Abstract
Aging and diabetes are associated with exacerbated expression of adhesion molecules. Given their importance in endothelial dysfunction and their possible involvement in the alteration of glomerular permeability occurring in diabetes, we have evaluated expression of the sialomucin-type adhesion molecule CD34 in renal glomerular cells of normal and diabetic animals at two different ages by colloidal gold immunocytochemistry and immunoblotting. CD34 labeling was mostly assigned to the plasma membranes of glomerular endothelium and mesangial processes. Podocyte membranes were also labeled, but to a lesser degree. Short- and long-term diabetes triggers a substantial increase in immunogold labeling for CD34 in renal tissues compared with young normoglycemic animals. However, the level of labeling in old diabetic and healthy control rats is similar, suggesting that the effect of diabetes and aging on CD34 expression is similar but not synergistic. Western blotting of isolated glomerular fractions corroborated immunocytochemical results. Increased expression of CD34 may reflect its involvement in the pathogenesis of glomerular alterations related to age and diabetes. Alterations present in early diabetes, resembling those occurring with age, strengthen the concept that diabetes is an accelerated form of aging.(J Histochem Cytochem 56:605–614, 2008)
Keywords: CD34, aging, diabetes, glomerular wall, immunocytochemistry, sialomucin, adhesion molecule
CD34 is a sialomucin-type glycophosphoprotein (Simmons et al. 1992; Krause et al. 1996) characterized for having an extracellular domain of 258 residues, a transmembrane domain of 23 residues, and a cytoplasmic domain of 73 residues. It contains two serine sites for protein kinase C phosphorylation and a potential tyrosine kinase phosphorylation site in the cytoplasmic portion (Simmons et al. 1992). CD34 is also found in a truncated form with only 16 residues in the cytoplasmic domain (Nakamura et al. 1993; Fackler et al. 1995; Krause et al. 1996).
CD34 was identified as a surface membrane molecule of the immature normal human hematopoietic progenitors and leukemic cells (Civin et al. 1984) and described as a surface marker in a variety of endothelial beds (Fina et al. 1990; Pusztaszeri et al. 2006).
CD34 has potentially important roles in blood vessel formation in both embryos and adults (Ito et al. 1995; Lin et al. 1995; Wood et al. 1997) and has been involved in the proliferation and/or maintenance of hematopoietic progenitor cells (Cheng et al. 1986). It is involved in cell adhesion processes in hematopoietic (Hu and Chien 1998) and endothelial cells (Fina et al. 1990) where, as a ligand of L-selectin, it has been proposed to mediate leukocyte trafficking (Baumheter et al. 2003). In addition, as a potential regulator of hematopoietic cell differentiation (Fackler et al. 1995), CD34 seems to act as a signaling molecule, interacting with the hematopoietic CrkL, which is an adaptor protein implicated in multiple signal transduction pathways (Felschow et al. 2001).
CD34, as well as other endothelial cell markers, appears to be modulated under pathological conditions (Pusztaszeri et al. 2006) and with age (Muller et al. 2002). Being an adhesion molecule and likely a signaling molecule, CD34 as other sialomucins (Kerjaschki et al. 1984; Letarte et al. 2005; Ballermann 2007) must play significant roles in the maintenance of glomerular function and in its alterations occurring with hyperglycemia and aging.
Aiming for a better understanding of the role of cell surface molecules in the regulation of the renal glomerular structure and function, we have studied the expression and distribution of CD34 in the normal glomerulus and during short- and long-term hyperglycemia. Several previous studies from this laboratory have already demonstrated changes in expression and distribution of glomerular membrane proteins and adhesion molecules during diabetes as well as during the aging process (Regoli and Bendayan 1997,1999; Yoon et al. 2001; Boucher et al. 2006), concurring with alterations of the glomerular basement membrane (GBM) composition and permselectivity.
Materials and Methods
Animals
One-month-old male Sprague Dawley rats weighing 100 g were obtained from Charles River Co. (St-Constant, Quebec, Canada). Animals were handled following the guidelines of the Canadian Council of Animal Care, kept in individual cages under a 12:12 hr light/dark cycle, and fed Standard Purina chow ad libitum. Experimental protocols were approved by the institutional “Comité de déontologie de l'expérimentation sur les animaux de l'Université de Montréal.”
Renal tissues from animals hyperglycemic for 3 and 12 months and from age-matched normoglycemic counterparts (three animals per group) were studied. The hyperglycemic state was induced by an IP streptozotocin injection (50–70 mg/kg body weight, in citrate buffer 10 mmol/liter, pH 4.5). Hyperglycemic state developed within 48 hr and was maintained during the lifetime of the animals. No insulin was administered to the animals. Glycosuria was evaluated using Uriscan test strips (YD Diagnostics, VWR; Montreal, Canada), and glycemia was evaluated with the AccuSoft Monitoring System (Roche Diagnostics; Laval, Canada). At the end of the study, glycemia averaged 4.3 ± 0.5 and 8.5 ± 0.7 mmol/liter for young and old control animals, respectively, and 21.2 ± 0.9 and 33.9 ± 4.0 mmol/liter for young and old diabetic animals, respectively. Body weight values averaged 157 ± 20 g and 295 ± 35 g for 3-month diabetic animals and their age-matched controls, respectively, and 370 ± 28 g and 750 ± 50 g for 12-month diabetic animals and age-matched counterparts, respectively. At the time of sacrifice, samples of sera and urine were collected and kept frozen at −20C.
