Abstract
Sphingolipids are most prominently expressed in the plasma membrane, but recent studies have pointed to important signaling and regulatory roles in the nucleus. The most abundant nuclear sphingolipid is sphingomyelin (SM), which occurs in the nuclear envelope (NE) as well as intranuclear sites. The major metabolic product of SM is ceramide, which is generated by nuclear sphingomyelinase and triggers apoptosis and other metabolic changes. Ceramide is further hydrolyzed to free fatty acid and sphingosine, the latter undergoing conversion to sphingosine phosphate by action of a specific nuclear kinase. Gangliosides are another type of sphingolipid found in the nucleus, members of the a-series of gangliotetraose gangliosides (GM1, GD1a) occurring in the NE and endonuclear compartments. GM1 in the inner membrane of the NE is tightly associated with a Na+/Ca2+ exchanger whose activity it potentiates, thereby contributing to regulation of Ca2+ homeostasis in the nucleus. This was shown to exert a cytoprotective role as absence or inactivation of this nuclear complex rendered cells vulnerable to apoptosis. This was demonstrated in the greatly enhanced kainite-induced seizure activity in knockout mice lacking gangliotetraose gangliosides. The pathology included apoptotic destruction of neurons in the CA3 region of the hippocampus. Ca2+ homeostasis was restored in these animals with LIGA-20, a membrane-permeant derivative of GM1 that entered the NE and activated the nuclear Na+/Ca2+ exchanger. Some evidence suggests the presence of uncharged glycosphingolipids in the nucleus.
Keywords: sphingolipid, sphingomyelin, sphingomyelinase, ceramide, sphingosine phosphate, gangliosides, nuclear calcium, ganglioside GM1
It is a pleasure to participate in this symposium honoring the contributions of Professor Herbert E. Carter to sphingolipid chemistry. His pioneering achievements laid the groundwork for the biochemical and biological breakthroughs that followed. This author (RWL) was privileged to participate in a symposium honoring Professor Carter nearly 40 years ago (1) at which Dr. Prostenik referred to the “explosive augmentation of experimental data and corresponding scientific conclusions,” which characterized the then-current phase of sphingolipid chemistry. Explosive growth in this field not only continued but has also accelerated into the present, characterized by elucidation of signaling pathways and other manifestations of biological activity. It is gratifying to know that, although in retirement, Professor Carter was able to witness and appreciate these developments that were based in no small degree on his pioneering achievements.
Nuclear sphingolipids encompass an area of research now experiencing rapid progress, as is the case for nuclear lipids in general. Lipids as a whole comprise only 5% or so by weight of most nuclei, resulting in high buoyant density that facilitates isolation in high purity. This has provided confidence in chemical composition, leading to identification of quantitatively minor lipids, including some sphingolipids. The nuclear envelope (NE) contains the large bulk of nuclear lipids, which, in addition to providing structural support, are also the source of numerous signaling reactions. Recent studies have shown that endonuclear loci are also the source of numerous phospholipid signaling reactions, consonant with their detection in chromatin (2), nucleolus (3), and nuclear matrix (4). An early misconception viewed the nucleus as an organelle having little intrinsic capacity for lipid metabolism and therefore dependent on extranuclear processes in conjunction with import mechanisms for its lipid components. This has been corrected in recent years by numerous studies showing extensive metabolism of lipids and other components, which likely account for the recent description of the nucleus as “a cell within a cell” (5). It is of interest that certain extracellular stimuli are able to induce lipid signaling in the nucleus only (6, 7), whereas other stimuli affect the nucleus and cytoplasm (8, 9). In addition to the signaling properties of the NE, much has been revealed on the metabolically active lipids of endonuclear compartments, which mediate a complex array of signaling reactions and modulatory mechanisms that exert major influences on cellular functioning. Each of the two membranes that comprise the NE is known to possess unique composition and metabolic/signaling patterns, including regulation of Ca2+ flux and other determinants of nuclear homeostasis. Sphingolipids contribute through direct and indirect mechanisms to this signaling network. Sphingomyelin (SM) is the most prominent member of this group, giving rise through metabolism to ceramide, sphingosine, sphingosine phosphate, and possibly other signaling entities. Glycosphingolipids comprise yet another group shown to influence nuclear events and provide cytoprotection through regulation of nuclear Ca2+. Most studies to date have dealt with nuclei from mammalian tissues and cell lines; and while certain patterns seem to prevail in virtually all nuclei, it is not known to what degree variations in nuclear lipid composition and signaling can occur among different cell types and animal species. This review summarizes some of the major developments in the area of nuclear sphingolipids, with emphasis on recent findings in regard to functional roles. For additional details on these and other aspects of nuclear lipids the reader is referred to a number of recent informative reviews (10–15).
NUCLEAR DOMAINS AND RELATED STRUCTURES
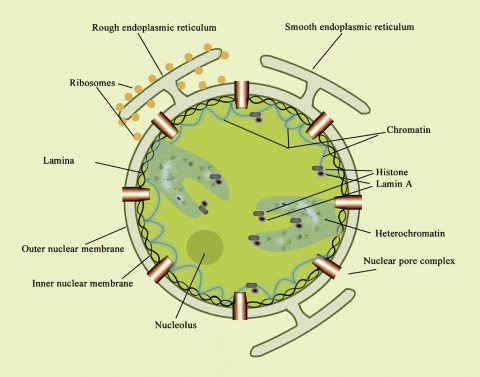
Nuclei of eukaryotic cells are now recognized as structurally well ordered, possessing a well-defined NE enclosure with less well-defined subnuclear domains (16) (Fig. 1). These intranuclear compartments have been described as diffuse and dynamically variable in relation to metabolic function (17). They are all believed to contain sphingolipids that contribute along with other lipid types to nuclear signaling. One such domain is chromatin, the major repository of nucleic acids, which is closely associated with the nuclear matrix or nucleoskeleton, whose main function is to organize chromatin. This matrix is considered analogous to the cellular cytoskeleton in maintaining nuclear shape; it is operationally defined as the components that remain insoluble after extraction of the nuclei with nonionic detergents and salts and treatment with nuclease. Its composition is consequently dependent on isolation methodology and has been described as including the nuclear lamina, inner matrix, elements of the NE, and various structural links between the internal matrix and peripheral lamina (17, 18). Because of its dependence on isolation procedure, the nuclear matrix is not universally considered a distinct structural/functional domain. The nuclear lamina comprises a meshwork of intermediate filaments located on the endonuclear surface of the inner nuclear membrane. The so-called heterochromatin regions, which contain little DNA and are transcriptionally inactive, are nevertheless rich in specific nuclear proteins that regulate transcriptional activity. Heterochromatin can suppress the transcriptional activity of genes that are translocated adjacent to it (16). The ribosome-producing machinery is contained in the nucleolus, one of the better-defined structural units.
Fig. 1.
Representation of nuclear structure with endonuclear domains, as presently conceived. The outer nuclear membrane is continuous with the endoplasmic reticulum (ER), while the inner nuclear membrane is closely associated with the nuclear lamina and has a unique lipid composition. These two membranes are joined at the nuclear pore complexes that are distributed over the nuclear surface and permit passive flow of small molecules between cytoplasm and nucleoplasm. The lumenal space between the two membranes of the nuclear envelope (NE) is a storage site for Ca2+, continuous with the ER lumen. In addition to the NE, lipids have been shown to occur in intranuclear compartments such as nucleolus, chromatin, and heterochromatin. Reproduced from Fig. 1 of ref. 14 with permission.
