Abstract
In alcoholic patients, ethanol is often consumed in a repeated cyclic pattern of intoxication followed by abstinence and the emergence of withdrawal symptoms. Repeated cycles of ethanol intoxication and withdrawal lead to a sensitization of CNS hyperexcitability as a result of an imbalance between inhibitory GABAergic transmission and excitatory glutamatergic transmission. Symptoms of alcohol withdrawal are usually treated pharmacologically with either benzodiazepines or anticonvulsant medications. However, recent evidence suggests that inhibition of glutamate transmission by stimulation of presynaptic inhibitory metabotropic glutamate receptors (i.e., mGluR2/3 receptors) or inhibition of mGluR5 receptors produces anticonvulsant effects. Therefore, the present study was designed to determine the effects the mGluR2/3 agonist LY379268 and the mGluR5 antagonist MPEP on ethanol withdrawal-induced seizure activity. Adult male C3H/He mice received chronic 16 h of ethanol vapor exposure in inhalation chambers followed by 8 hr of withdrawal daily for 4 consecutive days. During the final (fourth) withdrawal cycle, mice were evaluated hourly for handling-induced convulsions (HIC), and were treated with vehicle, LY379268 (0.3, 1 and 3 mg/kg) or MPEP (1, 3 and 10 mg/kg) treatment at 4 and 8 hr into withdrawal. Significant reductions in overall HIC activity were not observed following administration of either compound. These results suggest that inhibition of glutamate transmission by mGluR2/3 agonists or mGluR5 antagonists does not alter HIC activity during withdrawal from repeated ethanol exposure, and as such these compounds may have limited usefulness in the treatment of CNS hyperexcitability during alcohol withdrawal.
Keywords: alcohol withdrawal, sensitization, seizure, handling-induced convulsion, metabotropic glutamate receptors, MPEP, LY379268
Introduction
Alcoholics frequently consume alcohol in a binge-like manner, which is typically characterized by episodes of heavy intoxication followed by periods of abstinence and the emergence of withdrawal symptoms (Epstein et al., 2004; Miller et al., 2004; Miller et al., 2007). Preclinical and clinical studies have shown that repeated cycles of ethanol intoxication and withdrawal can result in a sensitization or “kindling” of CNS hyperexcitability, in which overactivity of the nervous system progressively intensifies over repeated withdrawal episodes (Ballenger and Post, 1978; Becker et al., 1997b; Becker and Hale, 1993). CNS hyperexcitability during alcohol withdrawal is primarily a result of an imbalance between excitatory and inhibitory neurotransmission brought on by chronic ethanol exposure. Pharmacological treatment of the symptoms of ethanol withdrawal typically entails the use of CNS depressants such as benzodiazepines or anticonvulsant medications (Johnson, 2004; Kiefer and Mann, 2005; Krystal et al., 2006).
Alcohol inhibits the function of the N-methyl-D-asparate (NMDA) subtype of glutamate receptors, and following chronic ethanol exposure NMDA receptor expression and function are increased (Dodd et al., 2000; Gass and Olive, 2007; Hoffman, 2003; Krystal et al., 2003; Tsai and Coyle, 1998). In addition, ethanol withdrawal is characterized by heightened extracellular levels of glutamate in rodents and humans (Dahchour and De Witte, 1999, 2003b; Tsai et al., 1998). Thus, dampening either NMDA receptor function or excess glutamate release might be an appropriate pharmacological approach to reducing the severity of the alcohol withdrawal syndrome (Becker and Redmond, 2003; Grant et al., 1990; Veatch and Becker, 2005).
Recent evidence suggests that pharmacological targeting of metabotropic glutamate receptors (mGluRs), which exert slower modulatory effects on glutamate transmission, might be a novel avenue of research for treating various aspects of alcoholism (Bachteler and Spanagel, 2005; Gass and Olive, 2007; Olive, 2005). For example, it has been shown that stimulation of presynaptic mGluR2/3 receptors suppresses glutamate release (Attwell et al., 1998b; Battaglia et al., 1997; Xi et al., 2002a), and that blockade of mGluR5 receptors, which are structurally and biochemically linked to NMDA receptors, inhibits NMDA receptor function (Homayoun and Moghaddam, 2006; Homayoun et al., 2004; Lea et al., 2002). Accordingly, mGluR2/3 receptor stimulation (Attwell et al., 1998a; Attwell et al., 1998b; Dalby and Thomsen, 1996; Klodzinska et al., 2000; Klodzinska et al., 1999; Moldrich et al., 2001a; Moldrich et al., 2001b) and mGluR5 receptor blockade (Barton et al., 2003; Chapman et al., 2000) have been shown to produce anticonvulsant effects in experimentally-induced seizures in rodents, and as such have been proposed to represent a potential new class of therapeutic agents to treat seizure-related disorders such as epilepsy (Alexander and Godwin, 2006; Moldrich et al., 2003).
