Abstract
Adaptation to alternating periods of desiccation and hydration is one of the lichen’s requirements for survival in high mountain environment. In the dehydrated state, respiration and photosynthesis of the foliaceous lichen Xanthoria elegans were below the threshold of CO2-detection by infrared gas analysis. Following hydration, respiration totally recovered within seconds and photosynthesis within minutes. In order to identify metabolic processes that may contribute to restart so quickly and efficiently lichen physiological activity, we analysed the metabolite profile of lichen thalli step by step during hydration/dehydration cycles, using 31P-and 13C-NMR. It appeared that the recovery of respiration was anticipated during dehydration by the accumulation of important stores of gluconate 6-P (glcn-6-P) and by the preservation of the nucleotide pools, whereas glycolysis and photosynthesis intermediates like glucose 6-P and ribulose 1,5-diphosphate disappeared. The important pools of polyols present in both X. elegans photo- and mycobiont likely contributed to protect cells constituents like nucleotides, proteins, and membrane lipids, and to preserve the intactness of intracellular structures during desiccation. Our data indicate that glcn-6-P accumulated due to the activation of the oxidative pentose phosphate pathway, in response to cell need for reducing power (NADPH) during the dehydration-triggered down regulation of metabolism. On the contrary, glcn-6-P was metabolised immediately after hydration, supplying respiration with substrates during the recovery of the pools of glycolysis and photosynthesis intermediates. Finally, the high net photo synthetic activity of wet X. elegans thalli at low temperature may help this alpine lichen to take advantage of short hydration opportunities such as ice melting, thus favouring its growth in the harsh high mountain climate.
Keywords: Altitude, Lichens, metabolism, physiology, Magnetic Resonance Spectroscopy, Photosynthesis
Keywords: lichen, Xanthoria elegans, extreme environment, alpine environment, stress adaptation, dessication, reviviscence, metabolism, energy metabolism, photosynthesis, glycolysis, polyols, oxidative pentose phosphate pathway, metabolite profiling, nuclear magnetic resonance, NMR spectroscopy
Introduction
Lichens are living organisms among the most resistant to extreme environments including the deserts and frigid areas of the five continents. In the alpine environment, these symbiotic organisms are exposed to harsh fluctuations of water supply, light intensity, and temperature (Kappen 1988; Körner, 2003). This is typically the case for Xanthoria elegans (Link) used in this study, whose growth on rocky surfaces mainly depends on atmospheric water input. X. elegans is a saxicolous desiccation-tolerant lichen of the Teloschistaceae family (Helms, 2003), containing an ascomycetous fungus and a unicellular green alga belonging to the Teloschistes and Trebouxia genus, respectively (Helms, 2003). In the place where they were harvested, thalli naturally undergo hydration/dehydration cycles, growing when they are hydrated by snow melting, rain, or dew.
Metabolic activity of lichen thalli appreciated via gas exchanges becomes almost undetectable when their water content decreases below 10–15% of their dry weight (Lange 1980; Schroeter et al. 1991). Nevertheless, lichens may take up sufficient amounts of water from the vapour in the atmosphere (Lange 1980) and thus maintain a significant metabolic activity above this rather low hydration threshold. Hydration is facilitated by high concentrations of polyols in both photo- and mycobiont (Rundel 1988) that lower water potential (Lange et al. 1990), thus allowing photosynthetic activity even under increasing degrees of desiccation (Nash et al. 1990). Immersion in water or rewetting in moist air triggers a process of reviviscence in dry desiccation-tolerant lichens (Smith and Molesworth 1973; Bewley 1979). Respiration and photosynthesis shortly resume normal activities upon hydration, indicating that cell damages induced by drying are rapidly repaired (Farrar and Smith, 1976). Indeed, according to different authors (Dudley and Lechowicz 1987; Longton 1988), desiccated membranes are leaky, exposing cell contents to surrounding solution during rewetting, which leads to losses of organic and inorganic solutes. Thus, cell survival requires a rapid resealing of plasma membrane disruptions (McNeil and Steinhardt 1997; McNeil et al. 2003). Nevertheless, a delay for repairs and synthesis of lost metabolites could be expected.
Intense solar radiation is another environmental parameter that threatens lichens with the potential damaging effects of reactive oxygen species (ROS) production (Fridovich, 1999), in particular when photosynthetic water oxidation stops due to dehydration. In the plant kingdom, the ability to withstand light stress and desiccation of vegetative organs involves various mechanisms recently reviewed by Hoekstra et al. (2001), Rascio and La Rocca (2005), and Heber et al. (2005). In hydrated organs, these include increased energy dissipation by fluorescence at the PSII level and cyclic electron transport around PSI (Heber and Walker 1992; Manuel et al. 1999; Cornic et al. 2000). On the contrary, when they are dry, poikilohydric organisms do not emit light-induced fluorescence, revealing an inactivation of PSII function (Lange et al. 1989). These organisms minimise damage by activating exciton transfer to quenchers and converting excess energy to heat (Bilger et al. 1989; Heber et al. 2000). Interestingly, following hydration, a burst of intracellular production of ROS was reported in photo- and mycobiont of Ramalina lacera, and this burst modifies superoxyde dismutase, catalase, glutathione reductase, and glucose 6-P dehydrogenase activities (Weissman et al. 2005).
In desiccation-tolerant plants, the recovery of cell damages upon hydration, and the subsequent restoration of cell functions, involves various and complex inductive mechanisms including gene transcriptions and an increased need for protein turnover (Oliver et al. 2004). Therefore it requires a rather lengthy time ranging from a few hours to several days (Gaff, 1997). In contrast, we observed that the respiration and the photosynthetic activity of different high mountain lichens restarted almost immediately after rewetting. Therefore, we hypothesised that the rapid recovery of these organisms relies on the preservation/accumulation of key pools of metabolites during dehydration. We supposed, for example, that intermediates of energy metabolism like respiratory substrates, nucleosides, and pyridine nucleotides did not massively leak out photo- and mycobiont during hydration/desiccation cycles in lichens. To substantiate this hypothesis, we assessed in parallel the respiration and photosynthetic activities and the metabolite profiles of X. elegans thalli during hydration and dehydration. The main pools of metabolites were characterized in vitro and in vivo using 31P- and 13C-NMR as a convenient technique giving a precise overview of the soluble organic compounds present in plant materials (Bligny and Douce 2001; Streb et al. 2003).
Materials and methods
Lichens sampling and measurement of their dry and wet weight
X. elegans thalli were collected at 2800 m on limestone rocks situated above the Galibier pass (Hautes-Alps), an area characterised by a relatively continental and dry climate. The so-called “dry thalli” were taken under the sun, during the hottest hours of the day (rocks surface temperature, 30–35°C; relative air humidity, 20%). They still contained ca 8% water by comparison with oven-desiccated thalli (2 h at 110°C). The so-called “wet thalli” correspond to lichens collected in the same area either at dawn, in the dark, or just after a rain, in the light, as stated in text. The weight of wet thalli was measured in situ after removing interstitial water by straining between two layers of absorbent paper. The wet wt versus dry wt ratio was usually ca 2.5. Thalli were stored frozen until needed.
