Abstract
Skeletal remodeling is a finely orchestrated process coupling bone formation to bone resorption. The dynamics of coupling is regulated by the microenvironment at the bone remodeling site, which in turn is influenced by the intercellular communication between cells like osteoclasts and osteoblasts. Understanding the dynamics of coupling is important in devising new therapeutic approaches to the treatment of skeletal diseases characterized by disturbances in the bone remodeling process. In this study, we report the localization of bone morphogenetic proteins (BMPs) in osteoclasts generated from primary cocultures of bone marrow cells from mouse femur and tibia with mouse calvarial osteoblasts, using immunocytochemistry and in situ hybridization. Positive staining was seen in osteoclasts for BMP-2, -4, -6, and -7. Real-time PCR was used to quantitatively confirm the expression of transcripts for BMP-2, BMP-4, and BMP-6 mRNA in murine osteoclasts. Finally, the presence of BMP-2, -4, -6, and-7 proteins was confirmed in osteoclast lysates by Western blotting. Overall, our data suggest a possible direct role for osteoclasts in promoting bone formation via expression and synthesis of BMPs, which then would play an important role in promoting the recruitment, proliferation, and differentiation of osteoblasts at bone resorption sites. (J Histochem Cytochem 56:569–577, 2008)
Keywords: bone morphogenetic proteins, skeletal remodeling, coupling, osteoclasts, osteoblasts
Skeletal remodeling depends upon the coupling of bone formation with bone resorption in a dynamically regulated process involving intercellular communication between osteoblasts and osteoclasts. Osteoblasts are bone-forming cells that originate from mesenchymal stem cells, whereas osteoclasts are multinucleate, bone-resorbing cells that originate from hematopoietic precursors of the monocyte-macrophage lineage. The remodeling process (Parfitt 1982,2004) consists of (a) activation: preosteoclasts are stimulated to differentiate into osteoclasts under the influence of cytokines and other growth factors from cells of osteoblastic lineage; (b) osteoclastic resorption: osteoclasts resorb mineralized matrix at the site of a multicellular remodeling unit; (c) reversal: end of resorption; and (d) bone formation: preosteoblast migration to the resorption site (Schwartz et al. 2000) and differentiation into mature osteoblasts, followed by osteoid formation and mineralization. The equilibrium between bone formation and bone resorption at sites of bone remodeling is controlled by paracrine and autocrine growth factors including bone morphogenetic proteins (BMPs) (Wozney and Rosen 1998), transforming growth factor-β (TGF- β) (Mundy and Bonewald 1990), insulin-like growth factor (IGF-1) (Baylink et al. 1993), basic fibroblast growth factor (bFGF) (Iwaniec et al. 2003), platelet-derived growth factor (PDGF) (Mitlak et al. 1996), receptor activator of NF-κ B ligand (RANK-L) (Takahashi et al. 1999) and osteoprotegrin (OPG) (Hadjidakis and Androulakis 2006). Understanding the paracrine cross talk between osteoclasts and osteoblasts is critical in devising novel therapeutic approaches for diseases of unbalanced bone resorption (e.g., osteoporosis), or bone formation (e.g., Paget’s disease).
It is now well established that osteoclast differentiation is regulated by many systemic hormones and local cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), and parathyroid hormone (PTH) via stimulation of osteoblast-derived soluble and membrane-bound receptor activator of RANK-L (Yasuda et al. 1998), and macrophage colony-stimulating factor (M-CSF). Recently, recombinant BMPs have also been shown to support osteoclastic differentiation in cultures (Kaneko et al. 2000; Okamoto et al. 2006; Wutzl et al. 2006). The coupling of bone resorption to bone formation has been reported by many researchers, including Parfitt (1982,2004), Martin (Martin and Rodan 2001; Martin and Sims 2005), and Mundy (Mundy 1993; Mundy and Elefteriou 2006). Howard et al. (1981) were the first to propose the term “coupling factor” to link bone resorption by osteoclasts to subsequent bone formation by osteoblasts. According to the coupling concept, the coupling factor is derived from one or many growth factors such as IGF-I and -II and TGF-β that are released by osteoclastic proteolytic digestion of bone matrix during bone resorption, and are thus made available for stimulating osteoblast precursors to form osteoblasts and new bone (Centrella et al. 1991; Mohan and Baylink 1991; Martin and Rodan 2001). Only recently, a more direct anabolic role of osteoclasts in stimulating bone formation has been indicated, in which osteoclasts secrete anabolic growth factors that mediate osteoblast chemotaxis, proliferation, differentiation, and mineralization (Koh et al. 2005; Martin and Sims 2005; Henriksen et al. 2006; Karsdal et al. 2007). Some of the osteoclast-secreted factors that may enhance osteoblast activity include TGF-β (Robinson et al. 1996), IGF-1 (Hayden et al. 1995), TRAP (tartrate-resistant acid phosphatase) (Sheu et al. 2002,2003), and BMPs (Anderson et al. 2000; Dhanyamraju et al. 2003; Garimella et al. 2006; McCullough et al. 2007). BMPs are members of the TGF-β superfamily that have highly conserved seven-cysteine repeats in their carboxy terminus (Wozney 1992). BMPs have well-established roles in pattern formation, organogenesis, and skeletal morphogenesis during vertebrate development. At the cellular level, BMPs regulate cell proliferation, differentiation, and apoptosis in embryonic and postnatal chondrocytes, osteoblasts, and osteoclasts (Zhao 2003). BMP dimers bind to one of the two types of serine and threonine kinase membrane receptors, and ligand receptor binding initiates an intracellular signaling cascade mediated by Smad proteins, ultimately leading to regulation of target genes. BMPs are thought to be key regulators of embryonic skeletogenesis (Hogan 1996), endochondral ossification (Grimsrud et al. 1999), bone remodeling (Wozney and Rosen 1998; Groeneveld and Burger 2000), fracture repair (Reddi 1995; Onishi et al. 1998), and bone regeneration (Ripamonti and Duneas 1998).