Given these reports of anticonvulsant effects of mGluR2/3 agonists and mGluR5 antagonists, the goal of the present study was to test these compounds in an established model of CNS hyperactivity during withdrawal from repeated episodes of ethanol intoxication (Becker et al., 1997b; Becker and Hale, 1993). We hypothesized that the selective mGluR2/3 agonist (1R,4R,5S,6R)-4-Amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY379268; (Monn et al., 1999) and the selective mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine hydrochloride (MPEP; (Gasparini et al., 1999) would reduce seizure-like activity (i.e., handling-induced convulsions, HIC) during ethanol withdrawal.
Materials and Methods
Subjects
Adult male C3H/He mice (21-35 g at the start of the experiment, Charles River Laboratories, Portage, MI) were used as subjects. Mice were housed 3-4 per cage in an AAALAC-accredited facility under a 12-hr light/dark cycle (lights on at 0600) with ad libitum access to food and water. The colony room was maintained with temperature and humidity conditions within the guidelines of the National Institutes of Health. All procedures were approved by an Institutional Animal Care and Use Committee.
General Study Design
The experiments were designed to assess the effect of the mGluR2/3 agonist LY379268 and the mGluR5 antagonist MPEP on the withdrawal response following four cycles of chronic ethanol exposure and withdrawal, according to our previously published methods (Becker et al., 1997b; Becker and Hale, 1993; Becker and Veatch, 2002; Veatch and Becker, 2005). Briefly, mice were randomly selected to receive either 4 cycles of 16 hr continous ethanol vapor exposure in inhalation chambers separated by 8-hr periods of withdrawal, or assigned to a control group that was exposed only to air in control chambers. Both ethanol- and air-exposed animals received similar handling by the experimenter. In addition, within each treatment group (ethanol or air exposure), mice were randomly assigned to receive LY379268 (0, 0.3, 1.0 and 3.0 mg/kg) or MPEP (0, 1, 3 or 10 mg/kg) treatment during the final (fourth) withdrawal episode. Drug injections were administered 5 min prior to assessment of seizure activity at 4 and 8 hr into the final withdrawal period (see below).
Chronic Ethanol Exposure
Ethanol was chronically administered by the inhalation route, as previously described (Becker and Hale, 1993; Becker et al., 2006). Briefly, mice in their home cages were placed in Plexiglas chambers (60 × 36 × 60 cm). Fresh air was continuously delivered to the chamber at a rate of 10 l/min to provide for the respiratory needs of the animals. Ethanol was vaporized by pumping air (0.9–1.0 l/min) through an air stone submerged in 95% ethanol held in a 1-l flask. Addition of the vaporized ethanol to the fresh air maintained the ethanol concentration in the chamber in the range of 10–13 mg/l air. Control chambers were similarly configured, but with the absence of ethanol vapor. Prior to each entry into the ethanol vapor inhalation chamber, ethanol intoxication was initiated by administration of a loading dose of ethanol (1.6 g/kg; 8% w/v), and blood ethanol concentration (BEC) was stabilized by administration of the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg). Control (air-exposed) mice received saline injections along with pyrazole prior to all four cycles.
Ethanol Samples and Measurement
Chamber ethanol vapor concentrations were monitored daily. Air samples (2 ml) from the ethanol vapor inhalation chambers were collected with a 5-ml syringe through a port in the chamber wall. Samples were then transferred to Venoject™ tubes for later analysis using a modified spectrophotometric assay procedure (Becker and Hale, 1993). Blood samples were obtained from the retro-orbital sinus of mice using heparinized capillary tubes upon removal of the animals from the ethanol inhalation chambers at a time corresponding to the beginning of fourth withdrawal cycle. Whole blood samples (40 μl) were transferred to microcentrifuge tubes and centrifuged at 10,000 × g for 10 min for phase separation. Five microliter aliquots of plasma were injected into an Analox Instruments alcohol analyzer (Lunenburg, MA) for determination of ethanol concentration by an alcohol oxidase enzymatic procedure. Blood ethanol concentrations (BECs) are expressed as mg/dl blood and chamber ethanol concentrations are expressed as mg/l air.
Drug Preparation and Administration
LY379268 ((R,4R,5S,6R)-4-Amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid) was obtained as a generous gift from Eli Lilly Company (Indianapolis, IN) and dissolved in a vehicle of distilled water. Pyrazole and 2-methyl-6-(phenylethynyl)-pyridine hydrochloride (MPEP) were obtained from Sigma-Aldrich (St. Louis, MO) and were dissolved in a vehicle consisting of 0.9% w/v sodium chloride. All drugs were vortexed immediately prior to use and administered by intraperitoneal injection in a volume of 10 ml/kg body weight. Ethanol (95%) for vapor inhalation was obtained from AAPER Alcohol and Chemical Co. (Shelbyville, KY).