Measurements of respiration and photosynthesis activities
These activities were measured either via O2 or CO2 exchanges as stated in results section. CO2 exchanges were measured with an infrared gas analyser (IRGA) equipped with a 1,200 ml chamber (LI-COR 6200, Lincoln USA). The temperature inside the closed chamber was maintained by a thermostat between ~2.5°C and 30°C. Thalli (ca 0.5 g dry weight) were let attached to their support. O2 was monitored polarographically at 20°C in a 1-ml water chamber equipped with a Clark-type oxygen-electrode purchased from Hansatech Ltd (King’s Lynn, Norfolk, UK). The O2 concentration in air-saturated medium was taken as 210 μM at 20°C and 780 hPa in the alpine lab (2100 m) where measurements were done. In order to facilitate the sample stirring in the measurement chamber, thalli were fragmented into pieces of 1–2 mm2 by gentle grinding with mortar and pestle in liquid nitrogen. 50 mg of dry thalli was used for O2-electrode measurements. Controls were done to ensure that the lichen fragmentation at liquid nitrogen temperature did not modify the rates of respiration and photosynthesis after unfreezing and hydration. When utilised, illumination was maintained above saturation at a photosynthetically active photon flux density (PPFD) of 500 μmol m−2 s−1, using a Shott KL 1500 (Amilabo, France) light generator. The incubation medium contained 0.1 mM potassium bicarbonate, pH 6.8.
PCA extract preparation
Thalli (4 g dry weight) were quickly frozen in liquid nitrogen and ground to a fine powder with a mortar and pestle with 1 ml of 70% (vol/vol) PCA. The frozen powder was then placed at −10°C and thawed. The thick suspension thus obtained was centrifuged at 15,000 g for 10 min to remove particulate matter and the supernatant was neutralised with 2 M KHCO3 to about pH 5.2. The supernatant was then centrifuged at 10,000 g for 10 min to remove KClO4; the resulting supernatant was lyophilised and stored in liquid nitrogen. This freeze-dried material was dissolved in 2.5 ml water containing 10% D2O, and stored frozen.
31P- and 13C-NMR analyses of PCA extracts
Spectra were recorded on a Bruker NMR spectrometer (AMX 400, wide bore; Bruker Instruments, Inc., Billerica, MA) equipped with a 10-mm multinuclear probe tuned at 162 MHz or 100.6 MHz for 31P- or 13C-NMR studies respectively. The deuterium resonance of D2O was used as a lock signal.
31P-NMR acquisition conditions: 70° radio frequency pulses (15 μs) at 3.6 s intervals; spectral width 8200 Hz; 4096 scans; Waltz-16 1H decoupling sequence (with two levels of decoupling: 1 W during acquisition time, 0.5 W during delay). Free induction decays were collected as 8K data points, zero filled to 16K and processed with a 0.2 Hz exponential line broadening. 31P-NMR spectra are referenced to methylenediphosphonic acid (pH 8.9) at 16.38 ppm. Before 31P-NMR analyses, divalent cations were chelated by the addition of sufficient amounts of CDTA ranging from 100 to 150 μmol. The pH was buffered by the addition of 75 μmol Hepes and adjusted to 7.5.
13C-NMR acquisition conditions: 90° radio frequency pulses (19 μs) at 6 s intervals; spectral width 20,000 Hz; 3600 scans; Waltz-16 1H decoupling sequence (with two levels of decoupling: 2.5 W during acquisition time, 0.5 W during delay). Free induction decays were collected as 16K data points, zero filled to 32K, and processed with a 0.2-Hz exponential line broadening. 13C-NMR spectra are referenced to hexamethyldisiloxane at 2.7 ppm. Mn2+ was chelated by the addition of 2 μmol CDTA and the pH was adjusted to 7.5.
The assignments were made after running a series of standard solutions of known compounds at pH 7.5 and adding aliquots of these compounds to the PCA extracts as described previously (Roby et al. 1987). Identified compounds were quantified by comparison of the surface of their resonance peaks to the surface of resonance peaks of standards added to samples before grinding according to Aubert et al. (1996); NADP+ and NADPH were quantified as described by Pugin et al. (1997). Fully relaxed conditions during spectra acquisition (pulses at 20-s intervals) were used for quantification. The standards utilized were methylphosphonate and maleate for 31P- and 13C-NMR analyses, respectively.
In vivo 31P-NMR measurements
A perfusion system was utilized to optimize the signal-to-noise ratio as described earlier (Gout et al. 2001). Spectra were recorded on a Bruker spectrometer (AMX 400, wide bore) equipped with a 25-mm probe tuned at 162 MHz. 31P-NMR acquisition conditions: 50° radio frequency pulses (70 μs) at 0.6 s intervals; spectral width 9800 Hz; 6000 scans; Waltz-16 1H decoupling sequence (with two levels of decoupling: 2.5 W during acquisition time, 0.5 W during delay). Free induction decays were collected as 4K data points, zero filled to 8K and processed with a 2-Hz exponential line broadening. Spectra were referenced to a solution of 50 mM methylenediphosphonic acid (pH 8.9 in 30 mM Tris) contained in a 0.8 mm capillary itself inserted inside the inlet tube along the symmetry axis of the cell sample (Roby et al. 1987). The assignment of inorganic phosphate (Pi), phosphate esters, phosphate diesters, and nucleotides to specific peaks was carried out according to Roberts and Jardetzky (1981), Roby et al. (1987), Aubert et al. (1996), and from spectra of the PCA-extracts that contained the soluble low molecular weight constituents.
Results
As frequently observed with lichens (Smith and Molesworth 1973; Farrar and Smith 1976; Larson 1981), X. elegans can lose most of its constitutive water in dry atmosphere and become wet again as soon as it receives water. The dehydration of this lichen takes about 30 min when it is exposed to the summer sun. In a spectacular manner, we first observed that respiration, which was undetectable in dry thalli, started at full speed within the seconds following its rewetting.
In situ measurement of gas exchanges in X. elegans thalli
No gas exchanges were detected using the IRGA apparatus (detection threshold, ca 10 nmol CO2 min−1) in thalli containing less than 10% H2O, whatever the temperature and the light conditions i.e. dark or light (photon flux exceeding 1500 μmol m−2 s−1). This indicated that photosynthesis and respiration activities were negligible. On the contrary, CO2 uptake or loss, depending on light or dark conditions, was easily measured at different temperatures in wet thalli (Fig. 1). Interestingly, chlorophyll fluorescence measured according to Heber et al. (2000) was largely quenched in dry lichens, whereas it was detected under the light from the first min of hydration, increasing fast and reaching a steady state after 5–6 min (data not shown), thus confirming that e-transport was negligible in dry thalli.
Fig. 1.
