Abstract
Major histocompatibility complex (MHC) class II molecules (MHC-II) function by binding antigenic peptides and displaying these peptides on the surface of antigen presenting cells (APCs) for recognition by peptide-MHC-II (pMHC-II)-specific CD4 T cells. It is known that cell surface MHC-II can internalize, exchange antigenic peptides in endosomes, and rapidly recycle back to the plasma membrane; however, the molecular machinery and trafficking pathways utilized by internalizing/recycling MHC-II have not been identified. We now demonstrate that unlike newly synthesized invariant chain-associated MHC-II, mature cell surface pMHC-II complexes internalize following clathrin-, AP-2-, and dynamin-independent endocytosis pathways. Immunofluorescence microscopy of MHC-II expressing HeLa-CIITA cells, human B cells, and human DCs revealed that pMHC enters Arf6+Rab35+EHD1+ tubular endosomes following endocytosis. These data contrast the internalization pathways followed by newly synthesized and peptide-loaded MHC-II molecules and demonstrates that cell surface pMHC-II internalize and rapidly recycle from early endocytic compartments in tubular endosomes.
Major histocompatibility complex3 class II molecules (MHC-II) function by binding antigenic peptides and displaying these peptides on the surface of antigen presenting cells (APCs) for recognition by MHC-II-restricted, peptide-specific CD4 T lymphocytes (1). Antigenic peptides that bind to MHC-II are usually generated by proteolysis of foreign proteins in late endosomal/lysosomal antigen processing compartments in APCs. However, some antigenic peptides are generated in earlier endosomal compartments and are, in fact, destroyed in late, more acidic lysosomes (2-5). Therefore MHC-II must follow a trafficking pathway that takes them to lysosome-like antigen processing compartments but still allows access to early endosomes.
Newly synthesized MHC-II is targeted to antigen processing compartments as a consequence of its association with a chaperone protein termed Invariant chain (Ii). Ii association inhibits peptide binding to MHC-II (6) and enhances MHC-II folding and egress from the endoplasmic reticulum (7-9). The cytosolic domain of Ii also contains intracellular sorting signals that direct Ii-associated MHC-II complexes (MHC-II-Ii) to lysosome-like antigen processing compartments (10, 11). Once in these compartments Ii is degraded by proteolysis and antigenic peptides bind to Ii-free MHC-II molecules with the assistance of the “peptide editor” HLA-DM (reviewed in Ref. 12). Once loaded with peptides, MHC-II moves from the antigen processing compartments to the cell surface to display these pMHC-II to antigen-specific CD4 T cells.
Although the pathway followed by newly synthesized MHC-II-Ii complexes to access these compartments has been the subject of intense debate, there is now considerable data showing that most, if not all, Ii-associated MHC-II travels from the trans-Golgi network to the plasma membrane and then enters the endocytic pathway by clathrin-mediated endocytosis. Rapid internalization of MHC-II-Ii complexes from the cell surface is dependent on dileucine-based sorting signals in the cytosolic domain of Ii (10, 11, 13). Mutation of these sorting signals prevent efficient sorting of MHC-II to antigen processing compartments (14, 15) and leads to the accumulation of MHC-II-Ii complexes on the plasma membrane (16). Some of the dileucine sorting motifs, including those in Ii are responsible for the binding of cargo proteins to the clathrin-associated adaptor protein AP-2 (17, 18) and are thus required for clathrin-mediated endocytosis of these proteins from the cell surface. The use of specific small interfering RNA (siRNA) targeting clathrin and AP-2 profoundly inhibited Ii internalization and resulted in the accumulation of Ii at the surface of clathrin/AP-2 depleted cells (19, 20), highlighting the importance of AP-2-mediated clathrin-dependent endocytosis in MHC-II-Ii delivery to antigen processing compartments. In addition, overexpression of a dominant-negative mutant of the GTPase dynamin prevented MHC-II-Ii internalization and Ii degradation (21), indicating that dynamin is also required for efficient endocytosis of MHC-II-Ii complexes.
In contrast to the behavior of Ii-associated MHC-II, almost nothing is known about the molecular mechanisms regulating the fate of Ii-free, peptide-loaded MHC-II (pMHC-II) once at the cell surface. Ii-free MHC-II is known to rapidly internalize from the cell surface, however, unlike Ii-associated MHC-II (which traffics to antigen processing compartments), the majority of internalized Ii-free MHC-II rapidly recycles back to the plasma membrane (22-24). Recycling MHC-II can in fact exchange antigenic peptides in early endosomes in HLA-DM-dependent (25) or HLA-DM-independent manners (26) and it has been suggested that recycling MHC-II could represent a significant fraction of the total pool of endosomal/lysosomal MHC-II molecules (23). Because Ii-association prevents peptide binding to MHC-II (6), antigenic peptides generated in early endosomes cannot bind to Ii-associated MHC-II complexes that are en route to lysosome-like antigen processing compartments. Thus it is likely that rapid MHC-II recycling (and HLA-DM-catalyzed peptide-exchange) could serve to increase the repertoire of peptides available to T cell recognition by MHC-II molecules on the surface of APCs.
In this study we have investigated the mechanisms regulating MHC-II-Ii and pMHC-II endocytosis and recycling and find that pMHC-II and MHC-II-Ii complexes enter cells by completely different mechanisms. Unlike Ii-associated MHC-II, pMHC-II endocytosis is clathrin-, AP-2-, and dynamin-independent. Internalized pMHC-II enters elongated tubules that contain the small GTPases Arf6 and Rab35, whereas Ii-associated MHC-II does not. Because both Arf6 and Rab35 have been suggested to regulate clathrin-independent protein recycling (27, 28), our data highlights the different pathways of endocytosis and recycling of Ii-associated and Ii-free MHC-II molecules.
EXPERIMENTAL PROCEDURES
Cells and Reagents—HeLa cells (ATCC, Manassas, VA), JY B cells, HeLa cells expressing CIITA, and the KG-1 DC line (both gifts from Dr. Peter Cresswell, Yale University School of Medicine) were maintained as described previously (60, 61). Human DCs were generated from elutriated human monocytes obtained from the NIH Blood bank. Briefly, the cells were grown for 6 days in RPMI 1640 medium supplemented with 10% fetal bovine serum, 50 ng/ml human granulocyte macrophage-colony stimulating factor (Peprotech, Rocky Hill, NJ), 35 ng/ml human interleukin-4 (Peprotech), 2 mml-glutamine, 50 μg/ml gentamicin, 1 mm sodium pyruvate, 55 μm β-mercaptoethanol, and non-essential amino acids (Sigma). On day 6 the cells were activated using 1 μg/ml lipopolysaccharide for 24 h. Half of the media was changed every 2 days, adding fresh medium containing twice the concentration of the cytokines mentioned above.
The following antibodies were used in this study: anti-pMHC-II mAb L243 (BD Biosciences), anti-MHC-II-Ii mAb LL1 (33), Alexa 488-conjugated anti-HA (Covance, Denver, PA), anti-Lamp-1 mAb H4A3 and anti-Lamp-2 mAb H4B4 (Developmental Studies Hybridoma Bank, Des Moines, IA), AP-2 medium chain μ2 (AP50), anti-clathrin heavy chain (BD Biosciences), and anti-β-tubulin (Sigma). The anti-DR β-chain mAb XD5.All has been described previously (16). Alexa Fluor-conjugated secondary antibodies were obtained from Molecular Probes (Eugene, OR) and horseradish peroxidase-conjugated reagents were obtained from Southern Biotech (Birmingham, AL).
Plasmids encoding HA-tagged Arf6 and the Arf6 T27N GTPase mutant were provided by Julie Donaldson (NHLBI, National Institutes of Health), GFP-tagged Rab35 and the Rab35 S22N GTPase mutant were provided by Steve Shaw (NCI, National Institutes of Health), GFP-tagged EHD1 and GFP-CD63 were provided by Juan Bonifacino (NICHD, National Institutes of Health), HA-tagged dynamin (K44A) mutant was provided by Katherine Roche (NINDS, National Institutes of Health), and the dominant-negative fragment AP180C-EGFP was provided by Harvey T. McMahon (Cambridge, UK).