Assessment of Handling-Induced Convulsions
Behavioral signs of ethanol withdrawal-induced seizure activity were assessed by scoring handling-induced convulsions (HIC) on a 7-point scale, as previously described (Becker et al., 1997a; Becker et al., 1997b). The HIC response has proven to be a sensitive index of CNS hyperexcitability associated with withdrawal from ethanol and, in particular, is sensitive to the effects of repeated withdrawal experience (Becker, 1994; Becker and Hale, 1993; Veatch and Becker, 2002). HIC was assessed hourly for 10 hr following final removal of the animals from the inhalation chambers. HIC testing was conducted by a single experimenter who was unaware of the subjects' experimental history. Data are presented as hourly HIC scores and, as a measure of the overall withdrawal response, area under the 10-hr HIC withdrawal curve (AUC). AUC was calculated for each subject using the trapezoidal formula for integrating area under the HIC function, as previously described (Becker and Veatch, 2002). Briefly, AUC was calculated using the following formula: a1/2 + a2 + a3 + … a9 + a10/2, with a values representing each hour of HIC assessment.
Statistical Analyses
Blood ethanol concentration for each ethanol exposure and drug treatment group was analyzed by one-way analysis of variance (ANOVA). HIC score data at the 4 and 8 hr time points (immediately following drug treatment) was analyzed by non-parametric Kruskal-Wallis one-way ANOVA, with drug dose serving as the between-subjects factor. Area under the 10 hr withdrawal HIC curves (AUC) were calculated for each subject and tested for significance by one-way ANOVA followed by Holm-Sidak multiple comparisons procedures where appropriate. Separate analyses were performed to evaluate effects of LY379268 and MPEP. Statistical analyses were conducted using SigmaStat 3.0 (Systat, San Jose, CA), and statistical significance was set at p<0.05.
Results
Blood Ethanol Concentrations
The blood ethanol concentrations (BEC) from each treatment group, measured from samples taken immediately upon removal from the inhalation chamber prior to the fourth ethanol withdrawal episode, are shown in Table 1. Statistical analysis revealed no significant differences in BECs across any of the treatment groups (F(7,80)=1.01, p>0.05).
Table 1.
Blood ethanol concentrations (BEC) of different treatment groups prior to assessment of handling-induced convulsions.
| Treatment group | Sample size (n) | BEC (mg/dl) | 10-hr AUC |
|---|---|---|---|
| Value | |||
| Vehicle (dH2O) | 7 | 195.89 ± 5.55 | 26.29 ± 0.62 |
