Abstract
Ptf1a and Pdx1 are critical transcription factors of early pancreatic development, as shown by loss of function studies where lack of each gene alone causes almost complete pancreas agenesis. Ptf1a is particularly interesting because it is linked to a recently reported signature gene expression profile associated with the multipotent condition. Few useful antibody reagents have been available for consistent and reliable immunohistochemical visualization of Ptf1a protein expression in the early developing pancreas in which the level of production of this critical regulator seems to be very low. We describe a novel rabbit antibody raised against the c-terminal portion of the mouse Ptf1a protein and report immunodetection, for the first time, as early as embryonic day (e) 8.5–e8.75 in the dorsal and ventral buds of the mouse pancreas as well as in the neural tube at e10.0. Detailed confocal analysis identifies an abundant triple-positive (Ptf1a+/Nkx6.1+/Pdx1+) putative early multipotent pancreatic progenitor cell that marks the e9.5 dorsal pancreas and e10.5 ventral pancreas. Furthermore, expression patterns of Nkx6.1 vs Ptf1a subsequently segregate during branching morphogenesis (trunk vs tip), ending up marking two distinct cell populations of progenitors at e12.5. From e15.5 (mouse) and in adult pancreas (mouse, rat, and human), the Ptf1a antibody marks only acinar cell nuclei, as expected for its subsequent role in committing/maintaining cells in this differentiated state. In summary, this antibody is a novel tool to further characterize important early steps of pancreas differentiation. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 56:587–595, 2008)
Keywords: Ptf1a, p48, pancreas, whole mount, antibody, Nkx6.1, Pdx1, progenitor, stem cell
Pancreas transcription factor 1a (Ptf1a), also known as p48, was first described in 1989 (Roux et al. 1989) as a basic helix–loop–helix (bHLH) transcription factor that is part of the trimeric PTF1 complex. Using RT-PCR, Ptf1a mRNA was reported as detectable from embryonic day (e) 12 and by in situ hybridization (ISH) at e14 in the just-forming acinar cells (Krapp et al. 1996). In a later study, Ptf1a mRNA expression was already detected in the early pancreatic buds at e9.5–10.0 by ISH, as well as in a thin stripe of the dorsal part of the neural tube (Obata et al. 2001). The first global deletion of Ptf1a resulted in an apancreatic phenotype with endocrine pancreatic cells reported in the spleen (Krapp et al. 1998). Lineage tracing studies using pPtf1a CRE/R26R mice allowed detection of Ptf1a+ cells in the dorsal and ventral pancreas beginning at e10.0–e10.5 and, more importantly, demonstrated that Ptf1a is expressed in the multipotent pancreatic progenitors giving rise to both exocrine and endocrine pancreatic cells (Kawaguchi et al. 2002). Moreover, this latter Ptf1a ablation study was able to follow the progeny of Ptf1a-deficient cells and showed that although a small rudimentary pancreatic outgrowth formed from the dorsal pancreas bud, most of the Ptf1a-deficient progeny of the dorsal and ventral buds converted into duodenum (Kawaguchi et al. 2002). Ptf1a immunoreactivity has been localized to the nucleus of acinar cells of the adult mouse pancreas (Beres et al. 2006) using a polyclonal rabbit antibody raised against a synthetic amino acid (aa) peptide corresponding to the carboxyl-terminal 16 aa of mouse and rat Ptf1a (Rose et al. 2001). A rabbit antibody generated against a glutathione-S-transferase (GST)–Ptf1a (mouse) fusion protein gave prominent nuclear staining of most cells at e10.5 in the dorsal and ventral pancreas (Li and Edlund 2001; Jørgensen et al. 2007) as well as nuclear staining of adult acinar cells (Hart et al. 2003). Real's group also reported an affinity-purified polyclonal rabbit antibody for immunohistochemical application (Adell et al. 2000). Despite these published antibodies, it has remained difficult to detect with consistency and specificity the Ptf1a protein during its first phase of expression (Zhou et al. 2007). We now describe a novel rabbit antibody raised against GST–Ptf1a (mouse aa 11–237) with which we can robustly detect Ptf1a immunoreactivity as early as day e8.75 both in ventral and dorsal pancreatic buds of mouse. We also report specific antigen retrieval and immunodetection conditions in which robust signals can be obtained in both human and rodent tissues to facilitate detection of this protein by other members of the community. The antibody has been produced in a substantial quantity and affinity that will serve as an important tool to further characterize the early multipotent pancreatic progenitor cells and potentially be useful in defining specific qualities of cells generated by induction programs in differentiating embryonic stem (ES) cells in vitro.
Materials and Methods
Expression and Purification of GST–Ptf1a
The GST fusion protein plasmid (gift from Helena Edlund) (Li and Edlund 2001) represents an insertion of a 685 nt SmaI/NaeI fragment of mouse Ptf1a into the SmaI site of pGEX-3. Fusion protein production was IPTG induced in JM109 and purified according to the methods and citations in Candia and Wright (1996). Briefly, induced cells were lysed by sonication and cell debris removed by mid-speed centrifugation. Soluble protein extracts were mixed with glutathione–agarose beads to bind the fusion protein. Beads were collected by low-speed centrifugation, poured into a column, and washed extensively with PBS/0.1% Triton X-100. Fusion protein was eluted with 5 mM glutathione, quantitated by Bradford assay vs BSA standards, and analyzed by SDS-PAGE. Fusion protein was ∼25% full length with proteolytic cleavage to other intermediate-sized proteins; overall, about half the total purified protein was estimated to represent Ptf1a sequences, which was used to determine the amounts for each rabbit immunization.
