in preparation for bone marrow transplantation. Bone Marrow Transplant. 1997;20:1069–10747. doi: 10.1038/sj.bmt.1701022. [DOI] [PubMed] [Google Scholar]
- 9.Moulder JE, Fish BL, Cohen EP. ACE inhibitors and AII receptor antagonists in the treatment and prevention of bone marrow transplantation. Current Pharm Design. 2003;9:737–749. doi: 10.2174/1381612033455422. [DOI] [PubMed] [Google Scholar]
- 10.Jaggi JS, Seshan SV, McDevitt MR, et al. Mitigation of radiation nephropathy after internal alpha particle irradiation of kidneys. Int J Radiation Oncology Biol Phys. 2006;64:1503–1513. doi: 10.1016/j.ijrobp.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 11.Moulder JE, Fish BL, Cohen EP. Noncontinuous use of angiotensin converting enzyme inhibitors in the treatment of experimental bone marrow transplant nephropathy. Bone Marrow Transplant. 1997;19:729–735. doi: 10.1038/sj.bmt.1700732. [DOI] [PubMed] [Google Scholar]
- 12.Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin converting enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 13.Cohen EP, Fish BL, Moulder JE. Successful brief captopril treatment in experimental radiation nephropathy. J Lab Clin Med. 1997;129:536–47. doi: 10.1016/s0022-2143(97)90008-1. [DOI] [PubMed] [Google Scholar]
- 14.Cohen EP, Lawton CA, Moulder JE, et al. Clinical course of late onset bone marrow transplant nephropathy. Nephron. 1993;64:626–635. doi: 10.1159/000187412. [DOI] [PubMed] [Google Scholar]
- 15.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function- measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 16.Klein JP. Modeling Competing Risks in Cancer Studies. Statistics in Medicine. 2006;25:1015–1034. doi: 10.1002/sim.2246. [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 18.Cohen EP, Drobyski W, Moulder JE. Significant increase in ESRD after hematopoietic cell transplantation. Bone Marrow Transplant. 2007;39:571–572. doi: 10.1038/sj.bmt.1705643. [DOI] [PubMed] [Google Scholar]
- 19.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Springer BC, Butler B, et al. Factors influencing enrollment in clinical trials for cancer treatment. South Med J. 1999;92:1189–1193. doi: 10.1097/00007611-199912000-00011. [DOI] [PubMed] [Google Scholar]