| LY379268 0.3 mg/kg | 8 | 208.28 ± 12.76 | 21.38 ± 1.19 |
| LY379268 1 mg/kg | 8 | 217.63 ± 8.57 | 25.25 ± 1.63 |
| LY379268 3 mg/kg | 8 | 218.75 ± 2.25 | 25.13 ± 1.36 |
| Vehicle (saline) | 15 | 189.64 ± 12.76 | 20.60 ± 1.47 |
| MPEP 1 mg/kg | 15 | 173.06 ± 14.43 | 20.27 ± 1.23 |
| MPEP 3 mg/kg | 15 | 193.32 ± 18.17 | 18.77 ± 1.07 |
| MPEP 10 mg/kg | 14 | 191.59 ± 15.72 | 19.93 ± 1.32 |
Effects of LY379268 on Handling-Induced Convulsions during Ethanol Withdrawal
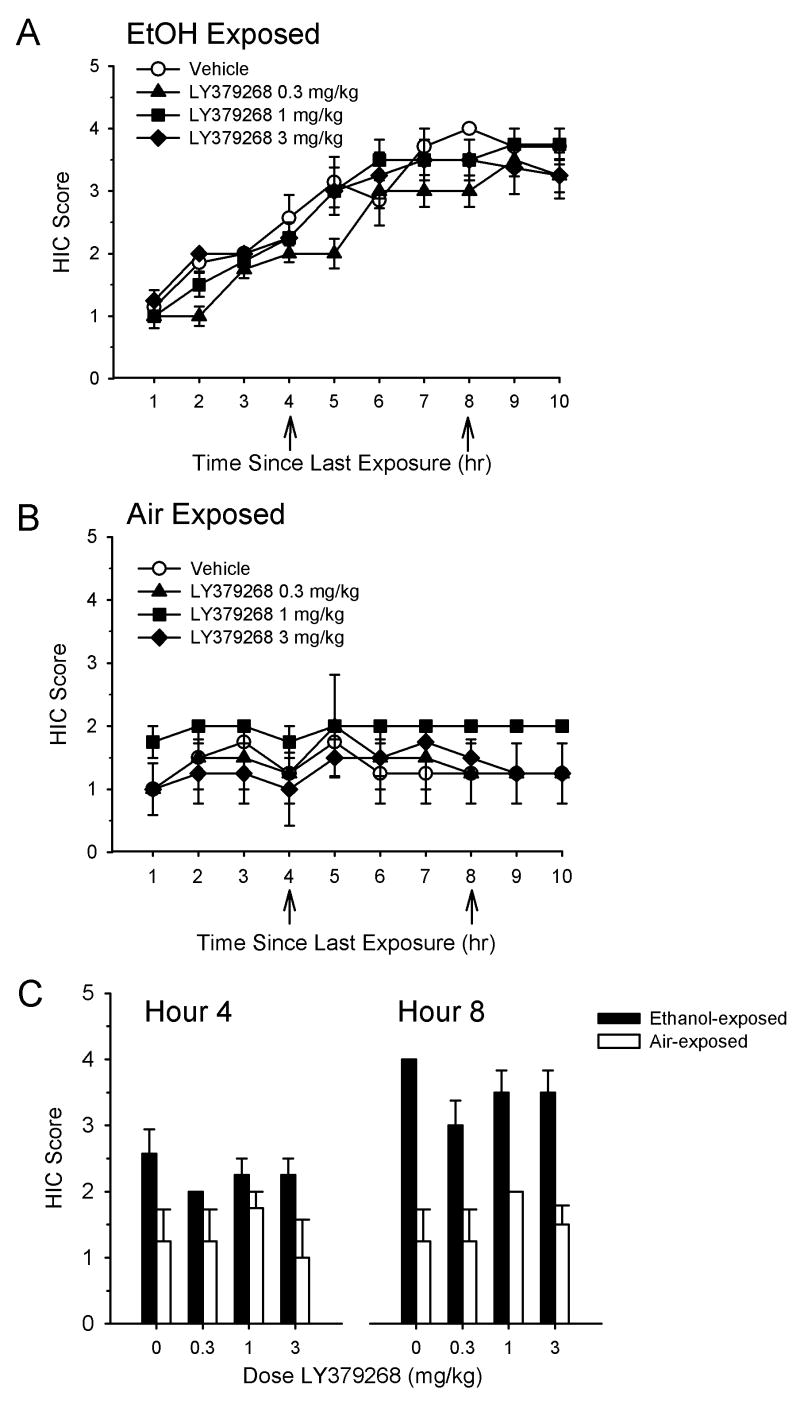
The effects of the mGluR2/3 agonist LY379268 on HIC when administered at 4 and 8 hr during ethanol withdrawal are shown in Figure 1. As can be seen, HIC activity progressively increased over time in all ethanol-exposed groups of mice (Figure 1A), consistent with previous findings (Becker et al., 1997a; Becker and Hale, 1993). In contrast, as shown in Figure 1B, HIC in control (air-exposed) groups remained relatively stable across the entire assessment period. HIC values for the individual time points when ethanol- and air-exposed animals were treated with LY379268 (i.e., 4 and 8 hr into the withdrawal period) are shown in Figure 1C. There was no significant main effect of LY379268 dose in ethanol-exposed animals at hour 4 (H(3)=2.63, p>0.05) or hour 8 (H(3)=4.73, p>0.05). In addition, there was no significant main effect of LY379268 on 10 hr AUC values (F(3,27)=2.81, p=0.059). AUC values for the 10 hr withdrawal period of ethanol-exposed animals treated with vehicle or LY379268 were significantly higher than those of similarly treated air-exposed animals (F(1,45)=52.29, p<0.001), indicating a significant effect of ethanol treatment on withdrawal seizures. There was no significant effect of LY379268 dose in air-exposed animals at hour 4 (H(3)=1.28, p=0.73) or hour 8 (H(3)=2.95, p=0.40).
Figure 1.
Effect of the selective mGluR2/3 agonist LY379268 on handling-induced convulsions (HIC) during ethanol withdrawal. Mice were subjected to four consecutive cycles of 16 hr of ethanol vapor inhalation, each followed by 8 hr of withdrawal. HIC assessment was performed hourly for 10 hr during the final (fourth) withdrawal period. Vehicle or drug treatments were administered at hours 4 and 8 in both ethanol-exposed mice (A) and air-exposed mice (B). HIC values at the time points following administration of LY379268 are shown in (C). Data are presented as mean ± SEM. Samples sizes are n=7-8 per treatment group in ethanol-exposed animals and n=4 per treatment group in air-exposed animals. Absence of error bars in panel C indicates the SEM values were equal to zero.