In this study, we report the localization and expression of BMPs in osteoclasts generated from primary cocultures of bone marrow cells with mouse calvarial osteoblasts, using immunocytochemistry, in situ hybridization, quantitative real-time PCR, and Western blotting. Our working hypothesis is that BMPs expressed by osteoclasts recruit osteoblasts to a bone resorption site, thus initiating the anabolic phase of bone remodeling.
Materials and Methods
Osteoclastogenesis in Cocultured Mouse Calvarial Osteoblasts and Bone Marrow (BM)
Primary osteoblast cultures were prepared from 1- or 2-day-old CD1 mouse calvaria (Ecarot-Charrier et al. 1983) according to the guidelines of the Institutional Animal Care and Use Committee, University of Kansas Medical Center. Briefly, small pieces of calvaria were incubated in 60-mm tissue culture dishes containing BGJb media supplemented with 10% FBS for 2 days at 37C with 5% CO2. During this period, the mature osteoblasts migrate off the calvaria, as determined by their ability to exhibit alkaline phosphatase enzyme activity and produce mineralizing nodules (data not shown). The mature osteoblasts were harvested by trypsinization and cultured in tissue culture slide wells (1 × 105 osteoblasts/8.6-cm2 surface area) in phenol red–free α-MEM mineralizing medium containing 10% FBS, 2.5 mM β-glycerophosphate, and 10−8 M 1α,25-(OH)2 vitamin D3 for 2 days prior to coculture with bone marrow cells. Multiple-passaged osteoblasts up to the fifth passage supported the formation of multinucleate, TRAP-positive osteoclasts from bone marrow. Bone marrow was obtained by aspiration from femurs and tibiae of 8-week-old CD1 mice. Adherent nucleated marrow cells (0.5 × 106/8.6-cm2 surface area) were cocultured with confluent osteoblast cultures in phenol red–free α-MEM medium containing 10% FBS and supplemented with 10−8 M 1α,25-(OH)2 vitamin D3 and 10−6 M prostaglandin E2 at 37C for 15 days in a humidified atmosphere of 5% CO2. The medium was changed every other day. After 15 days, the cocultures were prepared for TRAP staining, BMP-immunocytochemistry, and in situ hybridization.
Separation of Osteoclasts from Osteoblast-BM Cocultures
Upon the confirmation of osteoclast generation by TRAP staining, osteoclasts were separated from osteoblast-BM cocultures by gently exposing them to 0.025% EDTA for 10 min at 37C. During this time, osteoblasts get separated and detach themselves, while osteoclasts still remain adherent to the plastic surface of the tissue culture dish.
TRAP Staining
The cocultures were stained for TRAP, using a Sigma TRAP kit (no. 386-A; Sigma Chemicals, St. Louis, MO).
In Situ Hybridization
Digoxygenin-labeled cRNA probes were prepared by using an RNA labeling kit (Roche Molecular; Indianapolis, IN). Anti-sense mouse BMP-2 and BMP-4 probes were prepared by cleaving a partial mouse BMP-2 cDNA (1-kb fragment) with Eco RI and by cleaving the mouse BMP-4 cDNA (1.8-kb fragment) with Hind III. The anti-sense mouse BMP-6 probe was prepared by cleaving the mouse BMP-6 cDNA with Eco RI and Sac I (947 bp). In situ hybridization in osteoblast bone marrow cocultures was performed in RNase-free conditions. Before hybridization, the culture slides containing osteoblast bone marrow cocultures were fixed with 4% paraformaldehyde for 1 hr at 4C and later washed with PBS. The culture slides were then incubated with 2% glycine, and digested with proteinase-K to facilitate penetration of the probe. The cells were then acetylated by incubating the slides in 2.5% acetic anhydride/triethanolamine solution for 10 min. In situ hybridization was performed according to the procedure previously described (Feng et al. 2002; Ye et al. 2004). Digoxigenin-labeled BMP mRNAs were detected in an enzyme-linked immunoassay using anti-digoxigenin-alkaline phosphatase conjugate and in the presence of a chromogenic detection system: 4-nitroblue tetrazolium/5 bromo, 4-chloro-3 indolyl phosphate (Gibco-BRL; Carlsbad, CA) (Ye et al. 2004).
Real-time RT-PCR Analyses
Real-time PCR was performed in a two-step discontinuous procedure: (a) a reverse-transcription step leading to the synthesis of cDNA from total RNA (including the synthesis of BMP-2, -4, -6, and -7 cDNA); and (b) quantitative real-time PCR leading to the relative quantitation of BMP mRNA expression levels in osteoclasts and osteoblasts.