Immunoprecipitation and Immunoblotting—HeLa-CIITA cells were analyzed by immunoprecipitation and immunoblot analysis for expression of MHC-II, clathrin, and AP-2 using protocols described previously (62).
Transfection and siRNA Treatment—HeLa cells were transfected using Lipofectamine and Plus reagent (Invitrogen) and were assayed 24 h after transfection. RNA interference was carried out using Lipofectamine 2000 (Invitrogen). The siRNA constructs (20 μm) were added to HeLa cells in suspension and assayed 72 h after transfection. The sequences for the siRNA were as follows: clathrin heavy chain (5′ → 3′) GGGAAGTTACATATTATTGtt (Ambion, Foster City, CA), AP-2-μ2 (5′ → 3′) GTGGATGCCTTTCGGGUCAtt (Qiagen, Valencia, CA), and negative control siRNA (obtained from Qiagen (catalog number 1022076)). The sequences for the clathrin heavy chain siRNA was determined by Ambion (catalog number 16706), whereas the AP-2 medium chain siRNA sequences have been described previously (19, 20).
Immunofluorescence Microscopy—HeLa-CIITA cells were fixed in 4% paraformaldehyde in phosphate-buffered saline for 12 min at room temperature as described (63). For surface blocking, a mixture of goat anti-mouse IgG and Alexa 633-conjugated goat anti-mouse was incubated on ice with cells that had been stained with primary mAb. The cells were then fixed, permeabilized with 0.1% saponin in phosphate-buffered saline at room temperature, and incubated with Alexa 488- or Alexa 546-conjugated secondary antibodies to detect internalized mAb. Human DCs were incubated for 30 min at room temperature on polylysine-coated glass slides prior to fixation and immunostaining. All cells were imaged utilizing a Zeiss LSM 510 META confocal microscope using a ×63 oil immersion objective lens (NA 1.4). Z-stack sections were collected at 0.4-μm intervals for three-dimensional reconstruction and 0.7 μm for kinetic analyses.
Live cell imaging was performed in HeLa-CIITA cells by incubating cells with a trace amount of Alexa 488-conjugated mAb L243 (Biolegend, San Diego, CA) on a heated stage at 37 °C and immediately acquiring stacks of images at 2-min intervals. Complete Dulbecco's modified Eagle's medium without phenol red was used as imaging medium. All images were acquired in the mid-plane of the cells. Videos were generated by combining sequential images using LSM 510 WS (release version 3.5) software (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Computer animations of MHC-II distribution in cells were generated by combining individual z-stacks (thickness 0.7 μm) using LSM 510 WS software and ImageJ software (National Institutes of Health).
Flow Cytometry-based Internalization and Recycling Assays—Cells were briefly trypsinized and washed twice in FACS staining buffer (HBSS containing 2% fetal bovine serum). Primary mAb were incubated with cells (∼5 × 106 cells/ml) for 20 min on ice and washed twice in ice-cold FACS staining buffer. Alexa-conjugated specific secondary antibodies were incubated with the labeled cells for 20 min on ice, washed twice, and cells were then fixed in 1% paraformaldehyde in phosphate-buffered saline. For all internalization experiments the cells were first stained with the primary mAb on ice, washed twice in ice-cold FACS buffer, and re-cultured at 37 °C for various times. After two more washes in ice-cold buffer, the amount of primary antibody remaining on the cell surface was identified by staining cells with Alexa-conjugated secondary antibodies on ice. The cells were washed in ice-cold FACS staining buffer and then fixed in 1% paraformaldehyde in phosphate-buffered saline at room temperature. Intracellular HA-dynamin was detected by staining of fixed- and saponin-permeabilized cells with Alexa 488-conjugated anti-HA mAb. Expression of each antibody was determined by flow cytometry using a FACSCalibur (BD Biosciences). The median fluorescence intensity was determined for each FACS profile and expressed as a percentage of the value present on cells kept on ice for the duration of the internalization assay. In other experiments the amount of each antigen present on the plasma membrane at steady-state after transfection was expressed as a -fold change relative to mock-transfected cells.
MHC-II recycling assays were performed by incubating either KG-1 DCs or HeLa-CIITA cells with anti-pMHC-II mAb L243 at 37 °C for 30 min, washing the cells extensively in ice-cold FACS staining buffer, and then blocking surface-exposed L243 using an excess of goat anti-mouse IgG and Alexa 633-conjugated goat anti-mouse on ice. The cells were then washed again in ice-cold FACS buffer and then re-cultured in complete medium at 4 °C. At various times aliquots of each culture were removed and added to pre-warmed medium and incubated at 37 °C for various times. The cells were again washed in ice-cold FACS staining buffer and re-expressed pMHC-II present on the surface detected by staining cells with Alexa 488-conjugated goat anti-mouse antibody on ice. Cells were analyzed by flow cytometry and the amount of MHC-II recycling was expressed as a fraction of the amount of pMHC-II reappearance detected after culture at 37 °C for 30 min.
Cell Surface Biotinylation—HeLa-CIITA cells were seeded out in separate 6-well plates 1 day prior to cell surface biotinylation. Briefly, the cells were washed with ice-cold HBSS, and surface proteins were biotinylated on ice using 0.5 mg/ml EZ-Link Sulfo-NHS-SS-Biotin (Pierce) for 30 min. Free biotin was quenched with two washes with 50 mm glycine in HBSS. The cells were then “chased” for various times in complete Dulbecco's modified Eagle's medium at 37 °C or kept on ice in complete Dulbecco's modified Eagle's medium. The cells were washed in cold HBSS and the surface biotin was reduced by incubating the cells with 150 mm reduced glutathione (Calbiochem) in buffer containing 75 mm NaCl, 10 mm EDTA, and 75 mm NaOH for two treatments of 15 min each. Free glutathione was then quenched using 5 mg/ml iodoacetamide in HBSS. In some samples the biotinylated cells were not incubated with glutathione, thus representing the maximum amount of biotinylated pMHC-II present in the experiment. The cells were lysed, pMHC-II was isolated by immunoprecipitation using mAb L243 and the immunoprecipitates, and analyzed by blotting using streptavidin-horseradish peroxidase (Southern Biotech, Birmingham, AL) or using the DR β-chain mAb XD5.A11.
RESULTS
pMHC-II and MHC-II-Ii Complexes Internalize and Recycle in HeLa-CIITA and Professional APCs—To identify the machinery regulating endocytosis of Ii-associated MHC-II and Ii-free pMHC-II complexes we have examined MHC-II transport in CIITA-expressing HeLa cells and a variety of primary and immortalized APCs. Stable expression of CIITA in HeLa cells induces expression of endogenous MHC-II and allows us to examine the distribution and trafficking of pMHC-II and MHC-II-Ii in a cell that possesses very well characterized endocytic and recycling pathways. Furthermore, these cells can function to stimulate antigen-specific CD4 T cells (29), showing that they possess the cellular machinery required for antigen processing and presentation.
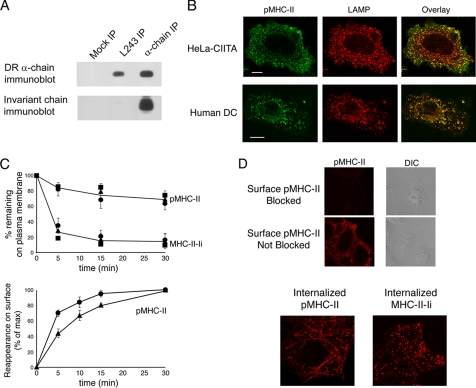
The ability to differentiate between Ii-associated MHC-II and Ii-free pMHC-II is critical to examine the pathways of internalization followed by these two distinct forms of MHC-II. The specificity of the anti-pMHC-II mAb L243 used in this study for Ii-free MHC-II (30) was confirmed by immunoprecipitation/immunoblot analysis. MHC-II was immunoprecipitated using either mAb L243 or the anti-HLA-DR α-chain mAb DA6.147 and the amount of Ii and MHC-II present in each immunoprecipitate was determined by immunoblot analysis. Whereas an anti-α-chain immunoprecipitate contained large amounts of Ii (bound as MHC-II-Ii complexes), there was no Ii present in the mAb L243 immunoprecipitate (Fig. 1A), demonstrating that L243 does not react with immature, Ii-associated forms of MHC-II in HeLa-CIITA cells.