Various cytochemical, biochemical, and autoradiographic methods have been employed for characterizing lipids and lipid-metabolizing enzymes within these nuclear domains. The two membranes of the NE can be individually isolated (19) and were shown to differ significantly in regard to lipid composition. Cholesterol, for example, was detected in the outer but not the inner nuclear membrane (20, 21), whereas gangliosides GM1 and GD1a occur in both (22). The outer membrane is continuous with the endoplasmic reticular membrane and shares certain properties with the latter. Early studies comparing NE with endoplasmic reticulum (ER) revealed the presence of similar lipids but at different relative concentrations (23, 24). Detailed comparison of lipid composition of the two membranes of the NE has not yet been reported. The two membranes are joined at the nuclear pores by the pore membranes, which are associated with the nuclear pore complexes. The latter are distributed over the entire nuclear surface and consist of multiprotein assemblies of ∼1,000 polypeptides that allow passive transfer of small and middle-sized molecules (<50 kDa) between cytoplasm and nucleoplasm. This accords with the frequently observed equilibration of such substances as Ca2+ between these two compartments. However, the possibility of independent regulation of Ca2+ in the nucleus has been suggested (see later discussion). Passage of larger molecules through the pores is energy dependent and generally requires a nuclear localization signal. Rapid progress is now being reported in the area of chromatin organization as related to such factors as transcription regulation, RNA splicing, and nuclear transport mechanisms (25–28).
SM: a major nuclear sphingolipid
SM was shown in early studies to occur in the NE of hepatic cell nuclei (23, 24), findings that were confirmed by later studies that also demonstrated SM occurrence in the nuclear matrix (29) and chromatin (30). The latter study showed SM to comprise a significant part of chromatin phospholipids, although its concentration was approximately a third of that in the nuclear matrix; cholesterol showed a similar ratio in the two compartments (31). SM and cholesterol increased at the beginning of the S phase during liver regeneration, whereas phosphatidylcholine decreased (31). That study demonstrated independence of nuclear matrix lipids from chromatin lipids and also suggested that the higher cholesterol-SM/phosphatidylcholine ratio in the matrix created a less-fluid environment in relation to DNA synthesis. An additional contribution to reduced fluidity in endonuclear domain(s) is the apparent enrichment of saturated fatty acid–containing phosphatidylcholine (32).
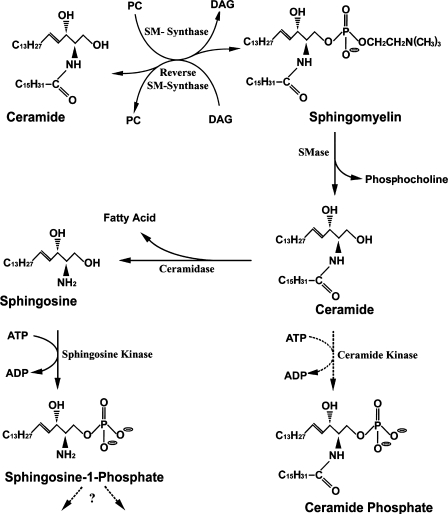
SM is metabolized to ceramide (CER) by sphingomyelinase (SMase), an enzyme first detected in the nuclear matrix of rat ascites hepatoma AH 7974 cells (33), and subsequently in the NE (34), chromatin (35), and nuclear matrix (29, 35) of rat liver nuclei. Intact nuclei were proposed to express this enzyme in the NE, with translocation to the nuclear matrix in regenerating/proliferating rat liver (34). However, the NE and nuclear matrix enzymes are believed to represent different isoforms, because neutral SMase 1 was identified biochemically and immunocytochemically as unique to the nuclear matrix and absent from the NE, chromatin, and plasma membrane (36). The latter report indicated that neutral SMase 1 possessed a nuclear export signal but no nuclear localization signal. If SMase is translocated from NE to nuclear matrix during DNA synthesis (34), this would imply an isoform other than SMase 1. The CER product can undergo further reactions in the nucleus (Fig. 2). SM synthase was detected in chromatin and NE, the latter activity being significantly greater. The two enzymes showed distinctive properties in regard to Km and pH optimum (37). An enzyme that carries out the reverse reaction of SM synthase was recently described in rat liver chromatin catalyzing reaction of (14C)SM with diacylglycerol (DAG) and resulting in transfer of (14C)phosphocholine from SM to DAG with formation of phosphatidylcholine (38). SM synthase activity in chromatin was 7.5 times that of reverse SM synthase, and it was unclear whether the two reactions are catalyzed by the same or different enzymes. The reverse reaction thus elevates CER, as does SMase, but with the important difference that it also reduces DAG while increasing phosphatidylcholine. As a result, the CER/DAG ratio, viewed as a form of regulatory control, is somewhat higher in chromatin than in the NE. It was proposed that perturbation of CER-DAG equilibrium in the nucleus might be a key factor that initiates proliferation or apoptosis, depending on the direction of change (11).
Fig. 2.
Sphingomyelin (SM), the major sphingolipid of nuclei, and its metabolic pathways in mammalian nuclei. All indicated reactions have been shown to occur in the nucleus, with the exception of pathways with dashed arrows, viz., ceramide (CER) kinase. In addition, possible catabolism of sphingosine-1-phosphate (S-1-P) via S-1-P phosphatase and/or S-1-P lyase, known to occur in other subcellular compartments, has not yet been detected in the nucleus. A single fatty acid component (C16) of CER is shown, but other chain lengths are possible. DAG, diacylglycerol; PC, phosphatidylcholine; SMase, sphingomyelinase.
SMase and SM synthase in their various isoforms and loci are thus considered to function as autonomous mediators of SM-induced nuclear signaling reactions that regulate metabolic and functional reactivities of the nucleus; reverse SM synthase may also participate (Fig. 2). The activity of neutral SMase in ligated rat liver nuclei increased prior to onset of apoptosis, coincident with increase of CER, and this was followed by elevation of ceramidase and sphingosine in the nucleus (39). This was thought to reflect NE activity, in the absence of related changes in the plasma membrane. Study of chromatin of liver cell nuclei from rats subjected to ciprofibrate, an agent promoting hepatocyte proliferation, revealed SMase increase in contrast to SM synthase that was depressed; these changes occurred selectively in the chromatin (40). Following drug withdrawal, the same hepatocytes underwent apoptosis with a resulting increase in chromatin SM synthase and SM. Experiments with whole nuclei of an embryonic hippocampal cell line subjected to serum deprivation-induced apoptosis showed that as these cells entered the G1 phase, nuclear SMase was activated and SM synthase was inhibited along with CER increase and SM reduction (41). These changes likely reflected the more active enzymes of the NE, perhaps behaving in an opposite manner to those of chromatin (40). Nuclear SMase activation, presumably in the NE, was shown to have a role in radiation-induced apoptosis of radio-sensitive TF-1 cells (42).
A series of cytochemical, biochemical, and ultrastructural investigations suggested association of nuclear phospholipids with RNA-containing structures that involved phospholipid localization near RNA in decondensed chromatin (43–45). The observed equivalent levels of SM and cholesterol suggested a complex of those lipids with proteins in the chromatin (46). Study of RNA-phospholipid interaction suggested to the investigators that SM might represent a bridge between the two RNA strands of double-stranded RNA, providing protection from RNase action (47). SMase was part of the complex, and after this enzyme acted on colocalized SM, the associated RNA became RNase sensitive. Possibly related to these phenomena as well as DNA replication was the finding that phospholipids detectable in the nucleus underwent significant concentration reduction in all steps of the S phase (48), consistent with their conversion to signaling metabolites.