Total RNA was extracted from adherent osteoclasts that were separated from osteoblasts as described above, using RNeasy Mini Kits (Qiagen; Santa Clara, CA). Total RNA was also extracted from mouse calvarial primary osteoblasts, and served as positive control. Reverse transcription reactions were performed in a 20-μl reaction volume containing 10× PCR buffer, MgCl2, dNTPs, murine leukemia virus reverse transcriptase, oligo dT, total RNA (1 μg) using the reaction conditions specified for the reverse transcription kit (Promega; Madison, WI). The cDNA was stored at −20C until further use.
Real-time quantitative PCR was performed in a 20-μl reaction volume using the standard protocols of Applied Biosystems 7500 Sequence Detection System and software (Applied Biosystems; Foster City, CA) using 1:10 diluted cDNA, Universal PCR Master Mix kit, SYBR green, and 300–900 nM of each primer. Primer pairs of BMP-2, BMP-4, BMP-6, and BMP-7 were obtained using a search in Prime Bank (BMP-2 ID#, 6680794a1; BMP-4 ID#, 6680796a2; BMP-6 ID#, 6680798a2; BMP-7 ID#, 31982487a2) (Wang and Seed 2003) and for GAPDH as endogenous control (forward: 5′-AAC GAC CCC TTC ATT GAC-3′, reverse: 5′-TCC ACG ACA TAC TCA GCA C-3′). Primers were identified using the BLAST program (www.ncbi.nlm.nih.gov). Relative quantitation of target mRNA expression, normalized to an endogenous control and relative to a calibrator, was calculated using the mathematical expression for fold change, i.e., 2−ΔΔCt (fold), as described by Livak and Schmittgen (2001), where ΔCt = Ct of the calibrator for the target gene − Ct of the endogenous control gene (GAPDH), and ΔΔ Ct = ΔCt of the samples for target gene − ΔCt of the calibrator for the target gene.
Immunocytochemistry
The cocultures adherent on the slide wells were fixed in 4% paraformaldehyde and preincubated in 0.3% Triton-X in PBS at room temperature for 15 min. After Triton-X pretreatment, the cells were washed several times in deionized Millipore-filtered H2O and briefly incubated with 3% H2O2 for 10 min at room temperature to block endogenous peroxidase activity. The adherent cells on the slides were gently washed with deionized water and PBS before blocking with 1.5% blocking serum for 1 hr followed by overnight incubation in primary antibody solution at 4C. Primary antibodies (polyclonal goat anti-human BMP-2, -4, -6, and -7) were obtained from Santa Cruz (Santa Cruz, CA), diluted in PBS (BMP-2, -4, and -6 = 1:50; BMP-7 = 1:100), and BMP 2-7 expression was detected using peroxidase-conjugated secondary antibody and a peroxidase substrate (3,5-diaminobenzidine). A non-immune goat serum in place of the primary antibody (at the same concentration as the primary antibodies used for the respective BMPs) was used as a negative control.
Western Blotting
Murine osteoclast cell lysate was obtained from osteoclasts generated in bone marrow coculture with osteoblasts. Lysates from isolated osteoclasts, containing 50–100 μg protein, were loaded in each lane and separated by SDS/PAGE on 10–20% gradient gels. The gels were transferred onto a polyvinylidene difluoride membrane and immunoblotted using a 1:200 dilution of anti-BMP antibody (Santa Cruz). Recombinant BMP-2, -4, -6, and -7 were used as standards to confirm the specificity of bands corresponding to BMPs. The immunostained bands were visualized using an enhanced chemiluminescence detection system (Amersham Biosciences; Piscataway, NJ).
Statistical Analyses
Quantitative data are presented as mean +/−SEM. Statistical differences in BMP mRNA expression between osteoblast control and osteoclast were determined by paired t-test. p<0.05 was considered to be significant.
Results
TRAP Staining
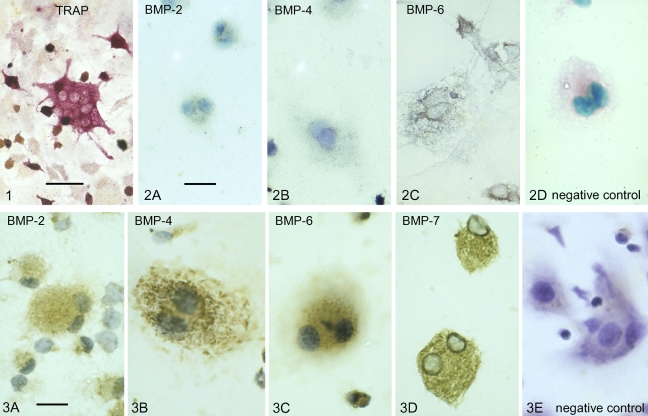
Osteoclasts generated from marrow cocultures with osteoblasts (as described in Materials and Methods), were recognized by their multinuclearity and positive TRAP staining (Figure 1).
Figure 1.
Photomicrograph showing the red staining associated with the presence of tartrate-resistant acid phosphatase (TRAP) in the cytoplasm of a multinucleated, culture-generated mouse osteoclast. Bar 5 20 μm.
In Situ Hybridization
To detect the presence of BMP-2, -4, and -6 transcripts in the osteoclasts, a non-radioactive in situ hybridization was utilized. Hybridization with an anti-sense probe to BMP-2 and BMP-4 showed a positive signal, indicating the presence of BMP-2 and -4 mRNA, whereas the BMP-6 anti-sense probe showed a more intense expression of BMP-6 mRNA in cultured osteoclasts (Figures 2A–2C).
