Abstract
In this study, we have used the PC12 cell model to elucidate the mechanisms by which sublethal doses of oxidants induce neuritogenesis. The xanthine/xanthine oxidase (X/XO) system was used for the steady state generation of superoxide, and CoCl2 was used as a representative transition metal redox catalyst. Upon treatment of purified protein kinase C (PKC) with these oxidants, there was an increase in its cofactor-independent activation. Redox-active cobalt competed with the redoxinert zinc present in the zinc-thiolates of the PKC regulatory domain and induced the oxidation of these cysteine-rich regions. Both CoCl2 and X/XO induced neurite outgrowth in PC12 cells, as determined by an overexpression of neuronal marker genes. Furthermore, these oxidants induced a translocation of PKC from cytosol to membrane and subsequent conversion of PKC to a cofactor-independent form. Isoenzyme-specific PKC inhibitors demonstrated that PKCε plays a crucial role in neuritogenesis. Moreover, oxidant-induced neurite outgrowth was increased with a conditional overexpression of PKCε and decreased with its knock-out by small interfering RNA. Parallel with PKC activation, an increase in phosphorylation of the growth-associated neuronal protein GAP-43 at Ser41 was observed. Additionally, there was a sustained activation of extracellular signal-regulated kinases 1 and 2, which was correlated with activating phosphorylation (Ser133) of cAMP-responsive element-binding protein. All of these signaling events that are causally linked to neuritogenesis were blocked by antioxidant N-acetylcysteine (both l and d-forms) and by a variety of PKC-specific inhibitors. Taken together, these results strongly suggest that sublethal doses of oxidants induce neuritogenesis via a direct redox activation of PKCε.
Understanding the signaling mechanisms involved in neuritogenesis resulting from a compensatory response to injury is crucial to the development of therapeutic agents for recovery after spinal cord and traumatic brain injuries (1, 2). The study of neuritogenesis requires the identification of molecular targets using a suitable experimental model. One of the best characterized cellular models for studying the neuronal pathways involved in neuritogenesis is the rat pheochromocytoma cell line PC12 (3). This cell line continues to be an important model system for the study of cell signaling mechanisms induced by a variety of stimuli, including neurotrophins, hormones, and oxidants (4, 5).
In addition to damage from mechanical forces, spinal cord and traumatic brain injuries may result from secondary mechanisms involving ischemia, excitotoxicity, cytokines, and an infiltration of neutrophils at the site of injury (6, 7). The inflammatory response results in the generation of reactive oxygen species (ROS),2 including superoxide and hydrogen peroxide, which can cause oxidative damage to tissues and lead to cell death (8). Paradoxically, sublethal doses of oxidants can also induce a variety of cellular processes, including cell growth, adhesion, invasion, and differentiation (9–11). Importantly, ROS are involved in the action of a variety of growth factors and neurotrophins, including nerve growth factor (NGF), which supports neuronal survival during development and promotes axonal regeneration after neuronal injury (12–15).
NGF induces neuritogenesis in PC12 cells by binding to and activating receptor-associated tyrosine kinase, TrkA (4, 5, 16). The associated signaling pathway leads to the activation of B-Raf (via Ras and Rap1), which is coupled to the activation of mitogen-activated protein kinase (MAPK) kinase. This activation leads to a rapid and sustained activation of extracellular signal-regulated kinases (ERKs). The activation of ERKs is both necessary and sufficient for neurite outgrowth in PC12 cells (17). Furthermore, TrkA activation is also coupled to phospholipase Cγ activation, which in turn leads to protein kinase C (PKC) activation (18). This is relevant, since PKC also contributes to the activation of ERKs via Raf. Many different stimuli act on PC12 cells to induce signaling pathways that may converge in the sustained activation of the MAPK pathway. This ultimately leads to phosphorylation-mediated activation of cAMP-responsive element-binding protein (CREB), which induces a set of genes involved in neuritogenesis (19). Although it has been established that NGF mediates its action through this protein kinase cascade, it is not known which protein kinases are regulated by sublethal doses of oxidants to induce neuritogenesis.