Ceramide and related enzymes/products
CER is the primary enzymatic product of SMase with a signaling role in the nucleus. It undergoes conversion to other signaling entities by the action of ceramidase, sphingosine kinase, and possibly CER kinase. As indicated above, the nuclear levels of CER and DAG are interrelated, and their ratio is viewed by some as an important determinant of nuclear signaling. In that regard it may prove analogous to cytosolic signaling, wherein protein kinase C is activated by DAG and inhibited by CER and sphingosine (49, 50). CER is also believed to activate certain protein kinases in the cytosol (51), but it is not known whether such reactions occur in the nucleus as well. As mentioned, induction of apoptosis in rat liver led to activation of SMase and ceramidase with concomitant increases of CER and sphingosine in the nucleus. Such changes were not observed in the plasma membrane and were presumably localized in the NE (39). Although controversial in some respects, the role of CER as an inducer of apoptosis has been described most fully in whole-cell studies (49, 50). Following the initial description of ceramidase activity in nuclei (39), a more detailed study of this enzyme in liver nuclear membrane was reported, including its possession of maximum activity over a broad neutral to alkaline range (52).
Sphingosine produced by ceramidase was shown to have modulatory properties in whole-cell studies and to be phosphorylated to sphingosine-1-phosphate (S-1-P) (50). The latter has been implicated as regulator of cell proliferation and antiapoptotic processes, stemming from its ability to act as intracellular messenger and extracellular ligand for a family of G protein-coupled receptors (53). Two major kinases are involved in the synthesis of S-1-P, and of these, sphingosine kinase-2 was shown to be localized in the nucleus due to a nuclear localization signal at the N-terminus (54). Expression of this kinase in various cell types caused cell-cycle arrest at the G1/S phase with resultant inhibition of DNA synthesis. On the other hand, Swiss 3T3 cells, when stimulated with platelet-derived growth factor, showed a significant increase in the nucleoplasm-associated kinase leading to S-1-P formation that correlated with progression of cells to the S phase and translocation of the kinase to the NE (10). That study also revealed that long-term exposure to platelet-derived growth factor caused activation of S-1-P formation in cytosol and translocation to nucleoplasm. It is not yet known whether hydrolase and lyase enzymes that metabolize S-1-P and that are known to occur in other loci also occur in the nucleus. The same may be said of CER kinase and its product, ceramide-1-phosphate, which in the context of whole-cell activity shows evidence of Ca2+ regulatory properties (55, 56) but to our knowledge has not yet been observed in the nucleus.
Nuclear gangliosides
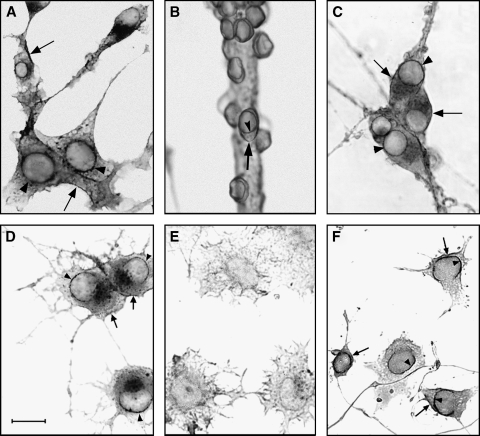
Several studies have indicated gangliosides to occur as intrinsic components of the nucleus, although systematic study of all classes of glycosphingolipids has yet to be reported for that organelle. An early report found them present in every subcellular fraction of bovine mammary gland and rat liver, the nuclear concentration being comparable to that in total liver homogenate (57). Another study of rat liver nuclear membrane indicated a ganglioside content ∼10% that of the plasma membrane, the major species being GM1 and GM3 (58). A subsequent investigation of isolated nuclei from bovine mammary gland reported the presence of gangliosides GM3, GD3, and GT1b, all of which inhibited to variable degrees protein kinase C extracted from the same nuclei (59). A more recent developmental study reported the presence of GM1 and other gangliotetraose gangliosides, along with lesser amounts of GM3 and c-series gangliosides, in large (presumably neuronal) nuclei from mature rat brain, and relatively more GM3 and GD3 in such nuclei from developing brain (60). The same study found smaller nuclei (presumably glial) to have significantly less total ganglioside. A frequent question in regard to all such studies concerns purity, viz. whether the isolated nuclei are truly free of microsomes, plasma membranes, and other membranous elements. This is a legitimate concern, for despite the high buoyant density of nuclei, which facilitates isolation by density gradient centrifugation, minor amounts of ganglioside-rich contaminants can lead to misleading results with ganglioside-poor nuclei and the various subfractions. Taking cognizance of this problem and the fact that greater purity can be achieved by employing cultured cells as opposed to whole tissue, nuclei were obtained from neuro2A cells with use of two successive high-density sucrose gradients and high purity verified by virtual absence of markers for plasma membrane, Golgi apparatus, and ER (61). GM1 and GD1a were found to comprise the large majority of nuclear gangliosides and were shown by biochemical analysis to occur primarily (though not exclusively) in the NE. This locus was confirmed for GM1 by cytochemical analysis with cholera toxin B subunit (Ctx B) linked to horseradish peroxidase (Fig. 3). In relation to function, it was notable that this ganglioside was elevated in the NE of cells that were stimulated to undergo axonogenesis (compare Fig. 3D and 3E), and was scarcely detectable in the nuclei of undifferentiated cells. Pimary neurons in culture yielded similar results.
Fig. 3.
Cytochemical evidence for copresence of GM1 and Na+/Ca2+ exchanger (NCX) in the NE of cultured neuronal cells. GM1 was detected with Ctx B–horseradish peroxidase, and NCX with anti-NCX antibody plus horseradish peroxidase–linked second antibody. GM1 expression was observed in the NE of differentiated neuro2A cells (A), rat cerebellar granular neurons (B), and rat superior cervical ganglion neurons (C). GM1 expression in the NE of differentiated (D) and undifferentiated (E) NG108-15 cells yielded scant GM1 in the NE of the latter and elevated GM1 in the NE of former. NCX expression was observed in the NE of differentiated NG108-15 cells (F). Arrowheads indicate staining of NE and arrows represent staining of plasma membrane. The bar represents 20 micrometers.
The nuclear ganglioside locus was further defined by subjecting isolated nuclei to mild treatment with sodium citrate solution to selectively remove and isolate the outer membrane of the NE (19, 62). The inner membrane was obtained from the resulting nuclear remnant, and both membrane components of the NE were shown to contain GM1 and GD1a (22). An unexpected finding in that study was that GM1 in the inner membrane occurred in tight association with a Na+/Ca2+ exchanger (NCX), a molecule not previously reported as an NE component. High-affinity binding of GM1 to NCX was indicated in their comigration during sodium dodecylsulfate-polyacrylamide gel electrophoresis. The GM1 in the outer nuclear membrane was not associated with NCX (nor was that in the plasma membrane). A possible function of GD1a in the inner membrane is that of GM1 reserve, undergoing conversion to the latter as needed by a sialidase present in the NE (63). A few studies have dealt with gangliosides in endonuclear domains, such as heterochromatin from mouse epithelial cells, which was suggested to contain GM1 due to binding of Ctx B and anti-GM1 antibody (64). Evidence for GD3 colocalization with nuclear chromatin was obtained with rat cortical neurons subjected to β-amyloid peptide, this occurring just prior to entry into S phase and apoptotic death (65). A recent study demonstrated translocation of GD3 from cytosol to nuclei in a manner strongly correlated with rapid phosphorylation of histone H1 shortly after induction of apoptosis (66). This was viewed as support for the hypothesis that nuclear GD3 influences apoptosis through posttranslational modification of histone H1 with activation/repression of specific genes.