Immunizations of Animals
Multiple animals (four mice, four guinea pigs, four rabbits) were immunized with GST–Ptf1a (mouse aa 11–237). However, only serum from rabbits resulted in positive reaction on sections. Rabbits were immunized SC biweekly with 50 μg GST–Ptf1a, the first immunization with Freund's complete adjuvant and the next three with Freund's incomplete adjuvant followed by monthly immunizations with 10 μg GST–Ptf1a with Freund's incomplete adjuvant. Animals were bled 10 days after each immunization. All animal experiments were performed according to national guidelines and approved by the national ethics committee.
Tissue
Several embryos from mouse (NMRI) and rat (Wistar) were fixed overnight in 4% fresh PFA at +5C. For whole-mount immunofluorescence (IF), mouse embryos were transferred to 100% methanol and stored at −20C until use. Individual time points are within ±e0.25 accuracy, and all embryos were beyond the embryonic turning stage. For IF on cryosections, mouse and rat embryos were cryoprotected overnight in 30% sucrose in PBS and embedded in Tissue-Tek (Sakura; Værlose, Denmark). Eight-μm sections were cut on a cryostat and stored at −80C until use. For IF on mouse paraffin sections, embryos were transferred to 70% ethanol for a few days at +5C and then paraffin embedded. Four-μm sections were cut on a microtome, and sections were stored at room temperature. One human adult non-pathological pancreas from a heart-beating, cadaveric, non-diabetic donor was procured at a European hospital associated with the Eurotransplant Foundation (Leiden, The Netherlands) and with the beta cell bank of the JDRF Center for Beta Cell Therapy of Diabetes, as approved by the ethical committee of the Free University of Brussels (“Commissie Medische Ethiek–VUB”, reference #2002/VS). Human tissue was fixed in 4% PFA/PBS for 4 hr.
Immunohistochemistry
Immunohistochemistry on sections was carried out as described in detail (Pedersen et al. 2006). Briefly, conditions were as described in Table 1.
Table 1.
Histological section pretreatments
| Pretreatment | Abcam8212 (proteolysis) | Ptf1a dilution | Detection | |
|---|---|---|---|---|
| (M) Embryonic cryosections | None | No | 1:5000 | Cy3 |
| (M) Adult cryosections | None | 5 min RT | 1:3000 | Cy3 |
| (M) Embryonic paraffin | TEG | 3 min RT | 1:6000 | Cy3 |
| (R) Adult cryosections | Citrate | 5 min RT | 1:12,000 | TSA-cy3 |
| (R) Embryonic cryosections | Citrate | No | 1:6000 | TSA-cy3 |
| (H) Adult paraffin | TEG | No | 1:10,000 | TSA-cy3 |
(M), mouse; (R), rat; (H), human; RT, room temperature.
Paraffin sections were deparaffinized and rinsed in PBS. Cryosections were rinsed in PBS. Pretreatment was microwaving in TEG (0.01 M Trizma base, 0.0005 M EGTA, pH 8.95–9.10) or citrate buffer (0.01 M citric acid, pH 6.0) for 4 min at 600 W in 200 ml buffer followed by 15 min at 250 W and finally left to cool for 20 min as outlined in Table 1. Antigen retrieval with ab8212 (Abcam; Cambridge, UK, discontinued but available as M34 from Biomeda; Burlingame, CA) was done according to this table. Sections were blocked with TNB (PerkinElmer; Hvidovre, Denmark), and rabbit Ptf1a antiserum was incubated overnight at room temperature at the dilution given in the table. Sheep anti-amylase was used (1:250, BP243; Acris Antibodies GmbH, Herford, Germany) and mouse anti-Nkx6.1 (1:150, F55A10; Beta Cell Biology Consortium, www.betacell.org). Secondary antibody combinations used were donkey anti-rabbit-cy3, donkey anti-sheep-cy5 or donkey anti-mouse-cy2, donkey anti-rabbit-cy3, or donkey anti-sheep-cy5 (Jackson ImmunoResearch; Soham, UK). Control for specific Ptf1a staining was carried out by antigen absorption where antigen (GST–Ptf1a or GST–Nkx6.1) was preincubated with the Ptf1a anti-serum for 2 hr at a final concentration of 50 μg/ml prior to staining.
Whole Mount
Whole-mount staining of embryonic mouse pancreas/gut region was done as described (Ahnfelt-Ronne et al. 2007). Antibody dilutions were as follows: goat anti-Pdx1 (Chris Wright lab, Vanderbilt University, Nashville, TN; this is a highly validated reagent with no nuclear signal on Pdx1 knockout tissue), 1:15,000; mouse anti-Nkx6.1 was a 1:1 mix of mouse anti-Nkx6.1 clone F55A10 and F64mA6B4, 1:1000 (Pedersen et al. 2006); rabbit anti-Ptf1a, 1:5000. Secondary antibodies used were donkey anti-goat-cy5, donkey anti-mouse-cy2, and donkey anti rabbit-cy3 (Jackson ImmunoResearch).
Western Blot
For Western blot of mouse e15.5 pancreas tissue, 35 pancreata were homogenized in PBS and dissolved in 200 μl loading buffer. Western blotting was carried out as described (Pedersen et al. 2006), and 4 μl buffer was loaded per lane. Antiserum was used: anti-Ptf1a, 1:5000; rabbit a-Nkx6.1 (Jensen et al. 1996) 1:1400. For specificity control, preincubation was done at a final concentration of 50 μg/ml with GST–Ptf1a or GST–Nkx6.1 for 2 hr in 50 μl buffer (TBST/2% milk) with anti-Ptf1a. This was then further diluted to 3 ml upon adding the dilution to the blot, resulting in the final Ptf1a antiserum (1:5000).