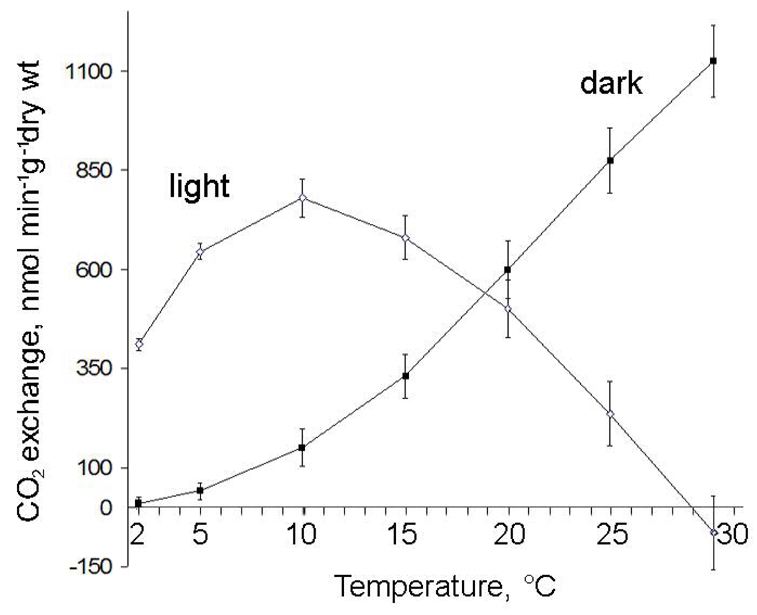
Respiration (●) and net photosynthesis (○) of wet X. elegans thalli in dark and light at different temperatures. Respiration and net photosynthesis were determined in situ via CO2 exchanges measured by IRGA, as described in Materials and methods. Values are referred to the dry weight of thalli dried at 110°C. Values are means ± SD (n=5)
Not surprisingly, the CO2 exchanges of wet thalli were temperature-dependent. In the dark, the respiration of wet thalli increased exponentially with temperature (Fig. 1), like that of higher plants (Bligny et al. 1985). For example, at 5°C, the emssion of CO2 was 25 ± 5 nmol O2 min−1 g−1 lichen dry wt, whereas it was 600 ± 90 at 20°C. Under the light, the uptake of CO2 first increased with temperature, reaching a maximum of 800 ± 80 nmol O2 min−1 g−1 lichen dry wt at 10–15°C, and then decreased continuously above 15 °C. Indeed, the CO2 uptake, called here net photosynthesis, was the resultant of the gross CO2 uptake due to photosyntheticactivity of alga and the emission of CO2 mainly due to fungal respiration if we admit that algal respiration was negligible in the light (Gans and Rebéillé, 1988; Tcherkez et al 2005). In illuminated wet thalli, the CO2 emitted by respiration compensated for the CO2 assimilated by photosynthesis at 28–29°C.
Recovery of respiration and photosynthesis activities of dry X. elegans thalli after hydration
In the following experiments, gas exchanges were measured on immersed thalli fragments with a Clark-type O2-electrode, at 20 °C, as indicated in Materials and methods. We preferred this technique because it was very difficult to stabilise in situ the temperature and relative humidity in the IRGA measurement chamber during dark/light cycles. The oxygraphic traces (Fig. 2) showed that: (i) the O2 uptake in the O2-electrode chamber started at a full speed in the dark, immediately after thalli fragments were introduced, and whatever their initial dry or wet status (traces A1 and B1). Oxygen was consumed at a rate comprised between 580 and 620 nmol min−1 g−1 lichen dry wt. These values were comparable with the rates of CO2 production measured in situ with the IRGA. (ii) A 1-min delay was first observed with initially wet thalli before O2 production stabilised under the light (Fig 2, trace B2). This delay was shorter when the duration of the preceding dark period was reduced (trace B2) and longer (2–3 min) when thalli were initially dry (trace A2). In all cases, the stabilised O2 emission was comparable (comprised between 520 and 640 nmol min−1 g−1 lichen dry wt); (iii)when s amples were then alternatively illuminated and darkened in successive dark/light sequences, respiration and net photosynthesis rates remained remarkably stable over several hours; (iv) assays done with lyophilised thalli containing less than 4% water or with thalli kept dry in the laboratory over one year gave similar results (not shown), thus showing the remarkable adaptation of this lichen to strong and prolonged desiccation.
Fig. 2.
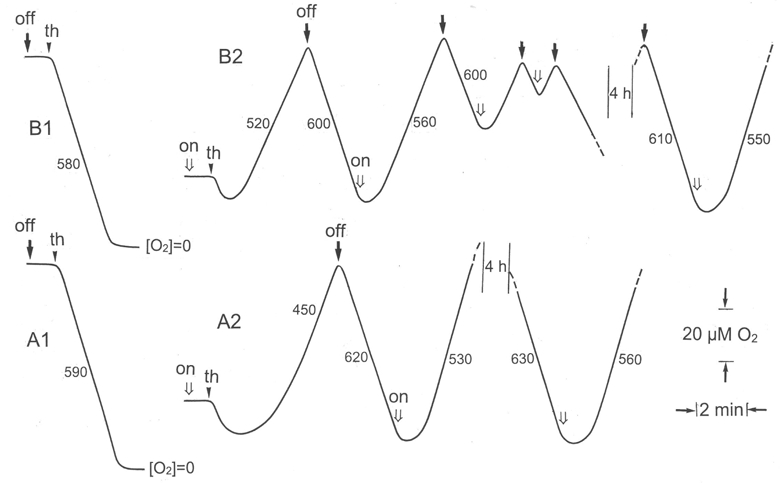
O2 exchanges in X. elegans thalli successively incubated in dark and saturating light. Theses traces were obtained with thalli fragments incubated in an oxygen-electrode chamber. Numbers on the traces refer to nmol of O2 consumed or produced min−1 g−1 lichen dry weight. Thalli (th) were introduced in the chamber as dry fragments in dark (A1) or light (A2), or as wet fragments in dark (B1) or light (B2). The incubation medium contained 0.1 mM bicarbonate at pH 6.8. Open arrows, light on; solid arrows, light off.
Taken together, these data indicated that the cell structures of the fungal and algal partners did not suffer irreversible desiccation-induced damages. Moreover, the fact that respiration recovered instantly suggested that key energy metabolism substrates, nucleotides, and pyridine nucleotides were not lost during a hydration/desiccation cycle. On the contrary, the delay for photosynthesis to recover suggested that key BBC cycle intermediates decreased strongly in the chloroplasts of photobiont during thalli dehydration. In order to verify these hypotheses, we analysed the metabolite profiles of X. elegans thalli taken dry or wet, and during rewetting and drying sequences.
Metabolite profiling of X. elegans thalli
Perchloric acid extracts of X. elegans thalli were analyzed using 31P- and 13C-NMR spectroscopy as described in “Materials and methods”. Representative spectra are shown in figures 3 and 5, and comparative data are given in Table 1.
Fig. 3.
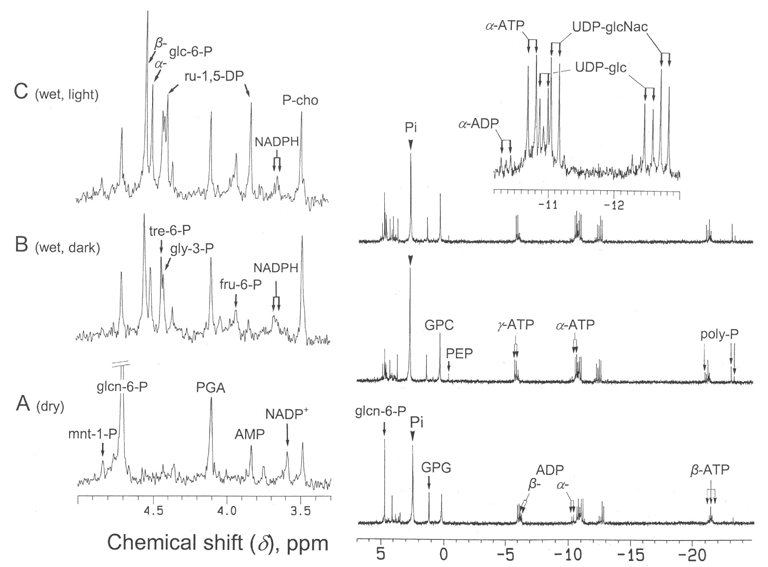
Proton-decoupled 31P-NMR spectra (161.93 MHz) of perchloric acid extracts of X. elegans thalli. Extracts were prepared from 4 g of thalli (on a dry weight basis) and analyzed by 31P-NMR. Thalli were collected as follows: A dry, in the light; B wet, in the dark; C wet, in the light. Peak assignments (from downfield to upfield): mnt-1-P, mannitol 1-P; glcn-6-P, gluconate 6-P; glc-6-P, glucose 6-P; tre-6-P trehalose 6-P; gly-3-P, glycerol 3-P; PGA, phosphoglycerate; fru-6-P, fructose 6-P; ru-1,5-DP, ribulose 1,5-diphosphate; AMP, adenosine monophosphate; P-cho, P-choline; GPG, glycerophosphoglycerol; GPC, glycerophosphocholine; PEP, phosphoenolpyruvate; UDP-glc, uridine 5′-diphosphate-α-D-glucose; UDP-glcNAc, uridine 5′-diphospho-N-acetylglucosamine; poly-P, polyphosphates. Spectra are representative of five independent experiments
Fig. 5.
