Abstract
Purpose
To evaluate the use of pulsed high-intensity focused ultrasound exposures to improve tissue plasminogen activator (tPA)-mediated thrombolysis in an in vitro model.
Materials and Methods
All experimental work was compliant with institutional guidelines and HIPAA. Clots were formed by placing 1 mL of human blood in closed-off sections of pediatric Penrose tubes. Four experimental groups were evaluated: control (nontreated) clots, clots treated with pulsed high-intensity focused ultrasound only, clots treated with tPA only, and clots treated with pulsed high-intensity focused ultrasound plus tPA. The focused ultrasound exposures (real or sham) were followed by incubations of the clots in tPA with saline or in saline only. Thrombolysis was measured as the relative reduction in the mass of the clot. d-Dimer assays also were performed. Two additional experiments were performed and yielded dose-response curves for two exposure parameters: number of pulses per raster point and total acoustic power. Radiation force-induced displacements caused by focused ultrasound exposures were simulated in the clots. A Tukey-Kramer honestly significant difference test was performed for comparisons between all pairs of experimental groups.
Results
The clots treated with focused ultrasound alone did not show significant increases in thrombolysis compared with the control clots. The clots treated with focused ultrasound plus tPA showed a 50% ([30.2/20.1]/20.1) increase in the degree of thrombolysis compared with the clots treated with tPA only (P < .001), further corroborating the d-dimer assay results (P < .001). Additional experiments revealed how increasing both the number of pulses per raster point and the total acoustic power yielded corresponding increases in the thrombolysis rate. In the latter experiment, simulations performed at a range of power settings revealed a direct correlation between increased displacement and observed thrombolysis rate.
Conclusion
The rate of tPA-mediated thrombolysis can be enhanced by using pulsed high-intensity focused ultrasound exposure in vitro.
Venous thromboembolism, which includes deep venous thrombosis and pulmonary embolism, accounts for about 250 000 hospitalizations per year in the United States (1). Venous thromboembolism is reportedly the third most common life-threatening cardiovascular disease in the United States (2). Complications of venous thrombosis include potentially fatal pulmonary embolism and chronic venous stasis in the legs.
For decades patients with venous thrombosis have been treated primarily with anticoagulation, which is usually effective at slowing further thrombus formation. However, anticoagulation is often inadequate for eliminating the source of subsequent emboli, alleviating the hemodynamic disturbances, preventing subsequent valvular damage, and preventing permanent impairment to the pulmonary vascular bed (3). For this reason, more aggressive therapy, such as thrombolysis or thrombectomy, is sometimes used.
Ultrasound has been studied as a treatment adjunct to thrombolytic drugs for thrombolysis, as well as an independent treatment method in various models (4-9). Catheter-based systems have been used with oscillating wires to cause direct thrombolysis (10-12). However, these methods are considerably more invasive than externally delivered ultrasound. Heat production via externally delivered ultrasound has been shown to increase thrombolysis (13). Microbubbles have been added during focused ultrasound exposure to increase acoustic cavitation for clot lysis (14,15). Focused ultrasound exposures that involve the use of thermal and cavitational mechanisms are limited by the potential for destructive side effects to the surrounding normal tissue and the blood vessels, which represents a major limitation in facilitating thrombolysis.
Pulsed high-intensity focused ultrasound has recently been used in animal models to improve the delivery of a magnetic resonance imaging contrast agent (16), a liposome-encapsulated chemotherapeutic agent and a large-molecular-weight fluorophore (17), and naked DNA (18) in a nondestructive and reversible manner. The purpose of our study was to evaluate the use of pulsed high-intensity focused ultrasound exposures to improve tissue plasminogen activator (tPA)-mediated thrombolysis in an in vitro model.
Materials and Methods
Our study was performed in compliance with approved institutional guidelines and the Health Insurance Portability and Accountability Act. All experiments were designed and supervised by three authors (V.F., M.H., K.C.P.L.).
Pulsed High-Intensity Focused Ultrasound System
A custom-built image-guided pulsed high-intensity focused ultrasound unit modified from the Sonoblate 500 system (Focus Surgery; Indianapolis, Ind) was used for our studies. The probe contained a 1-MHz therapeutic transducer and a 10-MHz colinear imaging transducer, both with a focal length of 4 cm. The therapeutic transducer was concave and spherical and had a diameter of 5 cm; the aperture of the imaging transducer was 0.8 cm. A maximum power output of 120 W was available with use of the therapeutic transducer. The focal zone of the therapeutic transducer was ellipsoid and had a radial diameter (-3 dB) of 1.38 mm and an axial length (-3 dB) of 7.2 mm. The overall focusing factor of the therapeutic transducer was approximately 1.3 × 103.Exposures were performed in a tank of degassed water with the temperature maintained at 37°C.