Various studies have shown an important role for PKC in neuritogenesis (20–22). Furthermore, PKC is also a unique target for oxidants (23). Depending on the type of oxidant, the site of oxidation, and the extent of modification, PKC can be either activated or inactivated by oxidation (24). The regulatory domain of PKC contains the C1 module with a cysteine-rich region coordinating four atoms of zinc (25). Selective modification of this autoinhibitory domain is achieved by treatment with a low concentration of oxidants and results in a cofactor-independent activation of PKC (23, 24). Alternatively, modification of the cysteine residues present within the catalytic domain is achieved by treatment with a higher concentration of oxidants and results in the inactivation of PKC. Since PKC is a family of more than 11 phospholipid-dependent serine/threonine protein kinases with variation in structure (26–28), there may be a difference in susceptibility to oxidants among the various isoenzymes.
The PKC isoenzymes are divided into three categories based upon the cofactors that are required for optimal catalytic activity (26–28). Conventional PKCs (α, β, and γ) are calcium-dependent and are stimulated by a second messenger, diacylglycerol. Novel PKCs (δ, ε, ç, and θ) are also activated by diacylglycerol but are calcium-independent. Atypical PKCs (ζ and λ/ι) require neither calcium nor diacylglycerol for optimal activity. Although most cells express more than one type of PKC, differences among the isoenzymes with respect to activation conditions and subcellular locations suggest that individual PKC isoenzymes mediate distinct cellular processes in a cell type-dependent manner (26–28). Therefore, it is important to identify the isoenzymes that are involved in oxidant-induced neuritogenesis, particularly in the phosphorylation-mediated regulation of growth-associated proteins that are intimately involved in this process.
The growth-associated protein GAP-43 (also known as neuromodulin or B-50) is abundant in developing and regenerating neurons and is believed to function in neuronal plasticity (29). The gene expression of GAP-43 is increased as a compensatory response in corticostriatal neurons after cortical lesion (30). In addition, synthesis of GAP-43 is enhanced by stabilization of its mRNA by a PKC-dependent pathway (31). GAP-43 is also a highly specific substrate for PKC (32). The PKC-mediated phosphorylation of GAP-43 has the ability to directly influence the structure of the actin cytoskeleton within the growth cone (33). Nevertheless, the mechanisms by which PKC-activating oxidants might influence the phosphorylation and function of GAP-43 in neurite outgrowth have not been previously described.
In this study, using the PC12 cell model, we show that the model oxidants COCl2 and xanthine/xanthine oxidase (X/XO) activate PKC by inducing the oxidative modification of zinc-thiolates present in its regulatory domain. Additionally, we show that oxidant-induced direct regulation of PKC (particularly PKCε) induces neuritogenesis through a sustained activation of the MAPK pathway and the phosphorylation of GAP-43 and CREB.
EXPERIMENTAL PROCEDURES
Materials—5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB), protein kinase A, phosphorylase kinase, xanthine oxidase, xanthine, N-acetyl-l-cysteine (NAC), aprotinin, leupeptin, dithiothreitol (DTT), pepstatin A, and monoclonal anti-GAP-43 antibodies were from Sigma. Myristoylated polypeptide PKC inhibitor residues 20–28, rottlerin, bisindolylmaleimide (BIM), bisindolylmaleimide V (BIM V), and PD98059 were from Alexis Biochemicals. PKCε translocation inhibitor and G418 were from Calbiochem. Mouse NGF was from Upstate Biotechnology, Inc. Anti-MAPK ERK1/2 antibodies and Anti-phosphoMAPK ERK1/2 (phospho-Thr202/Tyr204) antibodies were from New England Biolabs. Anti-phospho-Ser41-GAP-43 antibodies were from PhosphoSolutions. Phospho-CREB (Ser133) antibodies were from Cell Signaling Technology. Anti-CREB antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). N-Acetyl-d-cysteine was from Research Organics. [20-3H]phorbol 12,13-dibutyrate (specific activity 20 Ci/mmol) was from PerkinElmer Life Sciences, and [γ-32P]ATP (specific activity 20 Ci/mmol) was from ICN.