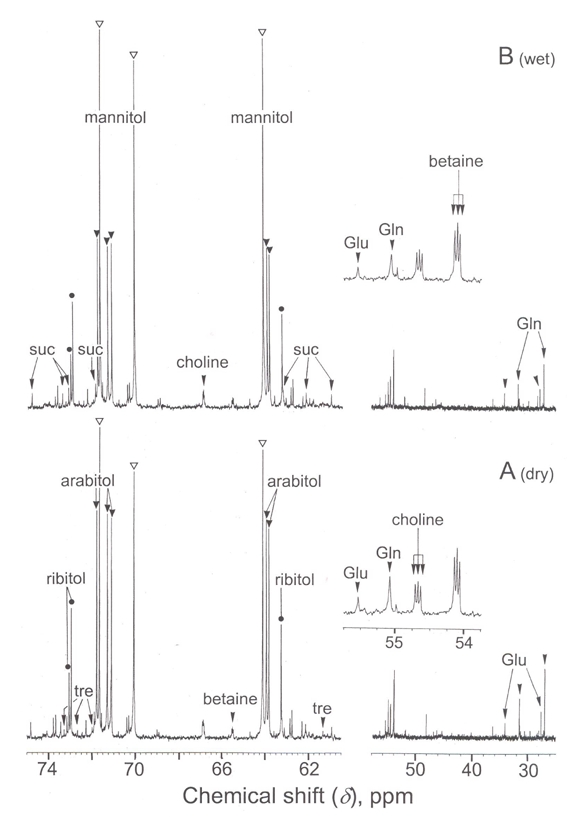
Proton-decoupled 13C-NMR spectra (100.6 MHz) of perchloric acid extracts of X. elegans thalli. PCA extracts were prepared from 4 g of thalli (on a dry weight basis) and analysed by 13C-NMR. Thalli were collected as follows: A dry, in the light; B wet, in the dark. Peak assignments (from downfield to upfield): sue, sucrose; tre, trehalose; Glu, glutamate; Gin, glutamine. Spectra are representative of five independent experiments.
Table 1.
Metabolic profiles of dry and wet X. elegans thalli. Dry thalli were collected in the light and wet thalli either in the dark (at dawn) or in the light. Metabolites were identified and quantified from PCA extracts, using maleate and methylphosphonate as internal standards for 13C- and 31P-NMR analyses, respectively, as described in Materials and methods. Values are given as μmol g−1 lichen dry wt. Abbreviations as indicated in the legends of figures 3 and 5; nd, not detected (<0.05 μmol). Values were obtained from a series of independent experiments and are given as mean ± SD (n=5).
| metabolite | Dry lichens (light) | Wet lichens (dark) | Wet lichen (light) |
|---|---|---|---|
| sucrose | 27 ± 3 | 29 ± 3 | 27 ± 3 |
| trehalose | 9 ± 2 | 5 ± 2 | 8 ± 2 |
| ribitol | 110 ± 10 | 90 ± 9 | 105 ± 10 |
| arabitol | 360 ± 30 | 240 ± 20 | 340 ± 30 |
| mannitol | 240 ± 20 | 290 ± 30 | 250 ± 20 |
| glutamate | 45 ± 5 | 40 ± 4 | 43 ± 5 |
| glutamine | 120 ± 12 | 71 ± 7 | 125 ± 12 |
| choline | 85 ±9 | 80 ± 8 | 88 ± 9 |
| betaine | 170 ± 15 | 150 ± 14 | 160 ± 15 |
| polyphosphates | 6.5 ± 1 | 6.4 ± 1 | 6.2 ± 1 |
| Pi | 2.5 ± 0.4 | 2.7 ± 0.4 | 3.6 ± 0.5 |
| mannitol 1-P | 0.06 ± 0.01 | 0.02 ± 0.01 | 0.5 ± 0.01 |
| gluconate 6-P | 0.91 ± 0.09 | 0. 18 ± 0.02 | 0.20 ± 0.02 |
| glucose 6-P | nd | 0.52 ± 0.05 | 0.81 ± 0.08 |
| trehalose 6-P | nd | 0.22 ± 0.02 | 0.22 ± 0.02 |
| glycerol 3-P | nd | 0.15 ± 0.02 | 0.17 ± 0.02 |
| ru-1,5-DP | nd | nd | 0.27 ± 0.03 |
| PGA | 0.27 ± 0.03 | 0.25 ± 0.03 | 0.27 ± 0.03 |
| fructose 6-P | nd | 0.8 ± 0.01 | 0.13 ± 0.02 |
| AMP | 0.12 ± 0.02 | 0.04 ± 0.01 | nd |
| NADPH | 0.02 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 |
| NADP+ | 0.05 ± 0.01 | nd | nd |
| P-choline | 0.15 ± 0.02 | 0.40 ± 0.04 | 0.33 ± 0.03 |
| GPG | 0.30 ± 0.03 | 0.25 ± 0.02 | 0.25 ± 0.02 |
| GPC | 0.28 ± 0.03 | 0.47 ± 0.05 | 0.47 ± 0.05 |
| PEP | nd | 0.07 ± 0.01 | 0.06 ± 0.01 |
| ATP | 0.38 ± 0.04 | 0.48 ± 0.05 | 0.53 ± 0.05 |
| ADP | 0.14 ± 0.02 | 0.08 ± 0.01 | 0.05 ± 0.01 |
| UDP-glc | 0.13 ± 0.02 | 0.33 ± 0.04 | 0.33 ± 0.04 |
| UDP-glcNAc | 0.39 ± 0.04 | 0.43 ± 0.04 | 0.46 ± 0.05 |
In dry thalli, the two most abundant metabolites measured by 31P-NMR (Fig. 3A) were inorganic phosphate (1.7 μmol g−1 dry wt) and gluconate 6-P (0.91 μmol g−1 dry wt). Other identified P-compounds were, from downfield to upfield: mannitol 1-P, glycerate 3-P, AMP, NADP+, P-choline, the two phosphodiesters glycerylphosphoryl-glycerol and -choline (GPG and GPC), nucleosides (mainly ATP and ADP), and nucleoside diphosphate sugars, UDP-glc and UDP-glcNAc. Polyphosphates were also detected in PCA extracts. However, since they largely precipitate during PCA extraction, we quantified these compounds from in vivo NMR analyses. Spectra like the one shown on Fig. 4 indicate that thalli contained a 6.5 μmol g−1 dry wt Pi equivalent of polyphosphates, which constitutes by far the largest phosphate pool in this lichen. In vivo assays also indicated that the distribution of Pi between alkaline (pH 7.5, cytoplasm) and acidic (pH 5.0–5.5, vacuoles) compartments was roughly 1 versus 2.