Picture Capturing and Handling
All IF images were recorded on a LSM510 confocal microscope (Carl Zeiss; Brock & Michelsen, Birkerød, Denmark). Western blots were recorded on a BioSpectrum 500 (UVP, LLC; AH Diagnostics, Aarhus, Denmark).
Picture data handling was done in Adobe Photoshop, and all manipulations are true to the original staining. Most pictures have minor adjustments done with contrast/brightness. In some panels, two or more optical sections have been overlaid. Optionally, such overlaid sections were cropped using the “Lasso tool” to ensure selection of the same crop area. To correct image distortions in scans of deeper tissue layers, some panels have been passed through a “Gaussian blur” filter of 0.3 (see figure legends for details). Raw optical section data can be viewed as movies in Supplemental Figure SM1A–J.
Alignment
Clustal W alignment was done at http://www.ebi.ac.uk/clustalw/ with default values.
Results
Following immunizations of a series of animals (four rabbits, four guinea pigs, four mice), only a single rabbit (2432A) mounted an unusually slowly developing immune response against GST–Ptf1a. Rabbit 2432A was followed by IF and following seven monthly immunizations a weak nuclear signal was detected combined with cytoplasmic signal in the developing acinar cells in e15.5 mouse pancreas tissue. After nine immunizations, a stronger nuclear signal was evident with no staining of the cytoplasm (data not shown). Continuous immunization with GST–Ptf1 and monthly bleedings has resulted in >100 ml of high-titer antiserum.
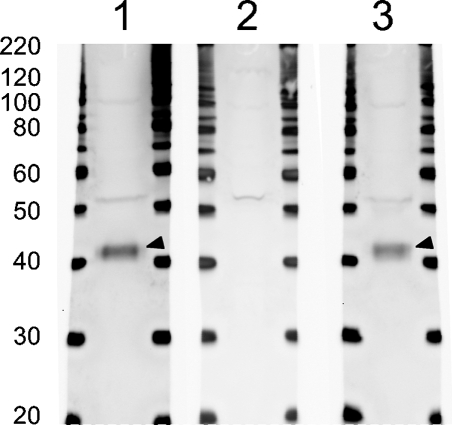
Rabbit Ptf1a Antiserum Detects a 42-kDa Protein on Whole Pancreas Western Blots
Specificity of the Ptf1a antiserum was tested by Western blot analysis on whole e15.5 mouse pancreas (Figure 1), which detected a band at 42 kDa (Figure 1, Lane 1, arrowhead) in agreement with a deduced molecular mass for Ptf1a of 38 kDa. GST–Ptf1a preabsorption of the antiserum completely blocked detection of this band (Figure 1, Lane 2), whereas preabsorption with GST–Nkx6.1 had no effect (Figure 1, Lane 3 and Supplementary Figure SF1A).
Figure 1.
Anti-Ptf1a serum recognizes a 42-kDa protein. Whole cell lysate from embryonic day (e) 15.5 pancreas was loaded in Lanes 1, 2, and 3. In Lane 1, the Ptf1a antiserum recognizes a weak band (∼52 kDa) and a strong band (arrowhead; ∼42 kDa) correlating well with the calculated molecular mass of 37.7 kDa of Ptf1a. In Lane 2, Ptf1a antiserum was preincubated with glutathione-S-transferase (GST)–Ptf1a and the intense 42-kDa band is not detected, whereas the weak 52-kDa band is still present. In Lane 3, the Ptf1a antiserum was preincubated with an unspecific control, GST–Nkx6.1, which does not interfere with the staining pattern as expected.
Anti-Ptf1a Stain Acinar Cell Nuclei of Exocrine Pancreas, Early Pancreas Progenitors, and Developing Neural Tube
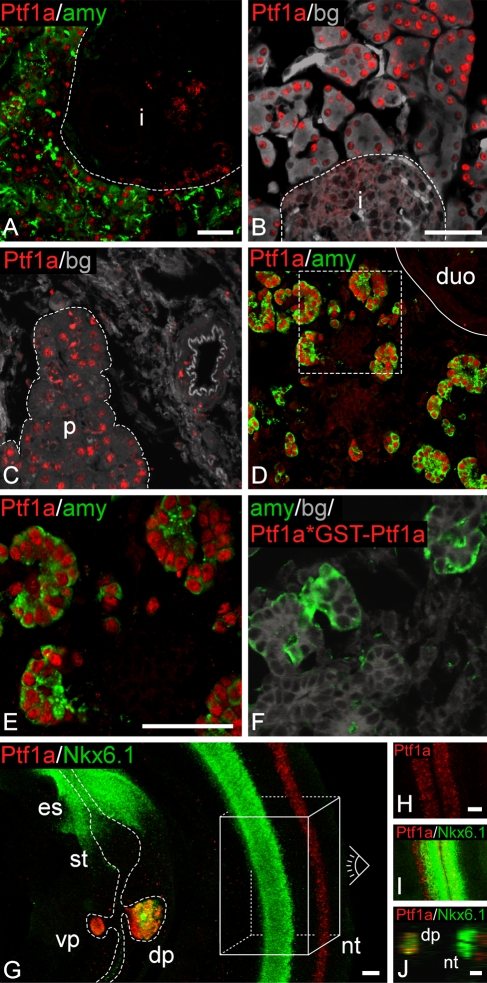
Localization of Ptf1a-like immunoreactivity to amylase-positive cell nuclei in adult was expected, but it was difficult to get a robust staining of amylase-positive cells on frozen sections of mouse pancreas (Supplementary Figure SF1B). However, with Abcam8212 antigen retrieval (now available as M34 from Biomeda), a reproducible and strong Ptf1a signal was observed in adult mouse (Figure 2A). Signal consistent with Ptf1a nuclear immunoreactivity was obtained in adult pancreatic acinar cells in both rat and human tissue (Figures 2B and 2C). Observed cross-reactivity toward rat, mouse, and human Ptf1a is supported by a high degree of amino acid sequence similarity over long stretches of the Ptf1a fragment used for immunization (Supplemental data SD1). Immunostaining of mouse e18.5 paraffin-embedded tissue also resulted, as expected, in strong nuclear Ptf1a-like immunoreactivity (Supplementary Figure SF1C) of acinar cells in mature pancreatic tissue. Similar strong nuclear immunoreactivity of amylase-positive cells was observed on frozen sections of mouse e15.5 pancreas, which did not require antigen retrieval (Figures 2D and 2E). At this time point, a few cells not expressing amylase were also labeled for nuclear Ptf1a (Figure 2E); these cells potentially represent a few remaining non-acinar committed progenitor type cells similar to those present in the