Clot Preparation
In a protocol approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases, venous blood was withdrawn from three healthy volunteers after they provided informed consent. One milliliter of blood was immediately placed in individual pre-cut 6-inch sections (diameter, 5/16 inch) of argyle latex Penrose tubes (Sherwood Medical, St Louis, Mo). The tubes were closed at each end with 110-mm dialysis tube closures (Sigma-Aldrich, St Louis, Mo). Two tubes were closed by using one set of closures. A small (1-cm) section of rubber hosing was placed between the two closures at each end to stabilize the clots and minimize torsion. Transpore tape (3M, St Paul, Minn) also was wrapped around each end of the closures to ensure that they did not accidentally open during handling. The tubes containing the blood (Fig 1) were then placed in a 37°C water bath for 90 minutes. (Results of preliminary trials have demonstrated that clots within tubes are completely formed after this time period.) For the clots exposed to pulsed high-intensity focused ultrasound, the front face of the closures was cut away to enable the tubes to be positioned within the focal zone of the transducer so as not to protrude and impede the transducer’s lateral scanning motion (Fig 1). This process was performed by two authors (J.O., M.P.).
Figure 1.

(a)Dialysis tube closures used to enclose blood samples in latex tubes. Altered closures are shown on the left; arrow points to the region that has been cut away. (b) Two blood samples enclosed by using dialysis closures in the latex tubes. (c) Clots with the enclosed tubes, which are held upright in a tank of degassed water directly opposite the high-intensity focused ultrasound transducer (arrow). A probe containing a digital thermometer used to monitor the tank water temperature is shown on the left.
Pulsed High-Intensity Focused Ultrasound Exposures
For those clots that were exposed to pulsed high-intensity focused ultrasound, one set of enclosed tubes (two tubes in total) was mounted horizontally on a custom-built holder and attached to a three-dimensional stage (Fig 1). The stage enabled manipulation of the clot in relation to the upright high-intensity focused ultrasound transducer so that the tubes could be positioned symmetrically within the transducer’s focal zone (Fig 2). During the focused ultrasound exposures, two unexposed clots were placed in the high-intensity focused ultrasound tank, outside the range of exposure. The pulsed high-in-tensity focused ultrasound exposure parameters that were kept constant throughout the study were duty cycle (10%, 100 msec/1000 msec) and pulse repetition frequency (1 Hz). Other parameters were varied, depending on the experiment. The two clots designated for focused ultrasound exposures received the exposures individually.
Figure 2.

B-mode ultrasonograms of clots (in latex tubes) lined up for pulsed high-intensity focused ultrasound exposure. Large arrows point to guide marks for the transducer’s focal zone; small arrow points to raster points spaced 2 mm apart. Lateral scan shows one clot. Vertical scan shows two clots, positioned one on top of each other, with the top clot marked for pulsed high-intensity focused ultrasound exposure.
tPA Treatments and Thrombolysis Assessment
After the clots were exposed to pulsed high-intensity focused ultrasound, the tube was cut open and the clots in all of the experimental groups were removed by using a spatula and placed in preweighed 14-mL test tubes that contained 2-mL solutions of either tPA in saline or saline only. The test tubes were then weighed again to determine the size of the clots. Depending on the experiment, 2 mg/mL preparations of tPA were diluted with saline (0.15 mol/L) at various concentrations. The clots were incubated in tPA at 37°C for various durations, depending on the experiment. After the incubations, the clots were removed from the test tubes and weighed on 1-inch square pieces of parafilm. Thrombolysis (T), expressed as a percentage, was defined as the relative reduction in the mass of the clot:
| (1) |
where Win is the initial weight of the clot and Wfi is the final weight of the clot. These processes were performed by two authors (J.O., V.F.).
d-Dimer Assays
A commercial d-dimer assay kit (Diagnostica Stago, Parsippany, NJ) was used to measure d-dimer levels in the serum after the clots were incubated. The assays were performed according to the directions provided by the manufacturer by two authors (M.P., M.H.).
Preliminary Experiments
Preliminary experiments were performed to determine the experimental conditions suitable for evaluating the potential effects of the pulsed high-intensity focused ultrasound exposures. For each experiment, four experimental clot groups were used: a control group of clots that were treated with neither pulsed high-intensity focused ultrasound exposure nor tPA; clots treated with high-intensity focused ultrasound only, clots treated with tPA only, and clots treated with high-intensity focused ultrasound and tPA. The pulsed high-intensity focused ultrasound parameters that were varied were total acoustic power (TAP), number of pulses per raster point, and number of raster points per clot. The tPA concentration and incubation time also were varied. Two clots were treated in each experiment group. This process was carried out by two authors (J.O., V.F.). The different focused ultrasound exposure and tPA incubation parameters used are summarized in Table 1.
