Abstract
In this first comparative in vitro study, linoleyl hydroxamic acid (LHA), a simple and stable derivative of linoleic acid, was tested as an inhibitor of several enzymes involved in arachidonic acid metabolism in mammals. The tested enzymes were human recombinant 5-lipoxygenase (h5-LO), porcine leukocyte 12-LO, rabbit reticulocyte 15-LO, ovine cyclooxygenases 1/2 (COX1/COX2), and human microsomal prostaglandin E synthase-1 (mPGES-1). Potato tuber and soybean lipoxygenases (ptLOX and sLOX, respectively) were studied for comparative purposes. LHA inhibited most of the tested enzymes with the exception of mPGES-1. The LHA inhibitory activity increased as follows: mPGES-1 (no inhibition)<<COX1 = COX2<h5-LO = sLOX = ptLOX<12-LO<<15-LO. The IC50 values for COX1/COX2, h5-LO, 12-LO, and 15-LO were 60, 7, 0.6, and 0.02 μM, respectively. sLOX was the only tested enzyme that was capable of aerobic oxygenation of LHA, producing 13-hydroperoxy-LHA. The enzyme rapidly inactivated during the reaction. Therefore, LHA could be used as an effective LO/LOX inhibitor without affecting COX1/COX2 and mPGES-1. Possible implications of this observation include treating diseases and pathological states that are caused by (or lead to) hyperproduction of LO-derived metabolites, e.g., inflammation, cardiovascular disorders, cancer, asthma, allergies, psoriasis, and stroke.
Keywords: human, animal, arachidonic acid, linoleic acid, enzyme inhibition, anti-inflammatory, anti-cancer, drug discovery
Lipoxygenase (LO) is the common name of a group of related enzymes that catalyze aerobic stereospecific oxidation of PUFAs with Z,Z-pentadienyl fragment in them (1). The major primary product of such a reaction is a hydroperoxide of the corresponding substrate. Two typical natural substrates of LOs are linoleic acid (octadeca-9Z,12Z-dienoic acid, LA) and arachidonic acid (eicosatetra-5E,8E,11E,14E-enoic acid, AA). AA is considered a quintessential substrate for LOs of mammalian origin, whereas LA is a standard substrate for plant LOs, also known as lipoxidases (LOXs). There are several subgroups of LO and LOX that are classified in part on the basis of their substrate specificity, and in part on the basis of their origin.
Depending on the point of insertion of molecular oxygen into AA, mammalian LOs are commonly classified into five major groups: 5-LO (EC 1.13.11.34), 8-LO (EC 1.13.11.40), 11-LO, (EC 1.13.11.45), 12-LO (EC 1.13.11.31), and 15-LO (EC 1.13.11.33). The primary products of corresponding reactions are hydroperoxides of AA (hydroperoxyeicosatetraenoic acids, or HPETEs). Thus, 5-LO produces mainly 5-HPETE, 15-LO, 15-HPETE, etc. No definite information on mammalian 9-LO is available at this time, although 9-hydroxyeicosatetraenoic acid (9-HETE), a product of 9-HPETE reduction, has been found in several tissues (2–5).
LOXs (EC 1.13.11.12) also differ in terms of their positional specificity toward their standard substrate, LA, producing either 9-, or 13-hydroperoxide of LA [9- or 13-hydroperoxyoctadecadienoic acid (HPODE)]. Complicating the classification is the fact that mammalian 15-LO oxidizes LA (6), and plant LOX can effectively oxidize AA (7). For example, under standard conditions, soybean LOX (sLOX) utilizes LA to produce 13(S)-HPODE (8), while making almost exclusively 15(S)-HPETE from AA (9). Potato tuber LOX (ptLOX), on the other hand, catalyzes the formation of various derivatives of LA (10), docosahexaenoic acid (11–13), and AA (14). (J. Whelan gathered and presented in his PhD dissertation (defended at Penn State University in 1988) a wealth of information on LOX-catalyzed oxidation of various polyunsaturated fatty acids.) A brief report by Hombeck, Pohnert, and Boland (15) on AA metabolism by the freshwater diatom Gomphonema parvulum implicated a 9-LO in the formation of 9-HPETE as a major product. Importantly, sLOX is considered a quintessential 15-LOX (9), whereas ptLOX is kinetically and mechanistically similar to human 5-LO (14). Both of the enzymes are currently used in de facto standard LO inhibitor assays produced by Cayman Chemical Co. (Ann Arbor, MI). sLOX was the first LOX ever to crystallize and still is the enzyme whose three-dimensional structure is used to predict structures of other LOs that have so far eluded crystallization. Remarkably, the only other enzyme of the LO/LOX family that has been successfully crystallized is rabbit reticulocyte 15-LO (16).
As key players in the formation of various physiologically active compounds of the leukotriene, lipoxin, and oxylipin families, LOs are in the center of lipid signaling pathways that regulate cell metabolism and apoptosis (17). Overproduction of certain LO metabolites has been shown to be associated with cancer development (18), stroke (19), myocardial infarction (20), ischemia and/or post-ischemic inflammatory response (21–23), arthritis (24, 25), asthma (26, 27), cancer (28), inflammation (29, 30), and many other diseases and pathological states. Therefore, LOs are justifiably among the most important targets for designing selective and safe inhibitors suitable for clinical use.
Among the large number of LO inhibitors, linoleyl hydroxamic acid (LHA) stands out as a simple chemical derivative of the naturally occurring LA. Earlier, we reported results of our preliminary experiments with sLOX (31), ptLOX (32, 33), and porcine leukocyte 12-LO (34). Inhibition of all the LOs was observed at micromolar concentrations of LHA, or below. In independent studies, LHA suppressed activity of a related enzyme, LA 8R-dioxygenase, albeit at rather high concentrations (35). Importantly, no information is available on the ability of LHA to inhibit other PUFA-metabolizing enzymes, e.g., cyclooxygenases 1 and 2 (COX1/COX2).
LHA was tested in vivo and was shown to decrease coronary perfusion pressure, systemic arterial pressure, and cardiac output in dogs under conditions of anaphylactic shock, presumably through downregulation of 5-LO-mediated leukotriene C4 biosynthesis (36). LHA also decreased lipid peroxidation during acute hypoxic hypoxia (37) and lessened the immune damage to the canine heart and coronary vessels in an experimentally induced coronary spasm of immune origin (36, 38). A decrease in free radical oxidation of lipoproteins and in formation of lipoid plaques was observed in rabbits once LHA was administered (39). For general information on hydroxamic acid, a review of Agrawal (40) is a good starting point.
Here, we report the results of a first comparative study of enzyme inhibition by LHA for human recombinant 5-LO (h5-LO), porcine 12-LO, rabbit 15-LO, ovine COX1 and COX2, and human microsomal prostaglandin E synthase-1 (mPGES-1), all of which are involved in oxidative transformations of PUFAs relevant to lipid signaling in mammals and humans.
MATERIALS AND METHODS
Reagents and equipment
Fatty acids were purchased from Nu-Chek Prep, Inc. (Elysian, MN). COX1/COX2 inhibitor assay kits, ptLOX, h5-LO-1, and 12-LO from porcine leukocytes were purchased from Cayman Chemical Co. (Ann Arbor, MI). 15-LO from rabbits was a product of Biomol (Plymouth Meeting, PA). Another sample of h5-LO (h5-LO-2) overexpressed in Escherichia coli and purified on ATP agarose as described earlier (41) was received from Dr. Olof Rådmark (Karolinska Institute, Stockholm, Sweden). HPLC and MS-grade solvents were products of Burdick and Jackson (Muskegon, MI). Other reagents were from Sigma-Aldrich (St. Louis, MO). Ultraviolet (UV) and visible spectra were taken on a Beckman DU800 spectrophotometer (Beckman-Coulter; Fullerton, CA). Chromatographic experiments were conducted on a Waters Alliance 2695 HPLC separations module equipped with a Waters 2996 diode array detector (Waters; Milford, MA). Mass spectra were taken on an LCQ Deca XP Max MSn ion trap spectrometer using Xcalibur software (Thermo Electron; San Jose, CA). NMR spectra were recorded on a Varian 400 MHz spectrometer (Varian Inc.; Palo Alto, CA). Melting point of LHA was determined using an Optimelt MPA100 melting point apparatus (Stanford Research Systems; Sunnyvale, CA).