FIGURE 1.
pMHC-II and MHC-II-Ii internalize with similar kinetics in both HeLa-CIITA and human B cells. A, HeLa-CIITA cells were lysed in Triton X-100 and aliquots of the lysate were subjected to immunoprecipitation using an isotype control IgG, anti-pMHC-II mAb L243, or the anti-MHC-II α-chain mAb DA6.147. The immunoprecipitates were then analyzed by immunoblot analysis for the total content of MHC-II α-chain (top panel) or Ii (bottom panel). B, HeLa-CIITA cells and monocyte-derived human immature DCs were fixed, permeabilized, and stained with mAb recognizing pMHC-II (green) and LAMP-2 (red). C, HeLa-CIITA cells (circles), JY B cells (squares), and KG-1 DCs (triangles) were incubated on ice with mAb recognizing pMHC-II (L243) or MHC-II-Ii (LL1). The cells were extensively washed and re-cultured at 37 °C. At different times the cells were washed on ice and the amount of tagged pMHC-II or MHC-II-Ii remaining on the cell surface was determined by incubating the cells with fluorescently labeled secondary antibodies on ice. The cells were analyzed by FACS analysis and representative histograms of pMHC-II and MHC-II-Ii expression on HeLa-CIITA cells are shown. The median fluorescence intensity of each time point was expressed as a fraction of the percentage of amount of tagged pMHC-II or MHC-II-Ii present on the cell surface at time 0. The data shown are the mean ± S.D. obtained from more than three independent experiments. HeLa-CIITA cells (circles) or KG-1 DCs (triangles) were incubated with anti-pMHC-II mAb L243 at 37 °C, washed, and the relative amount of internal pMHC-II recycling back to the cell surface was determined as described under “Experimental Procedures.” The data shown are the mean ± S.D. obtained from more than three independent experiments. D, upper panels, HeLa-CIITA cells were incubated with mAb recognizing pMHC-II for 30 min on ice. The cells were then fixed with paraformaldehye, pMHC-II molecules remaining on the surface were either “blocked” as described under “Experimental Procedures” or sham-blocked. Immunolabeled cell surface MHC-II was visualized using Alexa 546-conjugated secondary antibodies and confocal immunofluorescence microscopy. Lower panels, HeLa-CIITA cells were incubated with mAb recognizing pMHC-II (L243) or MHC-II-Ii (LL1) for 30 min on ice, extensively washed, and re-cultured at 37 °C for 15 min. The cells were then fixed with paraformaldehye, pMHC-II molecules remaining on the surface were blocked, and internalized pMHC-II or MHC-II-Ii visualized with Alexa 546-conjugated secondary antibodies after permeabilization.
Immunofluorescence microscopy using mAb L243 confirmed that a significant amount of the intracellular pMHC-II localizes to LAMP-1-positive lysosome-like compartments in HeLa-CIITA cells in a manner that is similar to that of the distribution of pMHC-II in monocyte-derived DCs (Fig. 1B). In addition, immunoelectron microscopy demonstrated that as in human and mouse DCs (31, 32), pMHC-II is present on late endosomal/lysosomal structures with multivesicular morphology in HeLa-CIITA cells (data not shown). These data strongly suggest that the machinery regulating pMHC-II trafficking is conserved in HeLa-CIITA cells and professional APCs.
The kinetics of MHC-II internalization and recycling has been examined in both transfected human fibroblasts and human B cells (22-24). To confirm that the rate of endocytosis of pMHC-II and MHC-II-Ii complexes are comparable in HeLa-CIITA cells and professional APCs we measured the kinetics of endocytosis of pMHC-II and MHC-II-Ii in various cell types by FACS analysis. pMHC-II internalizes rapidly in HeLa-CIITA cells, the JY B cell line, and the KG-1 DC line and reaches an equilibrium of 20% internalization after ∼15 min (Fig. 1C). This equilibrium has been attributed to efficient recycling of MHC-II from early endosomes to the cell surface (22). Immunofluorescence microscopy revealed the presence of internalized pMHC-II complexes in HeLa-CIITA cells (Fig. 1D) and recycling experiments confirmed that the internalized pMHC-II could traffic from these intracellular compartments to the plasma membrane (Fig. 1C). Essentially identical results to these were obtained when examining the kinetics of internalization of pMHC-II in monocyte-derived human DCs (results not shown). The internalization kinetics were similar when using whole antibody or the Fab fragment of the pMHC-II mAb L243, confirming that internalization was not a consequence of pMHC-II cross-linking (data not shown). Essentially all Ii molecules on the plasma membrane of human B cells (16) and HeLa-CIITA cells (29) are associated with MHC-II and we therefore used the Ii-specific mAb LL1 to tag surface MHC-II-Ii complexes (33). In agreement with previous studies (16, 34), we found that MHC-II-Ii is rapidly internalized in HeLa-CIITA cells (Fig. 1, C and D). These data demonstrate that pMHC-II internalizes and recycles in HeLa-CIITA cells and professional APCs with similar kinetics.
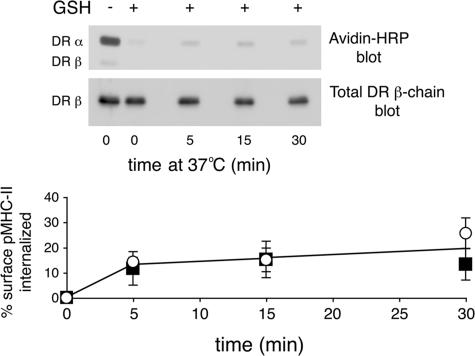
To confirm that the loss of pMHC-II mAb in the FACS-based endocytosis assay reflects internalization of pMHC-II protein we used an antibody-independent assay to examine pMHC-II endocytosis. Plasma membrane proteins on HeLa-CIITA cells were tagged with a reducible form of NHS-biotin and the resistance of protein reduction by extracellular glutathione used as an indication of protein endocytosis. When biotinylated HeLa-CIITA cells were kept on ice glutathione removed essentially all biotin moieties from surface pMHC-II. By contrast, culture of cells at 37 °C for various times before incubation of the cells with glutathione on ice revealed the presence of intracellular (reduction-resistant) pMHC-II (Fig. 2). Quantitative analysis showed that the rate of pMHC-II endocytosis measured using this antibody-independent assay almost exactly mirrored that obtained using the FACS-based internalization assay. These data validate the FACS-based assay as a faithful indicator of pMHC-II endocytosis.
FIGURE 2.
Kinetics of internalization of pMHC-II in HeLa-CIITA cells. HeLa-CIITA cells were biotinylated on ice using sulfo-NHS-SS-biotin as described under “Experimental Procedures.” The cells were kept on ice or incubated at 37 °C for various times and incubated with ice-cold reduced glutathione (or not) as indicated. The cells were lysed and pMHC-II isolated by immunoprecipitation using mAb L243. The immunoprecipitates were analyzed by SDS-PAGE and biotinylated pMHC-II detected using streptavidin-horseradish peroxidase and total pMHC-II present in the sample detected using the β-chain-specific mAb XD5.A11. The amount of biotin-labeled pMHC-II present in each sample was expressed as a fraction of the total amount of biotin-labeled pMHC-II present on cells before glutathione treatment.
