Abstract
Sustained nigrostriatal dopamine depletion increases the serine/threonine phosphorylation of multiple striatal proteins that play a role in corticostriatal synaptic plasticity, including Thr286 phosphorylation of calcium/calmodulin-dependent protein kinase IIα (CaMKIIα). Mechanisms underlying these changes are unclear, but protein phosphatases play a critical role in the acute modulation of striatal protein phosphorylation. Here we show that dopamine depletion for periods ranging from 3 weeks to 10 months significantly reduces the total activity of protein phosphatase (PP) 1, but not of PP2A, in whole lysates of rat striatum, as measured using multiple substrates, including Thr286-autophosphorylated CaMKIIα. Striatal PP1 activity is partially inhibited by a fragment of the PP1-binding protein neurabin-I, Nb-(146–493), because of the selective inhibition of the PP1γ1 isoform. The fraction of PP1 activity that is insensitive to Nb-(146–493) was unaffected by dopamine depletion, demonstrating that dopamine depletion specifically reduces the activity of PP1 isoforms that are sensitive to Nb-(146–493) (i.e. PP1γ1). However, total striatal levels of PP1γ1 or any other PP1 isoform were unaffected by dopamine depletion, and our previous studies showed that total levels of the PP1 regulatory/targeting proteins DARPP-32, spinophilin, and neurabin were also unchanged. Rather, co-immunoprecipitation experiments demonstrated that dopamine depletion increases the association of PP1γ1 with spinophilin in striatal extracts. In combination, these data demonstrate that striatal dopamine depletion inhibits a specific synaptic phosphatase by increasing PP1γ1 interaction with spinophilin, perhaps contributing to hyperphosphorylation of synaptic proteins and disruptions of synaptic plasticity and/or dendritic morphology.
Calcium/calmodulin-dependent protein kinase II (CaMKII)3 and protein phosphatase 1 (PP1) are critical for synaptic plasticity. Autophosphorylation of CaMKII at Thr286 is required for normal long term potentiation and hippocampus-based learning and memory (reviewed in Ref. 1). PP1 selectively dephosphorylates CaMKII that is associated with postsynaptic densities, whereas soluble CaMKII is primarily dephosphorylated by PP2A (2, 3). CaMKII has been shown to inhibit some forms of PP2A (4), but inhibition of PP1 via cAMP-dependent pathways promotes autophosphorylation at Thr286 and long term potentiation induction (5, 6). Spinophilin and neurabin are similar F-actin-binding proteins that selectively target the PP1γ1 isoform to dendritic spines, modulating spine morphology and synaptic plasticity (7–16). Thus, coordinated regulation of CaMKII and PP1 is critical for normal synaptic physiology.
Loss of nigrostriatal dopamine inputs in Parkinson disease or in parkinsonian animal models results in morphological alterations in striatal medium spiny neurons (MSNs) (17–22), which constitute >90% of the total striatal neuron population, and impairment of multiple forms of corticostriatal synaptic plasticity (23–26). Symptoms of Parkinson disease initially respond to dopamine replacement therapy, but as the disease progresses this approach generates debilitating side effects and/or loses efficacy. Studies of animal models, such as the 6-hydroxydopamine (6-OHDA) lesioned rat, have provided innumerable insights about terminal consequences of striatal dopamine depletion and the mechanisms underlying striatal deficits in Parkinson disease, resulting in improved therapeutic strategies (reviewed in Ref. 27).
Dopamine depletion increases Thr286 autophosphorylation of CaMKII, and this increase is reversed by dopamine replacement using levodopa (28, 29). Moreover, CaMKII inhibitors normalize dopamine depletion-induced alterations in both synaptic plasticity and behavior (29, 30), suggesting that altered regulation of CaMKII plays a critical role in the parkinsonian phenotype. The enhanced phosphorylation of CaMKII at Thr286 is sustained at similar levels for up to 18 months after inducing dopamine depletion (28). However, increased phosphorylation of a downstream CaMKII target, Ser831 in the α-amino-3-hydroxy-5-methyl-4-isoxazole propionate-type glutamate receptor GluR1 subunit, is detected 9–18 months, but not 3–6 weeks, after dopamine depletion (28). These data suggest complex interactions between the effects of long term dopamine depletion and aging that may have additional effects on striatal function.
Striatal dopamine depletion could increase CaMKII autophosphorylation by at least two potentially linked mechanisms. First, increased corticostriatal glutamatergic drive (21, 31) might activate N-methyl-d-aspartic acid receptors or voltage-gated Ca2+ channels, enhancing postsynaptic Ca2+ influx and CaMKII autophosphorylation. Second, reduced protein phosphatase activity(ies) might allow increased phosphorylation of CaMKII and other proteins. Many acute effects of dopamine are thought to require inhibition of striatal PP1 by the Thr34-phosphorylated form of DARPP-32 (32, 33), although reduced PP1 activity has not been directly demonstrated. Regulation of PP1 localization by spinophilin and neurabin also is critical for normal synaptic plasticity and dendritic spine morphology (9, 10, 13, 15, 16, 34, 35).
Here we report that chronic striatal dopamine depletion selectively decreases the activity of the PP1γ1 isoform, apparently by enhancing its association with spinophilin. This reduction in PP1 activity may allow enhanced phosphorylation of multiple striatal proteins, including CaMKII, thereby playing a key role in mediating changes in synaptic morphology and/or function following dopamine depletion.
EXPERIMENTAL PROCEDURES
6-OHDA Lesion Surgery—Male Sprague-Dawley rats (Harlan; Indianapolis, IN) were housed under a 12:12 light:dark cycle with food and water freely available. Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health), under the oversight of the Institutional Animal Care and Use Committee. Rats (3 months old) underwent unilateral 6-OHDA lesion surgery, or received sham (vehicle) injections, as detailed previously (28). At the times indicated after surgery, rats were lightly anesthetized with isoflurane, decapitated, and the brains removed. Data reported here were obtained from six batches of 6–8 rats sacrificed at times ranging from 3 weeks to 11 months following lesion surgery to assess the long term consequences of dopamine depletion. Rats in which a 6-OHDA lesion induced a >90% loss of tyrosine hydroxylase in the lesioned striatum relative to the intact contralateral striatum were considered to be completely dopamine-depleted. Rats with <90% loss of tyrosine hydroxylase in the lesioned hemisphere were considered to have a partial dopamine depletion, and data obtained from these animals were not included in quantitative analyses. The 6-OHDA lesioned rat is a well established model of essentially complete dopamine depletion and is well suited to the study of long term effects of striatal dopamine depletion. As such, it has proven to be a reliable animal model of Parkinson disease (27).