Synthesis and analyses of LHA
Because neither the analytical procedures for LHA characterization nor its properties have been described before in any detail, the following is a brief description of the methods utilized and the corresponding results. LHA was synthesized using procedures described earlier for polyfluorinated alcadiynoic hydroxamic fatty acids (42). The crude product was purified by normal-phase open-column silica gel chromatography (melting point of the final product, 39 ± 1°C). The purity and structure of LHA was checked by electrospray ionization mass spectrometry (ESI-MS), atmospheric pressure chemical ionization MS (APCI-MS), reverse-phase HPLC (RP-HPLC), and normal-phase HPLC (NP-HPLC), with APCI-MS detection of the analytes, and one-dimensional (1D) and two-dimensional (2D) proton (1H) and carbon (13C) NMR analyses.
ESI-MS analysis of LHA
The compound was dissolved in a MeOH-acetic acid (99:1; v/v) solvent mixture to a final concentration of 25 μM and was directly infused into the mass spectrometer using a syringe pump at a flow rate of 5 μl/min. The following settings were used: source voltage, 1.5 kV; sheath gas flow rate, ∼10 arbitrary units (au); capillary voltage, 40V; capillary temperature, 125°C.
APCI-MS analysis of LHA
A sample of 10 μM LHA in MeOH was directly infused at a flow rate of 7 μl/min using a syringe pump. The APCI-MS settings were as follows: source voltage, 4 kV; source current, 5 μA; vaporizer temperature, 350°C; sheath gas flow rate, 20 au; capillary voltage, 22V; capillary temperature, 300°C.
HPLC analyses of LHA
The compound was analyzed chromatographically using two different methods, NP-HPLC and RP-HPLC.
NP-HPLC analysis of LHA was conducted on a Phenomenex Diol HPLC column (3.2 mm × 150 mm, 5 μm) using isocratic elution with a solvent mixture composed of n-hexane, propan-2-ol, and glacial acetic acid (949:50:1; v/v/v). The effluent was continuously monitored using a tandem of a Waters 2996 diode array detector (DAD) and a mass spectrometer equipped with an APCI ion source. The DAD was set to record spectra in the range of 200 to 400 nm, and the mass spectrometer recorded spectra in either the positive- or the negative-ion modes in the range of m/z values between 200 and 1,000.
RP-HPLC analysis of LHA was performed on a 2.1 × 150 mm, 5 μm Hypersil GOLD reverse-phase column (Thermo Electron Corp.; San Jose, CA) in methanol-5 mM ammonium formate in water (70:30; v/v) at a flow rate of 0.25 ml/min and a temperature of 30°C. Detection of the analytes was performed essentially as described above for NP-HPLC experiments.
1D and 2D 1H and 13C NMR spectra
1H spectra of LHA in CD3OD (10 mg/0.6 ml) were taken at 399.78 MHz at room temperature. The 13C spectra were recorded at the spectrometer frequency of 75.46 MHz.
Compound stability
A dry sample of LHA stored under nitrogen in the dark at −20°C to −70°C has been stable for at least 8 years. No decomposition/isomerization products have been detected by UV-visible light spectrometry, NMR, or MS.
Kinetic and inhibitory studies
Enzymatic activities of COX1, COX2, 12-LO, and 15-LO were measured according to the manufacturers' recommendations with minor modifications (see below), whereas h5-LO, ptLOX, sLOX, and mPGES-1 were tested as described earlier (13, 41, 43, 44). To account for possible loss of enzymatic activity upon preincubation of the enzymes with LHA, control experiments were performed in which the enzymes were preincubated in the reaction buffers (without substrates) for the same period of time as in the experiments with the inhibitor(s). Then, fatty acid substrates were added and the remaining enzymatic activity was measured for each individual LO and LOX as described below. The calculated residual activity without inhibitor in the reaction media was used as control in the inhibition experiments.
Inhibition of h5-LO
Enzymatic activities of h-5LO-1 and h5-LO-2 were measured by using RP-HPLC with UV detection of the reaction products using an established procedure (13). Briefly, the reaction mixtures included 100 μM AA dissolved in 0.05 M Tris-HCl buffer, pH 7.6, 0.15 M NaCl, 1.2 mM EDTA × 2 Na+, 2 mM CaCl2, 2.3 mM ATP, 0.02 mM β-mercaptoethanol, 0.02 mg/ml phosphatidylcholine (from egg yolk), and 10 μM 13(S)-HPODE, in a final volume of 150 μl. The reactions were initiated by adding a concentrated stock solution of substrate (AA) and activator [13(S)-HPODE] to the mixture that already contained h5-LO. The reaction was carried out at 25°C ± 1°C for 20 min, then 350 μl of ice-cold stopping solution that contained acetonitrile and 2.5% glacial acetic acid (v/v) was added to the reaction mixture to stop the reaction and precipitate the proteins. The mixture was vortexed, left on ice for 10 min, and then centrifuged at 10,000 g at 4°C for another 10 min. The supernatant was transferred into a glass HPLC vial and stored at −20°C until the HPLC analysis. When LHA was being tested as 5-LO inhibitor, the compound was dissolved in the reaction buffer without AA and 13(S)-HPODE and was preincubated with the enzyme for 10 min. Then, the reaction was initiated by adding AA and 13(S)-HPODE and allowed to proceed for 20 min.
To analyze the main products of AA oxidation, [5(S)-HPETE and 5(S)-HETE], the final product mixture was separated by RP-HPLC on a 4.6 mm × 125 mm, 5 μm C8 Zorbax Eclipse (Agilent Technologies, Wilmington, DE) XDB-C8 column. The separation was performed at 30°C at a flow rate of 0.5 ml/min. The compounds were eluted in a water-acetonitrile solvent mixture using a previously described protocol (13). The elution profile was monitored at 236 ± 2 nm [the maximum of adsorption of conjugated dienes; molar extinction coefficient, εm = 23,000 M−1 × cm−1 (45)].
Inhibition of 12-LO
The enzymatic activity of 12-LO was measured with AA as suggested by the manufacturer, with two exceptions: use of Lubrol PX instead of Tween 20, and use of a UV-spectrophotometer set at 237 nm to monitor the product formation instead of an oxygen electrode. The control enzymatic activity was measured at 25°C in 1 ml of 0.1 M Tris-HCl buffer, pH 7.5, with 5 mM EDTA, 0.02% Lubrol PX, and 100 μM AA as substrate. First, the buffer (1 ml), placed in a 1 cm quartz spectrophotometric cuvette, was mixed with 5 μl of 20 mM stock solution of AA in ethanol. The reaction was started by adding an aliquot of the enzyme solution. The reaction mixture was briefly but vigorously mixed with a pipette, and then the product formation was recorded at 237 nm. In inhibitory studies, a concentrated stock solution of LHA (10 mM, in ethanol) or a blank (the same buffer) was added to the buffer first. Then, a 10 μl aliquot of 12-LO was added and incubated with LHA for 5 or 10 min. The remaining 12-LO activity was measured by adding the stock solution of AA, vigorous mixing, and recording the product formation at 237 nm.
Inhibition of 15-LO
The control enzymatic activity (2 μl of the enzyme stock solution, as supplied by the manufacturer) was measured in 1 ml of 0.05 M sodium phosphate buffer, pH 7.6, with 100 μM LA as substrate at 25°C, as recommended by the manufacturer. The molar absorptivity of the product at 324 nm was assumed to be the same as for AA (see above). The inhibitory studies were performed as described above for 12-LO, with 1, 5, or 10 min preincubation of the enzyme with LHA, followed by the addition of 5 μl of 20 mM stock solution of LA (final substrate concentration, 100 μM).