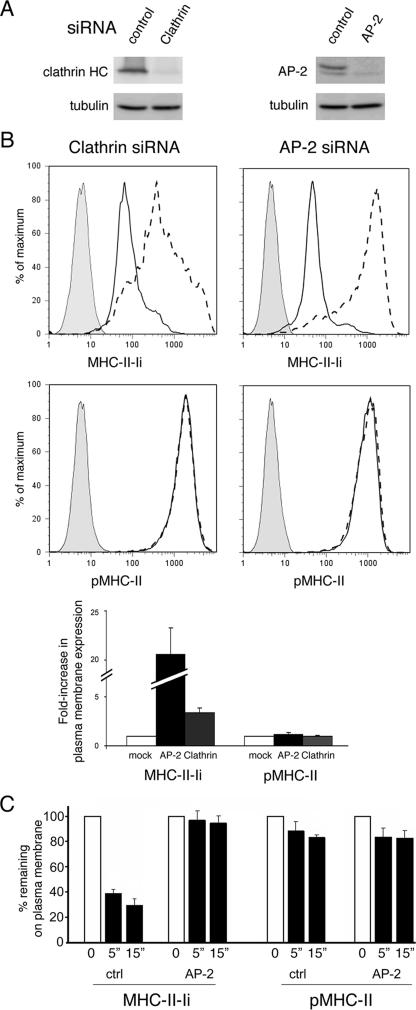
pMHC-II and MHC-II-Ii Complexes Internalize Using Different Machinery—Although it is well established that the internalization of MHC-II-Ii complexes proceeds via clathrin-dependent endocytosis (19, 20), the molecular mechanism regulating pMHC-II endocytosis has not been directly addressed. To directly examine the machinery required for internalization of pMHC-II and MHC-II complexes we have used siRNA to specifically deplete either clathrin or AP-2 in HeLa-CIITA cells. Immunoblot analysis revealed 90-95% knock down of the clathrin heavy chain and the AP-2 μ-subunit as compared with cells treated with control siRNA (Fig. 3A). The cells were examined for expression of cell surface pMHC-II or MHC-II-Ii 72 h after siRNA treatment (Fig. 3B). In agreement with previous studies (19, 20), Ii expression at the cell surface was dramatically increased when either clathrin or AP-2 were depleted from the cells, confirming the importance of clathrin and AP-2 in regulating MHC-II-Ii endocytosis. By contrast, depletion of either AP-2 or clathrin heavy chain for 3 days had no influence on the expression of pMHC-II on the cell surface, although, as reported previously depletion for 6 days did result in a slight decrease in the amount of pMHC-II present (19, 20).
FIGURE 3.
Depletion of clathrin or AP-2 inhibits MHC-II-Ii but not pMHC-II endocytosis. A, HeLa-CIITA cells were treated with control siRNA or siRNA targeted against clathrin or the μ2 subunit of AP-2. After 3 days the expression of clathrin or the μ2 subunit of AP-2 in the cell population was determined by immunoblot analysis. B, the expression of MHC-II-Ii (LL1) or pMHC-II (L243) on the plasma membrane of siRNA-treated cells was determined by FACS analysis. Representative histograms showing staining of isotype control antibody (filled gray), control siRNA (solid line), or either clathrin- or AP-2 siRNA (dashed line) are shown. The expression of pMHC-II or MHC-II-Ii on the cell surface under each condition is shown as a -fold increase in plasma membrane expression by normalizing the median fluorescence intensity of expression of the clathrin- or AP-2-siRNA-treated cells as compared with the expression in control siRNA-treated cells. C, HeLa-CIITA cells treated with control siRNA or AP-2 siRNA were incubated on ice with mAb recognizing MHC-II-Ii or pMHC-II. The cells were extensively washed and either kept on ice or re-cultured for either 5 or 15 min at 37 °C. The cells were washed on ice and the amount of tagged pMHC-II or MHC-II-Ii remaining on the cell surface was determined by incubating the cells with fluorescently labeled secondary antibodies on ice. The percentage of tagged MHC-II-Ii or pMHC-II remaining on the cell surface of control siRNA- or AP-2 siRNA-treated cells after 5 or 15 min of culture at 37 °C was expressed as a percentage of the total amount present on the surface on cells maintained on ice.
To examine whether the dramatic increase in MHC-II-Ii cell surface expression in AP-2-deficient cells was a consequence of diminished endocytosis we performed MHC-II internalization studies. These studies confirmed previous reports (19, 20) that the rapid internalization of MHC-II-Ii from the cell surface is blocked in AP-2-deficient cells (Fig. 3C). By contrast, we found no difference in the kinetics or extent of pMHC-II internalization in these cells, clearly demonstrating that pMHC-II and MHC-II-Ii internalize using different molecular machines in HeLa-CIITA cells.
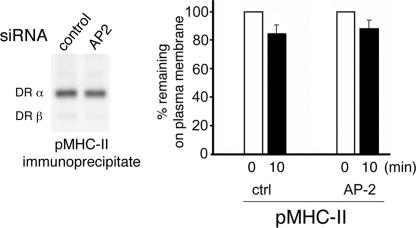
The effect of AP-2 depletion on pMHC-II endocytosis was also examined using our antibody-independent internalization assay. Plasma membrane proteins on HeLa-CIITA cells incubated with either control or AP-2 siRNA were tagged with reducible NHS-biotin and the cells were incubated either on ice or at 37 °C for 10 min prior to the addition of ice-cold glutathione. Depletion of AP-2 did not alter the amount of total biotinylated pMHC-II present at the plasma membrane and had no effect on the endocytosis of pMHC-II (Fig. 4). By contrast, AP-2 depletion dramatically increased the amount of biotinylated MHC-II-Ii complexes present on the cells and reduced the rate of endocytosis of these complexes (data not shown). These data demonstrate, using an antibody-independent internalization assay, that pMHC-II endocytosis is AP-2 independent.
FIGURE 4.
AP-2 depletion does not alter surface pMHC-II endocytosis. HeLa-CIITA cells treated with control siRNA or siRNA targeting the μ2 subunit of AP-2. After 3 days the cells were biotinylated on ice using sulfo-NHS-SS-biotin, washed, and either incubated on ice or cultured at 37 °C for 10 min. The cells were then incubated with reduced glutathione on ice to remove surface biotin, washed, and pMHC-II isolated by immunoprecipitation analysis. The immunoprecipitates were analyzed by SDS-PAGE and biotinylated pMHC-II detected using streptavidin-horseradish peroxidase. A representative gel indicating the amount of biotinylated pMHC-II on control or AP-2 siRNA-treated cells is shown. The percentage of biotinylated pMHC-II remaining on the plasma membrane after incubation of the cells at 37 °C was expressed as a fraction of the total amount of biotin-labeled pMHC-II present on cells before glutathione treatment. The results shown are representative of two independent experiments.
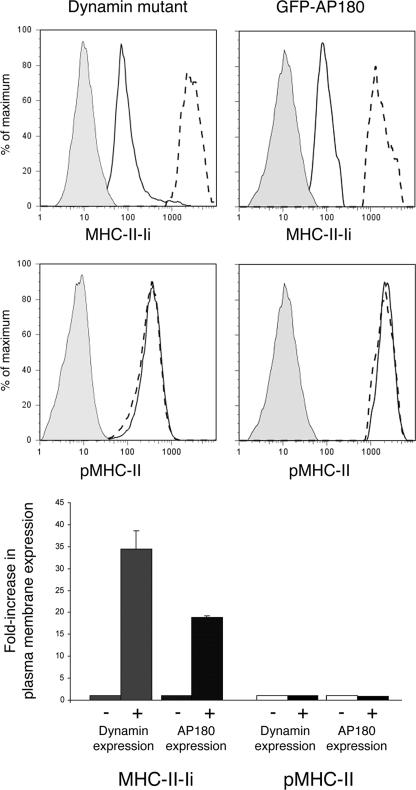
pMHC-II Internalization Is Dynamin- and AP180-independent in HeLa-CIITA Cells, B Cells, and DCs—Besides AP-2, there are a number of molecules that are involved in this process of clathrin-mediated endocytosis, including dynamin and AP180 (reviewed in Ref. 35). In addition, there are distinct clathrin-independent trafficking pathways that are either dynamin-dependent and dynamin-independent (reviewed in Ref. 36). To examine the role of dynamin and AP180 in pMHC-II internalization we expressed dominant-negative forms of HA-tagged dynamin or GFP-AP180 in HeLa-CIITA, human B cells, and KG-1 DCs. Each of these reagents has been shown to effectively block clathrin-mediated endocytosis and lead to an accumulation of cargo proteins at the cell surface (21, 37, 38). Similar to the depletion of clathrin or AP-2, introduction of either dominant-negative dynamin or AP180 into HeLa-CIITA cells dramatically enhanced expression of MHC-II-Ii at the cell surface (Fig. 5). By marked contrast, none of these treatments altered the expression of pMHC-II on the surface of HeLa-CIITA cells. To confirm that the results obtained in HeLa-CIITA cells were also valid for professional APCs, we introduced GFP-AP180 into JY B cells and KG-1 DCs. Sorting on GFP-AP180-positive or -negative cells (as a control) revealed that overexpression of AP180 resulted in an increase in MHC-II-Ii expression but had no effect on surface expression of pMHC-II (Fig. 6). As anticipated, overexpression of GFP alone had no effect on expression of MHC-II-Ii or pMHC-II (data not shown). These data demonstrate that pMHC-II internalization from the cell surface follows a pathway that is clathrin-, AP-2-, AP180-, and dynamin-independent.