Figure 2.
In situ hybridization showing the presence of bone morphogenetic protein (BMP) transcripts in cultured mouse osteoclasts. The bluish-gray stain indicates the presence of BMP-2 (A); BMP-4 (B); and BMP-6 (C). (D) Negative control (as indicated by red-staining cytoplasm and no bluish-gray/purple staining) using a non-complementary sense RNA probe. Bar = 4 μm.
Immunocytochemistry
Osteoclasts generated from marrow cocultures with osteoblasts showed positive immunostaining for BMP-2, -4, -6, and -7. Moderate to intense immunostaining for BMP-2, -4, -6, and -7 was detected within the cytoplasm of culture-generated osteoclasts (Figures 3A–3D). No immune reaction was detected in the presence of a non-immune serum-negative control (Figure 3E). The more conspicuous blue-violet color in the negative control is due to lack of peroxidase staining, whereas the brown peroxidase staining is indicative of a positive immune reaction, as shown in Figure 3.
Figure 3.
Immunocytochemistry of BMP proteins within cultured mouse osteoclasts. Brown peroxidase staining in the cytoplasm of multinucleated osteoclasts indicates the presence of BMP-2 (A), BMP-4 (B), BMP-6 (C), and BMP-7 (D). (E) Negative control (as indicated by no brown peroxidase staining. The blue-violet color is due to hematoxylin, the counterstain used in all preparations) using non-immune serum in place of primary antibody. Bar = 4 μm.
Real-time RT PCR
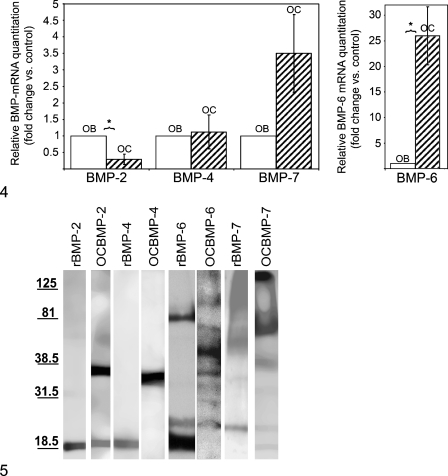
Real-time RT PCR analysis revealed that osteoclasts are able to synthesize mRNAs for BMP-2, -4, -6, and -7 (Figure 4). Expression of BMP-6 mRNA was the greatest of all the BMPs tested, at 18 times higher than expression in the osteoblast positive control (Figure 4).
Figure 4.
Real-time quantitative PCR analyses showing the expression of BMP-2, -4, -7 (left panel) and -6 transcripts (right panel) in osteoclasts (OC) versus in osteoblasts (OB). Results are reported as fold change or relative quantitation of target mRNA expression (2-ΔΔCt method), normalized to an endogenous control (GAPDH) and relative to a calibrator (osteoblast RNA sample). Error bars represent the standard error of the mean associated with the ΔΔCt value. *Statistically significant (p<0.05) difference in the expression of BMP -2, -4, -6, and -7 transcripts between osteoclast and osteoblast.
Western Blot Analyses
Western blots (Figure 5) confirmed the presence of BMPs at expected mature monomeric 18- to 21-kDa molecular mass (as seen in BMP-2, BMP-6, and BMP-7 expression in osteoclasts). Our finding of higher molecular forms of BMPs in osteoclasts might reflect the presence of proteolytically uncleaved precursor forms of BMPs or bands generated from posttranslational modifications such as glycosylation (partial or complete), as seen in other studies reporting BMP protein expression (Shoda et al. 1993; Kochanowska et al. 2002; Wordinger et al. 2002; Lories et al. 2003; Schwalbe et al. 2003). Another reason might be insufficient reduction by dithiotheritol of BMP multimers, at a concentration of 1 mM, which is routinely used in denaturing SDS gels. Two immunoreactive bands, one at 18 kDa (the monomeric form) and the other at 36 kDa (the dimeric form) were detected for BMP-2. A single immunoreactive band at 36 kDa was detected for BMP-4, probably representing a dimeric conformation of the mature peptide (Suzuki et al. 1993). For BMP-6, multiple bands were detected at 21 kDa (also detected in recombinant BMP-6), 23 kDa, 36 kDa, ∼45 kDa, 69 kDa, and 90 kDa in cultured osteoclasts, as previously reported in other cells (Boden et al. 1997; Rickard et al. 1998; Akiyoshi et al. 2004). For BMP-7, multiple bands were detected at 18 kDa (monomer), 23 kDa (glycosylated form), 40 kDa (pro-domain), 70 kDa, and an additional high-molecular-mass band (>125 kDa), as seen in other studies (Jones et al. 1994; Vukicevic et al. 1994; Chubinskaya et al. 2000).
Figure 5.
Western blot analyses for BMP-2, -4, -6, and -7 within lysates of cultured mouse osteoclasts (OC). BMP-2, BMP-4, BMP-6, and BMP-7 were detected within a 50–100-μg protein sample of osteoclast lysate. Recombinant BMP-2, -4, -6, and -7 were used as standards to assess the specificity of bands corresponding to BMPs.