Effects of MPEP on handling-induced convulsions during ethanol withdrawal
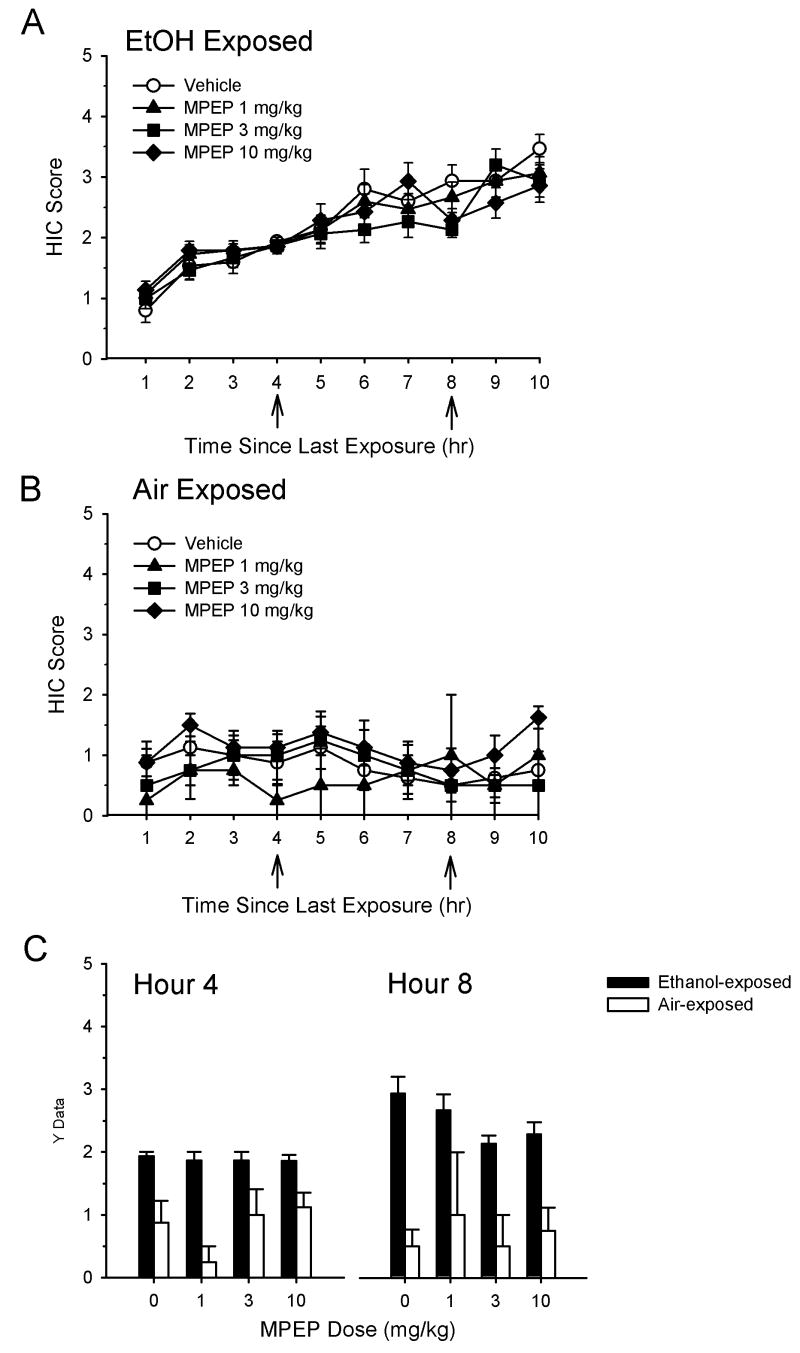
The effects of the mGluR5 antagonist MPEP on HIC during ethanol withdrawal are shown in Figure 2. As can be seen, HIC activity progressively increased over time in all ethanol-exposed groups of mice (Figure 2A), consistent with previous findings (Becker et al., 1997a; Becker and Hale, 1993). In contrast, as shown in Figure 2B, HIC in control (air-exposed) groups remained relatively stable across the entire assessment period. HIC values for the individual time points when ethanol- and air-exposed animals were treated with MPEP (i.e., 4 and 8 hr into the withdrawal period) are shown in Figure 2C. There was no significant main effect of MPEP dose in ethanol-exposed animals at hour 4 (H(3)=0.66, p=0.88) or hour 8 (H(3)=7.63, p=0.054). In addition, there was no significant main effect of MPEP on 10 hr AUC values (F(3,55)=0.39, p=0.76). AUC values of ethanol-exposed animals treated with MPEP were significantly higher than those of similarly treated air-exposed animals (F(1,81)=85.91, p<0.001), indicating a significant effect of ethanol treatment on withdrawal seizures. There was no significant effect of MPEP dose in air-exposed animals at hour 4 (H(3)=3.55, p=0.315) or hour 8 (H(3)=0.21, p=0.98)
Figure 2.
Effect of the selective mGluR5 antagonist MPEP on HIC during ethanol withdrawal. See legend for Figure 1 for experimental conditions. Vehicle or drug treatments were administered at hours 4 and 8 in both ethanol-exposed mice (A) and air-exposed mice (B). HIC values at the time points following administration of MPEP are shown in (C). Data are presented as mean ± SEM. Samples sizes are n=14-15 per treatment group in ethanol-exposed animals and n=4-8 per treatment group in air-exposed animals.
Discussion
Our findings demonstrate that the mGluR2/3 agonist LY379268 and the mGluR5 antagonist MPEP have minimal effects of ethanol withdrawal-induced seizures following repeated cycles of ethanol intoxication and withdrawal. These findings were contrary to our expectations, since these or similar ligands have been demonstrated to have anticonvulsant properties in other rodent models of seizure-like activity (Attwell et al., 1998a; Attwell et al., 1998b; Barton et al., 2003; Chapman et al., 2000; Dalby and Thomsen, 1996; Klodzinska et al., 2000; Klodzinska et al., 1999; Moldrich et al., 2001a; Moldrich et al., 2001b). BEC values obtained in the present experiments did not differ across treatment groups and were well within the range that we have found previously to produce dependence and withdrawal-related seizure activity (Becker et al., 1997a; Becker and Veatch, 2002; Veatch and Becker, 2002, 2005). However, a non-significant trend towards an increase in BEC levels was noted in animals treated with the high dose of LY379268, which may have made it more difficult to observe a reduction in withdrawal-related seizure activity with this compound. Nonethess, our data indicate limited potential clinical utility of such compounds as treatments for seizures occurring during alcohol withdrawal in human alcohol patients.