PKC isoenzymes α, β, γ, δ, and ε were partially purified from rat brains (24, 34, 35). In some cases, a mixture of Ca2+-dependent isoenzymes (α, β, and γ) was used, since these isoenzymes can be purified to homogeneity in higher amounts by Ca2+-dependent hydrophobic chromatography (24). The catalytic and regulatory domains of PKC isoenzymes (α, β, and γ) were separated by trypsin treatment and chromatographically isolated (36). Rabbit polyclonal antibodies against PKC isoenzymes were raised by injecting hemocyanin coupled with sequence-specific peptides from the variable regions in PKC isoenzymes using published procedures (37). These peptides and a PKC-specific substrate peptide corresponding to a neurogranin amino acid sequence (residues 25–43) were synthesized at the core facility of Norris Comprehensive Cancer Center.
Cell Culture and Treatments—PC12 cells, originally obtained from Dr. Christine Pike (University of Southern California), were grown in RPMI medium supplemented with 10% heat-inactivated horse serum, 5% fetal calf serum, 50 units/ml penicillin, and 0.05 mg/ml streptomycin. We used CoCl2 (50–150 μm) as a representative transition metal redox catalyst, and the X/XO system, composed of 0.25 mm xanthine and 1–5 milliunits/ml xanthine oxidase (Sigma), as a model for the steady state generation of oxidants (10). Inactive analogs of inhibitors were used as controls for inhibitors. When agents were dissolved in organic solvents, appropriate solvent controls were used.
Quantitative RT-PCR for Analyzing the Expression of Neuronal Marker Genes—Total RNA was extracted with TRIzol reagent (Invitrogen) and chloroform, precipitated with isopropyl alcohol, washed with 75% ethanol, and then dissolved in RNase-free water. Isolated RNA was then DNase-treated (Qiagen, Valencia, CA) and cleaned using a Qiagen RNeasy kit. RNA was quantified using the BioPhotometer (Eppendorf) and used for quantitative real time PCR (qRT-PCR) analysis of the mRNA for neurofilament-L, GAP-43, and SCG10 (superior cervical ganglion-10). qRT-PCR analysis was performed with the MyiQ single-color real time PCR detection system (Bio-Rad) using SYBR Green PCR Master Mix (Bio-Rad) according to the manufacturer's protocol. The amplification protocol consisted of one cycle at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The dissociation curve protocol was performed at the end of the amplification to confirm a single peak near the calculated melting temperature of each amplicon. All amplifications were run in triplicate. A standard curve of cycle thresholds using serial dilutions of cDNA samples was used to calculate the relative abundance. The difference in the initial amount of total RNA between the samples was normalized in every assay using a glyceraldehyde-3-phosphate dehydrogenase gene expression as an internal standard. Forward and reverse PCR primers, synthesized by Integrated DNA Technologies (Coralville, IA), consisted of the following: glyceraldehyde-3-phosphate dehydrogenase forward primer (5′-TGCACCACCAACTGCTTAG-3′) and reverse primer (5′-GGATGCAGGGATGATGTTC-3′), neurofilament-L forward primer (5′-TAGCGCCATGACGGGACACAATC-3′) and reverse primer (5′-TCTTCCTGGACGTGGCTGGTAT-3′), GAP-43 forward primer (5′-GATGGTGTCAAACCGGAGGAT-3′) and reverse primer (5′-CTTGTTATGTGTCCACGGAAGC-3′), and SCG-10 forward primer (5′-AGAAAGAGATGAATGGGAAGACAGA-3′) and reverse primer (5′-CGGCCCATTAGAAGGTTCAG-3′).
Immunofluorescence Staining of β-Tubulin III—PC12 cells were seeded at a low density on polylysine-coated culture slides (BD Biosciences, Bedford, MA) and were grown for 24 h. The cells were then treated with 150 μm CoCl2 or the X/XO system for 2 days. Cells were fixed with 4% paraformaldehyde in phosphate-buffered saline for 20 min were then washed three times in phosphate-buffered saline and subsequently permeabilized with 0.1% Triton X-100 for 30 min. Cells were blocked in 5% goat serum before incubation with β-tubulin III monoclonal antibody (1:200 dilution; Covance, Emeryville, CA) at 37 °C for 1 h. The cells were washed three times and incubated with Alexa Fluor 488 goat anti-mouse IgG secondary antibody (Invitrogen) for 1 h at room temperature. Cells were mounted with Vectashield (Vector Laboratories, Burlingame, CA) containing 4′,6-diamidino-2-phenylindole for nuclear staining and viewed on an LSM 510 laser-scanning microscope (Carl Zeiss, Thornwood, NY). No fluorescence was detected when the primary antibody was omitted.