Antibody
A mouse monoclonal antibody that recognizes the rat variant of CD34 was raised using a purified preparation of the luminal aspect of the rat lung endothelial plasma membrane (Ghitescu et al. 1999). This antibody, identified as 30B3 (IgG2 subtype), decorates the surface of a large number of micro- and macrovascular endothelial cells in light and electron microscopy, suggesting that the antigen recognized is probably pan-endothelial. In addition to endothelium, only fibroblast membranes were found to be labeled in situ by this antibody. Western blotting of the purified rat lung endothelial plasmalemma with the 30B3 antibody revealed a single 85-kDa polypeptide purified by a sequence of chromatography steps all performed in batch at 4C, as briefly described below. Rat lungs (Sprague Dawley, 125 g body weight) perfused in situ with cold PBS were homogenized at high speed (Potter Elvehjem; Cole Palmer, Montreal, QC, Canada) in 50 mM Na carbonate buffer, pH 11.0, containing 5 mM benzamidine and 1 mM phenylmethyl sulfonyl fluoride as protease inhibitors. A crude membrane fraction deprived of cytosol and most membrane peripheral proteins was obtained by centrifugation at 10,000 × g for 30 min and solubilized afterwards in 10 mM HEPES buffer, pH 7.2, containing 1% Triton X-100. The material was centrifuged to remove any particulate fractions, filtered through a 0.45-μm nitrocellulose filter, and diluted with 10 mM HEPES buffer to 0.1% Triton, final concentration (HEPES/TX-100 buffer) before being incubated with DEAE Sephacel (Sigma-Aldrich; Oakville, ON, Canada) equilibrated in the same buffer. Elution of the adsorbed proteins was performed in a gradient ranging in 100-mM steps from 0 to 1 M NaCl in HEPES/TX-100 buffer. The fraction containing the antigen recognized by the 30B3 antibody was identified as being eluted at 500 mM NaCl by immunoblotting all fractions at uniform protein load. This material was subsequently submitted to a lectin affinity chromatography step by incubating it with Concanavalin A–Sepharose (Sigma-Aldrich) equilibrated in HEPES/TX-100 buffer containing 1 mm each of MgCl2 and CaCl2. The lectin–Sepharose substrate was previously stabilized with glutaraldehyde to prevent leak of the Concanavalin subunits (Scher et al. 1989). The elution was performed in the same HEPES/TX-100 buffer without cations but supplemented with 400 mM methyl α-d-mannopyranoside. For the final immunoaffinity purification step, the 30B3 antibody was immobilized with dimethyl pimelimidate (Gerstern and Marchalonis 1978) on 1-ml protein G–Sepharose beads (GammaBind G Sepharose; Amersham Bioscience, Baie d'Urfé, QC, Canada) and incubated for 12 hr with the fraction issued from the lectin chromatography step. Adsorbed proteins were eluted in 300 μl of 0.1 M HCl–glycine buffer, pH 2.7, containing 0.1% Triton X-100 and then supplemented with 100 μl concentrated Laemmli buffer (4X) and resolved by SDS-PAGE. The antigen recognized by the 30B3 antibody is not stained by the regular Coomassie or Ag gel-staining protocols; only Stains-all known to impart a blue color to the highly sialylated proteins (Goldberg and Warner 1997) is able to locate it in the gel. This band was cut and submitted to LC-QT of mass spectrometry (MS) analysis after trypsin digestion. Alternatively, part of the gel carrying the eluate of the immunoaffinity column was electrotransferred on a PVDF membrane. A strip of this membrane was used to reveal the position of the antigen by immunoblotting with the 30B3 antibody, and the corresponding region was harvested from the rest of the membrane and submitted to the N-terminal amino acid analysis by Edman degradation performed on a 494-cLc-Procise HS sequencer (Applied Biosystems; Foster City, CA) (Hewick et al. 1981). Amino acid sequences obtained by MS or N-terminal analysis were used to identify the corresponding protein using the NCBI BLAST system.
Immunofluorescence
Rat renal tissues were fixed overnight in Carnoy's mixture and embedded in paraffin. Five-μm sections were dewaxed and quenched for 1 hr in 1% BSA in PBS containing 0.01% Tween 20 and 1% goat normal serum. After 2 hr incubation with the primary anti-CD34 antibody (diluted 1:20 in the quenching buffer), the sections were washed with PBS and overlaid with a 1:1000 dilution of Cy-3-conjugated goat anti-mouse secondary antibody for 1 hr. Control experiment consisted of removing the primary antibody step from the staining protocol.
Immunocytochemistry
Small pieces of renal cortex were sampled from the anesthetized animals (urethane, 1 g/kg body weight). Samples were immediately fixed by immersion in periodate–lysine–paraformaldehyde solution, dehydrated in graded methanol, and embedded in Lowicryl following protocols described previously (Bendayan 1995). Labeling was performed using the immunogold technique as previously described (Bendayan 1995). Briefly, grids carrying the ultrathin tissue sections were incubated on a drop of a saturated solution of sodium metaperiodate for 10 min, washed with distilled water, transferred to a drop of 0.15 M glycine for 10 min, and washed with PBS. Grids were then incubated on a drop of ovalbumin 1% for 5 min and transferred to the diluted anti-CD34 antibody (1:5) for 4 hr at room temperature. Grids were washed with PBS, incubated on a drop of goat anti-mouse IgG–gold complex (5 nm) for 30 min at room temperature, finally washed with PBS and distilled water, dried, and contrasted with uranyl acetate. Specificity of the immunolabelings was evaluated by control experiments, replacing the first antibody step with PBS and performing the immunolabeling with a non-related antibody.
Morphometrical Analysis
Electron micrographs of immunolabeled renal glomeruli were recorded in two regions: the glomerular wall and the mesangial area. For the glomerular wall, we measured the length of the endothelial luminal and abluminal plasma membranes, those of podocyte basal and apical plasma membranes, and then counted the number of gold particles delineating each membrane domain to calculate labeling density. Only transversal sections of the glomerular wall demonstrating the presence of the slit diaphragms between podocytes were selected for the evaluation. For mesangial cells, the plasma membrane at the level of the cell body and that of the mesangial, actin-rich processes were evaluated separately. Measurements were performed blinded by direct planimetry and particle counting, using an image processing system (Videoplan 2; Carl Zeiss, Toronto, Canada). Micrographs (at least 40 per animal, per region, two to three glomeruli per animal) were recorded at ×16,900 or ×21,000 and worked to a final magnification of ×40,600 and ×50,400. Results are reported as number of gold particles per μm of membrane (mean values ± SEM). Statistical comparisons were performed using the Mann–Whitney U test.