FIGURE 5.
Stable surface expression of pMHC-II is dynamin- and AP180-independent in HeLa-CIITA cells. HeLa-CIITA cells were transfected with plasmids encoding HA-dynamin mutant or GFP-AP180. After 2 days the cells were stained on ice with Alexa 548-conjugated mAb recognizing MHC-II-Ii (LL1) or pMHC-II (L243) and fixed with paraformaldehyde. Dynamin-transfected cells were then permeabilized and stained with Alexa 488-labeled anti-HA epitope antibody. The cell populations were analyzed by two-color FACS analysis and cells expressing HA-tagged dynamin mutant or GFP-AP180 were analyzed for MHC-II-Ii or pMHC-II expression and were compared with cells from the same transfections that did not express HA-tagged dynamin mutant or GFP-AP180. Representative histograms showing staining of isotype control antibody (filled gray), MHC-II-Ii or pMHC-II expression in dynamin- or GFP-AP180-negative cells (solid line), and MHC-II-Ii or pMHC-II expression in dynamin- or GFP-AP180-positive cells (dashed line) are shown. The expression of MHC-II-Ii or pMHC-II on the cell surface under each condition is shown as a -fold increase in plasma membrane expression by normalizing the median fluorescence intensity of expression of the dynamin mutant- or AP180-expressing cells as compared with the expression in non-expressing cells.
FIGURE 6.
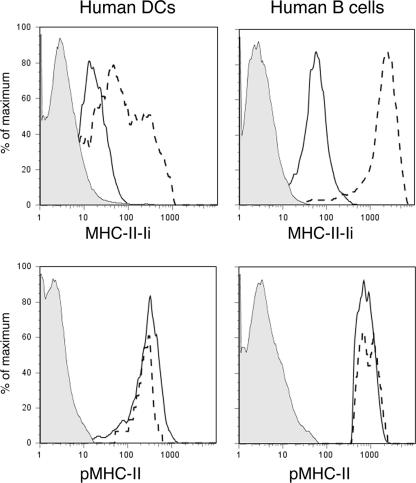
Stable surface expression of pMHC-II is dynamin- and AP180-independent in professional APCs. JY B cells and KG-1 DCs were transfected with a plasmid encoding GFP-AP180. After 2 days the cells were stained on ice with Alexa 548-conjugated mAb recognizing MHC-II-Ii (LL1) or pMHC-II (L243) and fixed with paraformaldehyde. The cell populations were analyzed by two-color FACS analysis and cells expressing GFP-AP180 were analyzed for MHC-II-Ii or pMHC-II expression and were compared with cells from the same transfections that did not express GFP-AP180. Representative histograms showing staining of isotype control antibody (filled gray), MHC-II-Ii or pMHC-II expression in GFP-AP180-negative cells (solid line), MHC-II-Ii or pMHC-II expression in GFP-AP180-positive cells (dashed line) are shown. The results shown are representative of those obtained in two independent experiments.
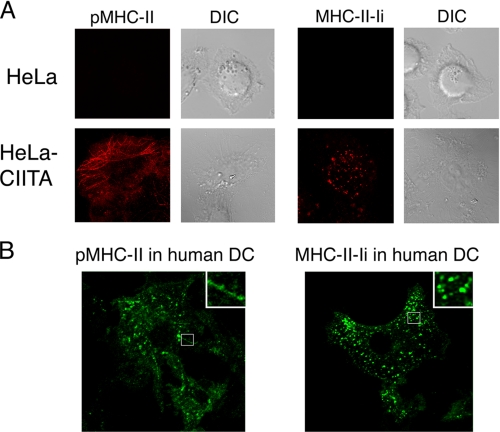
pMHC-II Internalizes into Tubular Endosomes—Surface tagging of pMHC-II or MHC-II-Ii with mAb on ice followed by a “chase” at 37 °C reveals the sites of accumulation of these molecules after the chase, whether they are at the cell surface or in lysosome-like antigen processing compartments. We therefore illuminated the entire endocytic/recycling pathway followed by pMHC-II or MHC-II-Ii complexes by culturing cells in the presence of pMHC-II or MHC-II-Ii-specific mAb continuously for 30 min at 37 °C, blocked access of secondary antibodies to bind to cell surface pMHC-II or MHC-II-Ii mAb, and examined the distribution of internalized pMHC-II or MHC-II-Ii complexes by confocal immunofluorescence microscopy. The uptake of each antibody required the expression of the appropriate antigen, as HeLa cells not expressing CIITA did not take up either anti-MHC-II mAb (Fig. 7A). Internalized MHC-II-Ii was present exclusively in vesicular structures in HeLa-CIITA cells, a finding that is consistent with our endocytosis data showing that MHC-II-Ii does not efficiently recycle. By contrast, a significant amount of the internalized pMHC-II antibody was present in long tubular structures that were adjacent to the plasma membrane as well as in small endocytic vesicles. Reconstruction of serial images from different planes of the cell clearly reveals the tubular morphology of the internalized pMHC-II structures (supplemental Video 1). These intracellular pMHC-II+ tubules were also observed in monocyte-derived human DCs (Fig. 7B), confirming that the pMHC-II tubules observed in HeLa-CIITA cells reflect a normal pathway of pMHC-II endocytosis/recycling that is also present in primary APCs.
FIGURE 7.
Internalized pMHC-II is present on tubular endosomes. Untransfected HeLa cells (panel A), HeLa-CIITA cells (panel A), and monocyte-derived human DCs (panel B) were incubated with mAb recognizing pMHC-II (L243) or MHC-II-Ii (LL1) for 30 min at 37 °C. The cells were then fixed with paraformaldehye, pMHC-II molecules remaining on the surface were blocked, and internalized MHC-II visualized using Alexa 546-conjugated secondary antibodies after permeabilization. Confocal microscopy reveals the presence of intracellular pMHC-II in tubular structures and MHC-II-Ii in perinuclear vesicles only in HeLa-CIITA cells and human DCs. Insets show higher magnification images of the indicated regions of the cells.