Antibodies—The following primary antibodies were used for immunoblotting (dilutions indicated) and/or for immunoprecipitation: whole serum and affinity-purified sheep and rabbit anti-PP1γ1 and anti-PP1β (7, 36, 37); mouse anti-tyrosine hydroxylase (ImmunoStar, 1:1000); mouse anti-PP2Ac (BD Transduction Laboratories, 1:4000); rabbit antibody to spinophilin (8) purified using protein A-Sepharose (Upstate); and mouse anti-spinophilin (BD Transduction Laboratories, 1:1000). Secondary antibodies were from Promega (goat anti-mouse HRP, 1:2000; goat anti-rabbit HRP, 1:4000), AlphaQuest (rabbit anti-goat HRP, 1:4000), or Santa Cruz Biotechnology (goat anti-mouse HRP or donkey anti-sheep HRP preadsorbed with human and/or mouse IgG, 1:5000).
Immunoblots—Dorsolateral striatum was isolated, processed for immunoblotting, and immunoblotted as described previously (28).
Tissue Homogenates for Phosphatase Activity Assays—Punches (1.15-mm inner diameter) of dorsolateral striatum were removed from both hemispheres of 1.0-mm-thick coronal slices, at the level of the crossing of the anterior commissure. Punches were immediately homogenized using a Kontes tissue homogenizer in ice-cold 7 mm Tris-HCl, pH 7.5, containing 0.2 mm EDTA, 0.2 mm EGTA, 320 mm sucrose, 1 mm benzamidine, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μm pepstatin, 50 mm sodium fluoride, 50 mm sodium β-glycerophosphate, 20 mm sodium pyrophosphate. Homogenates were stored at –80 °C and analyzed within 1–2 weeks.
Protein Phosphatase Activity Assays—Phosphatase activities were assayed using [32P]phosphorylase a, [32P]casein, or Thr286-autophosphorylated CaMKIIα substrates, as described previously (3). Briefly, extracts (10 μl of 0.1–0.2 mg/ml extract) were incubated at 30 °C in 50 mm Tris-HCl, pH 7.5, 0.1 m sodium chloride, 20 mm magnesium acetate, 2 mg/ml bovine serum albumin, 0.2 mm EGTA, 1 mm dithiothreitol for 30 min ([32P]phosphorylase a and [32P]casein) or 45 min (Thr286-autophosphorylated CaMKIIα) in a final volume of 50 μl. Caffeine (5 mm) was included with [32P]phosphorylase a, and assay blanks were incubated without extract. Trichloroacetic acid (50 μl of 40% w/v) was added to terminate the reaction and samples were centrifuged (10,000 × g, 10 min). Supernatants (80-μl aliquots) were analyzed in a liquid scintillation counter to quantify 32P released from the protein substrate.
Where indicated, diluted extracts were preincubated with the following inhibitors for ∼10 min on ice prior to initiating assays by addition of substrate. Nb-(146–453) and Nb-(146–493) were prepared as glutathione S-transferase (GST) fusion proteins and were characterized previously (12, 38). Nb-(146–453) does not contain the PP1 binding domain (residues 457–460) or the PP1γ1 selectivity domain (residues 473–479) that are present in Nb-(146–493). Inhibitor-2 (Calbiochem) and okadaic acid (LC Laboratories) were also used. PP2A activity was defined as the activity inhibited by 2.5 nm okadaic acid. PP1 activity was defined as the difference in activities measured using 2.5 nm and 2.5 μm okadaic acid. PP1γ1 activity was defined as the phosphatase activity inhibited by Nb-(146–493).
Immunoprecipitations—For phosphatase activity assays (Fig. 5A), native PP1γ1 and PP1β holoenzymes were isolated from extracts of whole striatum by immunoprecipitation. Briefly, freshly dissected whole striatal tissue was homogenized in 2 mm Tris-HCl, pH 7.5, containing 2 mm EDTA, 2 mm EGTA, 1 mm dithiothreitol, 1 mm benzamidine, 0.2 mm phenylmethylsulfonyl fluoride, 40 mg/liter soybean trypsin inhibitor, 10 mg/liter leupeptin, and 0.5% Triton X-100). Homogenates were centrifuged (10,000 × g, 10 min, 4 °C), and supernatants were diluted to 1 mg/ml in IP buffer (50 mm Tris-HCl, pH 7.5, 150 mm sodium chloride, 0.5% Triton X-100). Samples were precleared using gammabind-G-Sepharose (Amersham Biosciences) and then incubated at 4 °C for 1 h with sheep antisera to PP1γ1 or PP1β (5 μl each). After addition of gammabind-G-Sepharose (30 μl of a 50:50 slurry), incubation was continued overnight at 4 °C. Immunoprecipitates were sedimented, washed five times with 1 ml of IP buffer, suspended in homogenization buffer lacking Triton X-100, and immediately assayed.
FIGURE 5.
Nb-(146–493) is a selective inhibitor of striatal PP1γ1. A, PP1γ1 or PP1β were immunoprecipitated (IP) from whole striatal extracts. Upper panels show representative immunoblots confirming the immunoprecipitation specificity. Lower panels show activities in the immunoprecipitates measured using [32P]phosphorylase a in the presence of 2.5 nm okadaic acid (O.A.) alone or with the addition of 2.5 μm okadaic acid or 1 μm Nb-(146–493). Nb-(146–493) inhibited immunoprecipitated PP1γ1, but not immunoprecipitated PP1β. B, phosphatase activity in a control whole striatal extract was assayed using [32P]phosphorylase a in the presence of indicated concentrations of okadaic acid (open diamonds), inhibitor-2 (solid squares), Nb-(146–493) (solid circles), or Nb-(146–453) (open inverted triangles). Each point is the mean ± S.E. of 2–6 observations, plotted as a percentage of total activity (without inhibitor). PP1 accounts for 75–80% of the total activity when estimated using okadaic acid or inhibitor-2 (see “Results”), but Nb-(146–493) inhibits only ≈48% of the total activity.
For co-immunoprecipitations, intact (control) and lesioned (dopamine depleted) dorsolateral striatal punches (see above) were homogenized separately in the same buffer containing phosphatase inhibitors (50 mm sodium fluoride, 50 mm β-glycerophosphate, 20 mm sodium pyrophosphate). Aliquots (0.5 ml) of diluted/precleared soluble extracts were immunoprecipitated using rabbit antibodies to PP1γ1 (5 μl of affinity-purified), spinophilin (5 μl of protein A-purified), or control IgG in IP Buffer containing the same phosphatase inhibitors. Washed immunoprecipitates and aliquots of the input and immune supernatants were mixed with sample buffer and analyzed by immunoblotting using enhanced chemiluminescence detection. X-ray films exposed in the linear range were scanned and quantified using Image J (rsb.info.nih.gov). PP1γ1 and spinophilin signals in immune complexes were normalized to the corresponding input signal and then expressed as a ratio between the dopamine-depleted (lesioned) and intact hemispheres.
Statistical Analyses—Statistical comparisons were made by paired or unpaired Student's t test or Wilcoxon signed rank test, as appropriate.