Discussion
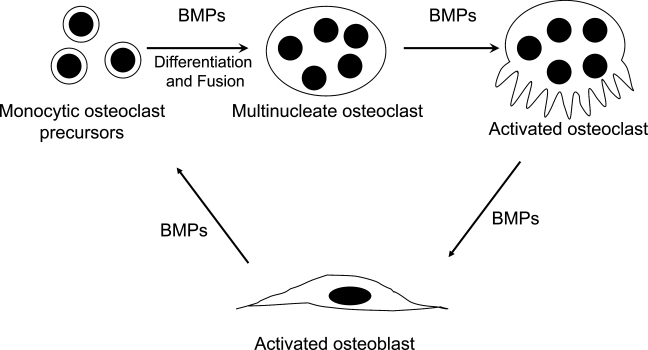
Recently, evidence has begun to emerge for a direct anabolic role of osteoclasts (via non-matrix–derived bone-inducing factors) in stimulating bone formation (Henriksen et al. 2006; Karsdal et al. 2007). The mechanism of osteoclast-mediated osteoblastic bone formation is not well understood. Bone anabolic factors that have so far been reported in osteoclasts include TGF-β (Robinson et al. 1996), IGF-1 (Hayden et al. 1995), IL-6 (Sims et al. 2004), and BMPs (Anderson et al. 2000; Dhanyamraju et al. 2003; Garimella et al. 2006; McCullough et al. 2007). Of these, only BMPs are known to be capable of inducing new bone formation (Wozney and Rosen 1998). Previously, there have been reports of immunohistochemical localization of BMP-2, -4, and -6 in the osteoclasts of metaphyseal bone (Anderson et al. 2000) and BMP-3 and -6 in osteoclasts during osteophyte formation (Zoricic et al. 2003). BMP receptors IA and II have been reported to be present in mature osteoclasts (Kaneko et al. 2000). However, no comprehensive study of BMP expression and localization in murine osteoclasts has been done to date. Our present studies confirm expression and localization of BMPs in osteoclasts using in situ hybridization, real-time PCR, immunocytochemistry, and Western blotting; and thus add support to the hypothesis that osteoclast BMPs are involved in initiation of the anabolic phase of bone remodeling, which involves active recruitment of osteoblasts to a bone resorption site (Figure 6).
Figure 6.
Schematic diagram of proposed osteoblast–osteoclast cross talk as mediated via BMPs.
Our data also suggest that there is a preferential expression of BMP-6 mRNA by osteoclasts versus the expression of BMP-2, -4, and -7 (Figure 4). This increased expression of BMP-6 by osteoclasts might play a dual role in not only stimulating osteoblastic precursors to undergo osteoblastic differentiation (Friedman et al. 2006) but also enhancing osteoclastogenesis from bone marrow cells (Wutzl et al. 2006). Previous studies have reported that BMP-6 is the most effective inducer of osteogenic differentiation from hematopoietic mesenchymal stem cells (Ahmed et al. 2001; Friedman et al. 2006). Sammons et al. (2004) showed that BMP-6 increased osteogenic differentiation and mineralization, induced by PTH and vitamin D3 in mesenchymal stem cell cultures. Solloway et al. (1998) reported that BMP-6 knockout mice are viable and show a pronounced delay in the ossification of the sternum but not other bones. Simic et al. (2005) reported that BMP-6−/− mice have decreased bone volume/trabecular volume as compared with wild-type mice. Those authors also demonstrated that systemic administration of human recombinant BMP-6 in osteoporotic rats and mice induced new bone formation, restored bone microarchitecture, and enhanced overall quality of the skeleton (Simic et al. 2006). Thus, the relatively higher BMP-6 expression by osteoclasts might be important in regulating bone homeostasis.
Our working hypothesis that osteoclastic BMPs might contribute to the anabolic path of bone remodeling fits very well with previous reports on osteoclastic stimulation of osteoblasts. Marrow monocytes, which are the precursors of osteoclasts, are capable of stimulating osteoblastic differentiation by marrow stromal osteoprogenitor cells in coculture (Aubin 1999). Another example suggesting paracrine stimulation of osteoblasts by osteoclasts is that of juvenile Paget’s disease, in which increased osteoclastic activity due to OPG deficiency is followed by an increase in osteoblastic activity, with the rapid formation of woven bone, and osteosclerosis (Whyte et al. 2002). In another study, Dai et al. (2004) investigated the regulation of osteoblastic bone formation by osteoclasts, using Csf1r−/− mice. They reported that an osteoclast deficiency in these mice resulted in disorganized matrix, reduced mineralization, and abnormal osteoblast function, leading to impaired lamellar bone formation in developing bone.
Sims et al. (2004) reported that genetically engineered mice with inactivated gp-130-SHP2/ras/MAPK pathway displayed increased osteoclast number and activity, with a concomitant increase in osteoblastic bone formation. Furthermore, those authors suggest that the increase in bone formation coupled to bone resorption is mediated by IL-6 derived from resorbing osteoclasts (Sims et al. 2004). Nakamura et al. (2003) reported that the increased bone formation seen in OPG−/− mice is probably due to stimulation by increased osteoclast numbers. Those mice also displayed high serum alkaline phosphatase activity and osteocalcin concentration, both of which were reduced to wild-type levels upon risedronate injection. Udagawa et al. (2002) suggested that osteoblast stimulation is osteoclast derived rather than emanating from the bone matrix, based on their in vitro studies demonstrating differentiation of mesenchymal cells into ALP-expressing cells in the presence of conditioned medium from osteoclast cultures. Recently, Henriksen et al. (2006) reported that conditioned medium derived from human osteoclast cultures grown on plastic or bone dose-dependently induced bone formation by osteoblasts. Further identification and characterization of osteoclast-derived anabolic factors will be very important in understanding the mechanism of coupling in the bone remodeling cycle, and thus will be valuable in devising new therapeutic modalities for treating bone remodeling disorders.