Preparation of Glomerular Fractions and Western Blotting
The CD34 molecule was also revealed in isolated glomerular fractions by Western blotting. Animals were anesthetized and kidneys were removed, decapsulated, and cut into small pieces. Renal glomeruli were obtained using the sieving method with 125-, 180-, and 106-μm-mesh filters (Spiro 1967; Regoli and Bendayan 1997). Glomeruli were resuspended in cold Tris-buffered saline (TBS) and centrifuged (500 rpm) four times in a Beckman TJ-6 centrifuge (Beckman-Coulter; Fullerton, CA) at 4C. Isolated glomeruli were resuspended in the lysis buffer [50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.25% deoxycholate, 1 mM sodium orthovanadate, 1% nonidet 40, 2 μg aprotinin/ml, 1 mM PMSF], homogenized, and kept on ice for 1 hr. Finally, homogenates were centrifuged at 2500 × g for 20 min at 4C to remove non-solubilized material.
Protein concentration was determined by the bicinchoninic acid colorimetric assay. Samples were separated into aliquots and stored at −80C. For Western blotting, the glomerular fraction homogenates, sera, and urines were thawed; boiled for 5 min at 100C in Laemmli buffer; and resolved by SDS-PAGE in 10% acrylamide minigels. Proteins were then transferred to nitrocellulose and blots were quenched with blocking buffer (5% BSA in 0.01% Tween–TBS) for 1 hr and incubated with the anti-CD34 antibody (1:200) overnight at 4C. After several washing steps, blots were incubated with goat anti-mouse IgG tagged with peroxidase. CD34 was finally revealed by the enhanced chemiluminescent kit (Roche Diagnostics). Band intensity was analyzed by densitometry using Scion Image software (Scion Corporation; Frederick, MD). Density of β-actin bands was taken as loading controls.
Results
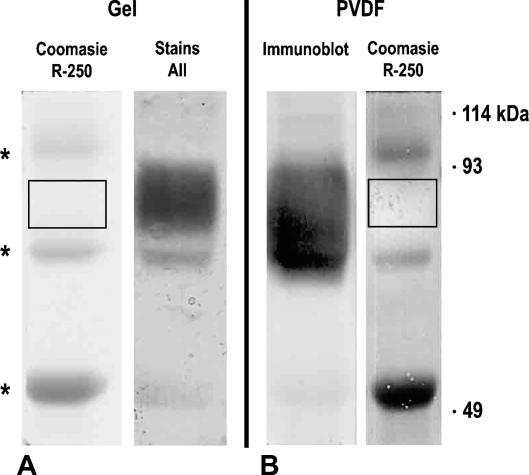
Specificity of our 30B3 antibody to the rat variant of the CD34 molecule was demonstrated by immunopurification to virtual homogeneity of this antigen. The final product of the isolation protocol contains only one band (Figure 1) stained in gel by Stains-all (Figure 1A) and on PVDF membrane by immunoblotting with the 30B3 antibody (Figure 1B). The only contaminants are the faint bands stained in gel by Coomassie identified by MS as being mouse IgG leaked in small amounts from the immunoaffinity column. Both Edman degradation and MS analysis yielded amino acid sequences unambiguously matching the primary structure of rat CD34 (Figure 2) known so far as a conceptual translation of genomic data only (NCBI database, accession #XP-223083). In this structure, the first amino acid of the N-terminal sequence analysis is located at position 37. The search for the presence of a putative signal peptide with the Internet-available SignalP Server 3.0 (Bendtsen et al. 2004) shows that the CD34 precursor contains such an initial peptide and that its cleavage site is situated with highest probability between aa 36 and 37.
Figure 1.
SDS-PAGE profile of the antigen recognized by the 30B3 antibody. (A) Alternative staining in gel with Coomassie Brilliant Blue or Stains-all. (B) The same material electrotransferred on PVDF membrane and alternatively probed by immunoblotting with the 30B3 antibody (left) or stained by Coomassie (right). Asterisks mark the position of mouse IgG fragments leaking from the column and contaminating the antigen preparation. Note that the 30B3 antigen is not stained by Coomassie. Its position is revealed by Stains-all in gel or immunoblotting on the nitrocellulose membrane.
Figure 2.
Position of the amino acid sequences obtained by N-terminal Edman degradation (in the rectangle) and mass spectrometry (underlined) in the primary sequence of the rat CD34 derived as a conceptual translation from the NCBI genomic database
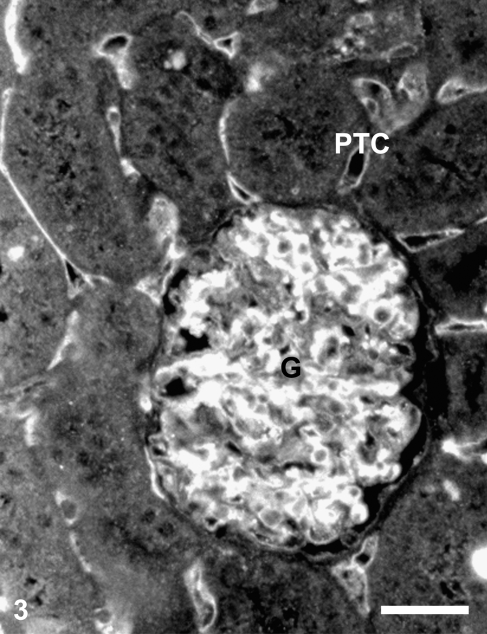
At the light microscope level, CD34 immunostaining of rat renal cortex appears to be concentrated in glomeruli, mostly in mesangial areas and endothelial surfaces; the peritubular capillaries were also labeled (Figure 3). Control experiments performed by omitting the specific antibody resulted in a total absence of staining (results not shown).
Figure 3.
CD34 immunofluorescence of the renal cortex of a control rat. Staining is intense in the glomerulus (G) and in peritubular capillaries (PTC). Bar = 50 μm.
At the electron microscope level, ultrastructural features of the renal corpuscle displayed the different characteristic morphological alterations related to age and hyperglycemic conditions, which correspond to those previously well established (Osterby and Gundersen 1975; Wehner and Petri 1983; Bendayan et al. 1986), namely, thickening of the GBM and expansion of the mesangial matrix.