RESULTS
Phosphorylation of protein phosphatase catalytic or regulatory subunits can regulate their activity and/or localization. Therefore, we developed conditions that limit dephosphorylation of putative regulatory sites following homogenization of striatal samples but allow for detection of phosphatase activities in diluted extracts. Homogenization in the presence of organic phosphatase inhibitors such as microcystin LR is most effective in blocking protein dephosphorylation, but no phosphatase activity was detected when striatal homogenates containing microcystin LR were diluted (data not shown), presumably because this inhibitor is effectively irreversible. However, inclusion of a mixture of inorganic phosphatase inhibitors in the homogenization buffer (see “Experimental Procedures”) allowed the detection of PP1 and PP2A activity if striatal homogenates were diluted 10-fold immediately prior to the assay.
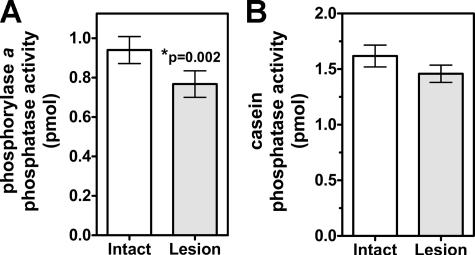
Total Phosphatase Activity Is Decreased Following Dopamine Depletion—We measured phosphatase activities in extracts of dorsolateral striatum ipsilateral or contralateral to 6-OHDA lesion of the substantia nigra. Extracts were prepared from different batches of animals 3–4 weeks or 10–11 months after lesion surgery to assess the consequences of long term dopamine depletion. Using [32P]phosphorylase a as a model substrate, activity in the dopamine-depleted striatum was significantly decreased by ≈18% when measured 3–4 weeks after lesion surgery (Fig. 1A) or by ≈14% when measured 10–11 months after surgery (data not shown; n = 13, p = 0.04). In contrast, there was no significant difference in total phosphatase activities at either time point if [32P]casein was used as the model substrate (Fig. 1B and data not shown).
FIGURE 1.
Total striatal phosphatase activity is decreased following dopamine depletion. Dorsolateral striatal tissue ipsilateral (Lesion) or contralateral (Intact) to the 6-OHDA lesion was collected 3–4 weeks after surgery, and whole extracts were assayed using [32P]phosphorylase a or [32P]casein as model substrates. A, total [32P]phosphorylase a phosphatase activity was decreased by ≈18% in the lesioned hemisphere (n = 8, paired t test). B, there was no significant difference in the total [32P]casein phosphatase activity between striatal hemispheres (n = 8).
Dopamine Depletion Selectively Decreases PP1 Activity—The choice of exogenous protein substrate affects the specific phosphatases that can be detected in assays of whole tissue homogenates. Generally, [32P]phosphorylase a is considered a PP1-selective substrate but also detects PP2A activity, whereas [32P]casein is an effective PP2A substrate (reviewed in Ref. 39). To more specifically compare PP1 and PP2A activities in control and dopamine-depleted striatal extracts, we measured phosphatase activities toward [32P]phosphorylase a and [32P]casein in the presence of 0, 2.5 nm, or 2.5 μm okadaic acid. Okadaic acid selectively inhibits PP2A-like enzymes at low nanomolar concentrations, but will also inhibit PP1 at micromolar concentrations (39). Our previous studies defined PP2A activity as the activity inhibited by 2.5 nm okadaic acid and PP1 activity as the difference in activities measured at 2.5 nm and 2.5 μm okadaic acid (3). PP1 activity detected using [32P]phosphorylase a was significantly decreased by 22% at 3–4 weeks following lesion surgery (Fig. 2A) and by ≈16% at 10–11 months after surgery (data not shown; n = 13, p = 0.014). However, PP2A activity detected using either [32P]phosphorylase a or [32P]casein substrate was not significantly different between the striatal hemispheres when measured 3–4 weeks (Fig. 2B) or 10–12 months (data not shown; n = 13) after lesion surgery.
FIGURE 2.
Dopamine depletion selectively decreases PP1 activity. Phosphatase activities in extracts of rat dorsolateral striatal tissue ipsilateral (Lesion) or contralateral (Intact) to the 6-OHDA lesion collected 3–4 weeks after surgery were assayed using [32P]phosphorylase a or [32P]casein in the presence of either 0, 2.5 nm, or 2.5 μm okadaic acid. A, dopamine depletion significantly decreased by ≈22% the PP1 activity measured using [32P]phosphorylase a (difference in activities measured in the presence of 2.5 nm and 2.5 μm okadaic acid) (n = 8, paired t test). B, there was no significant difference in PP2A activity measured using [32 P]casein (difference in activities measured in the absence and presence of 2.5 nm okadaic acid) (n = 8).
Dopamine Depletion Inhibits PP1-mediated Dephosphorylation of CaMKIIα—To ascertain whether dopamine depletion affects dephosphorylation of a physiologically relevant substrate, we assayed phosphatase activity toward exogenous Thr286-autophosphorylated CaMKIIα in the presence of various concentrations of okadaic acid. As reported previously in whole forebrain extracts (3), ∼80% of the total activity could be assigned to PP2A based on the sensitivity to 2.5 nm okadaic acid. However, this PP2A activity was unaffected by dopamine depletion. In contrast, PP1 activity toward [32P-Thr286]CaMKIIα (defined as the difference in activities at 2.5 nm and 2.5 μm okadaic acid) was significantly decreased by ≈19% after dopamine-depletion surgery (Fig. 3).
FIGURE 3.
Dopamine depletion decreases PP1-mediated dephosphorylation of [32P-Thr286]CaMKIIα. Phosphatase activities in extracts of rat dorsolateral striatal tissue ipsilateral (Lesion) or contralateral (Intact) to the 6-OHDA lesion collected 3–4 weeks after surgery were assayed using [32P-Thr286]CaMKIIα in the presence of 0, 2.5 nm, or 2.5 μm okadaic acid. The graph plots contributions of PP1 and PP2A (calculated as under “Experimental Procedures”) as the percentage of total activity in the intact hemisphere. Dopamine depletion had no significant effect on the PP2A-mediated dephosphorylation of [32P-Thr286]CaMKIIα but significantly decreased PP1-mediated dephosphorylation by ≈19% (n = 8).
