Abstract
Epoxyeicosatrienoic acids (EETs) are cytochrome P450 metabolites of arachidonic acid, which function in the brain to regulate cerebral blood flow and protect against ischemic brain injury. EETs are converted by soluble epoxide hydrolase (sEH) to the corresponding inactive diol metabolites. Previous animal studies have indicated that sEH gene deletion or treatment with sEH inhibitors results in increased levels of EETs and protection against stroke-induced brain damage. To begin elucidating the underlying mechanism for these effects, we sought to determine the distribution, expression, and activity of sEH in human brain samples obtained from patients with no neurological changes/pathologies. Immunohistochemical analyses showed the distribution of sEH mainly in the neuronal cell bodies, oligodendrocytes, and scattered astrocytes. Surprisingly, in the choroid plexus, sEH was found to be highly expressed in ependymal cells. Vascular localization of sEH was evident in several regions, where it was highly expressed in the smooth muscles of the arterioles. Western blot analysis and enzyme assays confirmed the presence of sEH in the normal brain. Our results indicate differential localization of sEH in the human brain, thus suggestive of an essential role for this enzyme in the central nervous system. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 56:551–559, 2008)
Keywords: soluble epoxide hydrolase, epoxyeicosatrienoic acids, central nervous system, cerebral blood flow, cytochrome P450 epoxygenase
Soluble epoxide hydrolase (sEH) is a phase I xenobiotic metabolizing enzyme shown to have a broad distribution in human tissues (EnayetAllah et al. 2004). sEH belongs to the family of epoxide hydrolases (EHs) that converts epoxides to their corresponding diols. The EH family also includes microsomal EH, leukotriene A4 hydrolase, and cholesterol 5,6-oxide hydrolase (Spector et al. 2004).
In addition to its C-terminal EH domain, sEH also contains an N-terminal phosphatase domain (Cronin et al. 2003; Newman et al. 2003). These two domains have functionally distinct catalytic sites (Newman et al. 2003). Likely, endogenous substrates of the C-terminus include arachidonic acid epoxides [epoxyeisocsatrienoic acids (EETs)] and linoleic acid epoxides, which have been shown to regulate cardiovascular, renal, and inflammatory functions (Node et al. 1999; Capdevila and Falck 2001; Roman 2002; Sun et al. 2002). Endogenous substrates for the N-terminal phosphatase are thought to be phosphorylated lipid metabolites, some of which act as precursors in the cholesterol biosynthesis pathway and in isoprenylation (Newman et al. 2003; Tran et al. 2005; EnayetAllah and Grant 2006).
EETs are potent vasodilators in the brain (Ellis et al. 1990) and are released from astrocytes after glutamate receptor activation (Alkayed et al. 1996,1997). Inhibitors of EET synthesis block functional hyperemia in brain (Alkayed et al. 1997; Peng et al. 2002,2004), suggesting that EETs may play a role in neurovascular coupling (Harder et al. 1998). Besides their role as vasodilators, EETs are considered neuroprotective agents because of their anti-inflammatory, antipyretic, antithrombotic, and proangiogenic effects (Larsen et al. 2006). EETs are formed from arachidonic acid through the enzymatic action of CYP-450 epoxygenases (Zeldin 2001), and all four regioisomers of EETs are found in the rodent brain: 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET (Amruthesh et al. 1992; Roman 2002). Studies using CYP-450 epoxygenase inhibitors in the brain showed a 30% reduction in blood flow (Alkayed et al. 1997), thus suggesting a vital role of EETs for regulating cerebral blood flow. Studies in mice showed that sEH inhibition was protective against ischemic brain damage (Zhang et al. 2007). DHETs, the metabolites formed by the hydrolysis of EETs, are generally less potent in their activity compared with EETs; thus, sEH is thought to attenuate EET action (Spector and Norris 2007). The CYP epoxygenase isoforms in the rat brain include CYP 2C11, 2C12, 2D8, 2D9, 2C29, 2C38, and 2C39 (Roman 2002). Immunocytochemical studies suggest that epoxygenase expression in rat brain is localized mainly in astrocytes (Alkayed et al. 1996,1997).
Several polymorphisms of human sEH affecting the C-terminus have been previously reported (Sandberg et al. 2000; Przybyla-Zawislak et al. 2003), and recently, sEH polymorphisms have been linked to neuronal survival after ischemic injury (Koerner et al. 2007). Sato et al. (2004) showed an association between the Arg287Gln variant and high plasma cholesterol levels in familial hypercholesterolemia patients, thus suggestive of a role for sEH in the isoprenoid/cholesterol biosynthesis pathway (Sato et al. 2004).