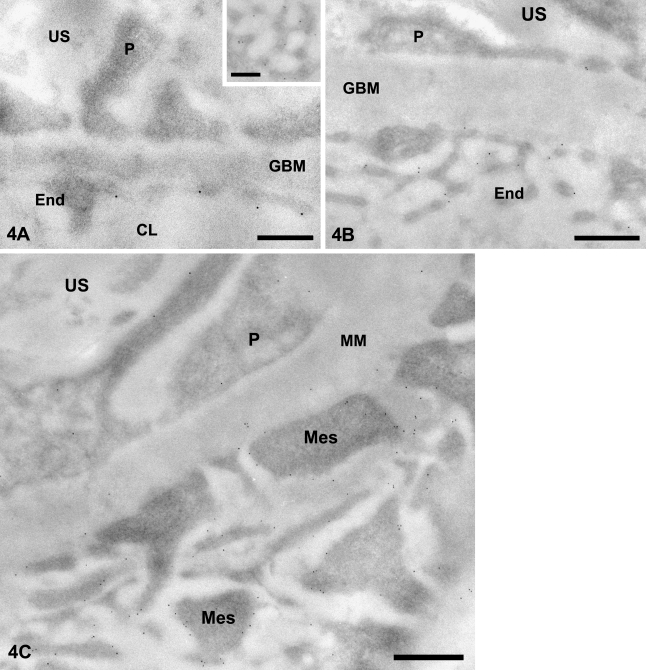
In the tissues of young control animals (Figure 4A), immunogold labeling for CD34 shows that this antigen is distributed on both the luminal and abluminal fronts of the fenestrated glomerular endothelium. Labeling is unevenly distributed and en face views of the glomerular loops indicate that CD34 is equally associated with the endothelial fenestrations (Figure 4A, inset). Within the endothelial cells, the endoplasmic reticulum, mitochondria, and nuclei are devoid of labeling. GBM shows no labeling. Podocytes show scattered gold particles on their plasma membrane, whereas the cytoplasm and organelles are free of labeling. Tissues from the 3-month diabetic animals exhibited a similar distribution of CD34 but with a consistently higher labeling intensity.
Figure 4.
CD34 immunogold labeling in glomeruli of control and diabetic rats. (A) Young normoglycemic rat. Gold particles revealing CD34 antigenic sites are associated with the endothelial (End) membrane, particularly on the luminal side. Association of the labeling with endothelial fenestrations (en face view) is clearly illustrated in the inset. (B) Old diabetic rat. Gold particles decorate luminal and abluminal endothelial (End) membranes and, less intensely, podocyte (P) membranes. Glomerular basement membrane (GBM) is thickened. (C) Old diabetic rat. Mesangial region. Labeling is intense over the plasma membrane of mesangial cell processes (Mes). Few gold particles are present over the mesangial matrix (MM). US, urinary space; CL, capillary lumen. Bars: A, inset = 0.25 μm; B,C = 0.5 μm.
Within the normoglycemic lot, when the glomeruli of old rats were compared with those of young animals, an increased GBM thickness and proliferative mesangium were recorded, and podocytes displayed numerous lysosomes. CD34 labeling dramatically increased along the plasma membranes of endothelial cells, podocytes, and mesangial cells. A similar increase of CD34 labeling was found in tissues of 12-month hyperglycemic animals (Figures 4B and 4C). In this case, the thickened GBM displays a sparse labeling.
In mesangial cells, CD34 is located mainly at the plasma membrane of the cell processes, the mesangial cell body membrane being almost devoid of labeling. Labeling increased in the 12-month diabetic animals (Figure 4C).
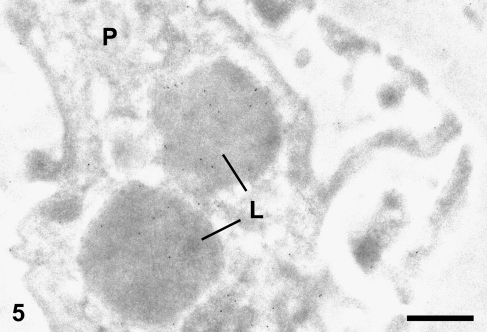
Gold particles were also present within the podocyte lysosomes (Figure 5). In all cases, only very few gold particles were detected in capillary lumina and urinary space. In control experiments, by omitting the primary antibody or replacing it with a non-related antibody, the labeling was virtually abolished with very few gold particles randomly distributed over the glomerular profile (results not shown).
Figure 5.
Old diabetic rat. CD34 immunogold labeling in glomerular podocytes (P). Labeling is present in lysosomes (L). Bar = 0.5 μm.
Morphometrical evaluation of the CD34 presence at the locations described above are shown in Table 1. In the glomeruli of all animals from all experimental groups, the highest labeling density for CD34 was recorded over the plasma membrane of the mesangial cell processes and the endothelium. Three or 12 months of diabetes, as well as 12 months of life under normoglycemic conditions, all substantially and significantly (p<0.05) increase the amount of CD34 detected at the endothelial, podocyte, and mesangial cell surfaces. Unexpectedly, there were no differences in labeling intensity for CD34 in the glomerular compartments considered between animals belonging to the 12-month diabetic and normoglycemic lots.
Table 1.