We did note a trend towards a significant effect of the low (0.3 mg/kg) dose of LY379268 on overall 10 hr AUC, as well as reductions in HIC values by 3 and 10 mg/kg of MPEP following the second administration 8 hr into the withdrawal period. However, because these observations did not meet the criteria for statistical significance (p<0.05), post-hoc tests were not conducted. The reasons for lack of effects of LY379268 and MPEP on ethanol withdrawal-induced seizures, particularly in light of the aforementioned studies showing positive effects on seizure activity, are not clear at the present time. One possible explanation is that the demonstration of preclinical efficacy of any potential anticonvulsant appears to be highly dependent on the seizure model used. For example, Klodzinska and colleagues showed that the mGluR2/3 agonist LY354740 dose-dependently inhibited seizures produced by the convulsant agents pentylenetetrazole and picrotoxin (Klodzinska et al., 2000; Klodzinska et al., 1999), but were ineffective against seizure induced by NMDA (Klodzinska et al., 2000). Other investigators have found that mGluR2/3 agonists such as (2S,3S,4S)-alpha-(carboxycyclopropyl)glycine, (1S,3R)-1-aminocyclopentane dicarboxylic acid or (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate were protective against sound-induced seizures but ineffective in protecting against seizures induced by various chemical agents or electrical brain stimulation (Dalby and Thomsen, 1996; Moldrich et al., 2001b). With regards to the ligands used in the present study, it has previously been demonstrated that LY379268 inhibits sound-, electrical- and chemically-induced seizures in mice but did not inhibit sound-induced seizures in genetically epilepsy prone rats (Barton et al., 2003; Moldrich et al., 2001a). In addition, MPEP has been reported to reduce seizures produced by sound and 6 Hz electrical brain stimulation in mice (Barton et al., 2003; Chapman et al., 2000), but a more recent study showed that the more selective mGluR5 antagonist [2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) was ineffective against seizures induced by amygdala kindling in rats (Loscher et al., 2006). Thus, it appears that LY379268 and MPEP exert anticonvulsant effects in some, but not all behavioral paradigms. The present findings suggest that these compounds are ineffective in reducing seizures induced by repeated cycles of chronic ethanol exposure and withdrawal in mice.
Another possible reason for the lack of apparent anticonvulsant efficacy of LY379268 and MPEP in our model is that repeated cycles of ethanol exposure and withdrawal might produce neuroadaptive changes in mGluR2/3 or mGluR5 receptor function that render these receptors ineffective in protecting against withdrawal-induced seizures. For example, ethanol consumption and exposure causes a number of adaptive changes in glutamatergic transmission in the brain, including an up-regulation of NMDA receptor expression or functionality (Dodd et al., 2000; Gass and Olive, 2007; Hoffman, 2003; Krystal et al., 2003; Tsai and Coyle, 1998), changes in glutamate release (Dahchour and De Witte, 1999, 2003a, b; Tsai et al., 1998) and alterations in glutamate uptake (Melendez et al., 2005). We know of no reports published to date on alterations in mGluR2/3 or mGluR5 receptor expression or function produced by repeated ethanol exposure and withdrawal, although such alterations have been shown to occur following chronic cocaine administration (Ghasemzadeh et al., 1999; Xi et al., 2002b). Therefore, it is possible that ethanol- and/or withdrawal-induced changes in mGluR2/3 or mGluR5 expression or function may reduce the ability of mGluR2/3 agonists or mGluR5 antagonists to exert anticonvulsant effects. Further studies are needed to explore this possibility.
Despite our largely negative findings, we are confident that the doses of LY379268 and MPEP used in this study were sufficient to significantly stimulate central mGluR2/3 receptor function and inhibit mGluR5 receptor function, respectively. For example, it has been shown that systemic administration of 10 mg/kg s.c. of LY379268 reaches extracellular concentrations of 600-800 nM in the brain (Schoepp et al., 2001), which are well above the reported EC50 values of 3-6 nM for the ability of this compound to stimulate mGluR2/3 receptors (Monn et al., 1999; Imre, 2007). However, because high doses of this compound can produce motor impairments, the dose range for LY379268 compound was selected as 0.3, 1 and 3 mg/kg, which is commonly used in behavioral studies with this compound (Imre, 2007). Likewise, a 10 mg/kg i.p. dose of MPEP produces rapid and full occupancy of brain mGluR5 receptors in mice (Anderson et al., 2003), and therefore this was chosen as the maximum dose, since higher doses have the potential to produce off-target effects (Lea and Faden, 2006). Therefore, the full dose range was 1, 3 and 10 mg/kg, which is commonly used in behavioral studies with MPEP (Lea and Faden, 2006).
One potential limitation of this present study is that HIC scores were assessed on an hourly basis, and several studies have shown that the central effects of LY379268 and MPEP may dissipate by as much as 50% within this time frame following systemic administration (Anderson et al., 2003; Schoepp et al., 2001). It is therefore possible that more significant effects of these two ligands might have been observed if HIC scores were assessed at more frequent intervals. Further studies are needed to examine this possibility.