In human brain, the mean concentration of unesterified cholesterol is higher than in any other body tissue (23 mg/g), and the majority of this pool is in the myelin sheath of axons (Dietschy and Turley 2004). Cholesterol is produced mainly by oligodendrocytes, a type of glia in the central nervous system (CNS). Currently there is no evidence of net transfer of sterols from blood into the brain or spinal cord, implying that the brain relies solely on de novo synthesis to meet its cholesterol requirements (Bjorkhem and Meaney 2004). Thus, sEH may play a role in regulation of intermediates in the cholesterol/isoprenoid pathway in the CNS.
Relatively little is known about cytochrome P-450 catalyzed arachidonic acid metabolism and the biological effects of the resulting eicosanoids in humans. sEH expression is evident from activity measurements and localization in human liver, kidney, and placental subcellular fractions (Pacifici et al. 1988; EnayetAllah et al. 2004). A previous study in rodents (Zhang et al. 2007) showed that sEH was localized in neuronal cell bodies and processes. Currently, rodent models are extensively used to study the potential role of sEH in hypertension and stroke; however, there is considerable variation between the activity and expression of sEH between rodents and humans (Oesch et al. 1986; Jung et al. 2005). This study focuses on the distribution, expression, and activity of sEH in the human CNS as a means for understanding the possible neural function of sEH.
Materials and Methods
Tissues and Antibodies
Postmortem normal brain tissue samples (frozen and formalin-fixed paraffin-embedded) and CNS tissue microarrays were obtained from the Cooperative Human Tissue Network–Mid-Atlantic Division (Charlottesville, VA). In most cases, samples were provided with background information (e.g., age, sex), which are listed in Table 1. Frozen tissues were kept at −80C until used.
Table 1.
Background information of donors (age and sex)
| Donor | Age (years) | Sex |
|---|---|---|
| 1 | 45 | Female |
| 2 | 36 | Male |
| 3 | 78 | Female |
| 4 | 72 | Male |
| 5 | 44 | Female |
| 6 | 78 | Female |
| 7 | 72 | Male |
| 8 | 75 | Male |
| 9 | 31 | Male |
For IHC procedures, 4-μm sections of paraffin-embedded tissues were used. Multiple sections were histologically evaluated with no pathological findings. Sections of the brain showing signs of freeze-thaw alterations were not used.
Liver sections were used as a positive control for the anti-human sEH (polyclonal rabbit anti-sEH, a gift from Dr. B. Hammock, University of California, Davis) antibody, and cerebellum was used as a positive control for the mouse anti-human glial fibrillar acidic protein (GFAP) antibody as recommended by the manufacturer (Sigma-Aldrich; St. Louis, MO).
The specificity of the anti-human sEH (hsEH) antibody was confirmed by preabsorption and immunoblot analysis. For preabsorption, we incubated the anti-hsEH antibody with excess purified hsEH protein and found no observable staining in brain tissues. Similarly, immunoblot analysis of brain tissues showed a single band at ∼62 kDa, corresponding to hsEH. These findings are consistent with previous studies in our laboratory using immunoblot analysis and preabsorption in a wide array of human tissues (EnayetAllah et al. 2004). The specificity of the GFAP antibody has been confirmed by the manufacturer (Sigma-Aldrich).
Single IHC
Sections on slides were deparaffinized and hydrated by passage through a series of xylene, ethanol, and distilled water washes. For staining with anti-sEH antibody, sections were heated to 95C in Retrieve-All (Signet; Dedham, MA) to unmask antigenic sites as recommended. The sections were washed in Tris buffer (0.096 M Tris-HCl, 0.029 M Tris base, 0.347 M NaCl with 0.025% Triton X-100, pH 7.6) for 10 min. Sections were treated with 3% hydrogen peroxide to quench endogenous peroxidase activity. Nonspecific binding was blocked with 10% normal goat serum and incubated with primary antibody against sEH (polyclonal rabbit anti-sEH, 1:400) for 1 hr. The sections were incubated with goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (1:350; Zymed Laboratories, South San Francisco, CA) for 30 min and visualized using the DAB kit (Zymed Laboratories). The DAB kit contains H2O2 solution, DAB solution, and substrate buffer and was used as per the manufacturer's recommendations. Tris buffer was used for washing. The sections were counterstained with hematoxylin, washed in distilled water, passed through a series of rapid dips in ethanol and xylene, and coverslipped. Negative control studies were done using preimmune rabbit serum or IgG for the sEH antibody at equivalent concentrations and incubation periods. All experiments were run in replicate (n=2–5 replicates per experiment), and experiments were repeated on different occasions to confirm constancy of staining in each sample. The expression level of enzyme in different tissues was evaluated blindly by three independent observers (PS, RS, and AE). Distinction between cell types, neuron, and glia was made based on cell size and morphology from both hematoxylin and eosin–stained slides and IHC slides.