Labeling densities in different renal glomerular plasma membrane domains
| Young control animals | Young diabetic animals | Old control animals | Old diabetic animals | |
|---|---|---|---|---|
| Endothelial membranes | ||||
| Luminal | 0.28 ± 0.04 (278 μm) | 0.55 ± 0.06* (308 μm) | 1.02 ± 0.1* (246 μm) | 1.19 ± 0.09*† (214 μm) |
| Abluminal | 0.33 ± 0.05 (181 μm) | 0.41 ± 0.05* (219 μm) | 0.79 ± 0.09* (179 μm) | 0.84 ± 0.08*† (146 μm) |
| Podocyte membranes | ||||
| Basal | 0.13 ± 0.03 (185 μm) | 0.27 ± 0.04* (233 μm) | 0.55 ± 0.06* (187 μm) | 0.56 ± 0.08*† (146 μm) |
| Apical | 0.06 ± 0.01 (488 μm) | 0.19 ± 0.03* (547 μm) | 0.27 ± 0.03* (427 μm) | 0.33 ± 0.04*† (358 μm) |
| Mesangial cell membranes | ||||
| Cell processes | 0.76 ± 0.09 (317 μm) | 0.99 ± 0.12 (255 μm) | 2.55 ± 0.19* (354 μm) | 2.81 ± 0.19† (392 μm) |
| Cell body | 0.56 ± 0.1 (92 μm) | 0.41 ± 0.12 (53 μm) | 0.59 ± 0.11 (78 μm) | 0.82 ± 0.23* (74 μm) |
| Mitochondrial membranes | 0 (14 μm) | 0 (10 μm) | 0.05 ± 0.05 (16 μm) | 0.07 ± 0.07 (15 μm) |
Values are expressed as number of gold particles per μm (mean ± SEM). Numbers in parentheses indicate the total membrane length evaluated. Statistically significant differences (p<0.05) are indicated by (*) when compared to the corresponding membrane domain in the 3-month normoglycemic control group and (†) when the reference group is the 3-month diabetic rats (n = 3 animals/group). No significant differences were found between old control and old diabetic animals.
Mitochondrial membranes, taken as internal negative control for the specificity of the CD34 labeling, display negligible values in all animal groups (Table 1). The same holds true for the control experiment where the primary antibody was omitted. In this case, labelings ranged between 0.01 and 0.06 particles/μm of plasma membrane in any of the evaluated glomerular cells.
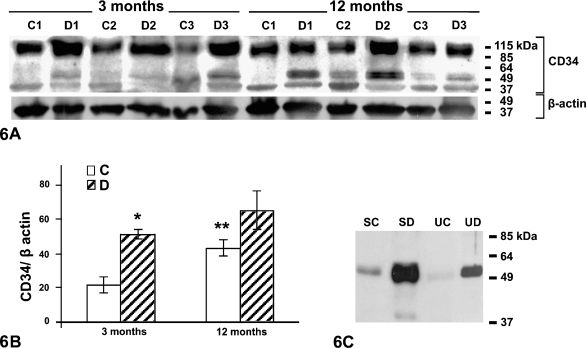
Semiquantitative detection of CD34 by Western blotting (Figure 6A) in all glomerular samples revealed a main band at 95 kDa, accompanied by fainter, lower molecular mass bands at 54 and 47 kDa. Analysis of the immunoblots by densitometry (Figure 6B) revealed an increase in the 95-kDa band with both age and diabetes, matching well the colloidal gold immunocytochemical data, whereas the immunochemical signal for β-actin (Figure 6A, lower panel) remained constant.
Figure 6.
Western blotting revealing CD34. (A) Glomerular homogenates of control and diabetic young and old animals. A major band of 95 kDa is observed in all samples. A minor band at 47 kDa is also observed in all samples, whereas a minor band at 54 kDa is particularly detected in tissues of young diabetic animals and those of normal and old diabetic animals. Molecular mass markers are indicated. In the lower panel, β-actin bands are shown as loading reference. (B) Densitometric analysis of the 95-kDa band from glomerular samples. Significant increase in intensity (asterisk) is observed for the young diabetic animals with respect to their age-matched controls and for older normoglycemic animals (double asterisk) with respect to young normoglycemic ones. C, control animals; D, diabetic animals; n=3. (C) Serum (S) and urine (U) samples of old control and old diabetic animals. A single 54-kDa band is detected in the serum of old control (SC) and diabetic animals (SD), being more intense in the latter (SD). Urine of old control (UC) and old diabetic (UD) animals also show a 54-kDa band, which is very faint in the former and strong in the latter. Molecular mass markers are indicated on the right side of the panel.
In the serum and urine of young normal and diabetic animals, CD34 is below the threshold of detection by Western blotting but increases above this limit in old rats. It is detected here at only an apparent 54-kDa band (Figure 6C), a result that demonstrates that this molecule circulates and is excreted in a truncated form. At equal protein load, significantly higher amounts of CD34 are found in the fluids harvested from old diabetic rats (Figure 6C).
Discussion
Cell adhesion molecules (CAMs) are plasma membrane proteins responsible for cell–cell and cell–extracellular matrix interactions that can trigger intracellular signaling cascades (Prozialeck and Edwards 2007). Inflammatory cytokines induce an enhanced expression of CAMs by endothelial cells. Chronic overexpression of these molecules, considered as a marker of endothelial dysfunction, leads to angiopathies (Hirata et al. 1998; Blüher et al. 2002). Age, as well as diabetes, has been related to impaired functions of blood vessels (Garlanda and Dejana 1997; Nakagawa 2007) and has been associated with an enhanced expression of cytokines and circulating CAMs (Brandes et al. 2005; Taddei et al. 2006). However, there is scarce literature concerning the topographic localization and changes in adhesion molecule expression in pathological conditions.
The immunocytochemical approach was used to localize a sialomucin-type CAM with high-resolution CD34 in the various cells of the rat glomerulus and to reveal changes in its expression along with age and diabetes. In the kidney, CD34 was assigned to rat glomeruli as well as to peritubular capillaries by immunofluorescence, as previously reported (Fina et al. 1990; Lin et al. 1995). On the other hand, at the electron microscope level, immunogold further revealed its presence at the plasma membrane of all glomerular cells including endothelial luminal and abluminal plasma membranes and podocyte basal and apical membranes, as well as the plasma membrane of mesangial processes and cell bodies. Morphometrical analysis of these labelings revealed moderate expression over all the membrane domains in tissues of young normoglycemic animals, although more intensely in endothelial luminal and mesangial membranes.
It has been well established that expression of endothelial cell markers in normal tissues varies among different vascular beds and even among blood capillaries in the same organ (Simionescu et al. 1981; Ghitescu and Robert 2002). In fact, different levels of CD34 have been reported in endothelial membranes of various tissues (Fina et al. 1990; Lin et al. 1995; Naruse et al. 2000; Pusztaszeri et al. 2006). In particular, studying human glomerulogenesis, Takano et al. (2007) demonstrated a significant expression of the CD34 protein in glomerular capillary endothelial cells in infants, an expression that gradually decreases to an almost complete loss in the adult (Naruse et al 2000). This coincides with our results that showed a relatively low expression of CD34 in glomerular cells of young normal animals.