Inhibition of sLOX
The reactions were carried out in a 1 cm quartz spectrophotometric cuvette. The enzyme (final concentration 2 μg/ml) was preincubated for 5 min with the indicated concentrations of LHA dissolved in 1 ml of 50 mM sodium borate buffer, pH 9.0, at 25°C. Then, an aliquot of an AA stock solution was added to the sLOX/LHA mixture (final concentration of AA was 100 μM), and an increase in the optical density at 237 nm was continuously monitored for up to 10 min. The remaining activity of the LHA-inhibited enzyme was calculated from the slope of the reaction progress curve.
Inhibition of COX1/COX2
Colorimetric COX (ovine) inhibitor screening assay kit (Cayman Chemical, catalog No. 760111) was used to measure enzyme activity and inhibition according to the manufacturer's protocols. SC-560 and DuP-697, standard inhibitors of, respectively, COX1 and COX2, were tested as positive controls.
Inhibition of mPGES-1
Effects of LHA on mPGES-1 were studied with PGH2 as substrate, as described by Thorén et al. (43) and Pettersson, Thorén, and Jakobsson (44). Briefly, 10 μM PGH2 and 2.5 mM GSH were dissolved in 1% Triton X-100 in 0.1 M sodium phosphate buffer (pH 7.5) containing 1% glycerol. The reactions were conducted on ice. The products were purified by solid-phase extraction on C18 silica gel cartridges, analyzed by RP-HPLC, and quantified using known amounts of internal standards (11-β-PGE2).
RESULTS
Synthesis and structure verification of LHA
LHA (C18H33NO2, theoretical monoisotopic mass 295.25) was prepared from LA as described earlier for other alkadiynoic hydroxamic fatty acids (42) and purified by open-column silica gel chromatography. The structure of the compound was elucidated by UV-visible light spectrometry, ESI- and APCI-MS, HPLC-MS, and NMR. Otherwise colorless, LHA developed a purple color (λmax = 527 nm) when mixed with FeCl3 in acetonitrile, proving the presence of a hydroxamic group in its structure. No conjugated double bonds were detected UV spectrophotometrically, because the compound did not have an absorption maximum at 230–238 nm typical of fatty acids with two conjugated double bonds.
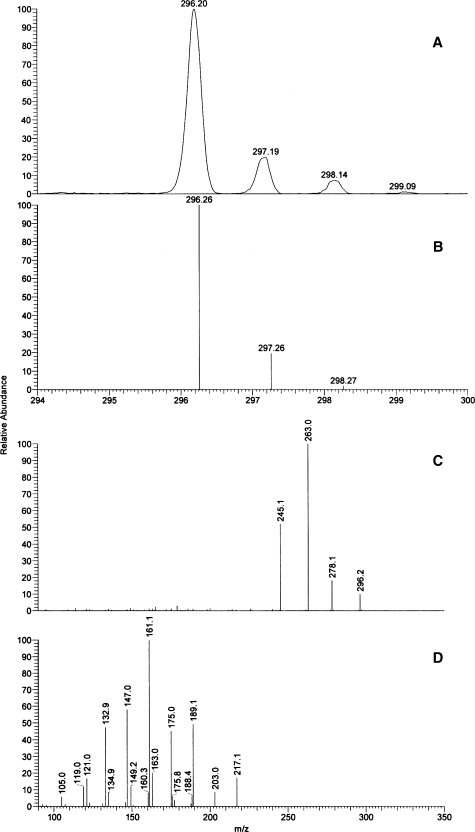
ESI-MS of the compound in a MeOH-glacial acetic acid (99:1; v/v) solvent mixture taken in the positive-ion zoom scan mode showed the presence of major ions with m/z values of 296.3 and 318.3 [(M+H)+ and (M+Na)+, respectively], whereas in the negative-ion mode, the major signal was at m/z 294.3 [(M−H)−] and 353.6 [(M+CH3COO)−]. Fragmentation of the ion with an m/z value of 296 produced the following ion chains: 296→278 (loss of H2O)→260 (loss of H2O) + 261 (loss of NH3); and 296.2→263.0 (loss of NH2OH)→245.1 (loss of H2O).
Positive-ion mode APCI-MS was used to verify the molecular mass of LHA (Fig. 1A). For comparison purposes, a theoretical MS spectrum of a compound with the molecular formula C18H34NO2 is presented in Fig. 1B. Fragmentation of the (M+H)+ ion in an MS2 experiment produced the following fragments: 296.2→278.1 (loss of H2O) + 263.0 (loss of NH2OH) + 245.1 (loss of NH2OH and H2O) (Fig. 1C). The MS data were consistent with a hydroxamic fatty acid with a molecular weight of 295.3 (C18H33NO2). An interesting minor ion with an m/z value of 280.4 that was observed in these experiments was attributed to an amide of LA formed in situ [theoretical m/z value of its (M+H)+ ion is 280.3]. In an MS3 experiment, the following ion chain was observed: 296.2 (MS1)→245.1 (MS2)→217.1 + 203.0 + 189.1 + 175.0 + 161.1 + 147.0 + 132.9 + 121.0 + 119.0 + 105.0 (MS3) (Fig. 1D). The latter fragments with m/z values from 270 to 105 were identical to those obtained during fragmentation of authentic LA [data not shown; (46)].
Fig. 1.
Atmospheric pressure chemical ionization mass spectra of LHA. A: A zoom mass spectrum of a proton adduct of linoleyl hydroxamic acid (LHA) [(M+H)+] taken in the positive-ion mode. B: Theoretical mass spectrum of a compound with molecular formula C18H34NO2. C: Fragmentation of ion m/z 296 in an MS2 experiment. D: Fragmentation of ion m/z 245 in an MS3 experiment.
NP-HPLC-APCI-MS analysis of LHA in the positive-ion mode showed only one HPLC peak, with retention time of ∼16.5 min (not shown) and a major signal with an m/z value of 296 and minor signals of m/z 280 and 278, discussed above. In the negative-ion mode, LHA produced a mixture of ions with m/z 294 and 340 (a free anion of LHA and an LHA adduct with formate, respectively; data not shown). When an acetate-based mobile phase was used instead of the formate-based one, the acetate adducts were the predominant species detected. RP-HPLC corroborated these findings, confirming the purity of the LHA preparation (not shown). In the negative-ion mode, the compound was detected as a single HPLC peak, exclusively as a (M+HCOO)− ion (m/z 340.3).
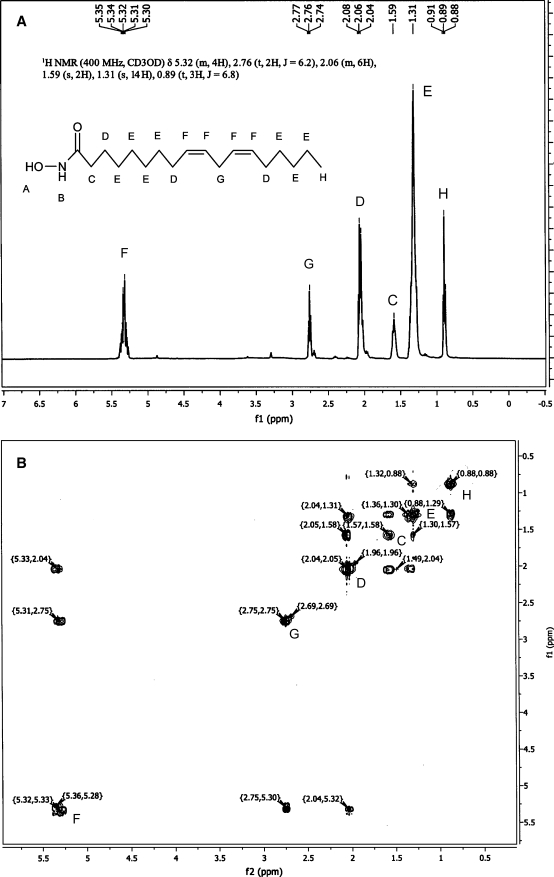
Analysis of the 13C NMR spectrum of LHA confirmed the presence of 18 distinctively different carbon atoms (Table 1). Integration of C2 to C18 atoms produced an expected ratio of the carbon atoms. The experimental 13C NMR spectrum of LHA was very close to those reported for LA (47, 48) and to a theoretical spectrum computed using the ChemDraw Ultra (v.10.0) software package (Table 1). The 1D 1H and 2D 1H-COSY NMR spectra of the compound (Fig. 2) were indicative of a C18:2 fatty acid with a nonconjugated octadeca-9Z,12Z-dienoic fragment. No signs of inadvertent Z→E isomerization of LHA during its synthesis were observed.