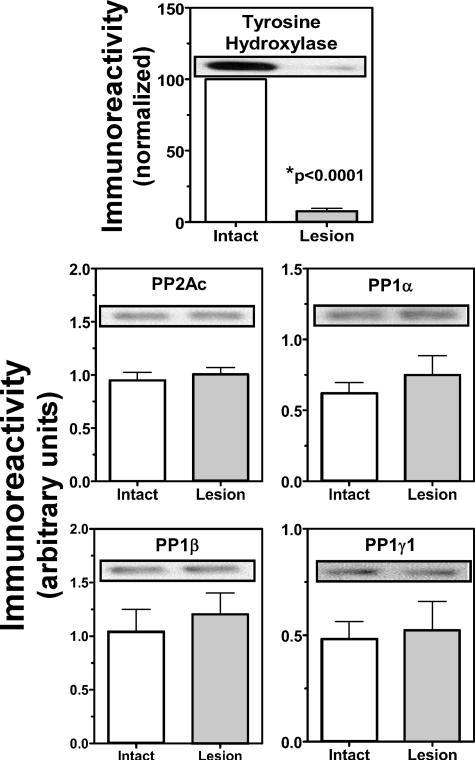
Phosphatase Isoform Levels Are Unchanged by Dopamine Depletion—The decreased phosphatase activity in dopamine-depleted striatum may be due to changes in the levels of specific phosphatase catalytic subunit isoforms, perhaps because of degeneration of dopaminergic terminals and/or the dendritic spines of MSNs. Therefore, aliquots of striatal extracts used in activity assays were immunoblotted for various PP1 catalytic subunit isoforms (α, β, and γ1) and for the PP2A catalytic subunit. These proteins are highly expressed in MSNs but are also present in presynaptic terminals. Despite the substantial degeneration of terminals, as reflected by the loss of >90% of tyrosine hydroxylase, there were no significant differences in levels of PP1 and PP2A catalytic subunit isoforms between intact and lesioned hemispheres (Fig. 4). This may reflect relatively low expression of these proteins in dopaminergic terminals and/or the small contribution of the terminals to total striatal tissue.
FIGURE 4.
PP1 catalytic subunit isoform expression is unaffected by dopamine depletion. Whole dorsolateral striatal extracts ipsilateral (Lesion) or contralateral (Intact) to the 6-OHDA lesion collected 10–11 months after surgery were immunoblotted for tyrosine hydroxylase, PP2Ac, and various isoforms of PP1 (α, β, and γ1). The efficacy of the 6-OHDA lesion was confirmed by the ≈90% decrease of tyrosine hydroxylase immunoreactivity (n = 11), but there was no significant difference in the levels of PP2Ac, PP1α, PP1β, or PP1γ1 between intact and lesioned hemispheres (n = 9–11, p > 0.240).
Nb-(146–493) Is a Novel, Selective Inhibitor of PP1γ1 in Striatal Extracts—Differential subcellular targeting of PP1 isoforms in neurons suggests that these proteins have different biological roles (7, 14). We sought to develop an assay that differentiates the contributions of PP1 isoforms to the total PP1 activity in extracts. Nb-(146–493) is a GST fusion protein that inhibits isolated brain PP1γ1 catalytic subunit ≈20-fold more potently than it inhibits isolated brain PP1β catalytic subunit (12). To determine whether this selectivity might be useful in discriminating PP1 isoform activities in tissue extracts, we investigated the effects of Nb-(146–493) (1 μm) on the activities of striatal PP1γ1 and PP1β holoenzyme complexes isolated by immunoprecipitation. Immunoblots of the isolated complexes confirmed the isoform specificity of the immunoprecipitations (Fig. 5A). Both isoform complexes displayed activity toward [32P]phosphorylase a in the presence of 2.5 nm okadaic acid, which could be completely blocked by 2.5 μm okadaic acid, consistent with the specific immunoprecipitation of active PP1. Significantly, Nb-(146–493) (1 μm) almost completely blocked activity in PP1γ1 immunoprecipitates but had no significant effect on PP1β activity (Fig. 5A). Thus, Nb-(146–493) is a highly selective inhibitor of striatal PP1γ1 holoenzymes over PP1β holoenzymes.
We extended these findings by comparing the effects of Nb-(146–493) and other phosphatase inhibitors on the activities of PP1 holoenzymes present in whole striatal extracts using [32P]phosphorylase a as a model substrate. About 20% of the activity detected was inhibited by nanomolar concentrations of okadaic acid, and the remaining activity was almost completely inhibited by micromolar concentrations of okadaic acid, suggesting that total PP1 activity accounted for about 80% of [32P]phosphorylase a phosphatase activity (Fig. 5B). Consistent with this assignment, a PP1 inhibitor with no known isoform selectivity (inhibitor-2) blocked ≈75% of the total activity with an apparent EC50% ≈30 nm (Fig. 5B). In contrast, Nb-(146–493) inhibited a maximum of only ≈48% of the total activity at the highest concentration tested (5 μm), with an apparent EC50% of ≈190 nm. However, Nb-(146–453), a GST-Nb fusion protein lacking the PP1 binding domain, had no significant effect on striatal phosphatase activity. Thus, Nb-(146–493) only partially inhibits striatal PP1 activity, presumably because of the isoform selectivity of Nb-(146–493) toward endogenous holoenzymes (Fig. 5A). Nb-(146–493)-insensitive activity may be due to PP1β and PP2A holoenzymes, whereas PP1γ1 holoenzyme complexes likely account for the Nb-(146–493)-sensitive activity.
Dopamine Depletion Selectively Decreases Striatal PP1γ1 Activity—To determine the effect of dopamine depletion on the activity of different PP1 isoforms, [32P]phosphorylase a phosphatase activity in dorsolateral striatal extracts from 6-OHDA-lesioned rats was assayed in the absence and presence of 1 μm Nb-(146–493) (Fig. 6). Dopamine depletion significantly reduced total phosphatase activity in the whole extract by ≈17% in extracts collected 10–11 months after 6-OHDA lesion surgery to this cohort of animals. However, there was no significant difference in Nb-(146–493)-insensitive activities in samples from the two hemispheres; thus, the Nb-(146–493)-sensitive PP1 activity was reduced by ≈27% (Fig. 6A). However, parallel analyses of PP1 activities in dorsolateral striatum from both hemispheres of control (shamoperated) animals revealed no significant difference in total protein phosphatase activities or Nb-(146–493)-sensitive PP1 activity (Fig. 6B). Moreover, there was no difference in Nb-(146–493)-sensitive PP1 activities between striatal hemispheres collected from rats with incomplete dopamine depletions, as defined by <90% loss of tyrosine hydroxylase (normalized Nb-(146-493)-sensitive PP1 activities of 45.3 ± 6.7 and 53.4 ± 7.3, respectively, n = 5, p = 0.44). However, Nb-(146–493)-sensitive PP1 activity was significantly reduced in dopamine-depleted striatum collected from a second batch of rats 3–4 weeks after 6-OHDA lesion surgery (18 ± 6% reduction, p = 0.03; data not shown). Thus, the selective loss of PP1γ1 activity is manifest within 3–4 weeks of striatal dopamine depletion and is sustained for several months.
FIGURE 6.
Dopamine depletion selectively decreases striatal PP1γ1 activity. A, extracts of dorsolateral striatum ipsilateral (Lesion) or contralateral (Intact) to the 6-OHDA lesion collected 10–11 months following surgery were assayed using [32P]phosphorylase a in the absence or presence of 1 μm Nb-(146–493). Dopamine depletion significantly reduces total protein phosphatase activity by ≈17% (p = 0.04), but activity that is insensitive to Nb-(146–493) was not significantly different between the two hemispheres. Thus, dopamine depletion significantly reduced Nb-(146–493)-sensitive PP1 activity (i.e. PP1γ1) by ≈27% (p = 0.02). B, dorsolateral striatal extracts from both brain hemispheres of sham-operated rats were separately analyzed as in A. No significant differences in activities of any protein phosphatases were detected.