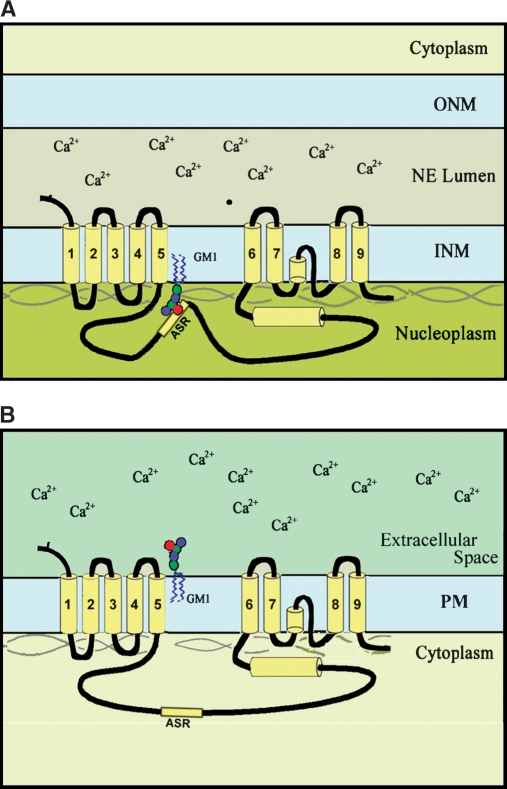
GM1 association with nuclear NCX results in potentiation of Na+/Ca2+ exchange, as demonstrated in Ca2+ uptake experiments with isolated nuclei incubated in the presence of 45Ca2+ (22). Calcium was transferred from nucleoplasm to the NE lumen, consistent with NCX/GM1 location at the inner membrane. Such activity was limited when GM1 was absent from the NE and could be blocked when it was present by binding with Ctx B. The potentiation effect was specific for GM1—that being the only ganglioside to potentiate activity upon addition to nuclei from undifferentiated cells. As with plasma membrane NCX, uphill transfer of Ca2+ from regions of low to high concentration is driven by a Na+ gradient, the required intraluminal Na+ buildup occurring by means of a Na+/K+ ATPase in the NE (67). This type of exchange, intrinsically reversible, mediates counter-transport across the plasma membrane of three Na+ ions for extrusion of one cytosolic Ca2+ (68). A topological requirement of this so-called “forward mode” in the plasma membrane is that the large polypeptide loop between transmembrane segments five and six of NCX reside on the low Ca2+ (cytosolic) side, which would place it on the plasma membrane side opposite to the GM1 oligosaccharide chain. Assuming the same topological requirement for the NE, this would place the loop in the nucleoplasm to facilitate transfer of nucleoplasmic Ca2+ across the inner nuclear membrane to the high Ca2+ concentration pool within the NE lumen. This would result in the oligosaccharide chain of GM1 extending on the same (nucleoplasmic) side as the NCX loop (Fig. 4), a substantially different orientation than for the plasma membrane. Support for this orientation of GM1 comes from the observed availability of GM1 oligosaccharide to Ctx B for cytochemical detection (Fig. 3) and blockage of Na+/Ca2+ exchange (22). In this orientation the negatively charged oligosaccharide is able to interact with the alternative splice region of the large inner loop of NCX, some of whose isoforms are enriched in basic amino acids (69). The existence of splice variants of the NCX1 subtype, which predominates in many neural cells (70, 71), suggests the possibility that specific isoforms are targeted to the NE and others to the plasma membrane.
Fig. 4.
Proposed topology of GM1 and Na+/Ca2+ exchanger (NCX) in the nuclear envelope (NE; A) and plasma membrane (PM; B). In both cases the large loop between transmembrane units 5 and 6 is located on the low Ca2+ side (i.e., cytoplasm for PM and nucleoplasm for NE). This accords with the demonstrated location of both GM1 and NCX in the inner membrane of the NE and occurrence of the large NCX loop in proximity to the GM1 oligosaccharide chain. The authors propose that the high-affinity association of GM1 with NCX arises from the negative charge of N-acetylneuraminic acid in GM1 interacting with the alternative splice region (ASR) of the NCX loop, some of whose isoforms are enriched in positively charged amino acids. Such association is not possible for the PM, because the NCX loop and GM1 oligosaccharide occur on opposite sides of the membrane. INM, inner nuclear membrane; ONM, outer nuclear membrane.
It is of interest to determine the variety of cell types that express NCX in the plasma and nuclear membranes. Plasma membrane expression of Na+/Ca2+ exchanger activity is most prominent in excitable cells such as neurons, cardiac myocytes, and secretory cells that experience rapid, several-fold elevation of intracellular Ca2+ (72, 73). However, they also occur at lower activity in certain nonexciteable cells such as astrocytes and C6 glioma cells (70, 71, 74). The latter cell line was found to express such activity only in the NE, in contrast to astrocytes that expressed it in NE and plasma membrane. In both cell types, nuclear NCX was associated with GM1 (74). The Jurkat T-cell line was shown to contain no NCX activity in either membrane, whereas HeLa and NCTC cells express it in both membranes (75). The same study revealed NCX expression in the plasma membrane and NE of some but not all human lymphocytes. These investigations, while limited at present, suggest widespread occurrence of the nuclear NCX/GM1 complex in many cell types of variable excitability.
Nuclear GM1 modulates nuclear Ca2+
Nuclear Ca2+ is regulated by a complexity of exchange, ion pump, and receptor-mediated mechanisms. Continuity of the NE lumen with the ER intermembrane space explains its role as a Ca2+ storage site. The outer membrane of the NE contains SERCA-type Ca2+-activated ATPase, similar to that in the ER, that pumps cytosolic Ca2+ into the NE lumen (76), whereas the inner membrane contains several Ca2+-release mechanisms regulated by Ins(1,4,5)P3, cADP-ribose, and NAADP (62, 77, 78). The existence of nuclear pore complexes that permit free diffusion of Ca2+ between cytosol and nucleoplasm would appear to preclude independent regulation of nuclear Ca2+. However, some studies have suggested the existence of nuclear-cytoplasmic Ca2+ gradients (79, 80), and the matter remains controversial (81). Nuclear Ca2+ regulation is critically important in relation to cell viability and signaling processes that govern virtually every aspect of cell behavior. Nuclear Na+/Ca2+ exchangers potentiated by GM1 could serve a cytoprotective role in shielding the nucleus against prolonged elevation of cytosolic Ca2+, a condition in which Ca2+ exit through nuclear pores would not serve as a protective strategy. Calcium is well known to have a critical role in apoptosis, the nucleus being especially vulnerable to prolonged elevation of nucleoplasmic Ca2+ (82). The protective role of gangliosides was suggested in studies of mice engineered to lack GM2/GD2 synthase, resulting in absence of GM2, GD2, and all gangliotetraose gangliosides such as GM1 (83). Cerebellar granule neurons from such mice, cultured in depolarizing medium, were shown to have lost the ability to regulate Ca2+ homeostasis, characteristic of wild-type cerebellar granule neurons; this resulted in apoptotic death (84). This finding could be due to an absence of GM1, because mutant cells were rescued from apoptosis-inducing levels of K+ and glutamate by bath application of this ganglioside. Significantly, LIGA-20, a semisynthetic analog of GM1 (Fig. 5), proved even more effective than GM1 itself (85). This correlated with the known efficacy of LIGA-20 to restore Ca2+ homeostasis in normal cerebellar granule neurons (86) and in the mutant cerebellar granule neurons as determined by fura-2 ratiometric determination of intracellular Ca2+ (85). In vivo studies suggested nuclear involvement because the above ganglioside-deficient knockout mouse, when administered kainic acid, developed temporal lobe seizures of significantly greater severity and duration than did normal mice (87). Kainate-induced seizures are associated with Ca2+ dysregulation (88), and LIGA-20 again proved significantly more effective than GM1 in attenuating such seizures. Experimental results suggested this was due to its greater membrane permeant properties with enhanced ability to cross the blood-brain barrier, enter brain cells, and insert into the NE (87). This was interpreted as functional replacement of the missing nuclear GM1 in the mutant cells with activation of the subnormally active nuclear NCX. LIGA-20 also reversed the kainite-induced apoptosis observed in the CA3 region of the hippocampus. The fact that exogenous gangliosides also exert multimodal neurotrophic effects at the plasma membrane (89) suggests the benefits incurred by LIGA-20 in this model may not be limited to the nucleus. A more detailed description of nuclear gangliosides and the cytoprotective role of GM1 are given in a recent review (90).