Fig. 4.
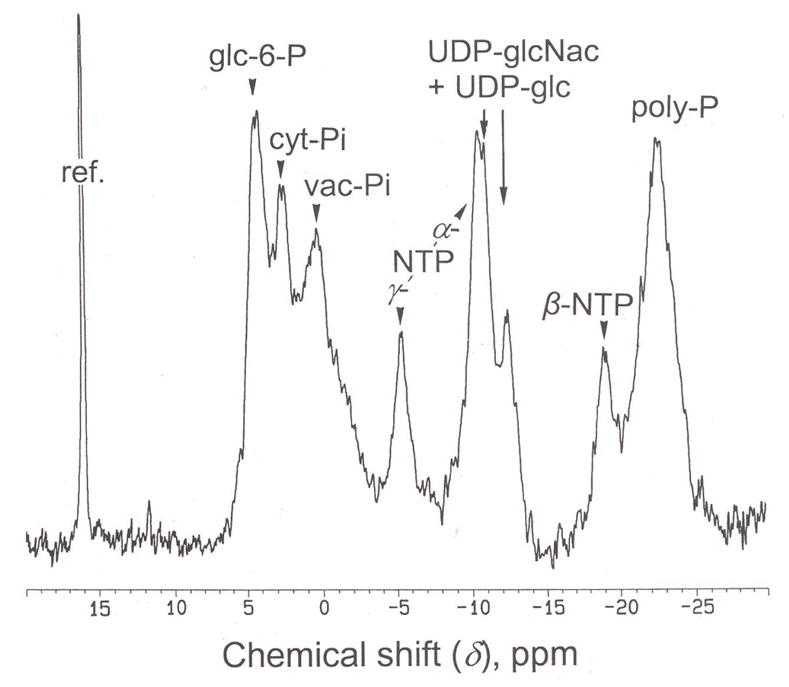
Proton-decoupled in vivo 31P- NMR spectrum of X. elegans thalli. Lichen fragments (4 g dry wt) were hydrated, packed in a 25 mm NMR tube as described in Gout et al. (2001), continuously perfused at a flow rate of 50 mL min−1 with a well oxygenated medium containing 0.2 mM Mops buffer (pH 6.2), at 20°C, and analyzed by 31P-NMR. Peak assignments as in Fig. 2; cyt-Pi, cytoplasmic phosphate; vac-Pi, vacuolar phosphate; ref, reference.
The metabolite profile of wet thalli harvested in the dark (at dawn) is strikingly different from that of dry ones (Fig. 3B). First, wet thalli contained important pools of various sugar phosphates including glucose 6-P, trehalose 6-P, and fructose 6-P. Glyceraldehyde 3-P and phosphoenolpyruvate were also detected. Second, on the opposite, gluconate 6-P was much less abundant (0.18 μmol g−1 dry wt). Third, they contained NADPH (NADP+ was not detected), P-Cho, GPC, and ATP (but less ADP and AMP), and their UDP-glc pool was multiplied by ca 3. PG, AGPG, and UDP-glcNAc pools were similar in both dry and wet thalli. The metabolite profiles of wet thalli harvested in the light (Fig. 3C) resemblethose of wet thalli harvested in the dark, except an important double peak corresponding to the BBC cycle intermediate ribulose 1,5-diphosphate (ru-1,5-DP) located in the chloroplasts of the algal partner. The fluctuations of Pi between samples may originate from partial hydrolyses of polyphosphates during PCA extraction.
13C-NMR spectra show much less differences in relation to the water status of thalli. Typically, dry thalli spectra (Fig. 5A) exhibit major resonance peaks corresponding to polyols, namely arabitol, mannitol, and ribitol (360, 240, and 110 μmol g−1 dry wt, respectively). The other detected compounds included two sugars, sucrose and trehalose, twoamin o acids, glutamine and glutamate, choline and its oxidation product betaine. The spectra of wet thalli collected under the light did not significantly differ from that of dry ones (not shown). In contrast, wet thalli collected at dawn contained more mannitol (290 μmol g−1 dry wt) but less arabitol and ribitol (240 and 90 μmol g−1 dry wt) (figure 5B), and nearly the same amounts of sucrose, choline, and betaine. Interestingly, arabitol disappeared completely in wet thalli stored several days at 20°C in the dark, in contrast with mannitol and ribitol (not shown). Wet thalli collected at dawn also contained less glutamine and glutamate, suggesting that a portion of these amino acids was utilised during the night to sustain the synthesis of nitrogen-containing compounds in wet tissues.
Time course changes of the metabolite profile of X. elegans thalli following hydration and during desiccation
Thalli fragments were immersd into deionised water at 20°C, in the light, and withdrawn with time for PCA extraction and 31P-NMR analysis. Figure 6 shows that the ATP pool was the first to fully recover, reaching a plateau during the first min. AMP and ADP decreased symmetrically. Glc-6-P (and also tre-6-P, gly-3-P, and fru-6-P), which was not detected in dry lichen, reached the value measured in wet lichens during the two following min. Interestingly, ru-1,5-DP started to accumulate after a 2–3 min delay corresponding to the time taken by photosynthesis to recover (Fig. 2, trace A2), and reached the level shown in Fig. 3C ca 5 min later. At the opposite, glcn-6-P decreased to ca one fifth of its initial value during the first 3 min before stabilising. NADP+ was reduced to NADPH from the first min (not shown on the graph). The pool of intermediates involved in membrane lipid syntheses, P-choline and GPC, significantly increased during the 5 first min following rewetting (not shown on the graph).
Fig. 6.