Table 1. Pulsed High-Intensity Focused Ultrasound Exposure and tPA Incubation Parameters Used in Preliminary Experiments.
| Trial No. | TAP (W) | No. of Raster Points* | No. of Pulses per Raster Point | tPA Concentration (mg/mL) | tPA Incubation Duration (min) |
|---|---|---|---|---|---|
| 1 | 20 | 5 × 1 | 60 | 0.10 | 120 |
| 2 | 40 | 6 × 1 | 60 | 0.10 | 120 |
| 3 | 40 | 6 × 2 | 60 | 0.10 | 120 |
| 4 | 60 | 6 × 2 | 60 | 0.10 | 120 |
| 5 | 60 | 6 × 3 | 40 | 0.10 | 120 |
| 6 | 60 | 6 × 2 | 60 | 0.10 | 120 |
| 7 | 60 | 6 × 2 | 60 | 0.02 | 120 |
| 8 | 60 | 6 × 2 | 60 | 0.01 | 60 |
| 9 | 60 | 6 × 2 | 60 | 0.01 | 30 |
The raster pattern was distributed uniformly across the clot (in horizontal [first number] times vertical [second number] pattern).
Standard Pulsed High-Intensity Focused Ultrasound Exposure and tPA Treatment
In this experiment, the same four experimental groups involved in the preliminary experiments were used; however, this time, eight clots were treated in each group. The pulsed high-intensity focused ultrasound exposures involved a TAP of 60 W, 60 pulses per raster point, and 12 raster points per clot. The clots were incubated in a tPA concentration of 0.01 mg/mL for 30 minutes. This set of experimental conditions was the same as that of preliminary experiment 9 (Table 1). d-Dimer assays for each clot in each group also were performed. This process was performed by two authors (J.O., V.F.).
Optimization Experiments
In each of two separate experiments, one of the pulsed high-intensity focused ultrasound exposure parameters was varied and all remaining parameters were kept constant at the levels used in the standard exposure experiments. For each experiment, two groups were used: clots treated with tPA only and clots treated with high-intensity focused ultrasound plus tPA. All of the focused ultrasound exposures except the one that was varied were the same as those used in the previous standard experiments. In the first experiment, TAP levels of 20, 40, 60, and 80 W were evaluated. In the second experiment, 20, 40, 60, and 80 pulses per raster point were evaluated. For all groups in both experiments, the tPA incubations were the same as those performed in the previous standard experiment. Five clots were treated in each group. These experiments were performed by two authors (J.O., V.F.).
Simulations of Local Displacement Induced by Pulsed High-Intensity Focused Ultrasound Exposures
The acoustic radiation force generated by high-intensity focused ultrasound exposures has been demonstrated to induce internal tissue motion. The acoustic radiation force —that is, the body force generated in tissue as a result of the energy gradient caused by acoustic energy absorption—is proportional to the in situ acoustic intensity (, expressed in watts per square centimeters), which is usually a function of space:
| (2) |
where is the attenuation coefficient (in Np · cm-1 · MHz-1), f is the frequency (in MHz), and c is the speed of sound in the medium (in cm · sec-1)(19). The displacement generated by the acoustic radiation force associated with pulsed high-intensity focused ultrasound is defined by using a set of equations for motion that relate displacement with tissue characteristics such as absorption coefficient and elastic properties (eg, Young modulus and Poisson ratio) (19). Our objective in performing the simulations was to investigate the correlation between the focused ultrasound exposure parameters and the internal tissue movements induced by the acoustic radiation force.
First, the spatial intensity profile of the high-intensity focused ultrasound beam generated by the transducer used in this experimental study was computed. A finite difference algorithm was then developed and used to numerically solve the equations for motion and compute the distribution of the displacement generated by the focused ultrasound beam with the specific exposure parameters used in this study (ie, frequency, TAP, pulse width, and pulse repetition frequency). The clots were assumed to have the general characteristics of a typical soft tissue. These simulations were performed by one author (C.D.).
Statistical Analyses
With use of the JMP software package (SAS, Cary, NC), a Tukey-Kramer honestly significant difference test was performed for comparisons between all pairs of experimental groups. Differences that yielded a P value of less than .05 were considered to be significant. These analyses were performed by one author (V.F.).