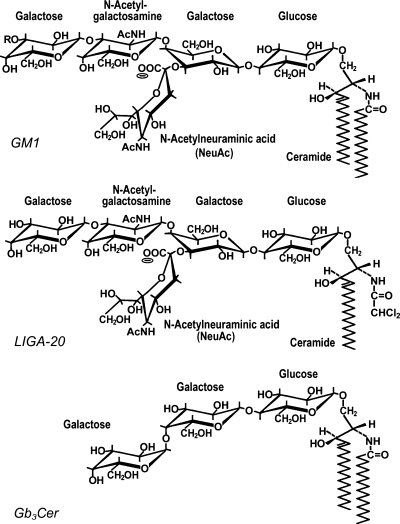
Fig. 5.
Glycosphingolipid structures. GM1, revealed as one of the major nuclear gangliosides in the authors' studies, has R = H. GD1a, the other major ganglioside of neural cells (not shown), has R = NeuAc. LIGA-20 is a semisynthetic derivative of GM1, more membrane permeant than the latter, in which the long-chain fatty acid of CER is replaced by dichloroacetyl. Globotriaosylceramide (Gb3CER) was suggested to occur in the NE (97–99), but this requires confirmation.
As counterpoint to the cytoprotective benefit in possessing the nuclear NCX-GM1 complex, it is worth considering the functional advantage that may accrue with absence of this mechanism in certain normal cells. During development of the nervous system, for example, the importance of programmed cell death as a universal feature of embryonic and postnatal neuroproliferative regions has been well established (91, 92), and the absence of nuclear GM1 at these early stages prior to neuronal differentiation (93) may be a factor rendering such cells vulnerable to programmed cell death. This might also pertain to the subpopulation of lymphocytes lacking the NCX/GM1 complex in the NE, analogous to Jurkat T cells (75). Calcium signaling in T cells is recognized as highly complex, Ca2+ entry being long-lasting and necessary for T-cell function (94, 95). It is necessary that immune effector cells disappear after eliminating foreign antigens, and the mechanism for this is unknown. One proposal is that return of the immune system to rest is mainly due to programmed cell death of activated lymphocytes (96). It remains to be determined whether such lymphocytes are among those shown to lack nuclear NCX/GM1 (75). To further speculate, absence of this complex in the NE might also be a factor in maintaining unresponsiveness or tolerance to self-antigens.
Most studies of nuclear glycosphingolipids have dealt with gangliosides; other categories such as neutral glycosphingolipids and sulfated species remain relatively unexplored. Indirect evidence has suggested the possible presence of globotriaosyl CER (Gb3; Fig. 5) in the NE of human astrocytoma and ovarian carcinoma cell lines, functioning as receptor for the B subunit of verotoxin/shigatoxin (97–99). This is based on the observation that intracellular targeting of the toxin following endocytosis was directed to ER and the perinuclear region, thus defining a new retrograde transport pathway from cell surface to nucleus. Interestingly, this targeting appeared dependent on fatty acid composition of the CER unit (97). This has been proposed as the basis for an improved method to effect nuclear targeting of exogeneous DNA in Gb3-positive cells (100). A systematic exploration of neutral glycosphingolipids as well as other types of acidic glycolipids in the nucleus would appear warranted at this juncture.
Acknowledgments
This review is derived in large part, with revisions and updating, from a recent review by the authors of Ref. 101. Figures from the latter have been utilized with permission from Elsevier.
Abbreviations
CER, ceramide
CGN, cerebellar granule neurons
Ctx B, cholera toxin B subunit
DAG, diacylglycerol
ER, endoplasmic reticulum
Gb3, globotraosylceramide
HRP, horseradish peroxidase
NCX, sodium calcium exchanger
NE, nuclear envelope
S-1-P, sphingosine phosphate
SM, sphingomyelin
SMase, sphingomyelinase
Published, JLR Papers in Press, March 9, 2008.
Footnotes
This study was supported by National Institutes of Health grant 2RO1 NS033912.
References
- 1.Chemistry and metabolism of sphingolipids. A collection of papers dedicated to Herbert E. Carter. 1970. Chem. & Phys. Lipids. 5: 1–300.
- 2.Goureau M. F., and J. Raulin. 1970. Unsaturation of exogenous fatty acids and composition of phospholipids linked to liver nucleus chromatin. Bull. Soc. Chim. Biol. (Paris). 52 941–953. [PubMed] [Google Scholar]
- 3.Cave C., and P. B. Gahan. 1970. A cytochemical and autoradiographic investigation of nuclear phospholipids. Cardiologia. 23 303–312. [Google Scholar]
- 4.Cocco L., N. M. Maraldi, and F. A. Mandzoli. 1980. Phospholipid interactions in rat liver nuclear matrix. Biochem. Biophys. Res. Commun. 9 890–898. [DOI] [PubMed] [Google Scholar]
- 5.Bkaily, G., D. Jacques, and P. D'Orleans-Juste. 2003. Receptors and channels of nuclear membranes as a new target for drug action. In Pathophysiology of Cardiovascular Disease. N. S. Dhalla, H. Rupp, A. Angel, and G. N. Pierce, editors. Kluwer Acad. Publ., Boston. 473–483.