TABLE 1.
13C-NMR spectra of LHA and LA
| LHA
|
|||
|---|---|---|---|
| #C | Predicteda | Experimentb | LAc |
| 1 | 169.9 | 172.08 (weak) | 180.545 |
| 13 | 130.3 | 129.74 (0.80) | 130.21 |
| 9 | 130.3 | 129.66 (0.83) | 130.02 |
| 10 | 127.3 | 127.85 (0.83) | 128.12 |
| 12 | 127.3 | 127.91 (0.81) | 129.95 |
| 2 | 32.5 | 32.61 (1.31) | 34.15 |
| 16 | 31.9 | 31.48 (1.10) | 31.58 |
| 7 | 29.9 | 29.54 (0.97) | 29.63 |
| 15 | 29.6 | 29.3 (0.90) | 29.19 |
| 6 | 29.7 | 29.13 (0.99) | 29.4 |
| 5 | 29.0 | 29.07 (0.97) | 29.12 |
| 4 | 28.6 | 28.99 (1.07) | 29.08 |
| 14 | 27.8 | 26.99 (1.01) | 27.25 |
| 8 | 27.8 | 26.97 (0.99) | 27.22 |
| 11 | 25.6 | 25.61 (1.06) | 25.67 |
| 3 | 25.6 | 25.34 (0.92) | 24.7 |
| 17 | 22.8 | 22.45 (0.91) | 22.62 |
| 18 | 14.1 | 13.26 (1.00) | 14.09 |
13C chemical shifts (δ, ppm) and number of carbon atoms determined by integration of the spectrum (in parentheses). #C, the carbon atom number in LHA [from 1 for (-CONHOH) to 18 for the terminal CH3]; LHA, linoleyl hydroxamic acid; LA, linoleic acid.
Chemical shifts predicted for neutral LHA using ChemDraw Ultra 10 software package.
Experimental data.
Fig. 2.
One-dimensional 1H-NMR (A) and two-dimensional 1H-COSY NMR (B) spectra of LHA. Detected proton resonances are labeled in accordance with the carbon atoms of LHA.
Inhibition of LOs
For measuring the activity of commercially available LO preparations, we used the conditions recommended by the manufacturers of the corresponding enzyme preparations. LHA effectively inhibited all of the tested human and mammalian LOs in reaction mixtures with physiologically relevant values of pH and PUFA substrate concentrations. In all cases, inhibition was found to be dose dependent.
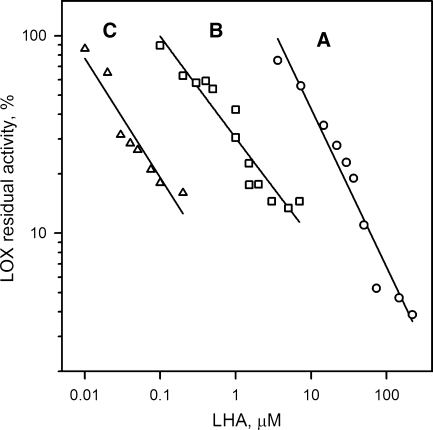
Two different preparations of h5-LO showed equal sensitivity toward LHA, with the IC50 values of ∼10 μM when 100 μM AA was used as substrate (Fig. 3, trace A). The length of preincubation of the enzyme with LHA prior to addition of the substrates had no effect on inhibition; a comparable decrease in h5-LO was observed for 1, 5, 10, and 15 min preincubation intervals. In a separate experiment, it was shown that h5-LO apparently did not catalyze oxygenation of LHA, inasmuch as no characteristic conjugated diene chromophore with λmax 230–240 nm was detected after incubation of the enzyme with the compound in the absence of AA, and no oxygenation product of LHA was detected using ESI- and/or APCI-MS analyses.
Fig. 3.
Inhibition of h5-lipoxygenase (h5-LO) (A), 12-LO (B), and 15-LO (C) by LHA. Activity of the enzymes without inhibitor was used as 100%.
12-LO showed a much higher sensitivity to LHA, with an IC50 value of ∼0.6 μM, as tested using UV-spectrophotometric analysis with 100 μM AA as substrate (Fig. 3, trace B). Similarly to h5-LO, 12-LO was not capable of oxygenating LHA. The length of preincubation of 12-LO with LHA also did not affect the efficacy of inhibition.
The highest degree of inhibition by LHA was detected for 15-LO. In the reaction of 100 μM LA oxidation (Fig. 3, trace C), the IC50 value was found to be ∼20 nM, regardless of the preincubation time. No oxidation products of LHA were detected after 10 min preincubation of LHA and 12-LO.
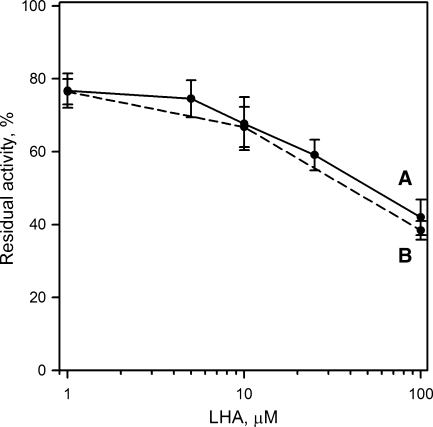
On the other hand, sLOX, which was tested at its pH optimum of 9.0 in two different types of experiments, was a notable exception. First, corroborating our previously published results (31), sLOX utilized LHA as a substrate, catalyzing direct aerobic oxygenation of the compound and producing 13-hydroperoxy-LHA (to be reported separately). During the reaction of LHA oxygenation, sLOX quickly inactivated. The initial rate of the reaction was close to that of sLOX-catalyzed oxidation of LA, but the reaction quickly stopped, in less than 5 min. Second, LHA inhibited sLOX-catalyzed oxidation of AA (IC50 ∼4 μM, Fig. 4). The enzyme was preincubated with LHA for 5 to 10 min before starting the reaction of AA oxidation.
Fig. 4.
Inhibition of soybean lipoxygenase by LHA in the reaction of arachidonic acid oxidation. Error bars indicate ± SEM (n = 4).
A preparation of ptLOX tested for comparison purposes with LA as substrate was expectedly (32, 33) inhibited by LHA with an IC50 of ∼5 μM (data not shown).
Inhibition of COX1/COX2 and mPGES-1
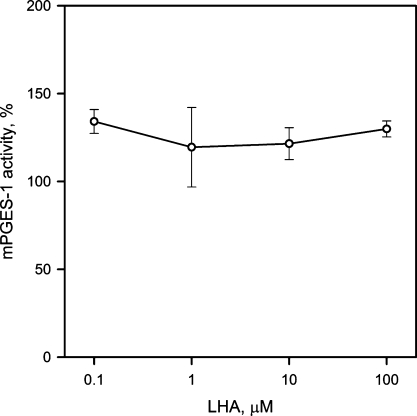
Unlike LOs, both the COX1 and COX2 enzymes showed high resistance to inhibition by LHA (Fig. 5). Their IC50 values of ∼60 μM were at least an order of magnitude higher than those of h5-LO and sLOX, two orders of magnitude higher than that for 12-LO, and three orders of magnitude higher than for 15-LO. Interestingly, dose-effect curves for COX1 and COX2 inhibition reached a plateau; there was little or no difference between the residual enzymatic activities measured above 100 μM concentrations of LHA. At the same time, standard COX1 and COX2 inhibitors SC-560 and DuP-697 suppressed enzymatic activities of the corresponding enzymes with expected efficacy (data not shown). Remarkably, mPGES-1 tested with 10 μM PGH2 as substrate showed no signs of inhibition, even in the presence of 100 μM LHA (Fig. 6).