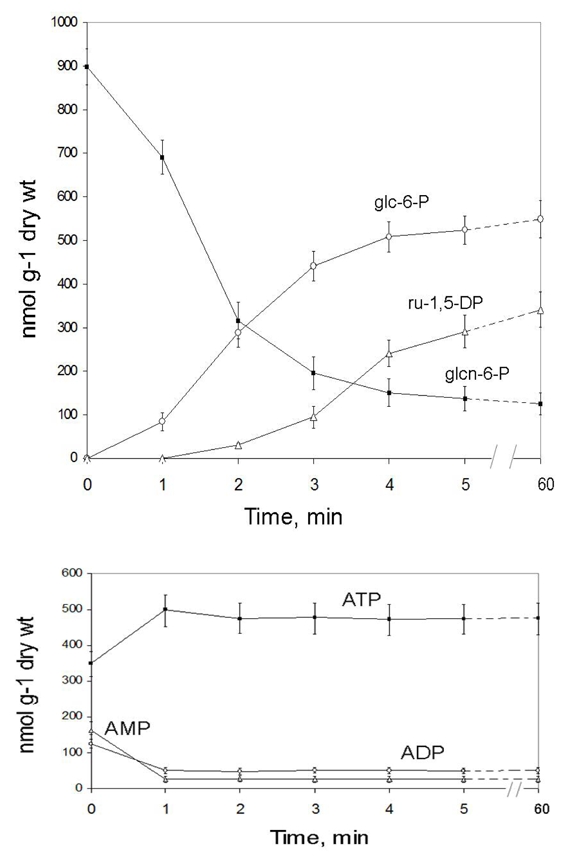
Time course evolution of gluconate 6-P, glucose 6-P, ribulose 1,5-diphosphate, ATP, ADP, and AMP in X. elegans thalli following hydration in the light. At time zero, thalli fragments were incubated in a well aerated liquid medium containing 0.2 mM Mops buffer (pH 6.2), at 20°C. Metabolites were quantified as indicated in Material and methods. Values are means ± SD (n=5)
Conversely, when hydrated lichens were let drying under natural conditions, which took ca 30 min, their metabolic profile met that of initially dry thalli. In particular, Glcn-6-P started to accumulate and glc-6-P to decrease as soon as the water content of lichens was dropping below 30–35% (results not shown). In parallel, NADPH decreased and NADP+ accumulated symmetrically, indicating that the cell need for redox power during dehydration was not met. Nevertheless, the accumulation of glcn-6-P suggested that the pentose phosphate pathway activity was stimulated by the accumulation of NADP+. In order to test this hypothesis, we decided to block the pentose phosphate pathway functioning prior to thalli dehydration. To this purpose, glc-6-P dehydrogenase, which converts glc-6-P into glcn-6-P, was inhibited using glucosamine 6-P (N-glc-6-P), a competitive inhibitor of the enzyme (Glaser and Brown, 1955). For this, wet thalli were incubated for 1 h in the dark in the presence of 5 mM glucosamine (N-glc) which was taken up bylichen cells and phosphorylated to N-glc-6-P (Table 2). As previously observed with tobacco cells (Pugin et al. 1997), glcn-6-P and NADPH decreased strongly, and NADP+ increased, whereas glc-6-P remained constant. When these thalli were subsequently dehydrated, NADPH was no more detected, like in the dry lichen (Table 1). However, in contrast to lichen dehydrated in the absence of N-glc, glcn-6-P did not increase (Table 2), showing that the accumulation of glcn-6-P was boosted by the cell dehydration-linked need for redox power.
Table 2.
Modifications of gluconate 6-P, NADPH, and NADP+ induced in X. elegans thalli by glucosamine treatment. Lichens were incubated for 1 h in the dark in the presence of 5 mM N-glc (wet thalli) and a fraction of them was subsequently let drying under natural conditions (dry thalli), prior to PCA extraction. Metabolites were identified and quantified as indicated in Table 1. Values are given as μmol g−1 lichen dry wt. Abbreviations as indicated in the legends of figure 3; nd, not detected (<0.05 μmol). Values were obtained from a series of independent experiments and are given as mean ± SD (n=5).
| metabolite | Wet thalli | Dry thalli |
|---|---|---|
| N-glucosamine 6-P | 0.38 ± 0.04 | 0.37 ± 0.04 |
| glucose 6-P | 0.62 ± 0.06 | nd |
| gluconate 6-P | 0.04 ± 0.01 | 0.03 ± 0.01 |
| NADPH | 0.02 ± 0.01 | nd |
| NADP+ | 0.07 ± 0.01 | 0.08 ± 0.01 |
Discussion
In this study, we present novel findings on the time course changes of the different pools of soluble metabolites of the foliaceous lichen X. elegans during hydration/dehydration cycles. The respiration, net photosynthesis, and metabolite profile of this lichen were analysed simultaneously, from the first minutes following hydration. The purpose of this approach was to look for the presence, in dry lichens, of preserved pools of metabolites playing key roles in the energetic metabolism, with the aim to determine how they contribute to sustain the instantaneous restart of respiration and the very efficient recovery of photosynthesis.
The first surprise was that dry thalli did not contain hexose phosphates, triose phosphates, and other intermediates of glycolysis, except PGA which could contribute to fuel respiration during the first seconds following hydration (Fig. 3) in the absence of tricarboxylic cycle intermediates like pyruvate, malate, succinate, or citrate (Fig. 5). However, in contrast with wet thalli and with most living material examined so far, they contained a very important pool of gluconate 6-P (Fig. 3) that can be converted into fructose 6-P and glyceraldehyde 3-P in the pentose phosphate pathway and subsequently contribute to fuel respiration. As a matter of fact, only one half of the glcn-6-P pool consumed during the first three minutes following hydration (ca 620 nmol g−1 lichen dry wt, Fig. 6), was sufficient to sustain respiration (580 nmol O2 min−1 g−1 lichen dry wt). The rest of metabolised glcn-6-P may contribute, via the pentose phosphate pathway, to the recovery of glc-6-P and ru-1,5-DP which increased symmetrically to the decrease of glcn-6-P. Finally, the recovery of the ATP pool after hydration was a clear indicator of the recovery of the energetic metabolism. ATP, which decreased by nearly 30% during dehydration, recovered totally at the expense of AMP and ADP during the first minute following hydration, indicating that adenylate kinase and ATP synthase activities restarted very rapidly (Roberts et al. 1997). Taken together these results suggest that the stores of glcn-6-P accumulated during dehydration contributed to sustain the rapid recovery of respiratory and photo synthetic activities in X. elegans thalli after rehydration.
The accumulation of glcn-6-P in X. elegans during dehydration, when the relative water content of thalli was decreasing below ca 30%, could originate from an increase of glucose-6-phosphate dehydrogenase activity, in relation with the production of ROS, as observed in other lichens (Weissman et al. 2005, Kranner and Grill 1994). Indeed, reducing power (NADPH) is required to limit the potential damaging effect of the ROS burst due to the impaired electron transport chains in water-stressed cells (Rascio and La Rocca, 2005). For example, the reduction of glutathione for the avoidance of reactive-oxygen production in chloroplasts, via the ascorbate-glutathione cycle (Asada 1994; Foyer et al.1994), requires NADPH. This was also observed in the alpine plant Soldanella alpina and in Pisum sativum exposed to cold-induced photoinhibition which accumulate glcn-6-P (Streb et al. 2003). On the opposite, when the pentose phosphate pathway was blocked after incubating wet thalli in the presence of glucosamine, NADP+ was no more reduced to NADPH and glcn-6-P did not accumulate during dehydration (Table 2), thus confirming the role of pentose phosphate pathway in the adaptation of X. elegans to hydration/dehydration cycles. Finally the fact that no other metabolite of the pentose phosphate pathway was detected in dry thalli, in particular ribulose 5-P, suggests that the functioning of 6-phosphogluconate dehydrogenase was blocked before that of glucose-6-phosphate dehydrogenase during cell dehydration.
The protection of many fungi and vascular plants against reactive oxygen species can also involve polyols which constitute alternative metabolic reserves, behave as osmoprotectants, and, like mannitol, are potent quenchers of ROS (Jennings et al. 1998). In this context, we observed that mannitol, when added to a solution of ATP, protected ATP from oxidation during desiccation when exposed to sun light (result not shown). More generally, polyols, sugars, and other compounds like glutamate, glycine-betaine, etc. stabilize proteins and protect intimate cellular structures against the potentially deleterious effect of dehydration (Hoekstra et al. 2001). Like many other lichens (Vincente and Legaz 1988; Honneger 1991), X. elegans contained high amounts of polyols in both photobiont (ribitol) and mycobiont (mannitol and arabitol). These polyol pools did not rapidly change after rehydration. Nevertheless, in accordance with previous results obtained by Farrar (1988), wet thalli collected at dawn contained more mannitol and less arabitol and ribitol (Fig. 5). Arabitol and mannitol originate from ribitol, a sugar alcohol synthesized by the photobiont, which moves from alga to fungus as demonstrated in X. aureola and X. calcicola (Richardson and Smith 1967; Lines et al. 1989). Table 1 showed that wet lichens under dark conditions contained lower levels of arabitol. Interestingly, under very long dark periods, arabitol was even completely metabolised in wet lichens suggesting that it contributed to sustain fungal respiration, whili mannitol remained nearly constant.