CD34 molecules have been mostly assigned to the endothelial luminal side (Fina et al. 1990; Sauter et al. 1998). However, along with our results, it was also reported on the abluminal endothelial membrane, particularly for skin lymphatic vessels (Sauter et al. 1998). The high resolution afforded by the immunogold allowed us to further demonstrate that CD34 is, in fact, associated with capillary fenestrations, an interesting observation considering the role of these structures in glomerular permeability (Bearer and Orci 1985; Ballermann 2007). Older animals, as well as diabetic animals, displayed increases in CD34 expression in all glomerular cell plasma membrane domains, which coincides with its increase upon injury and under pathological situations such as wound healing and tumor growth (Schlingemann et al. 1990; Ito et al. 1995). In glomerulonephritis, increased luminal and abluminal endothelial CD34 expression suggests a relationship with endothelial sprouting and proliferation (Sauter et al. 1998). Increased glomerular expression of CD34 occurring with age and diabetes, as found in the present study, may reflect a response to cell activation by growth factors and cytokines triggered by hyperglycemia and by the combined pathological factors that affect the aging endothelium (Brandes et al. 2005).
In what concerns the expression of CD34 by mesangial cells, it is first interesting to notice that the mesangial cell is the glomerular cell displaying the highest levels of CD34. Furthermore, the plasma membrane domain of the mesangial processes is the one carrying the molecule, labeling at the cell body membrane being significantly lower. Mesangial cells have contractile and phagocytic capabilities (Michael et al. 1980) and participate in basement membrane and mesangial matrix repair (Cheville et al. 1983). Based on studies using the anti-thymocyte-1 (Thy 1.1) nephritis model, it has been proposed that mesangial cells are required for reconstruction of capillary structures (Otani et al. 2006). Mesangial processes, rich in smooth muscle actin (SMA), have been suggested to play key roles in glomerular remodeling (Ichimura et al. 2006). The presence of CD34 in these SMA-rich processes concurs with the repairing properties assigned to CD34 and goes along with a possible role of CD34 in repair during glomerular diseases and aging. Furthermore, it has been established that hyperglycemia increases expression of transforming growth factor β1 (TGF-β1), a crucial modulator of mesangial cell proliferation and matrix production (Chen et al. 2003). Interestingly, TGF-β1 upregulates CD34 in hematopoietic cell lines, preventing cell differentiation (Pierelli et al. 2002). We could thus hypothesize that, during hyperglycemia, activated TGF-β1 induces proliferative phenotypes in glomerular cells with increased expression of cell surface CD34. The particular increase of CD34 on membranes of mesangial processes and in endothelial cells may be an indicator of cell activation and proliferation. Similarly, overexpression of CD34 in mesangial cells, as occurs in glomerulonephritis, has served as a marker of mesangial activation concurring with the severity of the disease (Naruse et al. 1999; Chebotareva et al. 2002).
Compared with endothelial and mesangial cells, the apical and basal plasma membranes of the glomerular podocyte showed low levels of CD34, which also increased with age and diabetes. The podocyte luminal membrane domain contains other sialomucins of the CD34 family, namely, podocalyxin and endoglin, which have also been assigned to the endothelial and mesangial cell membranes and are considered as important regulators of glomerular structure and function (Kerjaschki et al. 1984; Letarte et al. 2005; Ballermann 2007). Thus, CD34 appears to be part of the complex interplay of sialomucins contributing to the maintenance of glomerular function. Along this line, recent studies of Galeano et al. (2007) found that a genetic defect affecting sialic acid biosynthesis causes hematuria, proteinuria, and structural glomerular defects leading to animal death within days after birth.
Finally, a sparse labeling for CD34 is present in the GBM of the 12-month control and diabetic animals. These animals also displayed CD34 in epithelial podocyte lysosomes which, in these old animals, are quite numerous. The presence of CD34 in the extracellular space and in lysosomes suggests the existence of a soluble form of CD34. This form was also revealed in sera and urine samples of diabetic animals. Fernandez et al. (2000) demonstrated that myeloid cell lines release a soluble form of CD34 into the culture medium. Circulating soluble forms of other CAMs (VCAM, ICAM, E-selectin) have been reported in various pathological conditions (Hirata et al. 1998; Blüher et al. 2002). These adhesion molecules seem to undergo proteolytic cleavage of their membrane-anchored forms into soluble ones, by either serine, metallo-, or thiol proteases (Bazil and Strominger 1994; Budnik et al. 1996; Reiss et al. 2006). Our detection of CD34 in the glomerular extracellular matrix, blood, and urine of diabetic rats and old rats indicates the existence of similar processing.
We can thus conclude that CD34 cell surface expression appears to modulate glomerular cell physiology, highlighting the importance of adhesion molecules for the maintenance of glomerular function. Because changes occurring with age are similar to those of short-term diabetes, our results reinforce the previous proposition (Quagliano et al. 1993; Bendayan 1998; Boucher et al. 2006) that diabetes accelerates the renal aging process.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research and Diabète-Québec.
The authors thank Diane Gingras for excellent technical assistance.