Results
Preliminary Experiments
In the preliminary experiments, the pulsed high-intensity focused ultrasound parameters used, as well as the duration and concentration of the tPA incubations, were varied. In general, the TAP of the exposures was increased from 20 W to 60 W, and the number of raster points per clot was increased from five to 12. Combined, these parameter changes contributed to the total increase in the amount of acoustic energy used per clot. In addition, the tPA concentration was reduced by an order of magnitude, from 0.10 to 0.01 mg/mL, and the incubation time was reduced by 75%, from 2 hours to 30 minutes. These two reductions decreased the overall degree of tPA-mediated thrombolysis. Together, these changes were incorporated in consecutive trials and caused a trend of increased disparity in the degree of thrombolysis between the clots treated with focused ultrasound plus tPA and those treated with tPA only (Fig 3).
Figure 3.
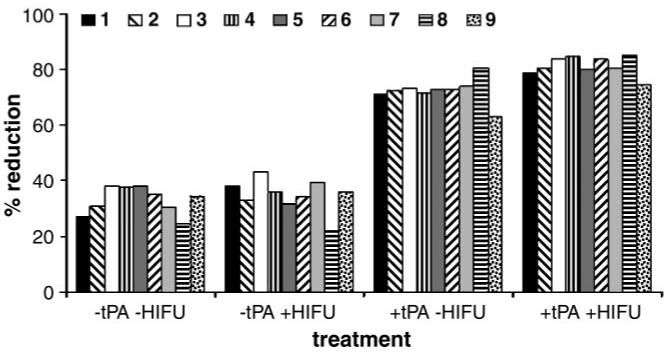
Graph shows results of nine trials performed in the preliminary experiments. (See Table 1 for pulsed high-intensity focused ultrasound [HIFU] exposure and tPA incubation details.) A trend of increasing disparity in the degree of clot size reduction was observed as the pulsed high-intensity focused ultrasound and tPA incubation parameter levels were varied from trials 1-9. Bars represent group means.
Standard Pulsed High-Intensity Focused Ultrasound Exposures and tPA Treatments
This experiment involved the use of the parameter levels of pulsed high-intensity focused ultrasound exposures and tPA incubations that were evaluated in the last trial (trial 9) of the preliminary experiments. Clots treated with focused ultrasound plus tPA incurred a 19% ([63.2/53.1]/53.1) greater degree of thrombolysis compared with clots treated with tPA only (P < .001). The degree of thrombolysis achieved in both of these groups was greater than that achieved in the control (untreated) and focused ultrasound exposure only groups; significant differences in thrombolysis between the two latter groups were not found. When the degree of thrombolysis achieved in the control and focused ultrasound only groups was subtracted from that achieved in the tPA only and focused ultrasound plus tPA groups, a 50% ([30.2/20.1]/20.1) increase in the degree of thrombolysis was observed in the clots treated with focused ultrasound plus tPA compared with the degree of thrombolysis observed in the clots treated with tPA only (P < .001).
The d-dimer assay results in this experiment mirrored the calculated degrees of thrombolysis achieved in the clots. The clots treated with focused ultrasound plus tPA released d-dimer concentrations that were 53% ([37 768/24 650]/24 650) higher than the d-dimer concentrations released by the clots treated with tPA only (P < .001). The d-dimer levels measured in the control (untreated) clots and in the clots treated with focused ultrasound only were less than two orders of magnitude lower than the d-dimer levels measured in both groups of clots treated with tPA (Fig 4).
Figure 4.

Graph shows results of the standard pulsed high-intensity focused ultrasound (HIFU) experiments. The degree of thrombolysis in clots treated with focused ultrasound plus tPA was significantly greater than the degree of thrombolysis in clots treated with tPA only. In both these groups, the degree of thrombolysis was significantly greater than that in the control (untreated) clots and the clots treated with focused ultrasound exposures only. Differing lowercase letters between mean levels of thrombolysis indicate a significant difference of at least P = .05. Bars represent group means with standard deviations. d-Dimer values for the four experimental groups appear inside the bars and directly reflect the results of thrombolysis.
Optimization Experiments
In these experiments, one pulsed high-intensity focused ultrasound exposure parameter was varied, with the remaining parameters kept constant at the levels used in the standard exposure experiments. The degree of thrombolysis in the clots exposed to pulsed high-intensity focused ultrasound at a TAP of 20 W was not significantly different from the degree of thrombolysis in the clots treated with tPA only. The degree of thrombolysis achieved at 40 W, however, was greater than that achieved at 20 W (P = .041). Increasing the TAP to 60 W caused a further increase in the degree of thrombolysis compared with the degree of thrombolysis achieved at 40 W (P = .045); however, an additional increase in the degree of thrombolysis was not observed at 80 W (Fig 5).
Figure 5.