- 6.Divecha N., H. Banfic, and R. F. Irvine. 1993. Inositides and the nucleus and inositides in the nucleus. Cell. 74 405–407. [DOI] [PubMed] [Google Scholar]
- 7.Cocco L., A. M. Martelli, R. S. Gilmour, S. G. Rhee, and F. A. Manzoli. 2001. Nuclear phospholipase C and signaling. Biochim. Biophys. Acta. 1530 1–14. [DOI] [PubMed] [Google Scholar]
- 8.Maraldi N. M., L. Cocco, S. Capitani, G. Mazzotti, O. Barnabei, and F. A. Manzoli. 1994. Lipid-dependent nuclear signaling: morphological and functional features Adv. Enzyme Regul. 34 129–143. [DOI] [PubMed] [Google Scholar]
- 9.Kleuser B., M. Maceyka, S. Milstien, and S. Spiegel. 2001. Stimulation of nuclear sphingosine kinase activity by platelet-derived growth factor. FEBS Lett. 503 85–90. [DOI] [PubMed] [Google Scholar]
- 10.Albi E., and M. P. Viola Magni. 2004. The role of intranuclear lipids. Biol. Cell. 96 657–667. [DOI] [PubMed] [Google Scholar]
- 11.Martelli A. M., R. Bortul, G. Tabellini, M. Aluigi, D. Peruzzi, R. Bareggi, P. Narducci, and L. Cocco. 2001. Re-examination of the mechanisms regulating nuclear inositol lipid metabolism. FEBS Lett. 505 1–6. [DOI] [PubMed] [Google Scholar]
- 12.Tamiya-Koizumi K. 2002. Nuclear lipid metabolism & signaling. J. Biochem. 132 13–22. [DOI] [PubMed] [Google Scholar]
- 13.Ledeen R. W., and G. Wu. 2004. Nuclear lipids: key signaling effectors in the nervous system and other tissues. J. Lipid Res. 45 1–8. [DOI] [PubMed] [Google Scholar]
- 14.Ledeen R. W., and G. Wu. 2006. Sphingolipids of the nucleus and their role in nuclear signaling. Biochim. Biophys. Acta. 1761 558–598. [DOI] [PubMed] [Google Scholar]
- 15.Irvine R. F. 1998. Nuclear lipid signaling. Nat. Rev. Mol. Cell Biol. 4 349–361. [DOI] [PubMed] [Google Scholar]
- 16.Lamond A. I., and W. C. Earnshaw. 1998. Structure and function in the nucleus. Science. 280 547–553. [DOI] [PubMed] [Google Scholar]
- 17.Maraldi N. M., N. Zini, S. Santi, A. Ognibene, R. Rizzoli, G. Mazzotti, and F. A. Manzoli. 1998. Cytochemistry of the functional domains of the nucleus in normal and in pathological conditions. Eur. J. Histochem. 42 41–53. [PubMed] [Google Scholar]
- 18.Vlcek S., T. Dechat, and R. Foisner. 2001. Nuclear envelope and nuclear matrix: interactions and dynamics. Cell. Mol. Life Sci. 58 1758–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilchrist J. S. C., and G. N. Pearce. 1993. Identification and purification of a calcium-binding protein in hepatic nuclear membranes. J. Biol. Chem. 268 4291–4299. [PubMed] [Google Scholar]
- 20.Alroy J., F. B. Merk, V. Goyal, and A. Ucci. 1981. Heterogeneous distribution of filipin-sterol complexes in nuclear membranes. Biochim. Biophys. Acta. 649 239–243. [DOI] [PubMed] [Google Scholar]
- 21.Kim J., and Y. Okada. 1983. Asymmetric distribution and temperature-dependent clustering of filipin-sterol complexes in the nuclear membrane of Ehrlich ascites tumor cells. Eur. J. Cell Biol. 29 244–252. [PubMed] [Google Scholar]
- 22.Xie X., G. Wu, Z-H. Lu, and R. W. Ledeen. 2002. Potentiation of a sodium-calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside. J. Neurochem. 81 1185–1195. [DOI] [PubMed] [Google Scholar]
- 23.Keenan T. W., R. Berezney, and F. L. Crane. 1972. Lipid composition of further purified bovine liver nuclear membranes. Lipids. 7 212–215. [DOI] [PubMed] [Google Scholar]
- 24.James J. L., G. A. Clawson, C. H. Chan, and E. A. Smuckler. 1981. Analysis of the phospholipid of the nuclear envelope and endoplasmic reticulum of liver cells by high pressure liquid chromatography. Lipids. 16 541–545. [DOI] [PubMed] [Google Scholar]
- 25.Xing Y., C. V. Johnson, P. R. Dobner, and J. B. Lawrence. 1993. Higher level organization of individual gene transcription and RNA splicing. Science. 259 1326–1330. [DOI] [PubMed] [Google Scholar]
- 26.Carter K. C., D. Bowman, W. Carrington, K. Fogarty, J. A. McNeil, F. S. Fay, and J. B. Lawrence. 1993. A three-dimensional view of precursor messenger RNA metabolism within the mammalian nucleus. Science. 259 1330–1335. [DOI] [PubMed] [Google Scholar]
- 27.Shopland L. S., and J. B. Lawrence. 2000. Seeking common ground in nuclear complexity. J. Cell Biol. 150 41–51. [DOI] [PubMed] [Google Scholar]
- 28.Casolari J. M., C. R. Brown, S. Komili, J. West, H. Hieronymus, and P. A. Silver. 2004. Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell. 117 427–439. [DOI] [PubMed] [Google Scholar]
- 29.Neitcheva T., and D. Peeva. 1995. Phospholipid composition, phospholipase A2 and sphingomyelinase activities in rat liver nuclear membrane and matrix. Int. J. Biochem. Cell Biol. 27 995–1001. [DOI] [PubMed] [Google Scholar]
- 30.Albi E., M. Mersel, C. Leray, M. L. Tomassoni, and M. P. Viola Magni. 1994. Rat liver chromatin phospholipids. Lipids. 29 715–719. [DOI] [PubMed] [Google Scholar]
- 31.Albi E., S. Cataldi, G. Rossi, and M. P. Viola Magni. 2003. A possible role of cholesterol-sphingomyelin/phosphatidylcholine in nuclear matrix during rat liver regeneration. J. Hepatol. 38 623–628. [DOI] [PubMed] [Google Scholar]
- 32.Hunt A. N., G. T. Clark, G. S. Attard, and A. D. Postle. 2001. Highly saturated endonuclear phosphatidylcholine is synthesized in situ and collocated with CDP-choline pathway enzymes. J. Biol. Chem. 276 8492–8499. [DOI] [PubMed] [Google Scholar]
- 33.Tamiya-Koizumi K., H. Umekawa, S. Yoshida, and K. Kojima. 1989. Existence of Mg2+-dependent, neutral sphingomyelinase in nuclei of rat ascites hepatoma cells. J. Biochem. 106 593–598. [DOI] [PubMed] [Google Scholar]
- 34.Alessenko A., and S. Chatterjee. 1995. Neutral sphingomyelinase: localization in rat liver nuclei and involvement in regeneration/proliferation. Mol. Cell. Biochem. 143 169–174. [DOI] [PubMed] [Google Scholar]
- 35.Albi E., and M. P. Viola Magni. 1997. Chromatin neutral sphingomyelinase and its role in hepatic regeneration. Biochem. Biophys. Res. Commun. 236 29–33. [DOI] [PubMed] [Google Scholar]
- 36.Mizutani Y., K. Tamiya-Koizumi, N. Nakamura, M. Kobayashi, Y. Hirabayashi, and S. Yoshida. 2001. Nuclear localization of neutral sphingomyelinase 1: biochemical and immunocytochemical analyses. J. Cell Sci. 114 3727–3736. [DOI] [PubMed] [Google Scholar]
- 37.Albi E., and M. P. Viola Magni. 1999. Sphingomyelin synthase in rat liver nuclear membrane and chromatin. FEBS Lett. 460 369–372. [DOI] [PubMed] [Google Scholar]
- 38.Albi E., R. Lazzarini, and M. P. Viola Magni. 2003. Reverse sphingomyelin-synthase in rat liver chromatin. FEBS Lett. 549 152–156. [DOI] [PubMed] [Google Scholar]
- 39.Tsugane K., K. Tamiya-Koizumi, M. Nagino, Y. Nimura, and S. Yoshida. 1999. A possible role of nuclear ceramide and sphingosine in hepatocyte apoptosis in rat liver. J. Hepatol. 31 8–17. [DOI] [PubMed] [Google Scholar]