Fig. 5.
Inhibition of cyclooxygenase 1 (COX1) (A) and COX2 (B) by LHA. Error bars indicate ± SEM (n = 3).
Fig. 6.
Inhibition of microsomal prostaglandin E synthase-1 by LHA. Error bars indicate ± SEM (n=2).
DISCUSSION
In this first comparative study, LHA inhibitory activity was found to increase in the following order: mPGES-1 (no inhibition)<<COX1 = COX2<h5-LO = sLOX = ptLOX<12-LO<15-LO. Earlier (36), we reported that intravenous injections of LHA in dogs with immunologically induced coronary spasm normalized otherwise increased levels of leukotriene C4, a 5-LO-derived metabolite of AA. Our current studies of isolated h5-LO (Fig. 3, trace A) provided firm evidence that LHA could directly inhibit the enzyme, thus revealing a mechanism through which LHA might have been exerting its activity in our earlier in vivo experiments. Because 5-LO was shown to produce increased amounts of pro-inflammatory metabolites of AA in various pathological conditions, including, for example, asthma (49), neuroinflammation (50), myocardial infarction, coronary artery disease, ischemia (51, 52), and cancer (53), its inhibition by LHA could be used to normalize levels of the metabolites under those conditions. Interesting observations, that efficacy of h5-LO inhibition by LHA was identical to that of ptLOX, and that neither of the enzymes was able to oxygenate LHA, corroborate earlier findings that ptLOX and h5-LO are similar in terms of their reaction mechanisms (14, 54).
12-LO has been repeatedly implicated in carcinogenesis (55–57), atherosclerosis (58–60), hypertension (61, 62), and stroke (63, 64). Increased biosynthesis of 12(S)-HPETE, the major 12-LO product of AA oxygenation, led, for example, to upregulation of protein kinase C-α via a receptor-mediated mechanism (65) and NF-κ-B (66) in certain types of cancer cells. Therefore, effective inhibitors of 12-LO were sought to develop novel anticancer treatments. In our in vitro experiments, submicromolar concentrations of LHA did effectively inhibit porcine leukocyte 12-LO (Fig. 3, trace B). The efficacy of inhibition was on par with or better than that of other known 12-LO inhibitors {5,8,11-eicosatriynoic acid [IC50 24 μM, (67)], baicalein [0.1 to 0.6 μM, (68, 69)], esculetin [2.5 μM, (70)], 3-methoxytropolone [MY3-469; 1.8 μM, (71)], and N-benzyl-N-hydroxy-5-phenyl-pentamid [BHPP; 0.2 to 1 μM, depending on the source of the enzyme, (72)]}. This, along with a relatively weak inhibition of the enzymes of the prostaglandin pathway (see below), suggests that LHA can be tried as a potential anticancer drug.
The most effective inhibition among LOs (IC50 about 20 nM) was observed with rabbit 15-LO (Fig. 3, trace C). This enzyme mediates atherosclerosis (58–60), brain oxidative stress (73), and Alzheimer's disease (74), and is a key player in oxidative stress in general. The efficacy of rabbit 15-LO inhibition by LHA far exceeded the reported efficacy of baicalein (IC50 between 1.6 and 38 μM) (69) and of other tested flavonoids (75). Anti-LO activity of LHA was on par with or better than that of ebselen (IC50 ∼60 nM with rabbit 15-LO) (76), a selenoorganic aromatic compound (77) with rather nonspecific glutathione peroxidase-like antioxidant activity (78). The effective in vitro inhibition of 5-, 12-, and 15-LO of mammals by LHA described above provides a mechanistic basis for the results of our in vivo experiments, in which LHA was proven to be a physiologically active compound with strong anti-LO activity (36–38).
LHA was found to be a relatively weak COX1/COX2 inhibitor, with an IC50 of ∼60 μM (Fig. 5). The compound also had no effect on mPGES-1 (Fig. 6). Therefore, it seems unlikely that LHA would impact biosynthesis of prostaglandins in vivo. COX1 and/or COX2 are implicated in the development of various diseases and pathological states, the discussion of which is beyond the scope of this manuscript. However, one should note that COX2 is the main target of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs), and it is its inhibition that is believed to be responsible for the most harmful side effects of NSAIDs. Currently, dual COX/LO inhibitors are a popular trend (79–82). This approach promises to simultaneously reduce biosynthesis of proinflammatory prostaglandins and leukotrienes, thus minimizing the risk of developing post-treatment complications such as ulceration of the gastrointestinal tract or cardiovascular disorders. The importance of such simultaneous inhibition of both LO and COX1/COX2 cascades was pointed out in earlier reports (83). However, a single drug that has dual anti-LO/anti-COX activity can hardly be flexible enough to accommodate all possible situations in which fine-tuning of AA metabolism is needed. More promising is an approach that would utilize two different drugs that have, respectively, pronounced anti-LO and anti-COX activities. Then, by varying the doses of both drugs, one could expect a better therapeutic outcome tailored for a particular disease or condition. Thus, a relatively low inhibitory activity of LHA toward COX1/COX2 and its high activity toward 5-, 12-, and 15-LO could be complemented by standard NSAIDs to achieve the desired therapeutic effect.
In conclusion, LHA was shown to be an effective inhibitor of mammalian LOs, with highest inhibitory activity toward 15- and 12-LOs, which makes this compound a possible candidate for treating conditions that are associated with hyperactivity of these enzymes.
Acknowledgments
I.A.B. thanks Drs. Marija Rakonjac and Staffan Thorén (Karolinska Institute, Stockholm, Sweden) for their help in conducting experiments with, respectively, hr5-LO-2 and mPGES-1 during his stay at the Karolinska Institute.
Abbreviations
AA, arachidonic acid (eicosatetra-5Z,8Z,11Z,14Z-enoic acid)
APCI, atmospheric pressure chemical ionization
au, arbitrary unit (unitless)
COX1/2, ovine cyclooxygenases 1 and 2
1D, one-dimensional
DAD, diode array detector
HETE, hydroxyeicosatetraenoic acid
ESI-MS, electrospray ionization mass spectrometry
HPETE, hydroperoxyeicosatetraenoic acid
HPODE, hydroperoxyoctadecadienoic acid
h5-LO, human recombinant 5-lipoxygenase
LA, linoleic acid (octadeca-9Z,12Z-dienoic acid)
LHA, linoleyl hydroxamic acid
LO, lipoxygenase (mammalian)
LOX, lipoxidase (plant)
mPGES-1, human microsomal prostaglandin E synthase-1
NP-HPLC, normal-phase HPLC
NSAID, nonsteroidal anti-inflammatory drug
ptLOX, potato tuber lipoxygenase (lipoxidase)
RP-HPLC, reverse-phase HPLC
sLOX, soybean lipoxygenase (lipoxidase)
Published, JLR Papers in Press, 27 February 2008.
Footnotes
The authors would like to acknowledge support from the Swedish Foundation for International Cooperation in Research and Higher Education, the National Institutes of Health (Grants 5P30AR-04194014 and EY-016664), and by an unrestricted grant from Research to Prevent Blindness, Inc. (New York, NY).