Graph shows results of the first optimization experiment. TAP levels were increased from 20 to 80 W, and all remaining exposure parameters were kept constant at the levels used in the standard exposure experiments. Differing lowercase letters between mean levels of thrombolysis indicate a significant difference of at least P = .05. Data points represent group means with standard deviations.
The degree of thrombolysis in the clots exposed to pulsed high-intensity focused ultrasound with 20 and 40 pulses per raster point was not significantly different from the degree of thrombolysis in the clots treated with tPA only. In the clots that received 60 pulses, however, the degree of thrombolysis was greater than that achieved at 40 pulses (P = .021). Increasing the number of pulses to 80 per raster did not cause a further increase in the degree of thrombolysis (Fig 6).
Figure 6.

Graph shows results of the second optimization experiment. The number of pulses per raster point was increased from 20 to 80, and all remaining exposure parameters were kept constant at the levels used in the standard exposure experiments. Differing lowercase letters between mean levels of thrombolysis indicate a significant difference of at least P = .05. Data points represent group means with standard deviations.
Simulations of High-Intensity Focused Ultrasound-induced Displacement
The effect of varying the high-intensity focused ultrasound TAP on displacement was investigated to demonstrate the influence of TAP on displacement distributions. The displacement simulations performed at power settings of 20, 40, 60, and 80 W—with the pulse width kept constant at 100 msec—revealed a nonuniform distribution of displacement in both the lateral and the axial dimensions (Fig 7), with maximal axial displacement (ie, high-intensity focused ultrasound beam propagation) occurring at the focus of the ultrasound beam. Shear stress and the resulting strain are expected to be highest in the region of the largest spatial gradient of displacement, such as the border region of the focal zone in both the lateral and the axial dimensions. The simulation results showed also a linear increase in peak displacement with increased TAP (Table 2).
Figure 7.

Simulation results achieved with a TAP of 20 W and a high-intensity focused ultrasound pulse of 100 msec in a clot show a nonuniform distribution of displacement in the lateral and axial dimensions. The peak displacement in the center of the focused ultrasound beam is just higher than 12 μm.
Table 2. Displacement Simulation Results.
| TAP (W) | Peak Axial Displacement (μm) |
|---|---|
| 20 | 12.5 |
| 40 | 27.4 |
| 60 | 44.8 |
| 80 | 59.1 |
Discussion
Various in vitro studies have been performed to determine whether focused ultrasound exposures can improve thrombolysis. Focused ultrasound exposures alone, with use of both vibrating wires (10,20) and external applications (8), have been shown to disrupt clots. The mechanisms involved in producing such effects are generally considered to involve acoustic cavitation (8), which can be extremely destructive to cell surfaces (21). Studies involving the use of focused ultrasound exposure combined with various thrombolytic drugs also have been performed (5,22,23). Although different in vitro clot models that were suited to the ultrasound sources were used, these studies revealed how combining the drugs with focused ultrasound exposure enhanced the thrombolysis achieved compared with that achieved by using the drugs alone. Regarding the mechanism underlying the degree of enhancement when ultrasound and drug treatments are combined (22), study results have suggested that various effects of acoustic cavitation (eg, bubble collapse and microstreaming) could have altered the structure of the fibrin fibers and consequently created new binding sites for the tPA molecules. Further evidence in support of this mechanism remains to be elucidated.
Although initial reports on the use of pulsed high-intensity focused ultrasound to enhance the delivery of thrombolytic agents in vivo have definitively shown the benefits of focused ultrassound exposures (16-18), an understanding of the mechanism that produced the results was less clear at the time of the reports. Bednarski et al (16) reasoned that the short high-energy pulses emitted induced focused regional shock waves that altered the vascular permeability without permanently damaging tissue. Shock waves in soft tissues can induce shearing and tearing and consequently produce microscopic lesions that grow and merge during repeated pulses (24). The resulting trauma (25), macroscopic tears in the vasculature (26), and hemorrhage (27) are generally considered to have negative effects on delivery enhancement, where induced vascular damage and stasis have been shown to decrease nanoparticle extravasation (28).
In the Yuh et al study (17), our findings demonstrated how pulsed high-intensity focused ultrasound exposures can enhance vessel permeability, and in Dittmar et al (18), we went on to observe how these exposures produce no detectable destructive effects in the exposed tissue. In Dittmar et al (18), we proposed a mechanism by which these exposures enhance delivery, where acoustic forces produced by short high-intensity focused ultrasound pulses create displacement in the tissues, similar to the displacements reported by Nightingale et al (29) and Lizzi et al (19). Nonuniform displacement in the region of the focal zone, as demonstrated in the Lizzi et al study (19), could produce shear forces and consequent strain that are capable of widening intercellular spaces; this process would ultimately facilitate improved extravasation of the macromolecules from the vasculature as well as improved diffusion throughout the parenchyma. Focused ultrasound exposures have been reported to create gaps between endothelial cells (30,31) and widen the intercellular spaces in epithelial tissue (32). Frenkel et al (33) showed that this latter effect could be used to increase both the depth of penetration and the effective diffusion rate of nanoparticles throughout the tissue.