- 40.Albi E., S. Pieroni, M. P. Viola Magni, and C. Sartori. 2003. Chromatin sphingomyelin changes in cell proliferation and/or apoptosis induced by ciprofibrate. J. Cell. Physiol. 196 354–361. [DOI] [PubMed] [Google Scholar]
- 41.Albi E., S. Cataldi, E. Bartoccini, M. P. Viola Magni, F. Marini, F. Mazzoni, G. Rainaldi, M. Evangelista, and M. Garcia-Gil. 2005. Nuclear sphingomyelin pathway in serum deprivation-induced apoptosis of embryonic hippocampal cells. J. Cell. Physiol. 9999 1–7. [DOI] [PubMed] [Google Scholar]
- 42.Jaffrezou J. P., A. P. Bruno, A. Moisand, T. Levade, and G. Laurent. 2001. Activation of a nuclear sphingomyelinase in radiation-induced apoptosis. FASEB J. 15 123–133. [DOI] [PubMed] [Google Scholar]
- 43.Zini N., N. M. Maraldi, A. M. Martelli, A. Antonucci, P. Santi, G. Mazzotti, R. Rizzoli, and F. A. Manzoli. 1989. Phospholipase C digestion induces the removal of nuclear RNA: a cytochemical quantitative study. Histochem. J. 21 491–500. [DOI] [PubMed] [Google Scholar]
- 44.Fraschini A., E. Albi, P. B. Gahan, and M. P. Viola Magni. 1992. TEM cytochemical study of localization of phospholipids in interphase chromatin in rat hepatocytes. Histochem. 97 225–235. [DOI] [PubMed] [Google Scholar]
- 45.Maraldi N. M., N. Zini, S. Squarzoni, R. Del Coco, P. Sabatelli, and F. A. Manzoli. 1992. Intranuclear localization of phospholipids by ultrastructural cytochemistry. J. Histochem. Cytochem. 40 1383–1392. [DOI] [PubMed] [Google Scholar]
- 46.Albi E., and M. P. Viola Magni. 2002. The presence and the role of chromatin cholesterol in rat liver regeneration. J. Hepatol. 36 395–400. [DOI] [PubMed] [Google Scholar]
- 47.Micheli M., E. Albi, C. Leray, and M. P. Viola Magni. 1998. Nuclear sphingomyelin protects RNA from RNase action. FEBS Lett. 431 443–447. [DOI] [PubMed] [Google Scholar]
- 48.Maraldi N. M., S. Santi, N. Zini, A. Ognibene, R. Rizzoli, G. Mazzotti, R. Di Primio, R. Bareggi, V. Bertagnolo, C. Pagliarini, et al. 1993. Decrease in nuclear phospholipids associated with DNA replication. J. Cell Sci. 104 853–859. [DOI] [PubMed] [Google Scholar]
- 49.Mathias S., and R. Kolesnick. 1993. Ceramide: a novel second messenger. Adv. Lipid Res. 25 65–90. [PubMed] [Google Scholar]
- 50.Spiegel S., D. Foster, and R. Kolesnick. 1996. Signal transduction through lipid second messengers. Curr. Opin. Cell Biol. 8 159–167. [DOI] [PubMed] [Google Scholar]
- 51.Mathias S., K. A. Dressler, and R. N. Kolesnick. 1991. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc. Natl. Acad. Sci. US. 88 10009–10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiraishi T., S. Imai, and Y. Uda. 2003. The presence of ceramidase activity in liver nuclear membrane. Biol. Pharm. Bull. 26 775–779. [DOI] [PubMed] [Google Scholar]
- 53.Le Stunff H., S. Milstein, and S. Spiegel. 2004. Generation and metabolism of bioactive sphingosine-1-phosphate. J. Cell. Biochem. 92 882–899. [DOI] [PubMed] [Google Scholar]
- 54.Igarashi N., T. Okada, S. Hayashi, T. Fujita, S. Jahangeer, and S. Nakamura. 2003. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem. 278 46832–46839. [DOI] [PubMed] [Google Scholar]
- 55.Colina C., A. Flores, C. Casstillo, M. del Rosario Garrido, A. Israel, R. DiPolo, and G. Benaim. 2005. Ceramide-1-P induces Ca2+ mobilization in Jurkat T-cells by elevation of Ins(1,4,5)-P3 and activation of a store-operated calcium channel. Biochem. Biophys. Res. Commun. 336 54–60. [DOI] [PubMed] [Google Scholar]
- 56.Mitsutake S., and Y. Igarashi. 2005. Calmodulin is involved in the Ca2+-dependent activation of ceramide kinase as a calcium sensor. J. Biol. Chem. 280 40436–40441. [DOI] [PubMed] [Google Scholar]
- 57.Keenan T. W., D. J. Morré, and C. M. Huang. 1972. Distribution of gangliosides among subcellular fractions from rat liver and bovine mammary gland. FEBS Lett. 24 204–208. [DOI] [PubMed] [Google Scholar]
- 58.Matyas G. R., and D. J. Morre. 1987. Subcellular distribution and biosynthesis of rat liver gangliosides. Biochim. Biophys. Acta. 921 599–614. [DOI] [PubMed] [Google Scholar]
- 59.Katoh N., T. Kira, and A. Yuasa. 1993. Protein kinase C substrates and ganglioside inhibitors in bovine mammary nuclei. J. Dairy Sci. 76 3400–3409. [DOI] [PubMed] [Google Scholar]
- 60.Saito M., and K. Sugiyama. 2002. Characterization of nuclear gangliosides in rat brain: concentration, composition, and developmental changes. Arch. Biochem. Biophys. 398 153–159. [DOI] [PubMed] [Google Scholar]
- 61.Wu G., Z-H. Lu, and R. W. Ledeen. 1995. Induced and spontaneous neuritogenesis are associated with enhanced expression of ganglioside GM1 in the nuclear membrane. J. Neurosci. 15 3739–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Humbert J-P., N. Matter, J. C. Artault, P. Köppler, and A. N. Malviya. 1996. Inositol 1,4,5-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear calcium signaling by inositol 1,4,5-trisphosphate. J. Biol. Chem. 271 478–485. [DOI] [PubMed] [Google Scholar]
- 63.Saito M., L. L. Fronda, and R. K. Yu. 1996. Sialidase activity in nuclear membranes of rat brain. J. Neurochem. 66 2205–2208. [DOI] [PubMed] [Google Scholar]
- 64.Parkinson M. E., C. G. Smith, P. B. Garland, and S. van Heyningen. 1989. Identification of cholera toxin-binding sites in the nucleus of intestinal epithelial cells. FEBS Lett. 242 309–313. [DOI] [PubMed] [Google Scholar]
- 65.Copani A., D. Melchiorri, A. Caricasole, F. Martini, P. Sale, R. Carnevale, R. Gradini, M. A. Sortino, L. Lenti, R. De Maria, et al. 2002. β-Amyloid-induced synthesis of the ganglioside Gd3 is a requisite for cell cycle reactivation and apoptosis in neurons. J. Neurosci. 22 3963–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tempera, I., B. Buchetti, E. Lococo, R. Gradini, A. Mastronardi, M. T. Mascellino, P. Sale, L. Mosca, M. d'Erme, and L. Lenti. 2008. GD3 nuclear localization after apoptosis induction in HUT-78 cells. Biochem. Biophys. Res. Comm. [DOI] [PubMed]
- 67.Garner M. H. 2002. Na,K-ATPase in the nuclear envelope regulates Na+: K+ gradients in hepatocyte nuclei. J. Membr. Biol. 187 97–115. [DOI] [PubMed] [Google Scholar]
- 68.Philipson K. D., and D. A. Nicoll. 2000. Sodium-calcium exchange: a molecular perspective. Annu. Rev. Physiol. 62 111–133. [DOI] [PubMed] [Google Scholar]
- 69.Kofuji P., W. J. Lederer, and D. H. Schulze. 1994. Mutually exclusive and cassette exons underlie alternatively spliced isoforms of the Na+/Ca2+ exchanger. J. Biol. Chem. 269 5145–5149. [PubMed] [Google Scholar]
- 70.He S., A. Ruknudin, L. L. Bambrick, W. J. Lederer, and D. H. Schulze. 1998. Isoform-specific regulation of the Na+/Ca2+ exchanger in rat astrocytes and neurons by PKA. J. Neurosci. 18 4833–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thurneysen T., D. A. Nicoll, K. D. Philipson, and H. Porzig. 2002. Sodium/calcium exchanger subtypes NCX1, NCX2, and NCX3 show cell-specific expression in rat hippocampus cultures. Brain Res. Mol. Brain Res. 107 145–156. [DOI] [PubMed] [Google Scholar]