In summary, our findings confirm that BMPs are expressed and translated in osteoclasts. We suggest that BMPs from osteoclasts might possibly be involved in the initiation of the anabolic phase of bone remodeling. Future research studies will focus on exploring the exact role of different osteoclastic BMPs, particularly BMP-6, in stimulating the differentiation of preosteoblasts and steering them to form a calcificable bone matrix.
Acknowledgments
This research was supported by United States Public Health Service, National Institutes of Health Grant DEO-5262, and University of Kansas Medical Center Bridging Funds.
References
- Ahmed N, Sammons J, Carson RJ, Khokher MA, Hassan HT (2001) Effect of bone morphogenetic protein-6 on haemopoietic stem cells and cytokine production in normal human bone marrow stroma. Cell Biol Int 25:429–435 [DOI] [PubMed] [Google Scholar]
- Akiyoshi T, Uchida K, Tateyama S (2004) Expression of bone morphogenetic protein-6 and bone morphogenetic protein receptors in myoepithelial cells of canine mammary gland tumors. Vet Pathol 41:154–163 [DOI] [PubMed] [Google Scholar]
- Anderson HC, Hodges PT, Aguilera XM, Missana L, Moylan PE (2000) Bone morphogenetic protein (BMP) localization in developing human and rat growth plate, metaphysis, epiphysis, and articular cartilage. J Histochem Cytochem 48:1493–1502 [DOI] [PubMed] [Google Scholar]
- Aubin JE (1999) Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell-cell interactions in osteoblast differentiation. J Cell Biochem 72:396–410 [PubMed] [Google Scholar]
- Baylink DJ, Finkelman RD, Mohan S (1993) Growth factors to stimulate bone formation. J Bone Miner Res 8(suppl 2):565–572 [DOI] [PubMed] [Google Scholar]
- Boden SD, Hair G, Titus L, Racine M, McCuaig K, Wozney JM, Nanes MS (1997) Glucocorticoid-induced differentiation of fetal rat calvarial osteoblasts is mediated by bone morphogenetic protein-6. Endocrinology 138:2820–2828 [DOI] [PubMed] [Google Scholar]
- Centrella M, McCarthy TL, Canalis E (1991) Transforming growth factor-β and remodeling of bone. J Bone Joint Surg Am 73:1418–1428 [PubMed] [Google Scholar]
- Chubinskaya S, Merrihew C, Cs-Szabo G, Mollenhauer J, McCartney J, Rueger DC, Kuettner KE (2000) Human articular chondrocytes express osteogenic protein-1. J Histochem Cytochem 48:239–250 [DOI] [PubMed] [Google Scholar]
- Dai XM, Zong XH, Akhter MP, Stanley ER (2004) Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone. J Bone Miner Res 19:1441–1451 [DOI] [PubMed] [Google Scholar]
- Dhanyamraju R, Sipe J, Zhang J, Xu Y, Osdoby P, Insogna K, Anderson HC (2003) Localization of bone morphogenetic proteins (BMPs) in osteoclasts. Transactions of 49th Annual Meeting of the Orthopaedic Research Society, New Orleans, LA
- Ecarot-Charrier B, Glorieux FH, van der Rest M, Pereira G (1983) Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J Cell Biol 96:639–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JQ, Zhang J, Dallas SL, Lu Y, Chen S, Tan X, Owen M, et al. (2002) Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J Bone Miner Res 17:1822–1831 [DOI] [PubMed] [Google Scholar]
- Friedman MS, Long MW, Hankenson KD (2006) Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J Cell Biochem 98:538–554 [DOI] [PubMed] [Google Scholar]
- Garimella R, Tague SE, Zhang J, Anderson HC (2006) Expression and synthesis of bone morphogenetic proteins (BMPs) in osteoclast cultures. Transactions of the 52nd Annual Meeting of the Orthopedic Research Society, Chicago, IL (Paper No: 1656)
- Grimsrud CD, Romano PR, D’Souza M, Puzas JE, Reynolds PR, Rosier RN, O’Keefe RJ (1999) BMP-6 is an autocrine stimulator of chondrocyte differentiation. J Bone Miner Res 14:475–482 [DOI] [PubMed] [Google Scholar]
- Groeneveld EH, Burger EH (2000) Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol 142:9–21 [DOI] [PubMed] [Google Scholar]
- Hadjidakis DJ, Androulakis II (2006) Bone remodeling. Ann N Y Acad Sci 1092:385–396 [DOI] [PubMed] [Google Scholar]
- Hayden JM, Mohan S, Baylink DJ (1995) The insulin-like growth factor system and the coupling of formation to resorption. Bone 17:93S–98S [DOI] [PubMed] [Google Scholar]
- Henriksen K, Pedersen AV, Christiansen C, Martin T, Karsdal M (2006) Osteoclasts secrete non-bone derived signals that induce bone nodule formation. J Bone Miner Res 21(suppl 1):M198 [Google Scholar]
- Hogan BL (1996) Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 10:1580–1594 [DOI] [PubMed] [Google Scholar]