In this study, we report, to our knowledge, the first evidence that acoustic radiation forces could be directly responsible for producing structural changes in tissue and consequently cause increased transport of macromolecules. In the first of two optimization experiments that we performed, increasing the TAP level from 20 to 40 W led to a significantly increased rate of thrombolysis, with the thrombolysis rates achieved at 20 W being no different from those achieved when no focused ultrasound exposures were used. A second increase in TAP from 40 to 60 W caused a further significant increase in thrombolysis. Increasing the TAP, or intensity, of the exposure (intensity equals TAP divided by the surface area of the focal zone) will cause a proportional increase in the radiation force (Eq [2]). Our simulations revealed a linear increasing dependence between the radiation force-induced displacement amplitude and the high-intensity focused ultrasound TAP in the range of 20-60 W.
Braaten et al (34) found that focused ultrasound exposures can reversibly disaggregate fibers in purified fibrin gels. Similar focused ultrasound exposures have been shown to significantly increase the flow of fluid through the gels (35). In a related study, clots were used in place of the gels and the penetration of radiolabeled tPA molecules, with their active sites blocked, was evaluated with and without focused ultrasound exposures (36). The results indicated that the uptake of tPA correlated with the duration of the exposures, with mean uptake levels being significantly greater in the clots exposed to ultrasound. In addition, both the mean and the median penetration depths of the tPA were greater in the clots exposed to ultrasound.
In view of the findings in the above-mentioned studies, it is not unreasonable to suggest that in the present study, pulsed high-intensity focused ultrasound exposures caused repetitive displacements in the clots, ultimately creating structural changes that enabled greater amounts of tPA to penetrate the clots and exposing additional binding sites and consequently increasing the thrombolysis rate. This proposed mechanism is supported by the direct correlation between the observed rates of thrombolysis and the predicted displacements caused by the focused ultrasound exposures.
These study results clearly demonstrate a significant improvement in tPA-mediated thrombolysis with the addition of pulsed high-intensity focused ultrasound exposures in vitro. The exposure parameters were optimized for the described in vitro system, although different exposure parameters may be optimal for in vivo models. Therefore, further optimization studies with in vivo models are necessary before the clinical use of the described focused ultrasound-tPA protocol. Although the described exposures previously have been shown to be safe for in vivo use (ie, not to have destructive effects on cells), their safety still needs to be further evaluated before clinical implementation. Furthermore, the possible effects of surrounding tissue and the pharmacokinetic properties of tPA in an in vivo system were not addressed in this in vitro study.
Practical applications
After years of research on ultrasound-enhanced thrombolysis, interest in this treatment method remains high, as evidenced by the recent publication of the results of a phase 2 trial (37). Focused ultrasound exposure used in combination with thrombolytic drugs has potential benefits for patients, including quicker resolution of the thrombus and a lower dose of thrombolytic agent, with a resultant reduction in associated bleeding risks. The delivery of focused ultrasound from an external source provides the opportunity to noninvasively treat thrombi in various anatomic areas. Parameters could be optimized to treat sites ranging from peripheral limb arterial or venous occlusions to intracranial thrombi during ischemic stroke. The thrombolysisenhancing effects of pulsed high-intensity focused ultrasound may also improve the effectiveness of pharmacologic thrombolysis in chronic clots, which are mature, organized, and often resistant to the current catheter-based thrombolytic treatments.
Acknowledgments
We thank Bill Draght of the technical support division of the National Institutes of Health for modifying the dialysis tube enclosures. We also thank Dr Sergio Dromi and Amir K. Durrani, B. Eng., for their technical assistance.
M.J.S. supported by the Research Scholars Program of the Howard Hughes Medical Institute/National Institutes of Health.
Abbreviations
- TAP
total acoustic power
- tPA
tissue plasminogen activator
Footnotes
Authors stated no financial relationship to disclose.