Dopamine Depletion Enhances PP1γ1 Association with Spinophilin—PP1γ1 is selectively associated with spinophilin in brain extracts, and spinophilin inhibits the activity of PP1γ1 in vitro (8). We investigated the interaction of PP1γ1 with spinophilin in control and dopamine-depleted dorsolateral striatal extracts by immunoprecipitation using anti-PP1γ1 or control IgG. Similar amounts of PP1γ1 were detected in immune complexes isolated from the two hemispheres, but no PP1γ1 was detected in control precipitations (Fig. 7). Quantitative analyses revealed a lesion:intact ratio that averaged 1.2 ± 0.5 (mean ± S.E., n = 7 animals) (Fig. 7), consistent with our findings that total levels of PP1γ1 are unaltered by dopamine depletion (28) (Fig. 4). Spinophilin was readily detected in PP1γ1 immune complexes but not in the control IgG samples (Fig. 7), confirming the specificity of co-immunoprecipitation seen previously (8, 12). Notably, levels of spinophilin were markedly higher in PP1γ1 immune complexes from dopamine-depleted tissue than in those from contralateral control tissue. The lesion:intact ratio for the amount of spinophilin in PP1γ1 immune complexes was 3.4 ± 0.8 (mean ± S.E., n = 7), significantly greater than the expected value of 1.0 if there was no change in the association of spinophilin with PP1γ1 (p = 0.029 by two-tailed, one sample t test) (Fig. 7).
FIGURE 7.
Dopamine depletion increases the association of PP1γ1 with spinophilin. Extracts of dorsolateral striatum ipsilateral (L) or contralateral (I) to the 6-OHDA lesion collected 3–7 months following surgery were immunoprecipitated using antibodies to spinophilin or PP1γ1 or control antibodies. Aliquots of the extract (Input) and the immunoprecipitates were immunoblotted for tyrosine hydroxylase (TH), spinophilin, and PP1γ1, as indicated. A >90% depletion of tyrosine hydroxylase in the dopamine-depleted hemisphere of this animal confirmed substantial degeneration of nigrostriatal dopaminergic neurons. Inputs from the two hemispheres contained comparable amounts of spinophilin and PP1γ1, and similar amounts of the cognate antigen were immunoprecipitated by each antibody from the two hemispheres. However, more spinophilin was detected in PP1γ1 immunoprecipitates from the lesioned hemisphere (L) than from the control (I) hemisphere. Similarly, more PP1γ1 was detected in the spinophilin immunoprecipitates from the lesioned hemisphere (L) than from the control (I) hemisphere. Quantitative analyses (right, plotted as mean ± S.E.) revealed statistically significant increases in the amount of spinophilin in PP1γ1 immune complexes (p = 0.029, n = 7 animals) and the amount of PP1γ1 in spinophilin immune complexes (p = 0.031, n = 7).
Separate aliquots of the control and dopamine-depleted striatal extracts were also immunoprecipitated with antibodies to spinophilin. Spinophilin was not detected in control immunoprecipitates, but similar levels were detected in spinophilin immune complexes from extracts of control or dopamine-depleted striatum (lesion:intact ratio = 1.6 ± 0.3, n = 7) (Fig. 7), consistent with our findings that total levels of spinophilin are unaltered by dopamine depletion (28). PP1β was not detected in spinophilin immune complexes (data not shown), consistent with our previous data (8, 12). However, PP1γ1 was present, and increased levels were detected in spinophilin immune complexes isolated from dopamine-depleted striatum than from control tissue (lesion:intact ratio = 5.7 ± 2.8, n = 7) (Fig. 7). This represents a significant increase over an expected value of 1.0 if this protein interaction is unaffected by dopamine depletion (p = 0.031 by Wilcoxon signed rank test) (Note: this test was used because these data did not pass the Shapiro-Wilk normality test.). In combination, these data show that dopamine depletion induced by the 6-OHDA lesion increases the interaction of spinophilin and PP1γ1 in the dorsolateral striatum.
DISCUSSION
Dopamine depletion has diverse effects on corticostriatal synaptic transmission and striatum-based behaviors in Parkinson disease and in parkinsonian animal models. The intracellular mechanisms underlying these effects are poorly understood, although altered protein phosphorylation in striatal MSNs is likely to be involved. Previous studies have linked changes in striatal protein phosphorylation following dopamine depletion to alterations in protein kinase activities (e.g. Refs. 29, 30, 40). However, protein phosphatases are believed to mediate many acute effects of dopamine in the striatum. It is widely accepted that dopamine acutely modulates PP1 activity and/or localization via phosphorylation/dephosphorylation of regulatory and targeting proteins such as DARPP-32 and spinophilin (33), although to the best of our knowledge changes in PP1 activity have not been directly demonstrated. We show here that chronic dopamine depletion significantly decreases striatal PP1 activity apparently because of regulation of the interaction between the PP1γ1 isoform and spinophilin. The decrease in PP1γ1 activity is observed within a few weeks of inducing dopamine depletion by 6-OHDA lesion surgery and is maintained for at least 11 months, with no evidence for progressive changes in PP1 activity over this time period. Decreased activity of this synaptically targeted PP1 isoform may be important in mediating chronic effects of dopamine depletion at corticostriatal synapses.
Dopamine Depletion Selectively Decreases PP1 Activity—Initial studies using model substrates showed that there was a significant reduction in phosphatase activity toward [32P]phosphorylase a but not toward [32P]casein (Fig. 1). Various concentrations of okadaic acid were used to confirm that PP1 activity was significantly decreased following dopamine depletion but that PP2A activity was unaffected (Fig. 2). Similar reductions in total PP1 activity were detected 3–4 weeks and 10–11 months after 6-OHDA lesion surgery (see “Results”). Moreover, PP1 activity toward the Thr286 autophosphorylation site in CaMKIIα (a physiologically relevant substrate in postsynaptic densities) was significantly decreased by dopamine depletion. Because PP1 is the major phosphatase responsible for dephosphorylation of PSD-associated CaMKII (3), the reduced PP1 activity may contribute to the enhanced autophosphorylation of striatal CaMKIIα at Thr286 following dopamine depletion (28, 29). Phosphorylation of a variety of synaptic receptors and other proteins is enhanced following dopamine depletion (see below), so it will be interesting to determine whether decreased PP1 activity also plays an important role in mediating these changes.
PP1γ1 Is Selectively Modulated Following Dopamine Depletion—The three major PP1 catalytic subunit isoforms present in the striatum (α, β, and γ1) are not known to differ in their fundamental enzymatic properties, but they are enriched in distinct subcellular compartments (7, 14, 41, 42). In particular, interactions of PP1γ1 with the actin-binding proteins spinophilin and neurabin are thought to be responsible for the selective localization of PP1γ1 in dendritic spines and at the PSD (8, 11, 14). Thus, PP1γ1 is poised to efficiently dephosphorylate synaptic substrates, such as CaMKII, and also to respond to activation of D1- and D2-like dopamine receptors that are localized on dendritic spine heads and necks.