Double IHC
Double immunostaining for sEH and GFAP was performed using HRP and alkaline phosphatase (AP) labeling systems, respectively. sEH antibody was polyclonal (rabbit), and the GFAP antibody was monoclonal (mouse). The sections were deparaffinized and pretreated in the same way as described above. They were incubated first with the primary antibody against sEH (1:400) for 1 hr, followed by the secondary antibody, goat anti-rabbit HRP (Zymed Laboratories) at room temperature for 30 min. After color development with DAB, the sections were washed with Tris buffer, blocked for 30 min, and incubated with mouse anti-human GFAP (1:600) primary antibody for 1 hr. The Vectastain Elite ABC avidin–biotin–alkaline phosphatase kit (Vector Laboratories; Burlingame, CA) was used for the second label with GFAP. The sections were incubated for 30 min with biotinylated goat anti-mouse secondary antibody followed by AP reagent for 30 min. To detect bound antibodies, 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium was used as substrate. No cross-labeling was detected in the negative controls where the first or second primary antibodies were replaced by preimmune rabbit serum or mouse IgG, confirming the efficiency of the blocking step for double IHC.
Sample Preparation
Subcellular fractions of frozen brain tissues (frontal lobe, parietal lobe, occipital lobe, temporal lobe, and thalamus) were prepared by homogenizing in a glass homogenizer. Approximately 500 mg of frozen tissue (stored at −80C) was transferred to 1 ml lysis buffer (10 mM HEPES, 10 mM NaCl, 1 mM KH2PO4, 5 mM NaHCO3, 5 mM EDTA, 1 mM CaCl2, 0.5 mM MgCl2, and protease inhibitor cocktail tablets) (Guillemin et al. 2005). All homogenates were centrifuged at 13,000 × g for 20 min at 4C. The supernatant obtained was centrifuged at 100,000 × g for 60 min at 4C. The resulting pellet yielded microsomes, and the supernatant yielded cytosol. Total protein was quantified on all samples with BCA reagent from Pierce (Rockford, IL) using BSA as the standard, according to the manufacturer's directions. Aliquots of the samples were frozen at −80C and stored for enzyme assays.
Enzyme Activity
Cytosolic fractions of brain tissue were isolated as described above and used to measure sEH activity using trans-[2-3H] 1, 3-diphenylpropene oxide (t-DPPO) as described previously (Borhan et al. 1995). Briefly, 1 μl of a 5 mM solution of [3H] t-DPPO in dimethylformamide was added to 100 μl of enzyme preparation in sodium phosphate buffer (0.1 M; pH 7.4) containing 0.1 mg/ml of BSA. The enzyme was incubated at 30C for 20 min, and the reaction was quenched by the addition of 60 μl of methanol and 200 μl of isooctane. Enzyme activity was determined based on the amount of radioactive diol formed in the aqueous phase using a scintillation counter. Assays were performed in triplicate.
Electrophoresis and Immunoblotting
Cytosolic fractions of frozen brain tissue (cerebral cortex, frontal lobe, occipital lobe, parietal lobe, and temporal lobe, and thalamus) were prepared as previously described. Twenty μl (15 μg total protein) of the supernatant fraction was added per lane for SDS-PAGE and Western blotting. Protein concentrations were measured by BCA reagent from Pierce as recommended. SDS-PAGE was performed using 12% resolving gels. Proteins were transferred to 0.2-μm polyvinylidene difluoride transfer membrane (Millipore; Bedford, MA) overnight at 30 V in Tris-glycine buffer with 0.037% SDS and 20% methanol. The membrane was blocked for 1 hr in 5% (w/v) non-fat dry milk plus 0.25% Tween-20 in PBS (pH 7.4) and incubated with polyclonal rabbit anti-human sEH (1:3000) for 2 hr. The secondary antibody, goat anti-rabbit–IgG–peroxidase conjugate (1:5000; Sigma) was incubated for 2 hr. Bound secondary antibodies were detected with supersignal chemiluminescent substrate as recommended (Pierce). Bands were visualized with a Kodak image station 440CF, with Kodak 1D software (Carestream Molecular Imaging; New Haven, CT). Human liver and His-tagged purified sEH were used as positive controls.