References
- 1.De Wet CJ, Pear RG. Postoperative thrombotic complications: venous thromboembolism—deep-vein thrombosis and pulmonary embolism. Anesthesiol Clin North Am. 1999;17:895–922. [Google Scholar]
- 2.Goldhaber S. Epidemiology of pulmonary embolism and deep vein thrombosis. In: Bloom AL, Forbes CD, Thombas DP, Tuddenham EGD, editors. Haemostasis and thrombosis. 3rd ed. Churchill Livingstone; New York, NY: 1994. pp. 1327–1333. [Google Scholar]
- 3.a National Institutes of Health Consensus Development Conference Thombolytic therapy in thrombosis. Ann Intern Med. 1980;93:141–144. doi: 10.7326/0003-4819-93-1-141. [DOI] [PubMed] [Google Scholar]
- 4.Tachibana K. Enhancement of fibrinolysis with ultrasound energy. J Vasc Interv Radiol. 1992;3:299–303. doi: 10.1016/s1051-0443(92)72029-6. [DOI] [PubMed] [Google Scholar]
- 5.Luo H, Steffen W, Cercek B, Arunasalam S, Maurer G, Siegel RJ. Enhancement of thrombolysis by external ultrasound. Am Heart J. 1993;125:1564–1569. doi: 10.1016/0002-8703(93)90741-q. [DOI] [PubMed] [Google Scholar]
- 6.Kashyap A, Blinc A, Marder VJ, Penney DP, Francis CW. Acceleration of fibrinolysis by ultrasound in a rabbit ear model of small vessel injury. Thromb Res. 1994;76:475–485. doi: 10.1016/0049-3848(95)90179-j. [DOI] [PubMed] [Google Scholar]
- 7.Kornowski R, Meltzer RS, Chernine A, Vered Z, Battler A. Does external ultrasound accelerate thrombolysis? results from a rabbit model. Circulation. 1994;89:339–344. doi: 10.1161/01.cir.89.1.339. [DOI] [PubMed] [Google Scholar]
- 8.Luo H, Nishioka T, Berglund H, et al. Effect of external ultrasound frequency on thrombus disruption in vitro. J Thromb Thrombolysis. 1996;2:63–66. doi: 10.1007/BF00226413. [DOI] [PubMed] [Google Scholar]
- 9.Riggs PN, Francis CW, Bartos SR, Penney DP. Ultrasound enhancement of rabbit femoral artery thrombolysis. Cardiovasc Surg. 1997;5:201–207. doi: 10.1016/s0967-2109(96)00093-2. [DOI] [PubMed] [Google Scholar]
- 10.Hong AS, Chae JS, Dubin SB, Lee S, Fishbein MC, Siegel RJ. Ultrasonic clot disruption: an in vitro study. Am Heart J. 1990;120:418–422. doi: 10.1016/0002-8703(90)90088-f. [DOI] [PubMed] [Google Scholar]
- 11.Rosenschein U, Roth A, Rassin T, Basan S, Laniado S, Miller H. Analysis of coronary ultrasound thrombolysis endpoints in acute myocardial infarction (ACUTE Trial) Circulation. 1997;95:1411–1416. doi: 10.1161/01.cir.95.6.1411. [DOI] [PubMed] [Google Scholar]
- 12.Rosenschein U, Gaul G, Erbel R, et al. Percutaneous transluminal therapy of occluded saphenous vein grafts. Circulation. 1999;99:26–29. doi: 10.1161/01.cir.99.1.26. [DOI] [PubMed] [Google Scholar]
- 13.Sakharov DV, Hekkenberg RT, Rijken DC. Acceleration of fibrinolysis by high-frequency ultrasound: the contribution of acoustic streaming and temperature rise. Thromb Res. 2000;100:333–340. doi: 10.1016/s0049-3848(00)00319-4. [DOI] [PubMed] [Google Scholar]
- 14.Cintas P, Nguyen F, Boneu B, Larrue V. Enhancement of enzymatic fibrinolysis with 2-MHz ultrasound and microbubbles. J Thromb Haemost. 2004;2:1163–1166. doi: 10.1111/j.1538-7836.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsui JM, Grayburn PA, Xie F, Porter TR. Drug and gene delivery and enhancement of thrombolysis using ultrasound and micro-bubbles. Cardiol Clin. 2004;22:299–312. doi: 10.1016/j.ccl.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Bednarski MD, Lee JW, Callstrom MR, Li KC. In vivo target-specific delivery of macromolecular agents with MR-guided focused ultrasound. Radiology. 1997;204:263–268. doi: 10.1148/radiology.204.1.9205257. [DOI] [PubMed] [Google Scholar]
- 17.Yuh EL, Shulman SG, Mehta SA, et al. Delivery of systemic chemotherapeutic agent to tumors by using focused ultrasound: study in a murine model. Radiology. 2005;234(2):431–437. doi: 10.1148/radiol.2342030889. [DOI] [PubMed] [Google Scholar]