The mechanisms of lichen tolerance to dehydration/rehydration cycles include an adaptive response of algal and fungal cells to ROS-induced peroxidation and de-esterification of glycerolipids that permeate membranes, and to mechanical constraints which lead to cell membrane disruptions. Hence, the capacity to rapidly reseal disrupted membranes plays a central role (McNeil and Steinhardt, 1997; McNeil et al 2003). In this context, our results have shown that, during rehydration, P-cho was multiplied by a factor 3 and GPC nearly doubled (Fig. 5; Table 1). P-cho and GPC are two precursors of phosphatidylcholine synthesis (van der Rest et al. 2002). Their increase may reflect the synthesis of phosphatidylcholine-rich membrane systems like plasma membrane or tonoplast, thus participating in the maintenance of cell structural integrity. On the contrary, the stability of GPG suggests that thylakoid membranes which contain most of the cell phosphatidylglycerol (Joyard et al. 1993) remained intact in the chloroplasts of photobiont during dehydration/rehydration cycles.
In summary, our data suggest that the very rapid recovery of X. elegans respiration and photosynthesis activities following rehydration was anticipated during dehydration by the accumulation of important stores of gluconate 6-P and by coordinated events associated with preventing oxidative damages and protecting cell components and structures. Glcn-6-P appeared to accumulate in response to different factors including the cell need for reducing power necessary to limit desiccation-generated ROS and the blockade of glcn-6-P metabolisation. The important pools of polyols present in both phyco- and mycobionts contributed to protect cells constituents like nucleotides and proteins and to preserve the intactness of intracellular structures. In lichen thalli, like in other poikilohydric organisms such as seeds, progressive dehydration modifies and finally stops metabolic activities. But, contrarily to seeds where dormancy is advantageous to avoid undesirable germination during momentarily good conditions (Bewley, 1997), the ability of lichen thalli to restart respiration and photosynthesis without delay permitsto take advantage of all reviviscence opportunities offered by the presence of both water and light, particularly at low temperature (Fig. 1). This is the case, for example, when ice is melting under the first rays of the sun. In such situations, the high net photosynthetic activities observed at low temperatures will favour the synthesis of carbohydrates within the minutes following rehydration. Finally, this work shows how the synthesis and preservation of sensitive components during dehydration-triggered down regulation of metabolism may contribute to the adaptation of lichens to the anhydrobiosis cycles imposed by high mountain climate.
Acknowledgments
Authors are indebt to Pr Ulrich Heber (Univ. Wuerzburg, Germany) for his kind encouragements and for his participation to fluorescence assays. We thank Fabrice Rébeillé and Peter Streb for their critical lecture of the manuscript. We also thank Odile and Roland Donzel (Café de la Ferme, Col du Lautaret) for their friendly help, Jean-luc Lebail for his dedicated technical assistance with the NMR spectrometer and perfusion system, and Juliette Asta for having introduced us to the world of lichens. Financial support from the Ministère de 1′Enseignement Supérieur et de la Recherche is gratefully acknowledged.
Abbreviation
- PCA
perchloric acid
- CDTA
1,2-cyclohexylenedinitrilotetraacetic acid
References
- Asada K. Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH, Mullineaux PM, editors. Causes of photooxidative stress and amelioration of defence systems in plants. CRC Press; Boca Raton: 1994. pp. 77–104. [Google Scholar]
- Aubert S, Bligny R, Gout E, Alabouvette J, Marty-Mazars D, Marty F, Douce R. Autophagy in higher plant cells submitted to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol. 1996;133:1251–1263. doi: 10.1083/jcb.133.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. Physiological aspects of desiccation tolerance. Ann Rev Plant Physiol. 1979;30:195–238. [Google Scholar]
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger W, Rimke S, Schreiber U, Lange OL. Inhibition of energy transfer to photosystem II in lichens by dehydration: different properties of reversibility with green and blue-green photobionts. J Plant Physiol. 1989;134:261–268. [Google Scholar]
- Bligny R, Rebeille F, Douce R. O2-triggered changes of membrane fatty acid composition have no effect on Arrhenius discontinuities of respiration in sycamore (Acer pseudoplatanus L.) cells. J Biol Chem. 1985;260:9166–9170. [PubMed] [Google Scholar]
- Bligny R, Douce R. NMR and plant metabolism. Curr Opin Plant Biol. 2001;4:191–196. doi: 10.1016/s1369-5266(00)00160-6. [DOI] [PubMed] [Google Scholar]
- Cornic G, Bukhov NG, Wiese C, Bligny R, Heber U. Flexible coupling between light-dependent electron and vectorial proton transport in illuminated leaves of C3 plants. Role of photosystem I-dependent proton pumping. Planta. 2000;210:468–477. doi: 10.1007/pl00008154. [DOI] [PubMed] [Google Scholar]
- Dudley S, Lechowicz MJ. Losses of polyol through leaching in subarctic lichens. Plant Physiol. 1987;83:813–815. doi: 10.1104/pp.83.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JF. Physiological buffering. In: Galun M, editor. Handbook of Lichenology II. CRC Press: Boca Raton, FL; 1988. pp. 101–105. [Google Scholar]
- Farrar JF, Smith DC. Ecological physiology of the lichen hypogymnia physodes. III. The importance of the rewetting phase. New Phytol. 1976;77:115–125. [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- Fridovich I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann N Y Acad Sci. 1999;893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- Gaff DF. Mechanisms of desiccation-tolerance in resurrection vascular plants. In: Basra AS, Basra RK, editors. Mechanisms of environmental stress resistance in plants. Harwood Academic Publishers; 1997. pp. 43–58. [Google Scholar]
- Gans P, Rébeillé P. Light inhibition of mitochondrial respiration in a mutant of Chlamydomonas reinhardtii devoid of ribulose-1,5-bisphosphate carboxylase/oxygenase activity. Arch Biochem Biophys. 1988;260:109–117. doi: 10.1016/0003-9861(88)90430-4. [DOI] [PubMed] [Google Scholar]
- Glaser BL, Brown DH. Purification and properties of D-glucose 6-phosphate dehydrogenase. J Biol Chem. 1955;216:67–79. [PubMed] [Google Scholar]
- Gout E, Boisson AM, Aubert S, Douce R, Bligny R. Origin of the cytoplasmic pH changes during anaerobic stress in higher plant cells. Carbon-13 and phosphorous-31 nuclear magnetic resonance studies. Plant Physiol. 2001;125:912–925. doi: 10.1104/pp.125.2.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U, Walker D. Concerning a dual function of coupled electron cyclic transport in leaves. Plant Physiol. 1992;100:1621–1626. doi: 10.1104/pp.100.4.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U, Bilger W, Bligny R, Lange OL. Phototolerance of lichens, mosses and higher plants in an alpine environment: analysis of photoreactions. Planta. 2000;211:770–780. doi: 10.1007/s004250000356. [DOI] [PubMed] [Google Scholar]