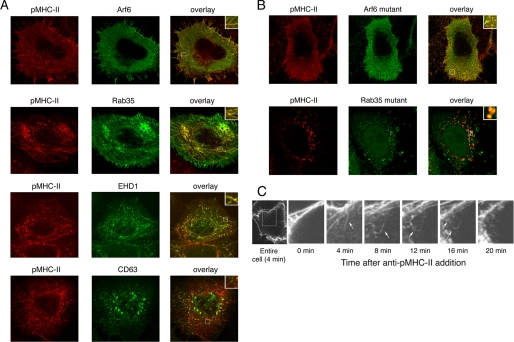
Internalized pMHC-II Is Present in Arf6+Rab35+ Tubular Endosomes—Arf6 is a small GTPase that marks a clathrin-independent cell surface recycling pathway that is used by CD59 and MHC class I molecules (reviewed in Ref. 27). Curiously, Arf6 compartments are also tubular, and we therefore introduced HA-tagged Arf6 into HeLa-CIITA cells to determine whether the pMHC-II tubules were Arf6-positive (endogenous levels of Arf6 are too low to be observed with the available antibodies and previous studies have shown that overexpression of wild-type Arf6 does not alter membrane endocytosis/recycling (39)). The pMHC-II containing tubules were clearly Arf6-positive (Fig. 8A). In addition, the small GTPase Rab35 and the Eps15-homology domain containing protein EHD1 have been reported to be present on tubular endosomes that mediate rapid early endosomal recycling to the cell surface (28, 40). Introduction of GFP-Rab35 or GFP-EHD1 into HeLa-CIITA cells confirmed that the pMHC-II tubules were Rab35- and EHD1-positive. By contrast to the distribution of internalized pMHC-II, MHC-II-Ii was only seen in vesicular structures and was never observed in Arf6-, Rab35-, or EHD1-positive tubules (data not shown). GFP-CD63 illuminated late endosomal/lysosomal vesicles and the pMHC-II-positive tubules did not contain CD63. However, the vesicular pool of internalized pMHC-II was present in CD63-positive structures, demonstrating that a small portion of internalized pMHC-II was sorted to late endosomes/lysosomes. In agreement with previous studies (28, 39), we found that expression of GTP binding mutants of either Arf6 (Arf6 T27N) or Rab35 (Rab35 S22N) disrupted the normal tubular endosome morphology. Most importantly, internalized pMHC-II was no longer found on tubular structures in these cells and instead co-localized with each of these markers on vesicular structures (Fig. 8B). Live cell imaging revealed the appearance of internalizing pMHC-II in tubules almost immediately and by 20 min the tubular pMHC-II structures were no longer observed, presumably due to fusion of the tubular recycling endosomes with the plasma membrane (Fig. 8C and supplementary Videos 2 and 3). These data demonstrate that unlike Ii-associated MHC-II, pMHC-II complexes internalize using a clathrin- and dynamin-independent endocytosis pathway and strongly suggests that these complexes recycle back to the plasma membrane in Arf6+Rab35+EHD1+ tubules.
FIGURE 8.
Internalized pMHC-II is present in Arf6+ Rab35+ endosomal tubules. HeLa-CIITA cells were transfected with plasmids encoding wild-type HA-Arf6, GFP-Rab35, GFP-EHD1, or GFP-CD63 (panel A) or the HA-Arf6 T27N mutant or GFP-Rab35 S22N mutant (panel B). The distribution of each endosomal protein (green) and internalized pMHC-II (red) was analyzed by confocal microscopy as described above. Insets show higher magnification images of the indicated regions of the cells. The overlays reveal considerable colocalization of internalized pMHC-II with Arf6-Rab35, and EHD1-containing tubules in cells expressing the wild-type proteins and colocalization with the disrupted, vesicular structures in cells expressing the mutant proteins. C, HeLa-CIITA cells were incubated with trace amounts of the Alexa 488-conjugated pMHC-II mAb L243 at 37 °C and immediately analyzed by confocal microscopy. Individual images were acquired every 2 min and videos were generated by overlaying successive images. Shown is a representative series of images (of a single focal plane) showing the movement of a bolus of internalizing pMHC-II along tubular internal structures back to the plasma membrane.
DISCUSSION
APCs such as mature DCs, macrophages, and activated B cells express a majority of their pMHC-II complexes on the plasma membrane. However, like all plasma membrane proteins even pMHC-II can rapidly internalize from the plasma membrane into early endosomes and then recycle back out to the plasma membrane (22-24, 41). In fact, recycling MHC-II has been shown to exchange one antigenic peptide for another in both HLA-DM-dependent (25) and HLA-DM-independent manners (26). Given the extremely large flux of MHC-II that can internalize in APCs, in 1990 Reid and Watts (24) made the prescient prediction that recycling MHC-II could represent a major pool of MHC-II capable of exchanging antigenic peptides to increase the diversity of ligands available to CD4 T cells (24).
In this study we have explored both molecular mechanisms leading to MHC-II internalization from the plasma membrane and the recycling pathway followed by internalized MHC-II. Ii-associated MHC-II molecules at the plasma membrane are rapidly internalized into late endosomal/lysosomal compartments in HeLa-CIITA cells (shown here), heterologous cells (16, 34), and APCs (16). Ii-dependent endocytosis of these complexes requires the recognition of dileucine motifs in the cytosolic domain of Ii with the clathrin-associated AP-2 adaptor (42). Because the β-chain in many alleles of mouse, rat, and human alleles of MHC-II contains a “dileucine-like” sequence in the cytosolic domain, it has been assumed that even Ii-free MHC-II internalizes by AP-2-dependent clathrin-mediated endocytosis (19, 20, 23, 43, 44). Using a highly specific pMHC-II mAb that does not recognize Ii-associated MHC-II we show that plasma membrane pMHC-II endocytosis is clathrin-, AP-2-, and dynamin-independent.
Whereas Ii is commonly referred to as an MHC-II chaperone that is required for MHC-II to access lysosome-like antigen processing compartments, it is clear that MHC-II (and even pMHC-II complexes from the plasma membrane) can reach these late endocytic compartments in the absence of Ii (45, 46). It is likely that Ii-associated MHC-II can more efficiently access these compartments than can Ii-free MHC-II, suggesting that Ii simply enhances the overall rate of MHC-II traffic to these compartments. In fact, we have found that the kinetics of cell surface pMHC-II arrival on CD63+ antigen processing compartments is significantly delayed relative to that of cell surface MHC-II-Ii complexes.4 We have also found that internalized MHC-II from the cell surface can traffic back to multivesicular bodies and be secreted on exosomes from B cells (47), demonstrated that the endocytosis pathway identified here leads to MHC-II delivery to multivesicular antigen processing compartments. Given the large number of pMHC-II on the cell surface at any given time, clathrin- and dynamin-independent endocytosis of Ii-free pMHC-II to both early endosomal and lysosome-like antigen processing compartments could be a major source of MHC-II for peptide loading (as discussed below). It is interesting to note that the distribution of MHC-II in immature DC derived from Ii-deficient mice is indistinguishable from that in wild-type mice (46), a fact that could be attributed to efficient MHC-II endocytosis and sorting into the relatively non-proteolytic antigen processing compartments in these cells (1).
Our internalization studies of surface-tagged pMHC-II in HeLa-CIITA and human DCs showed that pMHC-II internalizes and reaches a steady-state distribution of 80% plasma membrane, 20% intracellular and reaches this equilibrium within 5 min. This is in excellent agreement with other studies performed in human B cells and fibroblasts (22, 24). To maintain this distribution, cells must possess efficient machinery for recycling internalized molecules back to the plasma membrane. Following the entire pathway traversed by internalizing pMHC-II by continuous incubation with anti-pMHC-II mAb revealed pMHC-II in elongated tubular structures that appeared to emanate from the plasma membrane. These tubules also contained Arf6, Rab35, and EHD1, known regulators of membrane recycling. Expression of GTPase mutants of Arf6 and Rab35 prevented the appearance of these tubules, and internalized pMHC-II now co-localized with these proteins on fragmented vesicles. The tubular structures observed in our study appear identical to the clathrin- and dynamin-independent carriers that regulate cholera toxin endocytosis (48). Arf6 is present in tubulovesicular structures that mediate recycling of membrane proteins internalized by clathrin-independent endocytosis (27). Although not as well characterized as Arf6, Rab35 is also present on tubulovesicular structures and has been reported to regulate a rapid early endosomal membrane recycling pathway (28). It is important to note that whereas internalized pMHC-II can access CD63-positive lysosomes, the tubules we describe here are not themselves CD63-positive and do not represent the tubular lysosomes that have been described for the transport of pMHC-II from lysosomes to the plasma membrane of DCs (49, 50). Furthermore, pMHC-II+ tubular lysosomes are only observed after DC maturation (49), and in this study we have restricted our analysis to HeLa-CIITA cells, B cells, and immature (not activated) DCs. It has been suggested that MHC-II endocytosis is regulated by dileucine-like signals present in the cytosolic domain of the MHC-II β-chain (22, 51). However, we have never obtained any evidence to support this conclusion. We have found that truncation of the entire cytosolic domains of the MHC-II α- or β-chains in either mouse or human MHC-II proteins does not alter the kinetics of pMHC-II endocytosis and that even these mutant MHC-II molecules enter into the tubular endosomes shown in this study (data not shown). These observations are also consistent with previous reports that even tailless MHC-II can sort into lysosomal antigen processing compartments in the absence of Ii expression (14). This is not to say that the cytosolic domain of MHC-II does not contain targeting information, as it has been shown that the conserved leucine-based sorting signal in the tail of the β-chain was important for the intracellular distribution and polarized sorting of MHC-II (52) and for activation of certain antigen-specific T cells by antigen-pulsed APCs (51, 53).