- 18.Dittmar K, Xie J, Hunter F, et al. Pulsed high-intensity focused ultrasound enhances systemic administration of naked DNA in squamous cell carcinoma model: initial experience. Radiology. 2005;235(2):541–546. doi: 10.1148/radiol.2352040254. [DOI] [PubMed] [Google Scholar]
- 19.Lizzi FL, Muratore R, Deng CX, et al. Radiation-force technique to monitor lesions during ultrasonic therapy. Ultrasound Med Biol. 2003;29(11):1593–1605. doi: 10.1016/s0301-5629(03)01052-4. [DOI] [PubMed] [Google Scholar]
- 20.Rosenschein U, Bernstein JJ, DiSegni E, Kaplinsky E, Bernheim J, Rozensajn LA. Experimental ultrasonic angioplasty: disruption of atherosclerotic plaques and thrombi in vitro and arterial recanalization in vivo. J Am Coll Cardiol. 1990;15:711–717. doi: 10.1016/0735-1097(90)90651-5. [DOI] [PubMed] [Google Scholar]
- 21.Frenkel V, Kimmel E, Iger Y. Ultrasound-induced cavitation damage to external epithelia of fish skin. Ultrasound Med Biol. 1999;25(8):1295–1303. doi: 10.1016/s0301-5629(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 22.Everbach EC, Francis CW. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound Med Biol. 2000;26:1153–1160. doi: 10.1016/s0301-5629(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 23.Suchkova V, Carstensen EL, Francis CW. Ultrasound enhancement of fibrinolysis at frequencies of 27 to 100 kHz. Ultrasound Med Biol. 2002;28:377–382. doi: 10.1016/s0301-5629(01)00522-1. [DOI] [PubMed] [Google Scholar]
- 24.Howard D, Sturtevant B. In vitro study of the mechanical effects of shock-wave lithotripsy. Ultrasound Med Biol. 1997;23(7):1107–1122. doi: 10.1016/s0301-5629(97)00081-1. [DOI] [PubMed] [Google Scholar]
- 25.Kaude JV, Williams CM, Millner MR, Scott KN, Finlayson B. Renal morphology and function immediately after extracorporeal shockwave lithotripsy. AJR Am J Roentgenol. 1985;145(2):305–313. doi: 10.2214/ajr.145.2.305. [DOI] [PubMed] [Google Scholar]
- 26.Willis LR, Evan AP, Connors BA, Reed G, Fineberg NS, Lingeman JA. Effects of extra-corporeal shock wave lithotripsy to one kidney on bilateral glomerular filtration rate and PAH clearance in minipigs. J Urol. 1996;156(4):1502–1506. [PubMed] [Google Scholar]
- 27.Miller DL, Song J. Tumor growth reduction and DNA transfer by cavitation-enhanced high intensity focused ultrasound in vivo. Ultrasound Med Biol. 2003;29(6):887–893. doi: 10.1016/s0301-5629(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 28.Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 2001;61:3027–3032. [PubMed] [Google Scholar]
- 29.Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28:227–235. doi: 10.1016/s0301-5629(01)00499-9. [DOI] [PubMed] [Google Scholar]
- 30.Seidl M, Steinbach P, Wörle K, Hofstaädter F. Induction of stress fibres and intercellular gaps in human vascular endothelium by shockwaves. Ultrasonics. 1994;32(5):397–400. doi: 10.1016/0041-624x(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 31.Mesiwala AH, Farrell L, Wenzel HJ, et al. High-intensity focused ultrasound selectively disrupts the blood brain barrier in vivo. Ultrasound Med Biol. 2002;28(3):389–400. doi: 10.1016/s0301-5629(01)00521-x. [DOI] [PubMed] [Google Scholar]
- 32.Frenkel V, Kimmel E, Iger Y. Ultrasound-induced intercellular space widening in fish epidermis. Ultrasound Med Biol. 2000;26(3):473–480. doi: 10.1016/s0301-5629(99)00164-7. [DOI] [PubMed] [Google Scholar]
- 33.Frenkel V, Kimmel E, Iger Y. Ultrasound-facilitated transport of silver chloride (AgCl) particles in fish skin. J Control Release. 2000;68:251–261. doi: 10.1016/s0168-3659(00)00264-9. [DOI] [PubMed] [Google Scholar]
- 34.Braaten JV, Goss RA, Francis CW. Ultraound reversibly disaggregates fibrin fibers. Thromb Haemost. 1997;78:1063–1068. [PubMed] [Google Scholar]
- 35.Siddiqi F, Blinc A, Braaten J, Francis CW. Ultrasound increases flow through fibrin gels. Thromb Haemost. 1995;73(3):495–498. [PubMed] [Google Scholar]
- 36.Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol. 1995;21:419–424. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- 37.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]