- Howard GA, Bottemiller BL, Turner RT, Rader JI, Baylink DJ (1981) Parathyroid hormone stimulates bone formation and resorption in organ culture: evidence for a coupling mechanism. Proc Natl Acad Sci USA 78:3204–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniec UT, Magee KA, Mitova-Caneva NG, Wronski TJ (2003) Bone anabolic effects of subcutaneous treatment with basic fibroblast growth factor alone and in combination with estrogen in osteopenic ovariectomized rats. Bone 33:380–386 [DOI] [PubMed] [Google Scholar]
- Jones WK, Richmond EA, White K, Sasak H, Kusmik W, Smart J, Oppermann H, et al. (1994) Osteogenic protein-1 (OP-1) expression and processing in Chinese hamster ovary cells: isolation of a soluble complex containing the mature and pro-domains of OP-1. Growth Factors 11:215–225 [DOI] [PubMed] [Google Scholar]
- Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, Toyama Y, et al. (2000) Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 27:479–486 [DOI] [PubMed] [Google Scholar]
- Karsdal M, Martin T, Bollerslev J, Christiansen C, Henriksen K (2007) Are Non-Resorbing Osteoclasts Sources of Bone Anabolic Activity? J Bone Miner Res 22:487–494 [DOI] [PubMed] [Google Scholar]
- Kochanowska IE, Wlodarski K, Wojtowicz A, Kinsner A, Ostrowski K (2002) BMP-4 and BMP-6 involvement in the osteogenic properties of the HeLa cell line. Exp Biol Med (Maywood) 227:57–62 [DOI] [PubMed] [Google Scholar]
- Koh AJ, Demiralp B, Neiva KG, Hooten J, Nohutcu RM, Shim H, Datta NS, et al. (2005) Cells of the osteoclast lineage as mediators of the anabolic actions of parathyroid hormone in bone. Endocrinology 146:4584–4596 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔ C(T)). Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Lories RJ, Derese I, Ceuppens JL, Luyten FP (2003) Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast-like synoviocyte apoptosis. Arthritis Rheum 48:2807–2818 [DOI] [PubMed] [Google Scholar]
- Martin TJ, Rodan GA (2001) Coupling of bone resorption and formation during bone remodeling. In Marcus R, ed. Osteoporosis. San Diego, Academic Press, 361–372
- Martin TJ, Sims NA (2005) Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med 11:76–81 [DOI] [PubMed] [Google Scholar]
- McCullough KA, Waits CA, Garimella R, Tague SE, Sipe JB, Anderson HC (2007) Immunohistochemical localization of bone morphogenetic proteins (BMPs) 2, 4, 6, and 7 during induced heterotopic bone formation. J Orthop Res 25:465–472 [DOI] [PubMed] [Google Scholar]
- Mitlak BH, Finkelman RD, Hill EL, Li J, Martin B, Smith T, D’Andrea M, et al. (1996) The effect of systemically administered PDGF-BB on the rodent skeleton. J Bone Miner Res 11:238–247 [DOI] [PubMed] [Google Scholar]
- Mohan S, Baylink DJ (1991) The role of IGF-II in the coupling of bone formation to resorption. In Spencer EM, ed. Modern Concepts of Insulin-like Growth Factors. New York, Elsevier, 19–174
- Mundy GR (1993) Hormonal factors which regulate bone resorption. In Mundy GR, Martin TJ, eds. Handbook of Experimental Pharmacology: Physiology and Pharmacology of Bone. Heidelberg, Springer-Verlag, 215–238
- Mundy GR, Bonewald LF (1990) Role of TGF β in bone remodeling. Ann N Y Acad Sci 593:91–97 [DOI] [PubMed] [Google Scholar]
- Mundy GR, Elefteriou F (2006) Boning up on ephrin signaling. Cell 126:441–443 [DOI] [PubMed] [Google Scholar]
- Nakamura M, Udagawa N, Matsuura S, Mogi M, Nakamura H, Horiuchi H, Saito N, et al. (2003) Osteoprotegerin regulates bone formation through a coupling mechanism with bone resorption. Endocrinology 144:5441–5449 [DOI] [PubMed] [Google Scholar]
- Okamoto M, Murai J, Yoshikawa H, Tsumaki N (2006) Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res 21:1022–1033 [DOI] [PubMed] [Google Scholar]
- Onishi T, Ishidou Y, Nagamine T, Yone K, Imamura T, Kato M, Sampath TK, et al. (1998) Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in rats. Bone 22:605–612 [DOI] [PubMed] [Google Scholar]
- Parfitt AM (1982) The coupling of bone formation to bone resorption: a critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res 4:1–6 [DOI] [PubMed] [Google Scholar]
- Parfitt AM (2004) What is the normal rate of bone remodeling? Bone 35:1–3 [DOI] [PubMed] [Google Scholar]
- Reddi H (1995) Bone morphogenetic proteins. Adv Dent Res 9(suppl 3):13. [DOI] [PubMed] [Google Scholar]
- Rickard DJ, Hofbauer LC, Bonde SK, Gori F, Spelsberg TC, Riggs BL (1998) Bone morphogenetic protein-6 production in human osteoblastic cell lines. Selective regulation by estrogen. J Clin Invest 101:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripamonti U, Duneas N (1998) Tissue morphogenesis and regeneration by bone morphogenetic proteins. Plast Reconstr Surg 101:227–239 [DOI] [PubMed] [Google Scholar]
- Robinson JA, Riggs BL, Spelsberg TC, Oursler MJ (1996) Osteoclasts and transforming growth factor-β: estrogen-mediated isoform-specific regulation of production. Endocrinology 137:615–621 [DOI] [PubMed] [Google Scholar]