- 72.Blaustein M. P., and W. J. Lederer. 1999. Sodium/calcium exchange: its physiological implications. Physiol. Rev. 79 763–854. [DOI] [PubMed] [Google Scholar]
- 73.Quednau B. D., D. A. Nicoll, and K. D. Phillipson. 2004. The sodium/calcium exchanger family SLC. Pflugers Arch. Eur. J. Phys. 447 543–548. [DOI] [PubMed] [Google Scholar]
- 74.Xie X., G. Wu, and R. W. Ledeen. 2004. C6 cells express a sodium-calcium exchanger/GM1 complex in the nuclear envelope but have no exchanger in the plasma membrane: comparison to astrocytes. J. Neurosci. Res. 76 363–375. [DOI] [PubMed] [Google Scholar]
- 75.Xie X., G. Wu, Z-H. Lu, C. Rohowsky-Kochan, and R. W. Ledeen. 2004. Presence of sodium-calcium exchanger/GM1 complex in the nuclear envelope of non-neural cells: nature of exchanger-GM1 interaction. Neurochem. Res. 29 2135–2146. [DOI] [PubMed] [Google Scholar]
- 76.Gerasimenko O. V., J. V. Gerasimenko, A. V. Tepikin, and O. H. Petersen. 1995. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP- ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 80 439–444. [DOI] [PubMed] [Google Scholar]
- 77.Stehno-Bittel L., A. Lückhoff, and D. E. Clapham. 1995. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 14 163–167. [DOI] [PubMed] [Google Scholar]
- 78.Gerasimenko J. V., Y. Maruyama, K. Yano, N. J. Dolman, A. V. Tepikin, O. H. Petersen, and O. V. Gerasimenko. 2003. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J. Cell Biol. 163 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Mohanna F. A., K. W. T. Caddy, and S. R. Bolsover. 1994. The nucleus is insulated from large cytosolic calcium ion changes. Nature. 367 745–750. [DOI] [PubMed] [Google Scholar]
- 80.Badminton M. N., J. M. Kendall, C. M. Rembold, and A. K. Campbell. 1998. Current evidence suggests independent regulation of nuclear calcium. Cell Calcium. 23 79–86. [DOI] [PubMed] [Google Scholar]
- 81.Gerasimenko O. V., and J. Gerasimenko. 2004. New aspects of nuclear calcium signaling. J. Cell Sci. 117 3087–3094. [DOI] [PubMed] [Google Scholar]
- 82.Mattson M. P., and S. L. Chan. 2003. Calcium orchestrates apoptosis. Nat. Cell Biol. 5 1041–1043. [DOI] [PubMed] [Google Scholar]
- 83.Liu Y., R. Wada, H. Kawai, K. Sango, C. Deng, T. Tai, M. P. McDonald, K. Araujo, J. N. Crawley, U. Bierfreund, et al. 1999. A genetic model of substrate deprivation therapy for a glycosphingolipid storage disorder. J. Clin. Invest. 103 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu G., X. Xie, Z-H. Lu, and R. W. Ledeen. 2001. Cerebellar neurons lacking complex gangliosides degenerate in the presence of depolarizing levels of potassium. Proc. Natl. Acad. Sci. USA. 98 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu G., Z-H. Lu, X. Xie, and R. W. Ledeen. 2004. Susceptibility of cerebellar granule neurons from GM2/GD2 synthase-null mice to apoptosis induced by glutamate excitotoxicity and elevated KCl: rescue by GM1 and LIGA20. Glycoconj. J. 21 305–313. [DOI] [PubMed] [Google Scholar]
- 86.Manev H., M. Favaron, S. Vicini, A. Guidotti, and E. Costa. 1990. Glutamate-induced neuronal death in primary cultures of cerebellar granule cells: protection by synthetic derivatives of endogenous sphingolipids. J. Pharmacol. Exp. Ther. 252 419–427. [PubMed] [Google Scholar]
- 87.Wu G., Z. H. Lu, J. Wang, Y. Wang, X. Xie, M. F. Meyenhofer, and R. W. Ledeen. 2005. Enhanced susceptibility to kainite-induced seizures, neuronal apoptosis, and death in mice lacking gangliotetraose gangliosides, Protection with LIGA 20, a membrane-permeant analog of GM1. J. Neurosci. 23 11014–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ben-Ari Y., and R. Cossart. 2000. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 23 580–587. [DOI] [PubMed] [Google Scholar]
- 89.Mocchetti I. 2005. Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell. Mol. Life Sci. 62 2283–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ledeen R. W., and G. Wu. 2007. GM1 in the nuclear envelope regulates nuclear calcium through association with a nuclear sodium-calcium exchanger. J. Neurochem. 103 (Suppl. 1): 126–134. [DOI] [PubMed] [Google Scholar]
- 91.Blaschke A. J., J. A. Weiner, and J. Chun. 1998. Programmed cell death is a universal feature of embryonic postnatal neuroproliferative regions throughout the central nervous system. J. Comp. Neurol. 396 39–50. [DOI] [PubMed] [Google Scholar]
- 92.Yeo W., and J. Gautier. 2004. Early neural cell death: dying to become neurons. Developm. Biol. 274 233–244. [DOI] [PubMed] [Google Scholar]
- 93.Kozireski-Chuback D. F., G. Wu, and R. W. Ledeen. 1999. Developmental appearance of nuclear GM1 in neurons of the central and peripheral nervous systems. Dev. Brain Res. 115 201–208. [DOI] [PubMed] [Google Scholar]
- 94.Weiss A., J. Imboden, D. Shoback, and J. Stobo. 1984. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc. Natl. Acad. Sci. USA. 81 4169–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lewis R. S. 2001. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 19 487–521. [DOI] [PubMed] [Google Scholar]
- 96.Parijs L. V., and A. K. Abbas. 1998. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 280 243–248. [DOI] [PubMed] [Google Scholar]
- 97.Arab S., and C. A. Lingwood. 1998. Intracellular targeting of the endoplasmic reticulum/nuclear envelope by retrograde transport may determine cell hypersensitivity to verotoxin via globotriaosyl ceramfide fatty acid isoform traffic. J. Cell. Physiol. 177 646–660. [DOI] [PubMed] [Google Scholar]
- 98.Lingwood C. A., A. A. Khine, and S. Arab. 1998. Globotriaosyl ceramide (Gb3) expression in human tumour cells: intracellular trafficking defines a new retrograde transport pathway from the cell surface to the nucleus, which correlates with sensitivity to verotoxin. Acta Biochim. Pol. 45 351–359. [PubMed] [Google Scholar]
- 99.Khine A. A., M. Firtel, and C. A. Lingwood. 1998. CD77-dependent retrograde transport of CD19 to the nuclear membrane: functional relationship between CD77 and CD19 during germinal center B-cell apoptosis. J. Cell. Physiol. 176 281–292. [DOI] [PubMed] [Google Scholar]
- 100.Facchini L. M., and C. A. Lingwood. 2001. A verotoxin 1B subunit-lambda CRO chimeric protein specifically binds both DNA and globotriaosylceramide (Gb3) to effect nuclear targeting of exogenous DNA in Gb3 positive cells. Exp. Cell Res. 269 1117–1129. [DOI] [PubMed] [Google Scholar]
- 101.Ledeen R. W., and G. Wu. 2006. Sphingolipids of the nucleus and their role in nuclear signaling. Biochim. Biophys. Acta. 1761 588–598. [DOI] [PubMed] [Google Scholar]