References
- Ballermann BJ (2007) Contribution of the endothelium to the glomerular permselectivity barrier in health and disease. Nephron Physiol 106:19–25 [DOI] [PubMed] [Google Scholar]
- Baumheter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, Lasky LA (2003) Binding of L-selectin to the vascular sialomucin CD34. Science 262:436–438 [DOI] [PubMed] [Google Scholar]
- Bazil V, Strominger JL (1994) Metalloprotease and serine protease are involved in cleavage of CD43, CD44, and CD16 from stimulated human granulocytes. Induction of cleavage of L-selectin via CD16. J Immunol 152:1314–1322 [PubMed] [Google Scholar]
- Bearer EL, Orci L (1985) Endothelial fenestral diaphragms: a quick-freeze, deep-etch study. J Cell Biol 100:418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendayan M (1995) Colloidal gold post-embedding immunocytochemistry. Prog Histochem Cytochem 29:1–163 [DOI] [PubMed] [Google Scholar]
- Bendayan M (1998) Immunocytochemical detection of advanced glycated end products in rat renal tissue as a function of age and diabetes. Kidney Int 54:438–447 [DOI] [PubMed] [Google Scholar]
- Bendayan M, Gingras D, Charest P (1986) Distribution of endogenous albumin in the glomerular wall of streptozotocin-induced diabetic rats as revealed by high-resolution immunocytochemistry. Diabetologia 29:868–875 [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–795 [DOI] [PubMed] [Google Scholar]
- Blüher M, Unger R, Rassoul F, Ritcher V, Paschke R (2002) Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 45:210–216 [DOI] [PubMed] [Google Scholar]
- Boucher E, Mayer G, Londono I, Bendayan M (2006) Expression and localization of MT1-MMP and furin in the glomerular wall of short- and long-term diabetic rats. Kidney Int 69:1570–1577 [DOI] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R (2005) Endothelial aging. Cardiovasc Res 66:286–294 [DOI] [PubMed] [Google Scholar]
- Budnik A, Grewe M, Gyufko K, Krutmann J (1996) Analysis of the production of soluble ICAM-1 molecules by human cells. Exp Hematol 24:352–359 [PubMed] [Google Scholar]
- Chebotareva NV, Proppe D, Rudolf P, Kozlovskaia LV (2002) Clinical significance of expression of the smooth muscle actin-alpha and CD34 antigen in mesangial cells in glomerulonephritis. Ter Arkh 74:27–31 [PubMed] [Google Scholar]
- Chen S, Jim B, Ziyadeh FN (2003) Diabetic nephropathy and transforming growth factor-β: transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol 23:532–543 [DOI] [PubMed] [Google Scholar]
- Cheng J, Baumhueter G, Cacalano G, Carver-Moore K, Thibodeaux H, Thomas R, Broxmeyer HE, et al. (1986) Hematopoietic defects in mice lacking the sialomucin CD34. Blood 87:479–490 [PubMed] [Google Scholar]
- Cheville NF, Kumar V, Robbins SL (1983) Cell Pathology. 2nd ed. Ames, The Iowa State University Press
- Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH (1984) Antigen analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol 133:157–165 [PubMed] [Google Scholar]
- Fackler MJ, Krause DS, Smith OM, Civin CI, May WS (1995) Full-length but not truncated CD34 inhibits hematopoietic cell differentiation of M1 cells. Blood 85:3040–3047 [PubMed] [Google Scholar]
- Felschow DM, McVeigh ML, Hoehn GT, Civin CI, Fackler MJ (2001) The adapter protein CrKL associates with CD34. Blood 97:3768–3775 [DOI] [PubMed] [Google Scholar]
- Fernandez M, Simon V, Minguell JJ (2000) Production of soluble CD34 by human myeloid cells. Br J Haematol 111:426–431 [DOI] [PubMed] [Google Scholar]
- Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, et al. (1990) Expression of the CD34 gene in vascular endothelial cells. Blood 75:2417–2426 [PubMed] [Google Scholar]
- Galeano B, Klootwijk R, Manoli I, Sun M, Ciccone C, Darvish D, Starost MF, et al. (2007) Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest 117:1585–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Dejana E (1997) Heterogeneity of endothelial cells. Specific markers. Arterioscler Thromb Vasc Biol 17:1193–1202 [DOI] [PubMed] [Google Scholar]
- Gerstern DM, Marchalonis JJ (1978) A rapid, novel method for the solid-phase derivatization of IgG antibodies for immune-affinity chromatography. J Immunol Methods 24:305–309 [DOI] [PubMed] [Google Scholar]
- Ghitescu L, Jacobson BS, Crine P (1999) A novel, 85 kDa endothelial antigen differentiates plasma membrane macrodomains in lung alveolar capillaries. Endothelium 6:241–250 [DOI] [PubMed] [Google Scholar]
- Ghitescu L, Robert M (2002) Diversity in unity: the biochemical composition of the endothelial cell surface varies between the vascular beds. Microsc Res Tech 57:381–389 [DOI] [PubMed] [Google Scholar]
- Goldberg HA, Warner KJ (1997) The staining of acidic proteins on polyacrylamide gels: enhanced sensitivity and stability of “Stains-all” staining in combination with silver nitrate. Anal Biochem 251:227–233 [DOI] [PubMed] [Google Scholar]
- Hewick RM, Hunkapiller MW, Hood LE, Dreyer WS (1981) A gas-liquid solid phase peptide and protein sequenator. J Biol Chem 262:10035–10038 [PubMed] [Google Scholar]
- Hirata K, Shikata K, Matsuda M, Akiyama K, Sugimoto H, Kushiro M, Makino H (1998) Increased expression of selectins in kidneys of patients with diabetic nephropathy. Diabetologia 41:185–192 [DOI] [PubMed] [Google Scholar]
- Hu MC, Chien SL (1998) The cytoplasmic domain of stem cell antigen CD34 is essential for cytoadhesion signaling but not sufficient for proliferation signaling. Blood 91:1152–1162 [PubMed] [Google Scholar]
- Ichimura K, Kurihara H, Sakai T (2006) Involvement of mesangial cells expressing α-smooth muscle actin during restorative glomerular remodeling in Thy-1.1 nephritis. J Histochem Cytochem 54:1291–1301 [DOI] [PubMed] [Google Scholar]
- Ito A, Nomura S, Hirota S, Suda J, Suda T, Kitamura Y (1995) Enhanced expression of CD34 messenger RNA by developing endothelial cells of mice. Lab Invest 72:532–538 [PubMed] [Google Scholar]