- Sammons J, Ahmed N, El-Sheemy M, Hassan HT (2004) The role of BMP-6, IL-6, and BMP-4 in mesenchymal stem cell-dependent bone development: effects on osteoblastic differentiation induced by parathyroid hormone and vitamin D(3). Stem Cells Dev 13:273–280 [DOI] [PubMed] [Google Scholar]
- Schwalbe M, Sanger J, Eggers R, Naumann A, Schmidt A, Hoffken K, Clement JH (2003) Differential expression and regulation of bone morphogenetic protein 7 in breast cancer. Int J Oncol 23:89–95 [PubMed] [Google Scholar]
- Schwartz Z, Lohmann CH, Wieland M, Cochran DL, Dean DD, Textor M, Bonewald LF, et al. (2000) Osteoblast proliferation and differentiation on dentin slices are modulated by pretreatment of the surface with tetracycline or osteoclasts. J Periodontol 71:586–597 [DOI] [PubMed] [Google Scholar]
- Sheu TJ, Schwarz EM, Martinez DA, O’Keefe RJ, Rosier RN, Zuscik MJ, Puzas JE (2003) A phage display technique identifies a novel regulator of cell differentiation. J Biol Chem 278:438–443 [DOI] [PubMed] [Google Scholar]
- Sheu TJ, Schwarz EM, O’Keefe RJ, Rosier RN, Puzas JE (2002) Use of a phage display technique to identify potential osteoblast binding sites within osteoclast lacunae. J Bone Miner Res 17:915–922 [DOI] [PubMed] [Google Scholar]
- Shoda A, Murakami K, Ueno N (1993) Presence of high molecular weight forms of BMP-2 in early Xenopus embryos. Growth Factors 8:165–172 [DOI] [PubMed] [Google Scholar]
- Simic P, Culej JB, Orlic I, Grgurevic L, Borovecki F, Vukicevic S (2005) BMP-6 restores bone in osteoporotic aged rats and, unlike estradiol and PTH, restores trabecular bone in ovariectomized BMP-6 knockout mice. J Bone Miner Res 20(suppl 1):8 [Google Scholar]
- Simic P, Culej JB, Orlic I, Grgurevic L, Draca N, Spaventi R, Vukicevic S (2006) Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J Biol Chem 281:25509–25521 [DOI] [PubMed] [Google Scholar]
- Sims NA, Jenkins BJ, Quinn JM, Nakamura A, Glatt M, Gillespie MT, Ernst M, et al. (2004) Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. J Clin Invest 113:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ (1998) Mice lacking Bmp6 function. Dev Genet 22:321–339 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Nishimatsu S, Shoda A, Takebayashi K, Murakami K, Ueno N (1993) Biochemical properties of amphibian bone morphogenetic protein-4 expressed in CHO cells. Biochem J 291(Pt II):413–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Udagawa N, Suda T (1999) Role of osteoclast differentiation factor, the new member of the TNF ligand family, in osteoclast differentiation and function Seikagaku 71:241–253 [PubMed]
- Udagawa N, Itoh K, Li X, Ozawa H, Takahashi N (2002) Expression of osteoblast differentiation factor in mature osteoclasts. J Bone Miner Res 17:S344 [Google Scholar]
- Vukicevic S, Latin V, Chen P, Batorsky R, Reddi AH, Sampath TK (1994) Localization of osteogenic protein-1 (bone morphogenetic protein-7) during human embryonic development: high affinity binding to basement membranes. Biochem Biophys Res Commun 198:693–700 [DOI] [PubMed] [Google Scholar]
- Wang X, Seed B (2003) A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res 31:e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte MP, Obrecht SE, Finnegan PM, Jones JL, Podgornik MN, McAlister WH, Mumm S (2002) Osteoprotegerin deficiency and juvenile Paget’s disease. N Engl J Med 347:175–184 [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Agarwal R, Talati M, Fuller J, Lambert W, Clark AF (2002) Expression of bone morphogenetic proteins (BMP), BMP receptors, and BMP associated proteins in human trabecular meshwork and optic nerve head cells and tissues. Mol Vis 8:241–250 [PubMed] [Google Scholar]
- Wozney JM (1992) The bone morphogenetic protein family and osteogenesis. Mol Reprod Dev 32:160–167 [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V (1998) Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res 346:26–37 [PubMed] [Google Scholar]
- Wutzl A, Brozek W, Lernbass I, Rauner M, Hofbauer G, Schopper C, Watzinger F, et al. (2006) Bone morphogenetic proteins 5 and 6 stimulate osteoclast generation. J Biomed Mater Res A 77:75–83 [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, et al. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, et al. (2004) Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem 279:19141–19148 [DOI] [PubMed] [Google Scholar]
- Zhao GQ (2003) Consequences of knocking out BMP signaling in the mouse. Genesis 35:43–56 [DOI] [PubMed] [Google Scholar]
- Zoricic S, Maric I, Bobinac D, Vukicevic S (2003) Expression of bone morphogenetic proteins and cartilage-derived morphogenetic proteins during osteophyte formation in humans. J Anat 202:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]