In a new approach to selectively assess the contribution of PP1γ1 to total PP1 activity, we exploited a PP1-binding fragment of neurabin (Nb-(146–493)) that inhibits the purified PP1γ1 catalytic subunit more potently than it inhibits PP1β (12). We extended these findings by showing that Nb-(146–493) (1 μm) inhibits ≈90% of the activity of PP1γ1 holoenzymes immunoprecipitated from striatal extracts but does not inhibit immunoprecipitated PP1β holoenzymes (Fig. 5A). Moreover, Nb-(146–493) only partially inhibits the total PP1 activity in whole striatal extracts (Fig. 5B). The overall reduced inhibitory potency and enhanced selectivity of Nb-(146–493) in whole tissue extracts relative to our previous assays of purified catalytic subunits presumably reflects the fact that the added Nb-(146–493) fragment has to competitively displace endogenous regulatory subunits from the catalytic subunits in the extracts. Development of this tool allowed us to show that dopamine depletion has no effect on the phosphatase activity that is insensitive to Nb-(146–493) (Fig. 6), demonstrating that dopamine depletion selectively reduces the activity of the PP1 that is sensitive to Nb-(146–493), presumably the PP1γ1 isoform. Similar reductions in PP1γ1 activity were detected 3–4 weeks and 11 months after 6-OHDA lesion surgery.
We cannot formally exclude a contribution of PP1α to Nb-(146–493)-sensitive activity. Spinophilin and neurabin associate preferentially with PP1γ1 rather than PP1α (11), and PP1α is expressed in the striatum at ≈50% lower total levels than PP1γ1 (42, 43). In addition, PP1α and PP1γ1 are differentially distributed within dendritic spines, whereas PP1β is localized to dendritic shafts and the soma (14). Thus, it seems most likely that Nb-(146–493) preferentially inhibits PP1γ1. Incombination, our data demonstrate that dopamine depletion differentially affects the PP1 isoforms, apparently selectively reducing PP1γ1 activity. These observations represent the first direct evidence that activities of PP1 isoforms are differentially modulated in situ.
Mechanisms for Selective Regulation of Striatal PP1 Isoforms—PP1γ1 is selectively enriched in dendritic spines (associated with the postsynaptic density), as well as inconsistently (and to a lesser extent) in presynaptic terminals (14, 41). We considered the possibility that reduced activity of PP1γ1 following dopamine depletion directly resulted from the partial loss of spines containing PP1γ1 and other proteins from indirect pathway MSNs (18), or from the loss of dopaminergic terminals. However, it is likely that such an underlying mechanism would decrease total protein levels, in contrast to data showing no significant changes in levels of PP1γ1 (in whole extracts or in isolated PSD-enriched cytoskeletal fractions) or in total levels of several other postsynaptic or presynaptic proteins (Fig. 4) (28). Nevertheless, the reduced PP1γ1 activity may be associated with changes in its subcellular localization in specific subpopulations of striatal MSNs. Careful immunofluorescence and/or immunoelectron microscopic studies will be required to address this possibility. However, such studies would not address the activity in different compartments, and PP1γ1 activity may be altered without detectable changes in localization or vice versa.
We also considered the role of PP1γ1 interactions with other proteins that are known to play critical roles in mediating acute effects of dopamine signaling in the striatum. Most prominently, PP1 is inhibited by DARPP-32 that has been phosphorylated by cAMP-dependent protein kinase at Thr34 in response to D1 dopamine receptor activation. Phosphorylation of DARPP-32 at Thr75 in response to activation of D2 receptors interferes with Thr34 phosphorylation (reviewed in Ref. 33). However, there is no evidence that DARPP-32 is an isoform-selective modulator, and dopamine depletion has no significant effect on the total levels or Thr34 phosphorylation of DARPP-32, although Thr75 phosphorylation is substantially increased (25, 28, 44). Thus, DARPP-32 does not appear to be involved in selective suppression of PP1γ1 activity following dopamine depletion.
Most PP1 regulatory proteins are not known to exhibit isoform selectivity, and their roles in the striatum are poorly understood. Prominent exceptions are spinophilin and neurabin, related F-actin-binding proteins that are also localized to dendritic spines (reviewed in Ref. 45). Spinophilin and neurabin selectively interact with PP1γ1 (8, 11, 12) and are essential for dopaminergic modulation of corticostriatal synaptic plasticity (9, 15, 46). Chronic dopamine depletion does not change the total levels of spinophilin/neurabin or alter the amount of these proteins present in PSD-enriched cytoskeletal fractions (28). However, we show here that dopamine depletion increases the association of PP1γ1 with spinophilin (Fig. 7). Because spinophilin and neurabin selectively inhibit the activity of PP1γ1 catalytic subunits toward model substrates such as phosphorylase a, this increased interaction of PP1γ1 with spinophilin is the likely mechanism accounting for the decreased activity of PP1 detected in striatal lysates following dopamine depletion.
Implications of Altered Spinophilin-PP1γ1 Interaction Following Dopamine Depletion—Spinophilin and neurabin appear to function as classical scaffolding proteins with no intrinsic enzyme activity. Both proteins interact with a variety of signaling proteins in addition to PP1γ1. For example, spinophilin interacts with D2 dopamine receptors, p70 S6 kinase, and several small GTPase exchange factors that appear to pay key roles in regulating neuronal morphology (reviewed in Ref. 47). Moreover, alterations in spinophilin expression levels or overexpression of isolated domains from spinophilin results in altered neuronal morphology, including changes in dendritic spines. Interactions of spinophilin with F-actin are modulated by multiple protein kinases (46, 48), and some spinophilin-binding proteins are regulated by protein phosphorylation, including p70 S6 kinase and the rac GTPase exchange factor Tiam1 (49, 50). Moreover, PP1γ1 and p70 S6 kinase appear to bind neurabins in a competitive, mutually exclusive manner to modulate cell morphology (10). Although most previous studies focused on changes in neuronal morphology during relatively early postnatal development and/or using neuronal cultures, we suggest that the increased binding of PP1γ1 to spinophilin following dopamine depletion in the mature striatum reflects a re-organization of spinophilin signaling complexes, leading to altered protein phosphorylation/dephosphorylation. This reorganization might favor increased phosphorylation of PP1γ1 substrates, giving rise to diverse effects on striatal signaling and cell morphology. Indeed, dopamine depletion increases the phosphorylation of several known synaptic PP1 substrates, such as subunits of N-methyl-d-aspartic acid- and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate-type glutamate receptors and CaMKIIα (28–30, 51), which may be important in the disruptions of corticostriatal synaptic plasticity and behavior following dopamine depletion (23–26, 29, 30). However, it is possible that additional signaling proteins are either directly or indirectly affected by dopamine depletion, including kinases and other phosphatases.