pancreatic tissue at earlier stages. Specific staining of nuclear Ptf1a-like immunoreactivity was completely abolished by preabsorption to GST–Ptf1a (the fusion protein used as immunogen, Figure 2F compared with Figure 2E). This corresponds well to the Western blot data (Figure 1). We have also tested and verified that there is an absence of signal in Ptf1a homozygous null mutant mouse pancreas tissue (data not shown). Furthermore, freeze/thaw treatment of the antiserum did not compromise the staining quality (Supplementary Figures SF1D and SF1E), contrasting the behavior of a previous Ptf1a antiserum raised against the identical antigen (Li and Edlund 2001). Having shown that this Ptf1a antiserum stains cells as expected in the adult pancreas and embryonic pancreas at days 18.5 and 15.5 and gives a clean detection of a protein by Western blot analysis, whole-mount IF of mouse embryos at e10.0 was carried out to evaluate the ability to detect Ptf1a protein at early-stage pancreas tissue when the protein is present at much lower levels. Ptf1a-like immunoreactivity was evident in the dorsal and ventral pancreas buds and in the neural tube, whereas the rest of the mouse torso was negative for immunoreactivity (Figure 2G). In the neural tube, Ptf1a-like immunoreactivity was localized dorsal of the Nkx6.1 domain in two anterior–posterior stripes (Figures 2G–2I), in complete agreement with previous in situ hybridization mRNA detection (Obata et al. 2001). In the pancreas, surprisingly strong Ptf1a-like immunoreactivity was observed, also confirming published data (Li and Edlund 2001). This strong early staining signal at e10 prompted us to further investigate the onset of Ptf1a-like immunoreactivity in the pancreas in relation to that of Nkx6.1 and Pdx1.
Figure 2.
Ptf1a antiserum stains specifically in the pancreas and neural tube. (A) Double staining for Ptf1a and amylase on adult mouse cryosection. Robust immunoreactivity is observed for Ptf1a. Immunoreactivity is localized to the nucleus of amylase-positive cells and not in the islet outlined by the broken line. (B) Staining for Ptf1a on adult rat cryosection. Intense immunoreactivity for Ptf1a is evident in the nucleus of acinar cells of the pancreas. A negative islet is outlined with a broken line. (C) Ptf1a staining of adult human paraffin section. Ptf1a immunoreactivity marks acinar cells of the pancreas marked by the broken line. (D,E) Cryosection of e15.5 mouse section stained for Pft1a and amylase. Area marked by the broken line in D is shown in E. Full line marks the pancreas–duodenum boundary. In the gut region, Ptf1a-like immunoreactivity is observed only in the pancreas and is localized to the nucleus of the developing acinar cells. As observed in E, few Ptf1a-positive cells are negative for amylase at this time point. (F) Ptf1a antiserum preincubated with GST–Ptf1a (Ptf1a*GST–Ptf1a) prior to staining of an e15.5 mouse cryosection. Preincubating Ptf1a antiserum with GST–Ptf1a results in lack of Ptf1a immunoreactivity, compare with E. (G–I) Whole-mount immunofluorescence (IF) was performed on e10.5 mouse embryos for Ptf1a and Nkx6.1. (G) Ptf1a-like immunoreactivity is observed only in the pancreas and neural tube. In the pancreas, Ptf1a staining colocalizes with Nkx6.1 immunoreactivity. In the neural tube, Ptf1a reactivity is localized more dorsal than Nkx6.1 reactivity. Data in H and I are recorded looking from the dorsal side of an embryo down on the neural tube represented by the box and eye drawn in G. The more dorsal expression of Ptf1a compared with Nkx6.1 is also evident on the orthogonal section in J. Nkx6.1- and Ptf1a-like immunoreactivities are observed in both sides of the neural tube (H–J). Whole-mount double staining was done for Ptf1a and Nkx6.1 and stacks of optical sections recorded on a confocal microscope. Pictures shown in G–I represent two or more superimposed optical sections. This is done to present 3D information in a 2D format; thus, cells that appear to be overlapping are not necessarily so. (B,C,F) Gray/background is autofluorescence from cells to outline the tissue. i, islet; duo, duodenum; dp, dorsal pancreas; vp, ventral pancreas; nt, neural tube; st, stomach; es, esophagus. Identical magnifications for A and D, B and C, E and F, H and I. Bar = 50 μm.