Statistical Analysis
Differences in enzyme activity were analyzed by one-way ANOVA. The criterion for statistical significance was set at p≤0.05.
Results
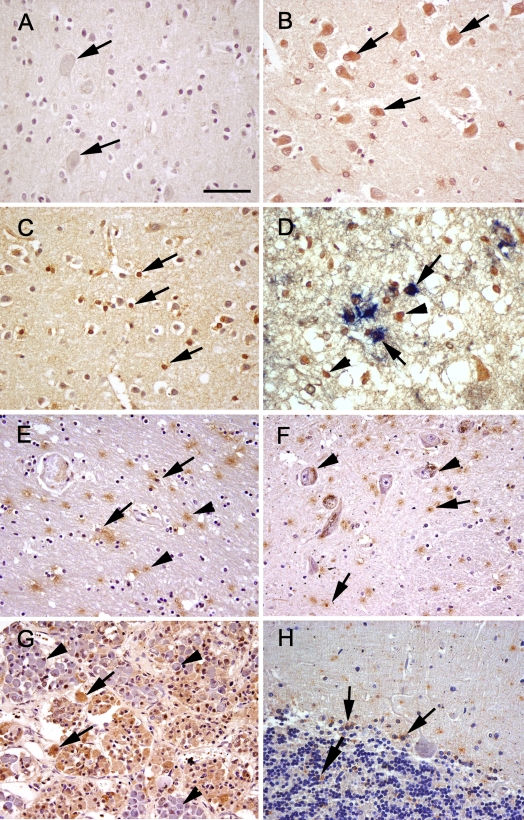
Immunoreactivity for sEH was detected in all brain regions evaluated; however, region- and cell-specific differences were apparent (Table 2). sEH was detected in vascular and non-vascular regions, with predominant expression in the glial subtype (thought to be oligodendrocytes as determined by double labeling), neuronal cell bodies, and neuropil surrounding the neurons. No staining was observed in the negative control slides (Figure 1A). Low-magnification images showing the overall distribution of sEH are provided as a supplementary figure (Supplementary Figure 1). In gray matter (cerebral cortex), sEH immunoreactivity was predominantly in the neurons, oligodendrocytes, and a few scattered astrocytes (Figures 1B–1D). On the other hand, in white matter (Figure 1E), there appeared to be marked immunoreactivity within and surrounding the oligodendrocytes. In regions such as the putamen and globus pallidus, there was punctate staining with distribution predominantly around the oligodendrocytes and astrocytes and occasional cytoplasmic staining of neurons in the latter region. In the substantia nigra (Figure 1F), thalamus, and hippocampus, the distribution was similar to putamen, with the exception of marked immunoreactivity surrounding and within the substantia nigral neurons, thalamic neurons, and hippocampal neurons. Marked immunoreactivity for sEH was detected in the pituitary (Figure 1G). There was diffuse, marked cytoplasmic staining in acidophils and chromophobes; however, no staining was observed in basophils. In the cerebellum, there was scattered staining of the granular cell layer and glial cells of the molecular layer (Figure 1H). In the pons and medulla oblongata (Figure 2A), staining was mainly observed in the neuronal cell bodies.
Table 2.