References
- 1.Peters-Golden M., and T. G. Brock. 2003. 5-Lipoxygenase and FLAP. Prostaglandins Leukot. Essent. Fatty Acids. 69 99–109. [DOI] [PubMed] [Google Scholar]
- 2.Camp R. D., A. I. Mallet, P. M. Woollard, S. D. Brain, A. K. Black, and M. W. Greaves. 1983. The identification of hydroxy fatty acids in psoriatic skin. Prostaglandins. 26 431–447. [DOI] [PubMed] [Google Scholar]
- 3.el Attar T. M., H. S. Lin, and J. Y. Vanderhoek. 1985. Biosynthesis of prostaglandins and hydroxy fatty acids in primary squamous carcinomas of head and neck in humans. Cancer Lett. 27 255–259. [DOI] [PubMed] [Google Scholar]
- 4.Kiss L., H. Schütte, K. Mayer, H. Grimm, W. Padberg, W. Seeger, and F. Grimminger. 2000. Synthesis of arachidonic acid-derived lipoxygenase and cytochrome P450 products in the intact human lung vasculature. Am. J. Respir. Crit. Care Med. 161 1917–1923. [DOI] [PubMed] [Google Scholar]
- 5.Yue H., S. A. Jansen, K. I. Strauss, M. R. Borenstein, M. F. Barbe, L. J. Rossi, and E. Murphy. 2007. A liquid chromatography/mass spectrometric method for simultaneous analysis of arachidonic acid and its endogenous eicosanoid metabolites prostaglandins, dihydroxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and epoxyeicosatrienoic acids in rat brain tissue. J. Pharm. Biomed. Anal. 43 1122–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salzmann-Reinhardt U., H. Kühn, R. Wiesner, and S. Rapoport. 1985. Metabolism of polyunsaturated fatty acids by rabbit reticulocytes. Eur. J. Biochem. 153 189–194. [DOI] [PubMed] [Google Scholar]
- 7.Wiseman J. S., M. T. Skoog, and C. H. Clapp. 1988. Activity of soybean lipoxygenase in the absence of lipid hydroperoxide. Biochemistry. 27 8810–8813. [DOI] [PubMed] [Google Scholar]
- 8.Hamberg M. 1971. Steric analysis of hydroperoxides formed by lipoxygenase oxygenation of linoleic acid. Anal. Biochem. 43 515–526. [DOI] [PubMed] [Google Scholar]
- 9.Hamberg M., and B. Samuelsson. 1967. On the specificity of the oxygenation of unsaturated fatty acids catalyzed by soybean lipoxidase. J. Biol. Chem. 242 5329–5335. [PubMed] [Google Scholar]
- 10.Galliard T., and D. R. Phillips. 1971. Lipoxygenase from potato tubers. Partial purification and properties of an enzyme that specifically oxygenates the 9-position of linoleic acid. Biochem. J. 124 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butovich I. A., M. Hamberg, and O. Rådmark. 2005. Novel oxylipins formed from docosahexaenoic acid by potato lipoxygenase—10(S)-hydroxydocosahexa-enoic acid and 10,20-dihydroxydocosahexaenoic acid. Lipids. 40 249–257. [DOI] [PubMed] [Google Scholar]
- 12.Butovich I. A. 2005. On the structure and synthesis of neuroprotectin D1, a novel anti-inflammatory compound of the docosahexaenoic acid family. J. Lipid Res. 46 2311–2314. [DOI] [PubMed] [Google Scholar]
- 13.Butovich I. A., S. M. Lukyanova, and C. Bachmann. 2006. Dihydroxydocosahexaenoic acids of the neuroprotectin D family: synthesis, structure, and inhibition of human 5-lipoxygenase. J. Lipid Res. 47 2462–2474. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu T., O. Rådmark, and B. Samuelsson. 1984. Enzyme with dual lipoxygenase activities catalyzes leukotriene A4 synthesis from arachidonic acid. Proc. Natl. Acad. Sci. USA. 81 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hombeck M., G. Pohnert, and W. Boland. 1999. Biosynthesis of dictyopterene A: stereoselectivity of a lipoxygenase/hydroperoxide lyase from Gomphonema parvulum (Bacillariophyceae). Chem. Commun. 3 243–244. [Google Scholar]
- 16.Sloane D. L., M. F. Browner, Z. Dauter, K. Wilson, R. J. Fletterick, and E. Segal. 1990. Purification and crystallization of 15-lipoxygenase from rabbit reticulocytes. Biochem. Biophys. Res. Commun. 173 507–513. [DOI] [PubMed] [Google Scholar]
- 17.Phillis J. W., L. A. Horrocks, and A. A. Farooqui. 2006. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res. Brain Res. Rev. 52 201–243. [DOI] [PubMed] [Google Scholar]
- 18.Fürstenberger G., P. Krieg, K. Müller-Decker, and A. J. Habenicht. 2006. What are cyclooxygenases and lipoxygenases doing in the driver's seat of carcinogenesis? Int. J. Cancer. 119 2247–2254. [DOI] [PubMed] [Google Scholar]
- 19.Jatana M., S. Giri, M. A. Ansari, C. Elango, A. K. Singh, I. Singh, and M. Khan. 2006. Inhibition of NF-kappaB activation by 5-lipoxygenase inhibitors protects brain against injury in a rat model of focal cerebral ischemia. J. Neuroinflammation. 11 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossoni G., A. Sala, F. Berti, T. Testa, C. Buccellati, C. Molta, R. Muller-Peddinghaus, J. Maclouf, and G. C. Folco. 1996. Myocardial protection by the leukotriene synthesis inhibitor BAY X1005: importance of transcellular biosynthesis of cysteinyl-leukotrienes. J. Pharmacol. Exp. Ther. 276 335–341. [DOI] [PubMed] [Google Scholar]
- 21.van Leyen K., H. Y. Kim, S. R. Lee, G. Jin, K. Arai, and E. H. Lo. 2006. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 37 3014–3018. [DOI] [PubMed] [Google Scholar]
- 22.Rubin P., and K. W. Mollison. 2007. Pharmacotherapy of diseases mediated by 5-lipoxygenase pathway eicosanoids. Prostaglandins Other Lipid Mediat. 83 188–197. [DOI] [PubMed] [Google Scholar]
- 23.Chu L. S., S. H. Fang, Y. Zhou, G. L. Yu, M. L. Wang, W. P. Zhang, and E. Q. Wei. 2007. Minocycline inhibits 5-lipoxygenase activation and brain inflammation after focal cerebral ischemia in rats. Acta Pharmacol. Sin. 28 763–772. [DOI] [PubMed] [Google Scholar]