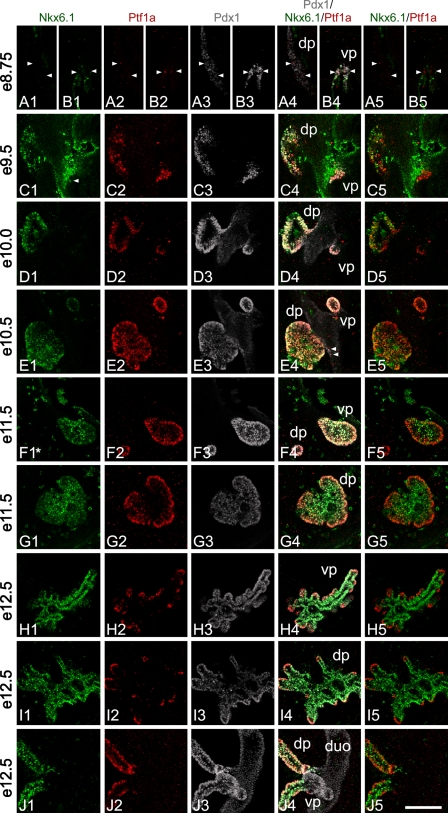
Dynamic Expression of Pft1a in Relation to Nkx6.1 and Pdx1 During Early Pancreas Development
A detailed triple whole-mount IF study for Ptf1a, Nkx6.1, and Pdx1 was carried out on a series of mouse embryos from day e8.5 to day e12.5 (Figure 3). The first cells weakly positive for Ptf1a-like immunoreactivity were observed from ∼e8.5 in the ventral pancreas, which derives from the nascent common bile duct tissue (data not shown). At e8.75, weak Ptf1a expression was observed in a few scattered cells of the dorsal pancreas (Figure 3A2), whereas in the ventral pancreas the numbers and staining intensity of Ptf1a-positive cells had increased (Figure 3B2). All cells positive for Ptf1a were coproducing Pdx1 (compare columns 2 and 3 in Figure 3).
Figure 3.
Ptf1a, Nkx6.1, and Pdx1 localization in the developing pancreas. Whole-mount triple staining was done for Ptf1a, Nkx6.1, and Pdx1 from e8.5 to e12.5 mouse embryos. Stacks of optical sections were recorded on a confocal microscope. Pictures shown in rows A,B,F,G,H,I are single optical sections from a stack, whereas pictures in rows C,D,E,J are composed of several cropped optical sections. This is done to present 3D information in a 2D way avoiding, at the same time, superimposing cells not in the same optical section. All optical sections can be viewed on supplementary movie SM1A–J. Asterisk in F1 indicates that the original Nkx6.1 signal has been bleached by the laser, whereas recording the dorsal pancreas seen in row G thus represents an artifact. (A1–5, B1–5) At ∼e8.75, the first weak Ptf1a-positive cells can be observed in the dorsal pancreas (arrowheads in A1–5). In the ventral pancreas (B1–5), immunoreactivity observed in scattered cells is stronger compared with positive cells in the dorsal pancreas. The first cells positive for Ptf1a appear at ∼e8.5 in the ventral pancreas (data not shown). The dorsal pancreas is devoid of Nkx6.1-positive cells (A1) and Ptf1a expression thus precedes that of Nkx6.1 in the dorsal pancreas. (C1–5) At e9.5, robust Ptf1a expression is observed in the dorsal and ventral pancreas. In the ventral pancreas, Ptf1a is expressed at levels comparable to what is observed later in development. Nkx6.1 is readily observed in the dorsal pancreas. In the ventral pancreas only very few and low Nkx6.1-expressing cells are observed (arrowhead) (see also supplemental movie SM1C). Thus, most Ptf1a-positive cells of the ventral pancreas are negative for Nkx6.1, whereas in the dorsal pancreas most Ptf1a-positive cells are also positive for Nkx6.1. In the dorsal pancreas, Ptf1a- and Nkx6.1-positive cells are found in what appears to be a disorganized pattern, whereas Ptf1a expression pattern in the ventral pancreas is much more uniform. (D1–5) At e10.0, cells positive for Ptf1 and Nkx6.1 are distributed in what appears to be a more organized epithelium compared with e9.5. In the dorsal pancreas, intense Ptf1 and Nkx6.1 immunoreactivity is observed at a level that is comparable to what is seen later in development. Ptf1a and Nkx6.1 positive cells are generally distributed in the bud in a random pattern however, clusters of single positive cells are also present. Nearest to the duodenum, cells tend to be more Ptf1a positive and Nkx6.1 negative/low compared with the rest of the bud, and in some bud areas cells that are Nkx6.1 positive and Ptf1a weak/negative are present. In the ventral pancreas, Ptf1a continues to be expressed at high levels, whereas Nkx6.1 is close to undetectable. (E1–5) At e10.5, Ptf1a, Pdx1, and Nkx6.1 are close to uniformly expressed in the ventral bud and to a large degree in the dorsal bud. Nkx6.1 immunoreactivity reappears in the ventral bud although immunoreactivity is weak. Ptf1a is strongly expressed in both buds. In the dorsal pancreas the tendency of having Ptf1a-positive, Nkx6.1-negative cells nearest to the duodenum continues. Clusters of cells negative for Ptf1a form holes in the otherwise uniform optical sections of Ptf1a-expressing dorsal bud cells. These cells are Nkx6.1 positive. Similar clusters are also observed budding off the Ptf1a-positive area. Such clusters are likely forming endocrine cells. In the rim of the bud, Pdx1 immunoreactivity is more intense compared with the other cells (E3). Furthermore, there is a small tendency for the very center of the dorsal bud to be more Nkx6.1 positive than Ptf1a positive. A few Ptf1a-positive cells can be observed in the duodenum outside the bud area (arrowheads in E4). (F1–5, G1–5) At e11.5, in the ventral pancreas Ptf1a, Nkx6.1, and