Semiquantitative assessment of sEH expression in the brain regions evaluated by three independent observers
| Regions of brain | Staining intensity | Comments |
|---|---|---|
| Prosencephalon | ||
| Thalamus | ++ | Staining of neuronal cell bodies, neuropil |
| Hypothalamus | ++ | Staining of neuronal cell bodies, neuropil |
| Cerebrum (cerebral cortex/gray matter) | ||
| Frontal lobe | ++ | Staining of glial cells especially oligodendrocytes (few glial cells colocalize with GFAP), staining of neuronal cell bodies |
| Occipital lobe | ++/+++ | Staining of glial cells especially oligodendrocytes (few glial cells colocalize with GFAP), staining of neuronal cell bodies |
| Parietal lobe | ++ | Staining of glial cells especially oligodendrocytes (few glial cells colocalize with GFAP), staining of neuronal cell bodies |
| Temporal lobe | ++ | Staining of glial cells especially oligodendrocytes (few glial cells colocalize with GFAP), staining of neuronal cell bodies |
| White matter | +++ | Intense cytoplasmic staining of glial cells (oligodendrocyte and few astrocytes) and surrounding neuropil |
| Hippocampus | ++ | Staining of neuronal cell bodies, neuropil |
| Basal ganglia (globus pallidus) | ++ | Random, punctate staining of glial cells and staining of neuronal cell bodies |
| Substantia nigra | ++ | Staining of neuronal cell bodies, neuropil |
| Putamen | ++ | Random, punctate staining of glial cells, staining of neuronal cell bodies |
| Pituitary | +++ | Diffuse, marked cytoplasmic staining of acidophils and chromophobes |
| Brain stem | ||
| Pons | ++ | Staining of neuronal cell bodies |
| Cerebellum | + | No staining in Purkinje neurons, random staining of granular cell layer, glial cells (mainly oligodendrocytes and few astrocytes) in molecular layer |
| Medulla oblongata | ++ | Staining of neuronal cell bodies |
| Spinal cord | +++ | Staining of neuronal cell bodies |
| Miscellaneous parts | ||
| Meningeal blood vessels | +++ | Marked staining of the endothelial cells lining arteries. Intense staining was also observed in the smooth muscles of arterioles |
| Choroid plexus | +++ | Diffuse staining of simple cuboidal, specialized ependymal cells |
The expression level of sEH in different regions was described as low (+), moderate (++), or high (+++). sEH, soluble epoxide hydrolase; GFAP, glial fibrillar acidic protein.
Figure 1.
Soluble epoxide hydrolase (sEH) immunoreactivity (brown) in sections of human brain. (A) Occipital lobe, negative control with no primary antibody. No immunoreactivity was present in the neurons (arrows). (B) Occipital lobe. There is diffuse sEH immunoreactivity of the neuronal cell bodies (arrows). (C) Parietal lobe. sEH immunoreactivity was observed in scattered glial cells (arrows). (D) Parietal lobe, double immunolabeling for sEH (brown) and GFAP (blue). Note there is colocalization of blue over brown (arrows) and some glial cells with sEH immunoreactivity alone (arrowheads). (E) White matter, immunoreactivity of sEH in the white matter, neuropil (arrowhead), and occasional staining of glial cells (arrows). (F) Substantia nigra. sEH immunoreactivity was observed in glial cells (arrows). Note the presence of dark brown granular neuromelanin in the cytoplasm of neurons (arrowheads). (G) Pituitary. There is marked sEH immunoreactivity in acidophils and chromophobes (arrows). However, there is no staining in basophils (arrowheads). (H) Cerebellum. There is scattered sEH immunoreactivity in the granular cell layer and molecular cell layer glia (arrows). Bar = 120 μm.
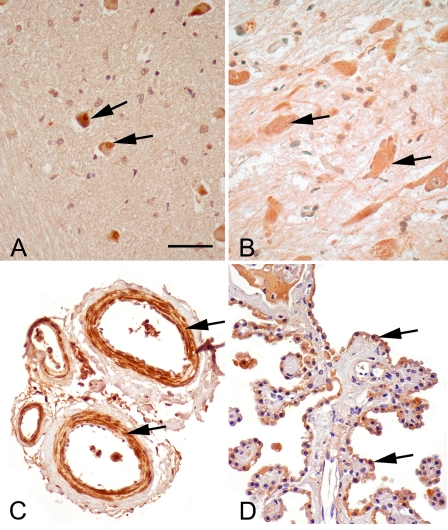
Figure 2.
sEH immunoreactivity (brown) in medulla, spinal cord, meningeal blood vessels, and choroid plexus. (A) Medulla oblongata, immunoreactivity of sEH in the neuronal cell bodies of medulla (arrows). (B) Spinal cord. sEH immunoreactivity was observed in neuronal cell bodies of spinal cord (arrows). (C) Meningeal blood vessels. There is intense immunoreactivity of the endothelial cells and the smooth muscles of arteries and arterioles (arrows). (D) Choroid plexus. The ependymal cells (arrows) lining the choroid plexus are immunoreactive. Bar = 120 μm.
There was robust staining of the neurons of the spinal cord and meningeal blood vessels (Figures 2B and 2C). Marked sEH reactivity was observed in the modified ependymal cells lining the choroid plexus (Figure 2D).
Double labeling of sEH with GFAP was used to differentiate between the two major glial cell populations: astrocytes and oligodendrocytes. sEH distribution was observed mainly in GFAP-negative cells, assumed to be oligodendrocytes based on cell size and morphology. However, occasional colocalization of sEH with GFAP was also observed, especially in regions of the cerebral cortex (Figure 1D).