Although we have not directly addressed this issue in our current study, it is intriguing to speculate as to the nature of pMHC-II internalization signal. One likely possibility is that association with lipid raft membrane microdomains serves as a “signal” for entry into the clathrin- and -dynamin-independent endocytosis pathways (as reviewed in Ref. 36). A considerable amount of cell surface pMHC-II is lipid raft associated (54) and it is also curious that deletion of the cytosolic domains of MHC-II does not affect pMHC-II raft association (55)5 or endocytosis from the plasma membrane. Furthermore, because lymphocytes do not express caveolin and do not possess structures that resemble traditional caveolae (56, 57), dynamin-dependent or -independent endocytosis of pMHC-II by caveolae seems extremely unlikely.
Although it has also been suggested that ubiquitination of the MHC-II β-chain regulates MHC-II internalization in DCs (43, 58), the kinetics of MHC-II endocytosis in B cells is unaltered in mice lacking the major MHC-II ubiquitin ligase March-I (59). In agreement with this, we find that mutation of the target lysine of MHC-II ubiquitination has no effect on the kinetics of pMHC-II endocytosis in HeLa-CIITA cells.4 Such findings could either highlight differences in MHC-II endocytosis pathways in DCs and B cells or indicate a more complex role for MHC-II ubiquitination than previously appreciated.
Our results demonstrate that cell surface MHC-II-Ii and pMHC-II complexes use completely different endocytic pathways and have different fates after entering the cell interior. MHC-II-Ii complexes internalize very quickly in clathrin-, AP-2-, and dynamin-dependent manners and efficiently reach lysosome-like antigen loading compartments with no evidence of entry into tubular recycling endosomes. Although the initial rate of pMHC-II endocytosis is also relatively rapid, endocytosis is clathrin-, AP-2-, and dynamin-independent and a significant amount of internalized pMHC-II efficiently recycles from endosomes back to the plasma membrane and only inefficiently reaches lysosome-like antigen loading compartments. This dichotomy of trafficking routes used by cell surface MHC-II could provide a critical advantage to an APC, because in addition to efficient targeting of Ii-associated MHC-II to lysosome-like antigen processing compartments pMHC-II will gain access to early endosomal antigens that are proteolytically sensitive and are unable to survive in the lysosomal antigen processing compartments. By utilizing both an early endosomal recycling pathway for pMHC-II and a late endosomal/lysosomal targeting pathway for MHC-II-Ii complexes, APCs are able to present a wider repertoire of peptide antigens to the T cells. In situations in which immunodominant epitopes are proteolytic sensitive, it is critical that MHC-II gain access to these peptides in early endocytic compartments before they are completely degraded in more proteolytic lysosome-like antigen processing compartments.
Supplementary Material
Acknowledgments
We thank Nicolas Barois, Steve Shaw, and David Segal for critical reading of the manuscript and our many colleagues who have provided materials for this study. We also thank Mike Kruhlak and the Experimental Immunology Branch microscopy facility for expert technical advice.
This work was authored, in whole or in part, by National Institutes of Health staff. This work was supported by the National Institutes of Health Intramural Program and the Norwegian Cancer Society (to O. B.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Videos 1-3.
Footnotes
The abbreviations used are: MHC, major histocompatibility complex; APC, antigen presenting cell; pMHC-II, peptide-loaded MHC-II; mAb, monoclonal antibody; GFP, green fluorescent protein; HA, hemagglutinin; FACS, fluorescence-activated cell sorter; HBSS, Hanks' balanced salt solution; siRNA, small interfering RNA; Ii, invariant chain.
E. Walseng, O. Bakke, and P. A. Roche, unpublished observations.
P. A. Roche, unpublished observations.
References
- 1.Trombetta, E. S., and Mellman, I. (2005) Annu. Rev. Immunol. 23 975-1028 [DOI] [PubMed] [Google Scholar]
- 2.Barnes, K. A., and Mitchell, R. N. (1995) J. Exp. Med. 181 1715-1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Delwig, A., Ramachandra, L., Harding, C. V., and Robinson, J. H. (2003) Eur. J. Immunol. 33 2353-2360 [DOI] [PubMed] [Google Scholar]
- 4.von Delwig, A., Musson, J. A., Shim, H. K., Lee, J. J., Walker, N., Harding, C. V., Williamson, E. D., and Robinson, J. H. (2005) Scand. J. Immunol. 62 243-250 [DOI] [PubMed] [Google Scholar]
- 5.Delamarre, L., Couture, R., Mellman, I., and Trombetta, E. S. (2006) J. Exp. Med. 203 2049-2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roche, P. A., and Cresswell, P. (1990) Nature 345 615-618 [DOI] [PubMed] [Google Scholar]
- 7.Anderson, M. S., and Miller, J. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 2282-2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikoff, E. K., Huang, L. Y., Episkopou, V., van Meerwijk, J., Germain, R. N., and Robertson, E. J. (1993) J. Exp. Med. 177 1699-1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viville, S., Neefjes, J., Lotteau, V., Dierich, A., Lemeur, M., Ploegh, H., Benoist, C., and Mathis, D. (1993) Cell 72 635-648 [DOI] [PubMed] [Google Scholar]
- 10.Bakke, O., and Dobberstein, B. (1990) Cell 63 707-716 [DOI] [PubMed] [Google Scholar]
- 11.Lotteau, V., Teyton, L., Peleraux, A., Nilsson, T., Karlsson, L., Schmid, S. L., Quaranta, V., and Peterson, P. A. (1990) Nature 348 600-605 [DOI] [PubMed] [Google Scholar]
- 12.Busch, R., Rinderknecht, C. H., Roh, S., Lee, A. W., Harding, J. J., Burster, T., Hornell, T. M., and Mellins, E. D. (2005) Immunol. Rev. 207 242-260 [DOI] [PubMed] [Google Scholar]
- 13.Pieters, J., Bakke, O., and Dobberstein, B. (1993) J. Cell Sci. 106 831-846 [DOI] [PubMed] [Google Scholar]
- 14.Simonsen, A., Stang, E., Bremnes, B., Roe, M., Prydz, K., and Bakke, O. (1997) J. Cell Sci. 110 597-609 [DOI] [PubMed] [Google Scholar]
- 15.Roche, P. A., Teletski, C. L., Karp, D. R., Pinet, V., Bakke, O., and Long, E. O. (1992) EMBO J. 11 2841-2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roche, P. A., Teletski, C. L., Stang, E., Bakke, O., and Long, E. O. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 8581-8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann, M. W., Honing, S., Rodionov, D., Dobberstein, B., von Figura, K., and Bakke, O. (1999) J. Biol. Chem. 274 36153-36158 [DOI] [PubMed] [Google Scholar]