Pdx1 are coexpressed in almost all cells. Pdx1 is expressed at similar intense levels throughout the bud, but Ptf1a and Nkx6.1 start to display opposite expression levels. This results in a cell population in the rim of the bud that expresses Ptf1a at elevated levels and coexpresses Nkx6.1 weakly, and cells in the center that express Ptf1a weakly and coexpress Nkx6.1 at an elevated level. Expression in the dorsal pancreas is like that of the ventral, just more distinct as Ptf1a expression is extinguished in the very center of the bud, but Nkx6.1 continues to be expressed weakly in the rim cells. In the forming stalk of the dorsal pancreas, Ptf1a and Pdx1 are strongly expressed, whereas Nkx6.1 expression is weaker. (H1–5, I1–5) At e12.5, expression levels and localization of Ptf1a and Nkx6.1 are almost reciprocal, whereas Pdx1 remains more uniformly expressed. Ptf1a is mainly localized to the tips of the branching pancreas where it is expressed at high levels. Nkx6.1 is mainly localized to all cells but the tip cells and the tip cells that do express Nkx6.1 do so at low levels. A few scattered cells express Pdx1 at an elevated level. Such cells are likely forming insulin cells. (J1–5) At e12.5, in the stalk regions Ptf1a-positive cells are localized to the rim of the stalks. Nkx6.1-positive cells are localized throughout the stalk with the weak-expressing cells in the rim and strong-expressing cells in the center. Pdx1 is uniformly localized and expressed. dp, dorsal pancreas; vp, ventral pancreas; duo, duodenum. Magnification is the same in all pictures. Bar = 100 μm.
Triple-positive Cell of the Dorsal Bud
As development progresses, intensity of Ptf1a immunoreactivity of the individual cells rapidly increases to that seen somewhat later in development. At e9.5, triple-positive cells (Ptf1a+/Nkx6.1+/Pdx1+) were observed in the dorsal bud (Figure 3C) in an apparently disorganized pattern intermingled with a significant number of Pdx1+/Ptf1a− cells. At e10.0 and e10.5, the triple-positive cell population of the dorsal bud appeared to be much more homogeneous, with the Pdx1+/Ptf1a− cell population having almost disappeared (Figures 3D and 3E). However, at e10.5, small areas of Nkx6.1+/Ptf1a− cells were apparently present (Figure 3E).
Triple-positive Cell of the Ventral Bud
It is noteworthy that Nkx6.1 is transiently expressed at e8.75 in the ventral pancreas and reappears at ∼e10.5, in agreement with Jørgensen et al. (2007) using another Nkx6.1 antibody. At this early time point, Nkx6.1-positive cell were found not to coproduce Ptf1a (Figure 3B). Ptf1a/Pdx1 double-positive cells that are negative for Nkx6.1 were detected at e9.5 and e10.0. In the ventral bud, triple-positive cells (Ptf1a+/Nkx6.1+/Pdx1+) were not detected until ∼e10.5 (Figure 3E).
Fate of Triple-positive Cells
In e11.5 tissue, most cells in the ventral bud were triple positive (Ptf1a+/Nkx6.1+/Pdx1+) similar to the dorsal bud, but Ptf1a became somewhat downregulated in the central-most cells (Figure 3F). This is in contrast to the dorsal bud in which the central-most cells were now devoid of Ptf1a-like immunoreactivity (Figure 3G). It was also observed that the Ptf1a signal was found in a graded fashion with the level of nuclear signal becoming progressively stronger toward the periphery (Figure 3G). In contrast, Nkx6.1 immunoreactivity was weak in the outer regions compared with more centrally located cells (Figure 3G). At e12.5, the Nkx6.1 and Ptf1a signals had become nearly completely complementary in both the ventral and dorsal buds (Figures 3H and 3I). Careful analysis over several embryonic pancreata at this stage led to the conclusion that the pattern of Nkx6.1 and Ptf1a expression in both the ventral and dorsal buds was very similar. The strongest Ptf1a immunoreactivity per nucleus was observed in cells toward the tips of the outgrowing epithelial branches where Nkx6.1 signal was absent or only weak, whereas strong Nkx6.1 signal remained in cells in the trunk regions of the pancreatic epithelium (where Ngn3-mediated endocrine cell specification occurs) (Jørgensen et al. 2007). Please note that all optical sections can be viewed on supplementary AVI format files. In e15.5 pancreas, when amylase was clearly detected in the forming acinar cells, a triple detection of Ptf1a, amylase, and Nkx6.1 was performed. At this time point, Nkx6.1- and Ptf1a-expressing cells showed no overlap at all, with Pft1a signal being evident in all amylase-positive cells and only a few Ptf1a-positive cells not showing amylase signal (Supplementary Figure SF1F).