- 24.Klickstein L. B., C. Shapleigh, and E. J. Goetzl. 1980. Lipoxygenation of arachidonic acid as a source of polymorphonuclear leukocyte chemotactic factors in synovial fluid and tissue in rheumatoid arthritis and spondyloarthritis. J. Clin. Invest. 66 1166–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Araico A., M. C. Terencio, M. J. Alcaraz, J. N. Domínguez, C. León, and M. L. Ferrándiz. 2007. Evaluation of the anti-inflammatory and analgesic activity of Me-UCH9, a dual cyclooxygenase-2/5-lipoxygenase inhibitor. Life Sci. 80 2108–2117. [DOI] [PubMed] [Google Scholar]
- 26.Burka J. F., and N. A. Paterson. 1980. Evidence for lipoxygenase pathway involvement in allergic tracheal contraction. Prostaglandins. 19 499–515. [DOI] [PubMed] [Google Scholar]
- 27.Berger W., M. T. De Chandt, and C. B. Cairns. 2007. Zileuton: clinical implications of 5-lipoxygenase inhibition in severe airway disease. Int. J. Clin. Pract. 61 663–676. [DOI] [PubMed] [Google Scholar]
- 28.Steele V. E., C. A. Holmes, E. T. Hawk, L. Kopelovich, R. A. Lubet, J. A. Crowell, C. C. Sigman, and G. J. Kelloff. 1999. Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol. Biomarkers Prev. 8 467–483. [PubMed] [Google Scholar]
- 29.Steinhilber D. 1999. 5-Lipoxygenase: a target for antiinflammatory drugs revisited. Curr. Med. Chem. 6 71–85. [PubMed] [Google Scholar]
- 30.Federico A., F. Morgillo, C. Tuccillo, F. Ciardiello, and C. Loguercio. 2007. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer. 121 2381–2386. [DOI] [PubMed] [Google Scholar]
- 31.Butovich I. A., V. P. Bridnya, and V. P. Kukhar. 1990. Linoleyl-hydroxamic acid—a suicide inhibitor of lipoxygenase. Biochemistry–Moscow. 55 908–912. [Google Scholar]
- 32.Butovich I. A., O. V. Kharchenko, L. B. Bondarenko, V. M. Babenko, and L. Livarchuk. 1994. Linoleyl hydroxamate as 5-lipoxygenase inhibitor. Biochemistry–Moscow. 59 597–600. [Google Scholar]
- 33.Butovich I. A., and C. C. Reddy. 2002. Inhibition of potato lipoxygenase by linoleyl hydroxamic acid: kinetic and EPR spectral evidence for a two-step reaction. Biochem. J. 365 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kharchenko O. V., V. N. Cernjuk, and I. A. Butovich. 1999. Inhibitory effect of linoleyl-hydroxamic acid on the oxidation of linoleic acid by 12-lipoxygenase from porcine leukocytes. Ukr. Biokhim. Zh. 71 33–37. [PubMed] [Google Scholar]
- 35.Brodowsky I. D., M. Hamberg, and E. H. Oliw. 1994. BW A4C and other hydroxamic acids are potent inhibitors of linoleic acid 8R-dioxygenase of the fungus Gaeumannomyces graminis. Eur. J. Pharmacol. 254 43–47. [DOI] [PubMed] [Google Scholar]
- 36.Moibenko A. A., L. A. Grabovskii, V. N. Kotsiuruba, V. N. Bulakh, L. V. Tumanovskaia, V. I. Azarov, and I. A. Butovich. 1994. The mechanisms of the changes in coronary vascular resistance in anaphylactic shock. Fiziol. Zh. Im. I M Sechenova. 80 77–82. [PubMed] [Google Scholar]
- 37.Kukoba T. V., M. M. Seredenko, I. A. Butovych, and O. O. Moibenko. 1998. The effect of linoleyl hydroxamic acid on lipid peroxidation processes and on the enzymatic activity of the antioxidant system in rats under hypoxia. Fiziol. Zh. 44 43–48. [PubMed] [Google Scholar]
- 38.Marchenko H. I., V. M. Kotsiuruba, I. A. Butovych, A. E. Sorochynskyi, V. K. Zrazhevs'ka, and L. V. Tumanovs'ka. 1994. The correction of disorders in arachidonic acid metabolism in coronary spasm of an immune origin. Fiziol. Zh. 40 81–87. [PubMed] [Google Scholar]
- 39.Chobot'ko H. M. 1998. Effects of modifications of lipoproteins by water soluble forms of lineoleic-hydroxamic acid on biochemical markers of development of atherosclerosis. Ukr. Biokhim. Zh. 70 64–68. [PubMed] [Google Scholar]
- 40.Agrawal Y. K. 1979. Hydroxamic acids and their metal complexes. Russ. Chem. Rev. 48 948–963. [Google Scholar]
- 41.Hammarberg T., Y. Y. Zhang, B. Lind, O. Radmark, and B. Samuelsson. 1995. Mutations at the C-terminal isoleucine and other potential iron ligands of 5-lipoxygenase. Eur. J. Biochem. 230 401–407. [DOI] [PubMed] [Google Scholar]
- 42.Butovich I. A., V. P. Kukhar, V. P. Bridnya, O. A. Radchenko, and A. Kotlinskaya. 1989. Polyfluorosubstituted alcadiynoic carboxylic and hydroxamic acids as new inhibitors of lipoxygenases. Proc. Natl. Acad. Sci. of Ukraine, ser. B. 5 55–58. [Google Scholar]
- 43.Thorén S., R. Weinander, S. Saha, C. Jegerschöld, P. L. Pettersson, B. Samuelsson, H. Hebert, M. Hamberg, R. Morgenstern, and P. J. Jakobsson. 2003. Human microsomal prostaglandin E synthase-1: purification, functional characterization, and projection structure determination. J. Biol. Chem. 278 22199–22209. [DOI] [PubMed] [Google Scholar]
- 44.Pettersson P. L., S. Thorén, and P. J. Jakobsson. 2005. Human microsomal prostaglandin E synthase 1: a member of the MAPEG protein superfamily. Methods Enzymol. 401 147–161. [DOI] [PubMed] [Google Scholar]
- 45.Gibian M. J., and P. Vandenberg. 1987. Product yield in oxygenation of linoleate by soybean lipoxygenase: the value of the molar extinction coefficient in the spectrophotometric assay. Anal. Biochem. 163 343–349. [DOI] [PubMed] [Google Scholar]
- 46.Butovich I. A., E. Uchiyama, and J. P. McCulley. 2007. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J. Lipid Res. 48 2220–2235. [DOI] [PubMed] [Google Scholar]
- 47.Aursand M., and H. Grasdalen. 1992. Interpretation of the 13C-NMR spectra of omega-3 fatty acids extracted from the white muscle of Atlantic salmon (Salmo salar). Chem. Phys. Lipids. 62 239–251. [Google Scholar]
- 48.Gunstone, F. D. 1993. High resolution of 13C-NMR spectroscopy of lipids. In Advances in Lipid Methodology, Vol. 2. W. W. Christie, editor. Oily Press, Dundee. 1–68.