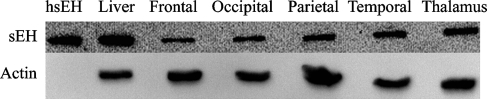
Expression of sEH in the human brain was confirmed by Western blot analysis. sEH immunoreactive protein was detected at the predicted molecular mass of ∼62 kDa (Figure 3). Purified His-tagged sEH was detected at a slightly higher molecular mass than 62 kDa as expected because of the presence of the His-tag. The level of sEH expression in the two brain regions (cerebral cortex and thalamus) was comparable, with little to negligible difference between the different lobes of the cerebral cortex.
Figure 3.
Western blot analysis of sEH expression in the human brain. There is sEH expression in the human frontal lobe, occipital lobe, parietal lobe, temporal lobe, and thalamus. His-tagged purified human sEH (hsEH) and human liver are used as positive controls. β-actin was used as a loading control for brain tissue samples.
sEH enzyme activity was measured in all brain tissues using the substrate t-DPPO. Hydration of t-DPPO by the cytosolic fraction of brain homogenates ranged from 1.7 to 2.1 nmol.min−1.mg protein−1 (Table 3). There was no significant difference in enzyme activity between the different brain regions evaluated (ANOVA, p=0.8491). However, the enzyme activity of the liver homogenate obtained from an age-matched individual showed that the liver (16.2 nmol.min−1.mg protein−1) had significantly higher sEH activity (ANOVA, p=0.002; Table 3).
Table 3.
Specific activity of sEH in different brain regions compared with the liver
| Human tissues | t-DPPO activity (nmol.min−1.mg protein−1) ± SEM |
|---|---|
| Liver | 16.2 ± 3.6 |
| Frontal lobe | 1.7 ± 0.4* |
| Occipital lobe | 1.7 ± 0.3* |
| Parietal lobe | 2.1 ± 0.2* |
| Temporal lobe | 1.8 ± 0.1* |
| Thalamus | 1.8 ± 0.1* |
Significant statistical difference from liver (p≤0.05).
Results are normalized to total protein concentration. sEH, soluble epoxide hydrolase; t-DPPO, trans-[2-3H] 1, 3-diphenylpropene oxide.
Discussion
This study provides the first description of the localization, distribution, and activity of sEH in the human brain. Previous reports have documented the presence of sEH in the mouse brain (Shin et al. 2005; Zhang et al. 2007). Since its documentation as a separate enzyme from the microsomal epoxide hydrolase (Ota and Hammock 1980), sEH has been widely studied to understand its expression profile, activity, and potential function. Recently, this enzyme has been implicated in brain pathology, especially in stroke (Zhang et al. 2007). In addition, by regulating cerebral blood flow, substrates of this enzyme (EETs) are thought to be neuroprotective. sEH is now known to contain a phosphatase domain suggested to play a role in sterol biosynthesis and isoprenylation (Tran et al. 2005; EnayetAllah and Grant 2006).
Although there is evidence indicating that sEH is present in the normal rodent brain (Shin et al. 2005; Zhang et al. 2007), its function in the nervous system is mainly attributed to levels of EETs and its implication to regulation of cerebral blood flow. This study documented the localization of this enzyme in the human nervous system, predominantly in the cytoplasm of neurons, oligodendrocytes, astrocytes, modified ependymal cells, and the smooth muscles of medium-sized arterioles. These results extend and confirm a previous report of localization of sEH in the mouse brain (Zhang et al. 2007) and suggest an important role for sEH in the human brain. The localization of this enzyme in vascular and non-vascular regions implies a potential for different functional roles (e.g., regulation of cerebral blood flow in blood vessels or localized biotransformation reactions in regions such as the choroid plexus).
Oligodendrocytes are responsible for myelin synthesis and maintenance of the integrity of myelinated axons. The localization of sEH in this cell type is suggestive of a role in myelin synthesis, possibly by regulating sterol synthesis. In addition, sEH was detected in certain cell types in the pituitary such as acidophils and chromophobes (Figure 2B). There have been previous reports suggesting the influence of EETs on the release of growth hormone (Snyder et al. 1989) and prolactin (Cashman et al. 1987), which are produced by acidophilic cells of the anterior pituitary. Thus, sEH maybe involved in hormone synthesis and regulation. Also, the pituitary is a region devoid of the blood–brain barrier and thus has higher xenobiotic metabolizing enzyme activities than other parts of the brain (Ghersi-Egea et al. 1992).