Discussion
Despite generation of several Ptf1a antibodies, very few reliable immunodetection protocols have been published that result in robust and consistent early detection of Ptf1a protein during pancreas development. Thus, visualization of Ptf1a gene expression at the cellular level has relied on in situ hybridization (mRNA) or staining for LacZ in reporter mice (Kawaguchi et al. 2002) or immunohistochemical detection of Ptf1a target genes such as carboxypeptidase A (CpaA) (Zhou et al. 2007). It was recently stated that the Ptf1a protein cannot be easily or reliably detected by antibody prior to day e12.5 (Zhou et al. 2007), i.e., at the stage where Ptf1a staining marks cells at the tip of the branching pancreatic tree, the region predicted to contain multipotent progenitor cells (Zhou et al. 2007). We have presented a novel high-affinity anti-Ptf1a serum that represents a powerful tool to monitor Ptf1a expression during pancreas development, beginning before any signs of bud formation and extending toward the time when adult acinar cells contain higher amounts of this protein. The antibody works for immunodetection on adult and embryonic tissue and, importantly and relevant to experiments that attempt to drive ES cells toward pancreatic endocrine cell differentiation for cell-based therapeutic approaches in the clinic, the antiserum detects Ptf1a protein in both rodent and human tissue. Furthermore, it works well in Western blotting, and our staining protocol has already been reproduced independently by several other laboratories (Semb Lab, University of Lund, Sweden, Gradwohl Lab, University of Strasbourg, France; personal communication).
We can now detect Ptf1a from ∼e8.5 in the ventral pancreas and, only slightly later, at ∼e8.75 in the dorsal pancreas and at e10.0 in the neural tube in whole-mount application. At e10.0–10.5, triple-positive Ptf1a+/Nkx6.1+/Pdx1+ cells are highly abundant in both the ventral and dorsal pancreas buds. Cells double positive for only two transcription factors were also clearly detected. But the largely congruent expression of Nkx6.1 and Ptf1a in the early triple-positive cell population apparently begins to diverge in both the dorsal and ventral bud at ∼e11.5 and e12.5, respectively. Ptf1a-positive cells start to become restricted to the tips of the branching pancreas epithelium and are negative or only weakly positive for Nkx6.1. Recently, these tip domains were suggested to be the location of multipotent pancreatic progenitor cells that can give rise to both endocrine and exocrine fates from e11.5–e13.3, but only exocrine acinar cells at later time points (Zhou et al. 2007). In the absence of a useful Ptf1a antiserum, these cells were studied by Zhou et al. (2007) by lineage tracing using CpaA as an indirect readout for Ptf1a function (Cpa1 is a likely downstream target of Ptf1a, even at the low levels of the latter seen in the early pancreatic progenitor cell types), and the endogenous Cpa1 locus was itself used to drive recombination-based lineage tracing via Cre–Lox activation of a reporter allele to test progenitor–progeny relationships. The ability to detect directly and cleanly the Ptf1a protein with this new antiserum should allow a set of complementary data to be produced toward the further detailed characterization of the transition between multipotent and acinar-committed progenitors, especially with respect to other transcriptional regulatory proteins involved in driving cells along the various differentiation pathways. Our data suggest that the major pancreas progenitor/stem cell pool present at e10.0–e10.5 in the vental and dorsal pancreas is characterized by the triple-positive cell (Ptf1a+/Pdx1+/Nkx6.1+), thus providing another level of understanding of the signature gene/protein expression profile associated with the multipotent progenitor cells that seed all mature cell types within the pancreas. We hypothesize that this cell population represents a true multipotent pancreatic progenitor cell and is in full agreement with earlier lineage tracing demonstrating that all pancreatic cell types can originate from Pdx1+ or Ptf1a+ progenitors (Kawaguchi et al. 2002; Gu et al. 2003) with the recent study of Zhou et al. (2007), as well as with the gain-of-function study demonstrating that ectopic expression of Ptf1a within the Pdx1 domain can induce ectopic pancreas formation (Afelik et al. 2006). We also hypothesize that the progressive spatial segregation of the Pft1a- and Nkx6.1-expressing cell populations illustrates the segregation of the exocrine and endocrine progenitor pools. Timed lineage tracing studies for Nkx6.1+ and for Ptf1a are in progress to more precisely document this process.
Few scattered Ptf1a-positive cells were consistently observed outside the pancreas bud domain (arrowheads in Figure 3E4). Furthermore, several cells within the tissue that connects the pancreas bud to the duodenum were often Ptf1a/Pdx1 positive, but Nkx6.1 negative. The significance of the slightly broader region of Ptf1a-immunoreactive cells compared with that of Nkx6.1-positive cells remains to be understood. To resolve more rigorously the potencies of such early pancreas cells, it may be possible to pulse-label cells and their progeny in transgenic or locus knock-in mice carrying the tamoxifen-inducible Cre-ERT protein driven by Ptf1a or Nkx6.1 cis-regulatory sequences, with intercrossing to floxed Ptf1a or Nkx6.1 mice to assess the developmental consequences of removing Ptf1a or Nkx6.1 protein at different time points from the putative pancreatic progenitor populations that are now beginning to be identified.