- Kerjaschki D, Sharkey DJ, Farquhar MG (1984) Identification and characterization of podocalyxin—the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol 98:1591–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Facler MJ, Civin CI, May WD (1996) CD34: structure, biology and clinical utility. Blood 87:1–13 [PubMed] [Google Scholar]
- Letarte M, McDonald ML, Li C, Kathirkamathamby K, Vera S, Pece-Barbara N, Kumar S (2005) Reduced endothelial secretion and plasma levels of transforming growth factor-β1 in patients with hereditary hemorrhagic telangiectasia type 1. Cardiovasc Res 68:155–164 [DOI] [PubMed] [Google Scholar]
- Lin G, Finger E, Gutierrez-Ramos JC (1995) Expression of CD34 in endothelial cells, hematopoietic progenitors and nervous cells in fetal and adult mouse tissues. Eur J Immunol 25:1508–1516 [DOI] [PubMed] [Google Scholar]
- Michael AF, Keane WF, Raij L, Vernier RL, Mauer SM (1980) The glomerular mesangium. Kidney Int 17:141–154 [DOI] [PubMed] [Google Scholar]
- Muller AM, Hermanns MI, Skrzynski C, Nesslinger M, Muller KM, Kirkpatrick CJ (2002) Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol 72:221–229 [DOI] [PubMed] [Google Scholar]
- Nakagawa T (2007) Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: an explanation for the paradoxical effects of VEGF in renal disease. Am J Physiol Renal Physiol 292:F1665–1672 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Komano H, Nakauchi H (1993) Two alternative forms of cDNA encoding CD34. Exp Hematol 21:236–242 [PubMed] [Google Scholar]
- Naruse K, Fujieda M, Miyazaki E, Hayashi Y, Kuroda N, Nakayama H, Kiyoku H, et al. (1999) CD34 expression as a novel marker of transformed mesangial cells in biopsied glomerular diseases. J Pathol 189:105–111 [DOI] [PubMed] [Google Scholar]
- Naruse K, Fujieda M, Miyazaki E, Hayashi Y, Toi M, Fukui T, Kuroda N, et al. (2000) An immunohistochemical study of developing glomeruli in human fetal kidneys. Kidney Int 57:1836–1846 [DOI] [PubMed] [Google Scholar]
- Osterby R, Gundersen HJ (1975) Glomerular size and structure in diabetes mellitus. I. Early abnormalities. Diabetologia 11:225–229 [DOI] [PubMed] [Google Scholar]
- Otani M, Zhang L, Aoyagi D, Chowdury R, Shigematsu H (2006) Postinflammatory glomerular recanalization is established under the accommodation of transformed mesangial cells. J Nephrol 19:449–457 [PubMed] [Google Scholar]
- Pierelli L, Marone M, Bonanno G, Rutella S, de Ritis D, Mancuso S, Leone G, et al. (2002) Transforming growth factor-β1 causes transcriptional activation of CD34 and preserves haematopoietic stem/progenitor cell activity. Br J Haematol 118:627–637 [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR (2007) Cell adhesion molecules in chemically-induced renal injury. Pharmacol Ther 114:74–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztaszeri MP, Seelentag W, Bossman FT (2006) Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand Factor, and Fli-1 in normal human tissues. J Histochem Cytochem 54:385–395 [DOI] [PubMed] [Google Scholar]
- Quagliano D, Fornieri C, Nanney LB, Davidson JM (1993) Extracellular matrix modification in rat tissues of different ages. Matrix 13:481–490 [PubMed] [Google Scholar]
- Regoli M, Bendayan M (1997) Alterations in the expression of the α3β1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia 40:15–22 [DOI] [PubMed] [Google Scholar]
- Regoli M, Bendayan M (1999) Expression of β1 integrins in glomerular tissue of Streptozotocin-induced diabetic rats. Biochem Cell Biol 77:71–78 [PubMed] [Google Scholar]
- Reiss K, Ludwig A, Saftig P (2006) Breaking up the tie: disintegring-like metalloproteinases as regulators of cell migration in inflammation and invasion. Pharmacol Ther 111:985–1006 [DOI] [PubMed] [Google Scholar]
- Sauter B, Foedinger D, Sterniczky B, Wolff K, Rappersberger K (1998) Immunoelectron microscopic characterization of human dermal lymphatic microvascular endothelial cells. Differential expression of CD31, CD34, and type IV collagen with lymphatic endothelial cells vs blood capillary endothelial cells in normal human skin, lymphangioma, and hemangioma in situ. J Histochem Cytochem 46:165–176 [DOI] [PubMed] [Google Scholar]
- Scher MG, Resneck WG, Bloch RJ (1989) Stabilization of immobilized lectin columns by crosslinking with glutaraldehyde. Anal Biochem 177:168–171 [DOI] [PubMed] [Google Scholar]
- Schlingemann RO, Rietveld FJ, de Waal RM, Bradley NJ, Skene AI, Davies AJ, Greaves MF, et al. (1990) Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest 62:690–696 [PubMed] [Google Scholar]
- Simionescu N, Simionescu M, Palade GE (1981) Differentiated microdomains on the luminal surface of the capillary endothelium. I. Preferential distribution of anionic sites. J Cell Biol 90:605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DL, Satterthwaite AB, Tenen DG, Seed B (1992) Molecular cloning of a cDNA encoding CD34, a sialomucin of human hematopoietic stem cells. J Immunol 148:267–271 [PubMed] [Google Scholar]
- Spiro RG (1967) Studies on the renal glomerular basement membrane. Preparation and chemical composition. J Biol Chem 242:1915–1922 [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Versari D, Salvetti A (2006) Endothelium, aging, and hypertension. Curr Hypertens Rep 8:84–89 [DOI] [PubMed] [Google Scholar]
- Takano K, Kawasaki Y, Imaizumi T, Matsuura H, Nozawa R, Tannji M, Suyama K, et al. (2007) Development of glomerular endothelial cells, podocytes and mesangial cells in the human fetus and infant. Tohoku J Exp Med 212:81–90 [DOI] [PubMed] [Google Scholar]
- Wehner H, Petri M (1983) Glomerular alterations in experimental diabetes of the rat. Pathol Res Pract 176:145–157 [DOI] [PubMed] [Google Scholar]
- Wood HB, May G, Healy L, Enver T, Morriss-Kay GM (1997) CD34 expression patterns during early mouse development are related to modes of blood vessel formation and reveal additional sites of haematopoiesis. Blood 90:2300–2311 [PubMed] [Google Scholar]
- Yoon S, Gingras D, Bendayan M (2001) Alterations of vitronectin and its receptor αv integrin in the rat renal glomerular wall during diabetes. Am J Kidney Dis 38:1298–1306 [DOI] [PubMed] [Google Scholar]