Quantitation of Neurite Outgrowth—PC12 cells were plated in polylysine-coated 24-well plates at a density of 1 × 104 cells/ml in a growth medium. After 16–24 h, cells were treated with CoCl2, X/XO, NGF, or other agents either alone or in combination for 4 days. The cells were then scored for the presence of neurites (10, 22). For each treatment, 200 cells in each of three separate fields were scored. Cells with outgrowths longer than two diameters of the cell body were scored positive for neurites and were expressed as a percentage of the total cell number.
PKC Assay—The assays of PKC as well as other protein kinases were carried out in 96-well plates with fitted filtration discs made of Durapore membrane (38). Briefly, PKC reaction samples containing 20 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 0.33 mm CaCl2, 0.1 mm [γ-32P]ATP (3 million cpm), histone H1 (0.1 mg/ml), 40 μm leupeptin, and 25 μl of PKC sample in a total volume of 125 μl were incubated at 30 °C for 5 min. After arresting the reaction with trichloroacetic acid and ultrafiltration, the radioactivity associated with the protein retained on the membrane was determined. In certain cases, histone H1 was replaced with 5 μM neurogranin substrate peptide (residues 25–43), and the same reactions were carried out in regular 96-well plates without filtration discs. The reaction was arrested with 10 μl of 1 m phosphoric acid, the samples were applied to Whatman P81 paper (2 × 2 cm), and the papers were washed four times with 75 mm phosphoric acid. Radioactivity retained in the washed paper was counted. PKC activity was expressed in units, where 1 unit of enzyme transfers 1 nmol of phosphate to histone H1 or neurogranin per min at 30 °C.
Since a PKC assay using histone H1 as a substrate is relatively simple, it was used for determining the activity of conventional PKC isoenzymes (α, β, and γ). Histone H1, however, is not a good substrate for measuring the activity of novel and atypical isoenzymes. On the other hand, the neurogranin substrate peptide is appropriate for determining the phosphotransferase activity of all PKC isoenzymes even in the presence of protein phosphatases (39). Therefore, we used this substrate for determining the activity of all PKC isoenzymes in cell extracts. Furthermore, there is significant homology between the phosphorylation domain of neurogranin and that of GAP-43 (39). Therefore, PKC assay using the neurogranin substrate peptide is an appropriate means for determining PKC activity in relation to GAP-43 phosphorylation.
Phorbol Ester Binding—Phorbol ester binding to isolated PKC was carried out by a multiwell filtration approach using [3H]phorbol 12,13-dibutyrate (PDBu) as a ligand (38). To determine phorbol ester binding in intact cells, PC12 cells were grown in polylysine-coated 6-well plates. The medium was changed to a serum-free medium, and then cells were treated with various concentrations of CoCl2 for 90 min. Then 37.5 nm [3H]PDBu (0.25 mCi) was added to the medium. To determine nonspecific binding, 10 μm unlabeled PDBu was included with radiolabel. After incubation for 30 min, cells were washed four times with ice-cold saline and lysed with 0.2 m NaOH. The radioactivity present in the cell extract was then determined. The specific binding was calculated by subtracting the nonspecific binding from the observed total binding (38).
Isolation of PKC from Cells Treated with CoCl2 or X/XO— Confluent PC12 cells in a serum-free medium were treated with CoCl2 or X/XO oxidase system for 15–120 min. From the oxidant-treated cells, both cytosol and detergent-solubilized membrane fractions were prepared (24). Unless otherwise indicated, mercapto compounds were omitted from all of the buffers used for cell homogenization and chromatographic isolation of PKC. The cell extracts were subjected to DEAE-cellulose chromatography, as described previously (24). PKC isoenzymes were eluted with 0.1 m NaCl (peak 1), and the constitutively active forms of isoenzymes were eluted with 0.25 m NaCl (peak 2).