Our observations in this animal model of parkinsonism are similar to emerging evidence for disruptions in the balance between kinase and phosphatase activities in other neurode-generative diseases or disease models, such as amyotrophic lateral sclerosis (52), multiple sclerosis (53), Charcot-Marie-Tooth disease (54), and Alzheimer disease (55). Perhaps most similarly, decreased protein phosphatase activity in a mouse model of Angelman mental retardation syndrome correlates with increased phosphorylation of hippocampal CaMKII and disruptions of synaptic plasticity, learning, and memory (56). Thus, we suggest that the development of strategies to increase the activity of critical protein phosphatases, including PP1γ1, might be a fruitful strategy to normalize protein phosphorylation and treat some of the symptoms of Parkinson disease, Angelman syndrome, and possibly other neurodegenerative diseases.
Final Summary—The present data demonstrate the specificity of cellular signaling via highly homologous PP1 isoforms in the striatum. Dopamine depletion selectively decreases the activity of the PP1γ1 isoform. Selective inhibition of a specific PP1 isoform that is precisely targeted in dendritic spines provides new insights into the long term changes in striatal signaling associated with Parkinson disease.
Acknowledgments
We thank Brian Wadzinski for PP1 isoform-specific antibodies, Pat Bauman for generation of GST fusion proteins, and Ariel Deutch, Danny Winder, and Leigh Carmody for critical comments.
This work was supported, in whole or in part, by National Institutes of Health Grants PO1-NS044282 and RO1-MH63232. This work was also supported by the Parkinson Foundation Center of Excellence at Vanderbilt University. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: CaMKII, calcium/calmodulin-dependent protein kinase II; 6-OHDA, 6-hydroxydopamine; GST, glutathione S-transferase; MSN, medium spiny neuron; PP1, protein phosphatase 1; PP2A, protein phosphatase 2A; HRP, horseradish peroxidase.
References
- 1.Lisman, J., Schulman, H., and Cline, H. (2002) Nat. Rev. Neurosci. 3 175–190 [DOI] [PubMed] [Google Scholar]
- 2.Strack, S., Choi, S., Lovinger, D. M., and Colbran, R. J. (1997) J. Biol. Chem. 272 13467–13470 [DOI] [PubMed] [Google Scholar]
- 3.Strack, S., Barban, M. A., Wadzinski, B. E., and Colbran, R. J. (1997) J. Neurochem. 68 2119–2128 [DOI] [PubMed] [Google Scholar]
- 4.Fukunaga, K., Muller, D., Ohmitsu, M., Bako, E., DePaoli-Roach, A. A., and Miyamoto, E. (2000) J. Neurochem. 74 807–817 [DOI] [PubMed] [Google Scholar]
- 5.Blitzer, R. D., Connor, J. H., Brown, G. P., Wong, T., Shenilikar, S., Iyengar, R., and Landau, E. M. (1998) Science 280 1940–1943 [DOI] [PubMed] [Google Scholar]
- 6.Brown, G. P., Blitzer, R. D., Connor, J. H., Wong, T., Shenolikar, S., Iyengar, R., and Landau, E. M. (2000) J. Neurosci. 20 7880–7887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strack, S., Kini, S., Ebner, F., Wadzinski, B. E., and Colbran, R. J. (1999) J. Comp. Neurol. 413 373–384 [PubMed] [Google Scholar]
- 8.MacMillan, L., Bass, M., Cheng, N., Howard, E., Tamura, M., Strack, S., Wadzinski, B. E., and Colbran, R. J. (1999) J. Biol. Chem. 274 35845–35854 [DOI] [PubMed] [Google Scholar]
- 9.Feng, J., Yan, Z., Ferreira, A., Tomizawa, K., Liauw, J. A., Zhuo, M., Allen, P. B., Ouimet, C. C., and Greengard, P. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 9287–9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver, C. J., Terry-Lorenzo, R. T., Elliott, E., Bloomer, W. A. C., Li, S., Brautigan, D. L., Colbran, R. J., and Shenolikar, S. (2002) Mol. Cell. Biol. 22 4690–4701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terry-Lorenzo, R. T., Carmody, L. C., Voltz, J. W., Connor, J. H., Li, S., Smith, F. D., Milgram, S. L., Colbran, R. J., and Shenolikar, S. (2002) J. Biol. Chem. 277 27716–27724 [DOI] [PubMed] [Google Scholar]
- 12.Carmody, L. C., Bauman, P. A., Bass, M. A., Mavila, N., DePaoli-Roach, A. A., and Colbran, R. J. (2004) J. Biol. Chem. 279 21714–21723 [DOI] [PubMed] [Google Scholar]
- 13.Terry-Lorenzo, R. T., Roadcap, D. W., Otsuka, T., Blanpied, T. A., Zamorano, P. L., Garner, C. C., Shenolikar, S., and Ehlers, M. D. (2005) Mol. Biol. Cell 16 2349–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bordelon, J. R., Smith, Y., Nairn, A., Colbran, R. J., Greengard, P., and Muly, E. C. (2005) Cereb. Cortex 15 1928–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen, P. B., Zachariou, V., Svenningsson, P., Lepore, A. C., Centonze, D., Costa, C., Rossi, S., Bender, G., Chen, G., and Feng, J. (2006) Neuroscience 140 897–911 [DOI] [PubMed] [Google Scholar]
- 16.Hu, X. D., Huang, Q., Roadcap, D. W., Shenolikar, S. S., and Xia, H. (2006) J. Neurochem. 98 1841–1851 [DOI] [PubMed] [Google Scholar]
- 17.Arbuthnott, G. W., Ingham, C. A., and Wickens, J. R. (2000) J. Anat. 196 587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day, M., Wang, Z., Ding, J., An, X., Ingham, C. A., Shering, A. F., Wokosin, D., Ilijic, E., Sun, Z., Sampson, A. R., Mugnaini, E., Deutch, A. Y., Sesack, S. R., Arbuthnott, G. W., and Surmeier, D. J. (2006) Nat. Neurosci. 9 251–259 [DOI] [PubMed] [Google Scholar]
- 19.Ingham, C. A., Hood, S. H., Taggart, P., and Arbuthnott, G. W. (1998) J. Neurosci. 18 4732–4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeill, T. H., Brown, S. A., Rafols, J. A., and Shoulson, I. (1988) Brain Res. 455 148–152 [DOI] [PubMed] [Google Scholar]