The presence of sEH in the smooth muscles on certain arterioles, especially of the meninges and spinal cord, reinforces previous findings of regulation of cerebral blood flow by degrading EETs, which are produced in the brain predominantly by astrocytes and endothelial cells (Alkayed et al. 1996,1997). EETs have been shown to be anti-inflammatory (Node et al. 1999) and thus important in the progression of stroke. Therefore, sEH may serve as a potential therapeutic target in the treatment of stroke and other neurodegenerative diseases that have inflammatory components.
The high level of sEH expression was striking in the choroid plexus. This distribution was similar to what was observed for CYP4X1 epoxygenase in the mouse brain (Al-Anizy et al. 2006). The choroid plexus is actively involved in the production and circulation of cerebrospinal fluid. Thus, sEH could be performing localized biotranformation reactions in these regions.
In summary, we showed that sEH is present in relatively high abundance in the human brain, with preferential expression in neuronal cell bodies, oligodendrocytes, astrocytes, meningeal blood vessels, and the choroid plexus, suggestive of a neuromodulatory role for sEH. Abundant sEH expression in smooth muscle cells of meningeal blood vessels is consistent with a role for sEH in the regulation of biologically active EET levels. The possibility that interindividual variation in sEH expression and function contributes to the underlying pathogenesis of diseases such as stroke requires further study using a larger sample size. These results are consistent with what is observed in rodents. However, in addition to distribution in neuronal cell bodies and processes, in the human brain, sEH was observed in oligodendrocytes, astrocytes, meningeal blood vessels, and the choroid plexus. This suggests that, in the human brain, sEH may be important in processes other than regulation of EET levels and effects on cerebral blood flow.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant ES-011630.
We thank Dr. B. Hammock (University of California, Davis) for providing the polyclonal anti-human soluble epoxide hydrolase rabbit serum. We also thank Denise Woodward, Ione Jackman, Lynn Howlett (Histology Laboratory, University of Connecticut), and Alex Bothell for advice and assistance during the completion of this work.
References
- Al-Anizy M, Horley NJ, Kuo CW, Gillett LC, Laughton CA, Kendall D, Barrett DA, et al. (2006) Cytochrome P450 Cyp4x1 is a major P450 protein in mouse brain. FEBS J 273:936–947 [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR (1997) Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke 28:1066–1072 [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR (1996) Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke 27:971–979 [DOI] [PubMed] [Google Scholar]
- Amruthesh SC, Falck JR, Ellis EF (1992) Brain synthesis and cerebrovascular action of epoxygenase metabolites of arachidonic acid. J Neurochem 58:503–510 [DOI] [PubMed] [Google Scholar]
- Bjorkhem I, Meaney S (2004) Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol 24:806–815 [DOI] [PubMed] [Google Scholar]
- Borhan B, Mebrahtu T, Nazarian S, Kurth MJ, Hammock BD (1995) Improved radiolabeled substrates for soluble epoxide hydrolase. Anal Biochem 231:188–200 [DOI] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR (2001) The CYP P450 arachidonic acid monooxygenases: from cell signaling to blood pressure regulation. Biochem Biophys Res Commun 285:571–576 [DOI] [PubMed] [Google Scholar]
- Cashman JR, Hanks D, Weiner RI (1987) Epoxy derivatives of arachidonic acid are potent stimulators of prolactin secretion. Neuroendocrinology 46:246–251 [DOI] [PubMed] [Google Scholar]
- Cronin A, Mowbray S, Durk H, Homburg S, Fleming I, Fisslthaler B, Oesch F, et al. (2003) The N-terminal domain of mammalian soluble epoxide hydrolase is a phosphatase. Proc Natl Acad Sci USA 100:1552–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD (2004) Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res 45:1375–1397 [DOI] [PubMed] [Google Scholar]
- Ellis EF, Police RJ, Yancey L, McKinney JS, Amruthesh SC (1990) Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol 259:H1171–H1177 [DOI] [PubMed] [Google Scholar]
- EnayetAllah AE, French RA, Thibodeau MS, Grant DF (2004) Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J Histochem Cytochem 52:447–454 [DOI] [PubMed] [Google Scholar]
- EnayetAllah AE, Grant DF (2006) Effects of human soluble epoxide hydrolase polymorphisms on isoprenoid phosphate hydrolysis. Biochem Biophys Res Commun 341:254–260 [DOI] [PubMed] [Google Scholar]