- 49.Jame A. J., P. M. Lackie, A. M. Cazaly, I. Sayers, J. F. Penrose, S. T. Holgate, and A. P. Sampson. 2007. Human bronchial epithelial cells express an active and inducible biosynthetic pathway for leukotrienes B4 and C4. Clin. Exp. Allergy. 37 880–892. [DOI] [PubMed] [Google Scholar]
- 50.Farooqui A. A., L. A. Horrocks, and T. Farooqui. 2007. Modulation of inflammation in brain: a matter of fat. J. Neurochem. 101 577–599. [DOI] [PubMed] [Google Scholar]
- 51.Takase B., A. Kurita, T. Maruyama, A. Uehata, T. Nishioka, K. Mizuno, H. Nakamura, K. Katsura, and Y. Kanda. 1996. Change of plasma leukotriene C4 during myocardial ischemia in humans. Clin. Cardiol. 19 198–204. [DOI] [PubMed] [Google Scholar]
- 52.Adamek A., S. Jung, C. Dienesch, M. Laser, G. Ertl, J. Bauersachs, and S. Frantz. 2007. Role of 5-lipoxygenase in myocardial ischemia-reperfusion injury in mice. Eur. J. Pharmacol. 571 51–54. [DOI] [PubMed] [Google Scholar]
- 53.Goossens L., N. Pommery, and J. P. Hénichart. 2007. COX-2/5-LOX dual acting anti-inflammatory drugs in cancer chemotherapy. Curr. Top. Med. Chem. 7 283–296. [DOI] [PubMed] [Google Scholar]
- 54.Butovich I. A., O. V. Harchenko, and V. M. Babenko. 1995. On the interfacial phenomena in lipoxygenase catalysis. Adv. Prostaglandin Thromboxane Leukot. Res. 23 159–161. [PubMed] [Google Scholar]
- 55.Chen F. L., X. Z. Wang, J. Y. Li, J. P. Yu, C. Y. Huang, and Z. X. Chen. 2008. 12-Lipoxygenase induces apoptosis of human gastric cancer AGS cells via the ERK1/2 signal pathway. Dig. Dis. Sci. 53 181–187. [DOI] [PubMed] [Google Scholar]
- 56.Fürstenberger G., P. Krieg, K. Müller-Decker, and A. J. Habenicht. 2006. What are cyclooxygenases and lipoxygenases doing in the driver's seat of carcinogenesis? Int. J. Cancer. 119 2247–2254. [DOI] [PubMed] [Google Scholar]
- 57.Nie D., S. Krishnamoorthy, R. Jin, K. Tang, Y. Chen, Y. Qiao, A. Zacharek, Y. Guo, J. Milanini, G. Pages, et al. 2006. Mechanisms regulating tumor angiogenesis by 12-lipoxygenase in prostate cancer cells. J. Biol. Chem. 281 18601–18609. [DOI] [PubMed] [Google Scholar]
- 58.Huo Y., L. Zhao, M. C. Hyman, P. Shashkin, B. L. Harry, T. Burcin, S. B. Forlow, M. A. Stark, D. F. Smith, S. Clarke, et al. 2004. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation. 110 2024–2031. [DOI] [PubMed] [Google Scholar]
- 59.Zhao L., D. Praticò, D. J. Rader, and C. D. Funk. 2005. 12/15-Lipoxygenase gene disruption and vitamin E administration diminish atherosclerosis and oxidative stress in apolipoprotein E deficient mice through a final common pathway. Prostaglandins Other Lipid Mediat. 78 185–193. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi Y., H. Zhu, and T. Yoshimoto. 2005. Essential roles of lipoxygenases in LDL oxidation and development of atherosclerosis. Antioxid. Redox Signal. 7 425–431. [DOI] [PubMed] [Google Scholar]
- 61.Quintana L. F., B. Guzmán, S. Collado, J. Clària, and E. A. Poch. 2006. A coding polymorphism in the 12-lipoxygenase gene is associated to essential hypertension and urinary 12(S)-HETE. Kidney Int. 69 526–530. [DOI] [PubMed] [Google Scholar]
- 62.Hao C. M., and M. D. Breyer. 2007. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 71 1105–1115. [DOI] [PubMed] [Google Scholar]
- 63.Musiek E. S., R. S. Breeding, G. L. Milne, G. Zanoni, J. D. Morrow, and B. McLaughlin. 2006. Cyclopentenone isoprostanes are novel bioactive products of lipid oxidation which enhance neurodegeneration. J. Neurochem. 97 1301–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khanna S., S. Roy, A. Slivka, T. K. Craft, S. Chaki, C. Rink, M. A. Notestine, A. C. DeVries, N. L. Parinandi, and C. K. Sen. 2005. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke. 36 2258–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu B., W. A. Khan, Y. A. Hannun, J. Timar, J. D. Taylor, S. Lundy, I. Butovich, and K. V. Honn. 1995. 12(S)-hydroxyeicosatetraenoic acid and 13(S)-hydroxyoctadecadienoic acid regulation of protein kinase C-alpha in melanoma cells: role of receptor-mediated hydrolysis of inositol phospholipids. Proc. Natl. Acad. Sci. USA. 92 9323–9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kandouz M., D. Nie, G. P. Pidgeon, S. Krishnamoorthy, K. R. Maddipati, and K. V. Honn. 2003. Platelet-type 12-lipoxygenase activates NF-kappaB in prostate cancer cells. Prostaglandins Other Lipid Mediat. 71 189–204. [DOI] [PubMed] [Google Scholar]
- 67.Hammarström S. 1977. Selective inhibition of platelet n-8 lipoxygenase by 5,8,11-eicosatriynoic acid. Biochim. Biophys. Acta. 487 517–519. [DOI] [PubMed] [Google Scholar]
- 68.Sekiya K., H. Okuda, and S. Arichi. 1982. Selective inhibition of platelet lipoxygenase by esculetin. Biochim. Biophys. Acta. 713 68–72. [PubMed] [Google Scholar]
- 69.Deschamps J. D., V. A. Kenyon, and T. R. Holman. 2006. Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorg. Med. Chem. 14 4295–4301. [DOI] [PubMed] [Google Scholar]
- 70.Neichi T., Y. Koshihara, and S. Murota. 1983. Inhibitory effect of esculetin on 5-lipoxygenase and leukotriene biosynthesis. Biochim. Biophys. Acta. 753 130–132. [PubMed] [Google Scholar]
- 71.Kitamura S., T. Iida, K. Shirahata, and H. Kase. 1986. Studies on lipoxygenase inhibitors. I. MY3–469 (3-methoxytropolone), a potent and selective inhibitor of 12-lipoxygenase, produced by Streptoverticillium hadanonense KY11449. J. Antibiot. (Tokyo). 39 589–593. [DOI] [PubMed] [Google Scholar]
- 72.Honn K. V., D. G. Tang, I. M. Grossi, C. Renaud, Z. M. Duniec, C. R. Johnson, and C. A. Diglio. 1994. Enhanced endothelial cell retraction mediated by 12(S)-HETE: a proposed mechanism for the role of platelets in tumor cell metastasis. Exp. Cell Res. 210 1–9. [DOI] [PubMed] [Google Scholar]
- 73.Chinnici C. M., Y. Yao, T. Ding, C. D. Funk, and D. Praticò. 2005. Absence of 12/15 lipoxygenase reduces brain oxidative stress in apolipoprotein E-deficient mice. Am. J. Pathol. 167 1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Praticò D., V. Zhukareva, Y. Yao, K. Uryu, C. D. Funk, J. A. Lawson, J. Q. Trojanowski, and V. M. Lee. 2004. 12/15-Lipoxygenase is increased in Alzheimer's disease: possible involvement in brain oxidative stress. Am. J. Pathol. 164 1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sadik C. D., H. Sies, and T. Schewe. 2003. Inhibition of 15-lipoxygenases by flavonoids: structure-activity relations and mode of action. Biochem. Pharmacol. 65 773–781. [DOI] [PubMed] [Google Scholar]
- 76.Walther M., H. G. Holzhütter, R. J. Kuban, R. Wiesner, J. Rathmann, and H. Kühn. 1999. The inhibition of mammalian 15-lipoxygenases by the anti-inflammatory drug ebselen: dual-type mechanism involving covalent linkage and alteration of the iron ligand sphere. Mol. Pharmacol. 56 196–203. [DOI] [PubMed] [Google Scholar]
- 77.Müller A., E. Cadenas, P. Graf, and H. Sies. 1984. A novel biologically active seleno-organic compound. I. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (ebselen). Biochem. Pharmacol. 33 3235–3239. [DOI] [PubMed] [Google Scholar]
- 78.Wendel A., M. Fausel, H. Safayhi, G. Tiegs, and R. Otter. 1984. A novel biologically active seleno-organic compound. II. Activity of PZ 51 in relation to glutathione peroxidase. Biochem. Pharmacol. 33 3241–3245. [DOI] [PubMed] [Google Scholar]
- 79.Khanapure S. P., D. S. Garvey, D. R. Janero, and L. G. Letts. 2007. Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Curr. Top. Med. Chem. 7 311–340. [DOI] [PubMed] [Google Scholar]
- 80.Leone S., A. Ottani, and A. Bertolini. 2007. Dual acting anti-inflammatory drugs. Curr. Top. Med. Chem. 7 265–275. [DOI] [PubMed] [Google Scholar]
- 81.Cicero A. F., G. Derosa, and A. Gaddi. 2005. Combined lipoxygenase/cyclo-oxygenase inhibition in the elderly: the example of licofelone. Drugs Aging. 22 393–403. [DOI] [PubMed] [Google Scholar]
- 82.Wallace J. M. 2002. Nutritional and botanical modulation of the inflammatory cascade – eicosanoids, cyclooxygenases, and lipoxygenases–as an adjunct in cancer therapy. Integr. Cancer Ther. 1 7–37. [DOI] [PubMed] [Google Scholar]
- 83.Burka J. F., and N. A. Paterson. 1980. Inhibition of antigen-induced release of prostaglandin-like material from guinea-pig trachea by antihistamines FPL55712 and atropine. J. Pharm. Pharmacol. 32 869–870. [DOI] [PubMed] [Google Scholar]