In summary, immunodetection of Ptf1a will be highly critical for understanding the nature of the early multipotent pancreatic progenitor pool and its subsequent segregation of specialized or committed progenitor pools toward exocrine and endocrine differentiation, as well as the potential detection of reemergent facultative multipotent progenitors (or plastic cell populations) during regeneration-inducing tissue lesions. Such knowledge will likely be of similar fundamental importance for the directed differentiation of ES cells toward therapeutic pancreatic cells, including β cells for clinical treatment of diabetes (Madsen 2005). Failure to fully replicate the normal process of pancreas ontogeny in vitro may explain the insufficiency of currently reported β-like cells (being polyhormonal and lacking glucose responsiveness) (D'Amour et al. 2006; Jiang et al. 2007). Our data also point to the potential utility of being able to define conditions that will allow for the directed differentiation of ES cells toward the triple-positive (Ptf1a+/Pdx1+/Nkx6.1+) stage of multipotent pancreatic progenitor cells. Moreover, such a stage may further provide a natural window for controlling expansion of cell mass prior to further differentiation.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Diabetes & Kidney Diseases of the National Institutes of Health, Bethesda, MD (Grants 1U19DK-61244-4 and 5U19 DK-42502-15) as part of the Antibody Core of the Beta Cell Biology Consortium. J.H. was supported by “MADBETA-Grant” (part of DK-42502-15). O.D.M. and P.S. were supported by the European Union 6th Framework Programme as part of the Juvenile Diabetes Research Foundation Center for Beta Cell Therapy of Diabetes.
The authors thank Anette Bjerregaard and Maria Lauritzen for expert technical assistance and Claude Rescan and Jan Nygaard Jensen for valuable input to the manuscript. A public release of the anti-Ptf1a antibody is planned via the Beta Cell Biology Consortium website (www.betacell.org).
References
- Adell T, Gomez-Cuadrado A, Skoudy A, Pettengill OS, Longnecker DS, Real FX (2000) Role of the basic helix-loop-helix transcription factor p48 in the differentiation phenotype of exocrine pancreas cancer cells. Cell Growth Differ 11:137–147 [PubMed] [Google Scholar]
- Afelik S, Chen Y, Pieler T (2006) Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue. Genes Dev 20:1441–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnfelt-Ronne J, Jorgensen MC, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J (2007) An improved method for 3D reconstruction of protein expression patterns in intact mouse and chicken embryos and organs. J Histochem Cytochem 55:925–930. Published online May 3, 2007. 10.1369/jhc.7A7226 [DOI] [PubMed] [Google Scholar]
- Beres TM, Masui T, Swift GH, Shi L, Henke RM, MacDonald RJ (2006) PTF1 is an organ-specific and Notch-independent basic helix-loop-helix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol Cell Biol 26:117–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia AF, Wright CV (1996) Differential localization of Mox-1 and Mox-2 proteins indicates distinct roles during development. Int J Dev Biol 40:1179–1184 [PubMed] [Google Scholar]
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, et al. (2006) Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24:1392–1401 [DOI] [PubMed] [Google Scholar]
- Gu G, Brown JR, Melton DA (2003) Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev 120:35–43 [DOI] [PubMed] [Google Scholar]
- Hart A, Papadopoulou S, Edlund H (2003) Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev Dyn 228:185–193 [DOI] [PubMed] [Google Scholar]
- Jensen J, Serup P, Karlsen C, Nielsen TF, Madsen OD (1996) mRNA profiling of rat islet tumors reveals Nkx 6.1 as a beta-cell-specific homeodomain transcription factor. J Biol Chem 271:18749–18758 [DOI] [PubMed] [Google Scholar]
- Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS (2007) Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 25:1940–1953 [DOI] [PubMed] [Google Scholar]
- Jørgensen MC, Ahnfelt-Rønne J, Hald J, Madsen OD, Serup P, Hecksher-Sørensen J (2007) An illustrated review of early pancreas development in the mouse. Endocr Rev 28:685–705 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV (2002) The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet 32:128–134 [DOI] [PubMed] [Google Scholar]
- Krapp A, Knöfler M, Frutiger S, Hughes GJ, Hagenbüchle O, Wellauer PK (1996) The p48 DNA-binding subunit of transcription factor PTF1 is a new exocrine pancreas-specific basic helix-loop-helix protein. EMBO J 15:4317–4329 [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Knofler M, Ledermann B, Burki K, Berney C, Zoerkler N, Hagenbuchle O, et al. (1998) The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev 12:3752–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Edlund H (2001) Persistent expression of Hlxb9 in the pancreatic epithelium impairs pancreatic development. Dev Biol 240:247–253 [DOI] [PubMed] [Google Scholar]
- Madsen OD (2005) Stem cells and diabetes treatment. APMIS 113:858–875 [DOI] [PubMed] [Google Scholar]
- Obata J, Yano M, Mimura H, Goto T, Nakayama R, Mibu Y, Oka C, et al. (2001) p48 subunit of mouse PTF1 binds to RBP-Jκ/CBF-1, the intracellular mediator of Notch signalling, and is expressed in the neural tube of early stage embryos. Genes Cells 6:345–360 [DOI] [PubMed] [Google Scholar]
- Pedersen IL, Klinck R, Hecksher-Sorensen J, Zahn S, Madsen OD, Serup P, Jorgensen MC (2006) Generation and characterization of monoclonal antibodies against the transcription factor Nkx6.1. J Histochem Cytochem 54:567–574 [DOI] [PubMed] [Google Scholar]
- Rose SD, Swift GH, Peyton MJ, Hammer RE, MacDonald RJ (2001) The role of PTF1-P48 in pancreatic acinar gene expression. J Biol Chem 276:44018–44026 [DOI] [PubMed] [Google Scholar]
- Roux E, Strubin M, Hagenbuchle O, Wellauer PK (1989) The cell-specific transcription factor PTF1 contains two different subunits that interact with the DNA. Genes Dev 3:1613–1624 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA (2007) A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell 13:103–114 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.