- 18.Bonifacino, J. S., and Traub, L. M. (2003) Annu. Rev. Biochem. 72 395-447 [DOI] [PubMed] [Google Scholar]
- 19.Dugast, M., Toussaint, H., Dousset, C., and Benaroch, P. (2005) J. Biol. Chem. 280 19656-19664 [DOI] [PubMed] [Google Scholar]
- 20.McCormick, P. J., Martina, J. A., and Bonifacino, J. S. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 7910-7915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, K., Peterson, P. A., and Karlsson, L. (1997) J. Biol. Chem. 272 17055-17060 [DOI] [PubMed] [Google Scholar]
- 22.Pinet, V., Vergelli, M., Martin, R., Bakke, O., and Long, E. O. (1995) Nature 375 603-606 [DOI] [PubMed] [Google Scholar]
- 23.Reid, P. A., and Watts, C. (1992) Immunology 77 539-542 [PMC free article] [PubMed] [Google Scholar]
- 24.Reid, P. A., and Watts, C. (1990) Nature 346 655-657 [DOI] [PubMed] [Google Scholar]
- 25.Pathak, S. S., Lich, J. D., and Blum, J. S. (2001) J. Immunol. 167 632-635 [DOI] [PubMed] [Google Scholar]
- 26.Sinnathamby, G., and Eisenlohr, L. C. (2003) J. Immunol. 170 3504-3513 [DOI] [PubMed] [Google Scholar]
- 27.Donaldson, J. G. (2003) J. Biol. Chem. 278 41573-41576 [DOI] [PubMed] [Google Scholar]
- 28.Kouranti, I., Sachse, M., Arouche, N., Goud, B., and Echard, A. (2006) Curr. Biol. 16 1719-1725 [DOI] [PubMed] [Google Scholar]
- 29.Stumptner-Cuvelette, P., Morchoisne, S., Dugast, M., Le Gall, S., Raposo, G., Schwartz, O., and Benaroch, P. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 12144-12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shackelford, D. A., Lampson, L. A., and Strominger, J. L. (1981) J. Immunol. 127 1403-1410 [PubMed] [Google Scholar]
- 31.Kleijmeer, M. J., Oorschot, V. M., and Geuze, H. J. (1994) J. Investig. Dermatol. 103 516-523 [DOI] [PubMed] [Google Scholar]
- 32.Kleijmeer, M. J., Ossevoort, M. A., van Veen, C. J., van Hellemond, J. J., Neefjes, J. J., Kast, W. M., Melief, C. J., and Geuze, H. J. (1995) J. Immunol. 154 5715-5724 [PubMed] [Google Scholar]
- 33.Hansen, H. J., Ong, G. L., Diril, H., Valdez, A., Roche, P. A., Griffiths, G. L., Goldenberg, D. M., and Mattes, M. J. (1996) Biochem. J. 320 293-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson, M. S., Swier, K., Arneson, L., and Miller, J. (1993) J. Exp. Med. 178 1959-1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maldonado-Baez, L., and Wendland, B. (2006) Trends Cell Biol. 16 505-513 [DOI] [PubMed] [Google Scholar]
- 36.Mayor, S., and Pagano, R. E. (2007) Nat. Rev. Mol. Cell. Biol. 8 603-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford, M. G., Pearse, B. M., Higgins, M. K., Vallis, Y., Owen, D. J., Gibson, A., Hopkins, C. R., Evans, P. R., and McMahon, H. T. (2001) Science 291 1051-1055 [DOI] [PubMed] [Google Scholar]
- 38.Gupta, S. N., Kloster, M. M., Rodionov, D. G., and Bakke, O. (2006) Eur. J. Cell Biol. 85 457-467 [DOI] [PubMed] [Google Scholar]
- 39.Naslavsky, N., Weigert, R., and Donaldson, J. G. (2004) Mol. Biol. Cell 15 3542-3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caplan, S., Naslavsky, N., Hartnell, L. M., Lodge, R., Polishchuk, R. S., Donaldson, J. G., and Bonifacino, J. S. (2002) EMBO J. 21 2557-2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinet, V. M., and Long, E. O. (1998) Eur. J. Immunol. 28 799-804 [DOI] [PubMed] [Google Scholar]
- 42.Rodionov, D. G., and Bakke, O. (1998) J. Biol. Chem. 273 6005-6008 [DOI] [PubMed] [Google Scholar]
- 43.van Niel, G., Wubbolts, R., Ten Broeke, T., Buschow, S. I., Ossendorp, F. A., Melief, C. J., Raposo, G., van Balkom, B. W., and Stoorvogel, W. (2006) Immunity 25 885-894 [DOI] [PubMed] [Google Scholar]
- 44.Santambrogio, L., and Strominger, J. L. (2006) Immunity 25 857-859 [DOI] [PubMed] [Google Scholar]
- 45.Simonsen, A., Momburg, F., Drexler, J., Hammerling, G. J., and Bakke, O. (1993) Int. Immunol. 5 903-917 [DOI] [PubMed] [Google Scholar]
- 46.Rovere, P., Zimmermann, V. S., Forquet, F., Demandolx, D., Trucy, J., Ricciardi-Castagnoli, P., and Davoust, J. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 1067-1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muntasell, A., Berger, A. C., and Roche, P. A. (2007) EMBO J. 26 4263-4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirkham, M., Fujita, A., Chadda, R., Nixon, S. J., Kurzchalia, T. V., Sharma, D. K., Pagano, R. E., Hancock, J. F., Mayor, S., and Parton, R. G. (2005) J. Cell Biol. 168 465-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boes, M., Cerny, J., Massol, R., Op den Brouw, M., Kirchhausen, T., Chen, J., and Ploegh, H. L. (2002) Nature 418 983-988 [DOI] [PubMed] [Google Scholar]
- 50.Chow, A., Toomre, D., Garrett, W., and Mellman, I. (2002) Nature 418 988-994 [DOI] [PubMed] [Google Scholar]
- 51.Zhong, G., Romagnoli, P., and Germain, R. N. (1997) J. Exp. Med. 185 429-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simonsen, A., Pedersen, K. W., Nordeng, T. W., von der Lippe, A., Stang, E., Long, E. O., and Bakke, O. (1999) J. Immunol. 163 2540-2548 [PubMed] [Google Scholar]
- 53.Nabavi, N., Ghogawala, Z., Myer, A., Griffith, I. J., Wade, W. F., Chen, Z. Z., McKean, D. J., and Glimcher, L. H. (1989) J. Immunol. 142 1444-1447 [PubMed] [Google Scholar]
- 54.Anderson, H. A., Hiltbold, E. M., and Roche, P. A. (2000) Nat. Immunol. 1 156-162 [DOI] [PubMed] [Google Scholar]
- 55.Becart, S., Setterblad, N., Ostrand-Rosenberg, S., Ono, S. J., Charron, D., and Mooney, N. (2003) J. Cell Sci. 116 2565-2575 [DOI] [PubMed] [Google Scholar]
- 56.Fra, A. M., Williamson, E., Simons, K., and Parton, R. G. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 8655-8659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fra, A. M., Williamson, E., Simons, K., and Parton, R. G. (1994) J. Biol. Chem. 269 30745-30748 [PubMed] [Google Scholar]
- 58.Shin, J. S., Ebersold, M., Pypaert, M., Delamarre, L., Hartley, A., and Mellman, I. (2006) Nature 444 115-118 [DOI] [PubMed] [Google Scholar]
- 59.Matsuki, Y., Ohmura-Hoshino, M., Goto, E., Aoki, M., Mito-Yoshida, M., Uematsu, M., Hasegawa, T., Koseki, H., Ohara, O., Nakayama, M., Toyooka, K., Matsuoka, K., Hotta, H., Yamamoto, A., and Ishido, S. (2007) EMBO J. 26 846-854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poloso, N. J., Denzin, L. K., and Roche, P. A. (2006) J. Immunol. 177 5451-5458 [DOI] [PubMed] [Google Scholar]
- 61.Ackerman, A. L., and Cresswell, P. (2003) J. Immunol. 170 4178-4188 [DOI] [PubMed] [Google Scholar]
- 62.Poloso, N. J., Muntasell, A., and Roche, P. A. (2004) J. Immunol. 173 4539-4546 [DOI] [PubMed] [Google Scholar]
- 63.Hiltbold, E. M., Poloso, N. J., and Roche, P. A. (2003) J. Immunol. 170 1329-1338 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.