In summary, our data suggest there is likely little potential therapeutic efficacy in the use of mGluR2/3 agonists or mGluR5 antagonists in the treatment ethanol withdrawal-related seizures. However, given that such ligands exert other potentially beneficial CNS effects such as anxiolysis and alleviation of depressive symptoms (Marino and Conn, 2006; Palucha and Pilc, 2007; Spooren and Gasparini, 2004; Swanson et al., 2005), they may be of use in the treatment of other symptoms of the ethanol withdrawal syndrome, such as elevated anxiety and depression.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism grants R01 AA013852 (MFO) and P50 AA10761 (HCB). The authors wish to acknowledge Laura Ralston for technical assistance, and Dr. Lynn Veatch for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GM, Godwin DW. Metabotropic glutamate receptors as a strategic target for the treatment of epilepsy. Epilepsy Res. 2006;71:1–22. doi: 10.1016/j.eplepsyres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Koumentaki A, Abdul-Ghani AS, Croucher MJ, Bradford HF. Specific group II metabotropic glutamate receptor activation inhibits the development of kindled epilepsy in rats. Brain Res. 1998a;787:286–291. doi: 10.1016/s0006-8993(97)01500-x. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Singh Kent N, Jane DE, Croucher MJ, Bradford HF. Anticonvulsant and glutamate release-inhibiting properties of the highly potent metabotropic glutamate receptor agonist (2S,2′R, 3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV) Brain Res. 1998b;805:138–143. doi: 10.1016/s0006-8993(98)00698-2. [DOI] [PubMed] [Google Scholar]
- Bachteler D, Spanagel R. Glutamatergic compounds: a perspective. In: Spanagel R, Mann K, editors. Drugs for Relapse Prevention of Alcoholism. Boston: Birkauser; 2005. pp. 205–216. [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barton ME, Peters SC, Shannon HE. Comparison of the effect of glutamate receptor modulators in the 6 Hz and maximal electroshock seizure models. Epilepsy Res. 2003;56:17–26. doi: 10.1016/j.eplepsyres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Monn JA, Schoepp DD. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY354740 in rats. Neurosci Lett. 1997;229:161–164. doi: 10.1016/s0304-3940(97)00442-4. [DOI] [PubMed] [Google Scholar]
- Becker HC. Positive relationship between the number of prior ethanol withdrawal episodes and the severity of subsequent withdrawal seizures. Psychopharmacology. 1994;116:26–32. doi: 10.1007/BF02244867. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Hale RL. Exacerbation of ethanol withdrawal seizures in mice with a history of multiple withdrawal experience. Pharmacol Biochem Behav. 1997a;57:179–183. doi: 10.1016/s0091-3057(96)00303-6. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997b;14:319–326. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Myrick H, Veatch LM. Pregabalin is effective against behavioral and electrographic seizures during alcohol withdrawal. Alcohol Alcohol. 2006;41:399–406. doi: 10.1093/alcalc/agl029. [DOI] [PubMed] [Google Scholar]
- Becker HC, Redmond N. Role of glutamate in alcohol withdrawal kindling. In: Herman BH, Frankenheim J, Litten R, Sheridan PH, Weight FF, Zukin SR, editors. Glutamate and Addiction. Totowa, NJ: Humana Press; 2003. pp. 375–387. [Google Scholar]
- Becker HC, Veatch LM. Effects of lorazepam treatment for multiple ethanol withdrawals in mice. Alcohol Clin Exp Res. 2002;26:371–380. [PubMed] [Google Scholar]
- Chapman AG, Nanan K, Williams M, Meldrum BS. Anticonvulsant activity of two metabotropic glutamate group I antagonists selective for the mGlu5 receptor: 2-methyl-6- (phenylethynyl)-pyridine (MPEP), and (E)-6-methyl-2-styryl-pyridine (SIB 1893) Neuropharmacology. 2000;39:1567–1574. doi: 10.1016/s0028-3908(99)00242-7. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol Clin Exp Res. 1999;23:1698–1703. doi: 10.1111/j.1530-0277.1999.tb04063.x. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effects of acamprosate on excitatory amino acids during multiple ethanol withdrawal periods. Alcohol Clin Exp Res. 2003a;27:465–470. doi: 10.1097/01.ALC.0000056617.68874.18. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur J Pharmacol. 2003b;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- Dalby NO, Thomsen C. Modulation of seizure activity in mice by metabotropic glutamate receptor ligands. J Pharmacol Exp Ther. 1996;276:516–522. [PubMed] [Google Scholar]
- Dodd PR, Beckmann AM, Davidson MS, Wilce PA. Glutamate-mediated transmission, alcohol, and alcoholism. Neurochem Int. 2000;37:509–533. doi: 10.1016/s0197-0186(00)00061-9. [DOI] [PubMed] [Google Scholar]
- Epstein EE, Labouvie E, McCrady BS, Swingle J, Wern J. Development and validity of drinking pattern classification: binge, episodic, sporadic, and steady drinkers in treatment for alcohol problems. Addict Behav. 2004;29:1745–1761. doi: 10.1016/j.addbeh.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, et al. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2007 doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Nelson LC, Lu XY, Kalivas PW. Neuroadaptations in ionotropic and metabotropic glutamate receptor mRNA produced by cocaine treatment. J Neurochem. 1999;72:157–165. doi: 10.1046/j.1471-4159.1999.0720157.x. [DOI] [PubMed] [Google Scholar]
- Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- Hoffman PL. NMDA receptors in alcoholism. Int Rev Neurobiol. 2003;56:35–82. doi: 10.1016/s0074-7742(03)56002-0. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Bursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: rate-dependent influence and interaction with NMDA receptors. Cereb Cortex. 2006;16:93–105. doi: 10.1093/cercor/bhi087. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Johnson BA. An overview of the development of medications including novel anticonvulsants for the treatment of alcohol dependence. Expert Opin Pharmacother. 2004;5:1943–1955. doi: 10.1517/14656566.5.9.1943. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Mann K. New achievements and pharmacotherapeutic approaches in the treatment of alcohol dependence. Eur J Pharmacol. 2005;526:163–171. doi: 10.1016/j.ejphar.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Klodzinska A, Bijak M, Chojnacka-Wojcik E, Kroczka B, Swiader M, Czuczwar SJ, Pilc A. Roles of group II metabotropic glutamate receptors in modulation of seizure activity. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:283–288. doi: 10.1007/s002109900197. [DOI] [PubMed] [Google Scholar]
- Klodzinska A, Chojnacka-Wojcik E, Pilc A. Selective group II glutamate metabotropic receptor agonist LY354740 attenuates pentetrazole- and picrotoxin-induced seizures. Pol J Pharmacol. 1999;51:543–545. [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D'Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, Harris RA, Gelernter J, Lappalainen J. γ-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63:957–968. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- Lea PM, Custer SJ, Vicini S, Faden AI. Neuronal and glial mGluR5 modulation prevents stretch-induced enhancement of NMDA receptor current. Pharmacol Biochem Behav. 2002;73:287–298. doi: 10.1016/s0091-3057(02)00825-0. [DOI] [PubMed] [Google Scholar]
- Loscher W, Dekundy A, Nagel J, Danysz W, Parsons CG, Potschka H. mGlu1 and mGlu5 receptor antagonists lack anticonvulsant efficacy in rodent models of difficult-to-treat partial epilepsy. Neuropharmacology. 2006;50:1006–1015. doi: 10.1016/j.neuropharm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ. Glutamate-based therapeutic approaches: allosteric modulators of metabotropic glutamate receptors. Curr Opin Pharmacol. 2006;6:98–102. doi: 10.1016/j.coph.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Miller JW, Gfroerer JC, Brewer RD, Naimi TS, Mokdad A, Giles WH. Prevalence of adult binge drinking: a comparison of two national surveys. Am J Prev Med. 2004;27:197–204. doi: 10.1016/j.amepre.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Chapman AG, De Sarro G, Meldrum BS. Glutamate metabotropic receptors as targets for drug therapy in epilepsy. Eur J Pharmacol. 2003;476:3–16. doi: 10.1016/s0014-2999(03)02149-6. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Jeffrey M, Talebi A, Beart PM, Chapman AG, Meldrum BS. Anti-epileptic activity of group II metabotropic glutamate receptor agonists (--)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate ( LY379268) and (--)-2-thia-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate ( LY389795) Neuropharmacology. 2001a;41:8–18. doi: 10.1016/s0028-3908(01)00044-2. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Talebi A, Beart PM, Chapman AG, Meldrum BS. The mGlu(2/3) agonist 2R,4R-4-aminopyrrolidine-2,4-dicarboxylate, is anti- and proconvulsant in DBA/2 mice. Neurosci Lett. 2001b;299:125–129. doi: 10.1016/s0304-3940(00)01732-8. [DOI] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, Harkness AR, Grutsch JL, Jr, Wright RA, Johnson BG, et al. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid ( LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- Olive MF. mGlu5 receptors: neuroanatomy, pharmacology, and role in drug addiction. Curr Psychiatry Rev. 2005;1:197–214. [Google Scholar]
- Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115:116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Spooren W, Gasparini F. mGlu5 receptor antagonists: a novel class of anxiolytics? Drug News Perspect. 2004;17:251–257. doi: 10.1358/dnp.2004.17.4.829052. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Ragan P, Chang R, Chen S, Linnoila VM, Coyle JT. Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am J Psychiatry. 1998;155:726–732. doi: 10.1176/ajp.155.6.726. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Becker HC. Electrographic and behavioral indices of ethanol withdrawal sensitization. Brain Res. 2002;946:272–282. doi: 10.1016/s0006-8993(02)02895-0. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Becker HC. Lorazepam and MK-801 effects on behavioral and electrographic indices of alcohol withdrawal sensitization. Brain Res. 2005;1065:92–106. doi: 10.1016/j.brainres.2005.10.047. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002a;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Devadoss J, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002b;308:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]