- 21.Meschul, C. K., Emre, N., Nakamura, C. M., Allen, C., Donohue, M. K., and Buckman, J. F. (1999) Neuroscience 88 1–16 [DOI] [PubMed] [Google Scholar]
- 22.Zaja-Milatovic, S., Schantz, A., Zhang, J., Montine, K. S., Samii, A., Deutch, A. Y., and Montine, T. J. (2005) Neurology 64 545–547 [DOI] [PubMed] [Google Scholar]
- 23.Centonze, D., Gubellini, P., Picconi, B., Calabresi, P., Giacomini, P., and Bernardi, G. (1999) J. Neurophysiol. 82 3575–3579 [DOI] [PubMed] [Google Scholar]
- 24.Centonze, D., Picconi, B., Gubellini, P., Bernardi, G., and Calabresi, P. (2001) Eur. J. Neurosci. 13 1071–1077 [DOI] [PubMed] [Google Scholar]
- 25.Picconi, B., Centzone, D., Hakansson, K., Bernardi, G., Greengard, P., Fisone, G., Cenci, M. A., and Calabresi, P. (2003) Nat. Neurosci. 6 501–505 [DOI] [PubMed] [Google Scholar]
- 26.Norman, E. D., Egli, R. E., Colbran, R. J., and Winder, D. G. (2005) Neuropharmacol. 48 311–321 [DOI] [PubMed] [Google Scholar]
- 27.Cenci, M. A., Whishaw, I. Q., and Schallert, T. (2002) Nat. Rev. Neurosci. 3 574–579 [DOI] [PubMed] [Google Scholar]
- 28.Brown, A. M., Deutch, A. Y., and Colbran, R. J. (2005) Eur. J. Neurosci. 22 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Picconi, B., Gardoni, F., Centzone, D., Mauceri, D., Cenci, M. A., Bernardi, G., Calabresi, P., and DiLuca, M. (2004) J. Neurosci. 24 5283–5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh, J. D., Vaughan, C. L., and Chase, T. N. (1999) Brain Res. 821 433–442 [DOI] [PubMed] [Google Scholar]
- 31.Jonkers, N., Sarre, S., Ebinger, G., and Michotte, Y. (2002) Brain Res. 926 149–155 [DOI] [PubMed] [Google Scholar]
- 32.Pisani, A., Centonze, D., Bernardi, G., and Calabresi, P. (2005) Movement Disorders 24 395–402 [DOI] [PubMed] [Google Scholar]
- 33.Svenningsson, P., Nishi, A., Fisone, G., Girault, J. A., Nairn, A. C., and Greengard, P. (2004) Annu. Rev. Pharmacol. Toxicol. 44 269–296 [DOI] [PubMed] [Google Scholar]
- 34.Yan, Z., Hsieh-Wilson, L., Feng, J., Tomizawa, K., Allen, P. B., Fienberg, A. A., Nairn, A. C., and Greengard, P. (1999) Nat. Neurosci. 2 13–17 [DOI] [PubMed] [Google Scholar]
- 35.Wu, L. J., Ren, M., Wang, H., Kim, S. S., Cao, X., and Zhuo, M. (2008) Plos ONE 3 e1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colbran, R. J., Bass, M. A., McNeill, R. B., Bollen, M., Zhao, S., Wadzinski, B. E., and Strack, S. (1997) J. Neurochem. 69 920–929 [DOI] [PubMed] [Google Scholar]
- 37.Colbran, R., Carmody, L., Bauman, P., Wadzinski, B., and Bass, M. (2003) Methods Enzymol. 366 156–175 [DOI] [PubMed] [Google Scholar]
- 38.Carmody, L. C., Baucum, A. J., II, Bass, M. A., and Colbran, R. J. (2008) FASEB J., in press [DOI] [PMC free article] [PubMed]
- 39.Cohen, P. (1991) Methods Enzymol. 201 389–398 [DOI] [PubMed] [Google Scholar]
- 40.Westin, J. E., Vercammen, L., Strome, E. M., Konradi, C., and Cenci, M. A. (2007) Biol. Psychiatry 62 800–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouimet, C. C., da Cruz e Silva, E. F., and Greengard, P. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 3396–3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.da Cruz E Silva, E. F., Fox, C. A., Ouimet, C. C., Gustafson, E., Watson, S. J., and Greengard, P. (1995) J. Neurosci. 15 3375–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shima, H., Hatano, Y., Chun, Y., Sugimura, T., Zhang, Z., Lee, E. Y. C., and Nagao, M. (1993) Biochem. Biophys. Res. Commun. 192 1289–1296 [DOI] [PubMed] [Google Scholar]
- 44.Chergui, K., Svenningson, P., and Greengard, P. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 2191–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen, P. B. (2004) Parkinsonism & Related Disorders 10 287–292 [DOI] [PubMed] [Google Scholar]
- 46.Hsieh-Wilson, L. C., Benfenati, F., Snyder, G. L., Allen, P. B., Nairn, A. C., and Greengard, P. (2003) J. Biol. Chem. 278 1186–1194 [DOI] [PubMed] [Google Scholar]
- 47.Sarrouilhe, D., di Tommaso, A., Metaye, T., and Ladeveze, V. (2006) Biochimie (Paris) 88 1099–1113 [DOI] [PubMed] [Google Scholar]
- 48.Grossman, S. D., Futter, M., Snyder, G. L., Allen, P. B., Nairn, A. C., Greengard, P., and Hsieh-Wilson, L. C. (2004) J. Neurochem. 90 317–324 [DOI] [PubMed] [Google Scholar]
- 49.Fleming, I. N., Elliott, C. M., Buchanan, F. G., Downes, C. P., and Exton, J. H. (1999) J. Biol. Chem. 274 12753–12758 [DOI] [PubMed] [Google Scholar]
- 50.Buchsbaum, R. J., Connolly, B. A., and Feig, L. A. (2003) J. Biol. Chem. 278 18833–18841 [DOI] [PubMed] [Google Scholar]
- 51.Dunah, A. W., Wang, Y., Yasuda, R. P., Kameyama, K., Huganir, R. L., Wolfe, B. B., and Standaert, D. G. (2000) Mol. Pharmacol. 57 342–352 [PubMed] [Google Scholar]
- 52.Strong, M. J., Kesavapany, S., and Pant, H. C. (2005) J. Neuropathol. Exp. Neurol. 64 649–664 [DOI] [PubMed] [Google Scholar]
- 53.Schneider, A., Araujo, G. W., Trajkovic, K., Herrmann, M. M., Merkler, D., Mandelkow, E., Weisser, R., and Simons, M. (2004) J. Biol. Chem. 279 55833–55839 [DOI] [PubMed] [Google Scholar]
- 54.Begley, M. J., and Dixon, J. E. (2005) Curr. Opin. Struct. Biol. 15 614–620 [DOI] [PubMed] [Google Scholar]
- 55.Liu, F., Grundke-Iqbal, I., Iqbal, K., and Gong, C. (2005) Eur. J. Neurosci. 22 1942–1950 [DOI] [PubMed] [Google Scholar]
- 56.Weeber, E. J., Jiang, Y. H., Elgersma, Y., Varga, A. W., Carrasquillo, Y., Brown, S. E., Christian, J. M., Mirnikjoo, B., Silva, A., Beaudet, A. L., and Sweatt, J. D. (2003) J. Neurosci. 23 2634–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]