- Heber U, Lange OL, Shuvalov VA. Conservation and dissipation of light energy as complementary processes : homoiohydric and poikilohydric autotrophs. J Exp Bol. 2005;57:1211–1223. doi: 10.1093/jxb/erj104. [DOI] [PubMed] [Google Scholar]
- Helms GWF. Taxonomy and symbiosis in associations of Physciaceae and Trebouxia. Dissertation zur Erlangung des Doktorgrades der Biologischen Fakultät der Georg-August Universität Göttingen; 2003. p. 141. [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001;6:431–438. doi: 10.1016/s1360-1385(01)02052-0. [DOI] [PubMed] [Google Scholar]
- Honegger R. Functional aspects of the lichen symbiosis. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:553–578. [Google Scholar]
- Jennings DB, Ehrenshaft M, Pharr DM, Williamson JD. Roles of Mannitol and Mannitol dehydrogenase in active oxygen-mediated plant defense. Proc Natl Acad Sci USA. 1998;95:15129–15133. doi: 10.1073/pnas.95.25.15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J, Block MA, Malherbe A, Maréchal E, Douce R. Origin of the synthésis of galactolipids and sulfolipid head groups. In: Moore TS Jr, editor. Lipid Metabolism in Plants. CRC Press; Boca Raton, FL: 1993. pp. 231–258. [Google Scholar]
- Kappen L. Ecophysiological relationships in different climatic regions. In: Galun M, editor. Handbook of Lichenology II. CRC Press; Boca Raton, FL: 1988. pp. 37–100. [Google Scholar]
- Körner C. Alpine plant life: functional ecology of high mountain ecosystems. Springer-Verlag; Berlin Heidelberg: 2003. [Google Scholar]
- Kraner I, Grill D. Rapid change of the glutathione status and the enzymes involved in the reduction of glutathionr-disulfide during the initial stage of wetting of lichens. Crypt Bot. 1994;4:203–206. [Google Scholar]
- Lange OL. Moisture content and CO2 exchange of lichens. Oecologia. 1980;45:82–87. doi: 10.1007/BF00346710. [DOI] [PubMed] [Google Scholar]
- Lange OL, Bilger W, Rimke S, Schreiber U. Chlorophyll fluorescence of lichens containing green and blue green algae during hydratation by water vapour uptake and by addition of liquid water. Bot Acta. 1989;102:306–313. [Google Scholar]
- Lange OL, Pfanz H, Kilian E, Meyer A. Effect of low water potential on photosynthesis in intact lichens and there liberated algal components. Planta. 1990;182:467–472. doi: 10.1007/BF02411401. [DOI] [PubMed] [Google Scholar]
- Lines CEM, Ratcliffe RG, Rees TAV, Southon TE. A 13C NMR study of photosynthate transport and metabolism in the lichen Xanthoria calcicola Oxner. New Phytol. 1989;111:447–456. doi: 10.1111/j.1469-8137.1989.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Longton RE. Studies in polar research. Cambridge University Press; Cambridge: 1988. The biology of polar bryophytes and lichens; p. 391. [Google Scholar]
- Manuel N, Cornic G, Aubert S, Choler P, Bligny R, Heber U. Protection against photoinhibition in the alpine plant Geum montanun. Oecologia. 1999;119:149–158. doi: 10.1007/s004420050771. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Loss, restoration, and maintenance of plasma membrane integrity. J Cell Biol. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Katsuya M, Vogel SS. The endomembrane requirement for cell surface repair. Proc Natl Acad Sci USA. 2003;100:4592–4597. doi: 10.1073/pnas.0736739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash TH, Reiner A, Demmig-Adams B, Kaiser WM, Lange OL. The effect of atmospheric desiccation and osmotic water stress on photosynthesis and dark respiration of lichens. New Phytol. 1990;116:269–276. [Google Scholar]
- Oliver MJ, Dowd SE, Zaragoza J, Mauget SA, Payton PR. The rehydration transcriptome of the desiccation-tolerant bryophyte Tortula ruralis: Transcript classification and analysis. BMC Genomics. 2004;5:89. doi: 10.1186/1471-2164-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugin A, Frachisse J-M, Tavernier E, Bligny R, Gout E, Douce R, Guern J. Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell. 1997;9:2077–2091. doi: 10.1105/tpc.9.11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascio N, La Rocca N. Resurrection plants: the puzzle of surviving extreme vegetative desiccation. Grit Rev in Plant Sci. 2005;24:209–225. [Google Scholar]
- Richardson DHS, Smith DC. IX. Carbohydrate movement from the Trebouxia symbiont of Xanthoria aureola to the fungus. New Phytol. 1968;67:61–68. Lichen Physiology. [Google Scholar]
- Roberts JKM, Jardetzky O. Monitoring of cellular metabolism by NMR. Biochim Biophys Acta. 1981;639:53–76. doi: 10.1016/0304-4173(81)90005-7. [DOI] [PubMed] [Google Scholar]
- Robert JKM, Aubert S, Gout E, Bligny R, Douce R. Cooperation and competition among adenylate kinase, nucleoside diphosphokinase, electron transport, and ATP synthase in plant mitochondria studied by 31P-nuclear magnetic resonance. Plant Physiol. 1997;113:1–7. doi: 10.1104/pp.113.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby C, Martin J-B, Bligny R, Douce R. Biochemical changes during sucrose deprivation in higher plant cells. II. Phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1987;262:5000–5007. [PubMed] [Google Scholar]
- Rundel PW. Water relations. In: Galun M, editor. Handbook of lichenology II. CRC Press; Boca Raton, FL: 1988. pp. 17–36. [Google Scholar]
- Schroeter B, Jacobsen P, Kappen L. Thallus moisture and microclimatic control of CO2 exchange of Peltigera aphthosa (L) Willd on Disco Island (West Greenland) Symbiosis. 1991;11:131–146. [Google Scholar]
- Smith DC, Molesworth S. Lichen Physiology. XIII. Effects of rewetting dry lichens. New Phytol. 1973;72:525–533. [Google Scholar]
- Streb P, Aubert S, Gout E, Bligny R. Cold- and light-induced changes of metabolite and antioxidant levels in two high mountain plant species Soldanella alpina and Ranunculus glacialis and a lowland species Pisum sativum. Physiol Plant. 2003;118:96–104. doi: 10.1034/j.1399-3054.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Cornic G, Bligny R, Gout E, Ghashgaie J. In vivo respiratory metabolism of illuminated leaves. Plant Physiol. 2005;138:1596–1606. doi: 10.1104/pp.105.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Rest B, Boisson A-M, Gout E, Bligny R, Douce R. Glycerophosphocholine metabolism in higher plant cells. Evidence of a new glyceryl-phosphodiester phosphodiesterase. Plant Physiol. 2002;130:244–255. doi: 10.1104/pp.003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente C, Legaz ME. Lichen enzymology. In: Galun M, editor. Handbook of lichenology I. CRC Press; Boca Raton, FL: 1988. pp. 239–284. [Google Scholar]
- Weissman L, Garty J, Hochman A. Characterization of enzymatic antioxidant in the lichen Ramalina lacera and their response to rehydration. Appl Environ Microbiol. 2005;71:6508–6514. doi: 10.1128/AEM.71.11.6508-6514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