- Ghersi-Egea JF, Leininger-Muller B, Minn A, Siest G (1992) Drug metabolizing enzymes in the rat pituitary gland. Prog Brain Res 91:373–378 [DOI] [PubMed] [Google Scholar]
- Guillemin I, Becker M, Ociepka K, Friauf E, Nothwang HG (2005) A subcellular prefractionation protocol for minute amounts of mammalian cell cultures and tissue. Proteomics 5:35–45 [DOI] [PubMed] [Google Scholar]
- Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ (1998) Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke 29:229–234 [DOI] [PubMed] [Google Scholar]
- Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, Busse R, et al. (2005) Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension 45:759–765 [DOI] [PubMed] [Google Scholar]
- Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, Alkayed NJ (2007) Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci 27:4642–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen BT, Gutterman DD, Hatoum OA (2006) Emerging role of epoxyeicosatrienoic acids in coronary vascular function. Eur J Clin Invest 36:293–300 [DOI] [PubMed] [Google Scholar]
- Newman JW, Morisseau C, Harris TR, Hammock BD (2003) The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci USA 100:1558–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, et al. (1999) Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285:1276–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch F, Schladt L, Hartmann R, Timms C, Worner W (1986) Rat cytosolic epoxide hydrolase. Adv Exp Med Biol 197:195–201 [DOI] [PubMed] [Google Scholar]
- Ota K, Hammock BD (1980) Cytosolic and microsomal epoxide hydrolases: differential properties in mammalian liver. Science 207:1479–1481 [DOI] [PubMed] [Google Scholar]
- Pacifici GM, Temellini A, Giuliani L, Rane A, Thomas H, Oesch F (1988) Cytosolic epoxide hydrolase in humans: development and tissue distribution. Arch Toxicol 62:254–257 [DOI] [PubMed] [Google Scholar]
- Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, et al. (2002) Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol 283:H2029–H2037 [DOI] [PubMed] [Google Scholar]
- Peng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC (2004) Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab 24:509–517 [DOI] [PubMed] [Google Scholar]
- Przybyla-Zawislak BD, Srivastava PK, Vazquez-Matias J, Mohrenweiser HW, Maxwell JE, Hammock BD, Bradbury JA, et al. (2003) Polymorphisms in human soluble epoxide hydrolase. Mol Pharmacol 64:482–490 [DOI] [PubMed] [Google Scholar]
- Roman RJ (2002) P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82:131–185 [DOI] [PubMed] [Google Scholar]
- Sandberg M, Hassett C, Adman ET, Meijer J, Omiecinski CJ (2000) Identification and functional characterization of human soluble epoxide hydrolase genetic polymorphisms. J Biol Chem 275:28873–28881 [DOI] [PubMed] [Google Scholar]
- Sato K, Emi M, Ezura Y, Fujita Y, Takada D, Ishigami T, Umemura S, et al. (2004) Soluble epoxide hydrolase variant (Glu287Arg) modifies plasma total cholesterol and triglyceride phenotype in familial hypercholesterolemia: intrafamilial association study in an eight-generation hyperlipidemic kindred. J Hum Genet 49:29–34 [DOI] [PubMed] [Google Scholar]
- Shin JH, Engidawork E, Delabar JM, Lubec G (2005) Identification and characterisation of soluble epoxide hydrolase in mouse brain by a robust protein biochemical method. Amino Acids 28:63–69 [DOI] [PubMed] [Google Scholar]
- Snyder GD, Yadagiri P, Falck JR (1989) Effect of epoxyeicosatrienoic acids on growth hormone release from somatotrophs. Am J Physiol 256:E221–E226 [DOI] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, Weintraub NL (2004) Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 43:55–90 [DOI] [PubMed] [Google Scholar]
- Spector AA, Norris AW (2007) Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol 292:C996–C1012 [DOI] [PubMed] [Google Scholar]
- Sun J, Sui X, Bradbury JA, Zeldin DC, Conte MS, Liao JK (2002) Inhibition of vascular smooth muscle cell migration by cytochrome p450 epoxygenase-derived eicosanoids. Circ Res 90:1020–1027 [DOI] [PubMed] [Google Scholar]
- Tran KL, Aronov PA, Tanaka H, Newman JW, Hammock BD, Morisseau C (2005) Lipid sulfates and sulfonates are allosteric competitive inhibitors of the N-terminal phosphatase activity of the mammalian soluble epoxide hydrolase. Biochemistry 44:12179–12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin DC (2001) Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem 276:36059–36062 [DOI] [PubMed] [Google Scholar]
- Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, et al. (2007) Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab 27:1931–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.