Quantitation of Cysteine Sulfhydryls in CoCl2-modified PKC—We used a mixture of PKC isoenzymes (α, β, and γ), which can be purified in large amounts. Highly purified PKC (0.5 nmol) free from thiol agents was incubated in triplicates with various amounts of CoCl2 (0.25–10 mol/mol of PKC) in 100 μl of 20 mm Tris-HCl (pH 7.5) and 1 mm CaCl2 for 25 min at 30 °C. The excess CoCl2 was removed by centrifuge gel filtration using Bio-Spin 6 (Bio-Rad) columns, and then the samples were transferred to a 96-well plate (40). Accessible sulfhydryls remaining after oxidation were quantitated by a modified DTNB method (36). To each well, 50 μl of 1.2 mm DTNB was added, and the absorbance was read at 405 nm. To measure the total titratable sulfhydryls present in PKC, 50 μl of 10% SDS was added to all of these samples, and the absorbance at 405 nm was read again after 5 min. NAC was used as the standard for sulfhydryl quantitation.
Western Blotting Analysis of Proteins—Cells were serum-starved overnight and treated with CoCl2. The cell pellet collected by centrifugation was resuspended in a cold lysis buffer consisting of 20 mm Tris-HCl (pH 7.4), 0.5% sodium deoxycholate, 2% Igepal CA-630, 0.2% SDS, 1 mm phenylmethylsulfonyl fluoride, 50 μg/ml aprotinin, 50 μm leupeptin, 150 nm pepstain A, and 0.5 mm sodium vanadate. The cell lysates were centrifuged at 10,000 × g for 10 min. The supernatants, after adding electrophoresis sample buffer, were subjected to SDS-polyacrylamide gel electrophoresis.
Electrophoretically separated proteins were transferred to a polyvinylidene fluoride membrane. The membranes were blocked with 5% dry milk and subsequently incubated with the indicated primary antibodies, as described below. Specific reactive bands were detected using goat anti-rabbit or goat anti-mouse secondary antibodies conjugated with horseradish peroxidase. The immunoreactive bands were visualized by the enhanced chemiluminescence Western blot detection kit (Amersham Biosciences). These bands were analyzed by densitometric scanning using Scion Imaging software.
Western Immunoblotting for PKC Isoenzymes—Both cytosol and membrane fractions were subjected to electrophoresis and blotted as described above. PKC isoenzymes in Western blots were detected using rabbit polyclonal antibodies. The specificity of the antibody for each PKC isoenzyme was determined by blocking immunoreactive staining with the corresponding peptide used for raising antiserum.
GAP-43 Phosphorylation in Intact Cells—PKC-mediated phosphorylation of GAP-43 at Ser41 was determined with rabbit anti-phospho-Ser41-GAP-43 antibodies. Total GAP-43 (phosphorylated and unphosphorylated) was determined using mouse monoclonal anti-GAP-43 antibodies. The total GAP-43 staining served to confirm equal loading of protein in all electrophoretic lanes.
Measuring Activation of MAPKs ERK1/2—Activation of MAPK was determined by Western immunoblotting using anti-phospho-MAPK ERK1/2 (phospho-Thr202/Tyr204) rabbit antibodies. Total ERK1/2 (unphosphorylated and phosphorylated forms) was determined using anti-MAPK ERK1/2 antibodies, which served to confirm equal loading of protein in all electrophoretic lanes.
Activating Phosphorylation of CREB—Phosphorylation of CREB(Ser133) was determined by Western immunoblotting using rabbit polyclonal phospho-CREB(Ser133) antibodies. Total CREB was determined using rabbit polyclonal antibodies to CREB. This staining served to confirm equal loading of protein in all electrophoretic lanes.
Stable Transfection of PKCε—The metallothionein expression vector (41) used in these experiments was a kind gift from Wayne Anderson (NCI, National Institutes of Health, Bethesda, MD). The cells were transfected with either a metallothionein-driven PKCε expression vector (to overexpress PKCε) or an empty vector (as a control) using Lipofectamine 2000 according to the manufacturer's recommended procedure. One day after transfection, the cells were plated at a lower density and grown in a selection medium containing 450 μg/ml G418. After 4 weeks in the selection medium, single colonies were picked, expanded, and screened for the presence of PKCε using Western blot analysis. Cadmium chloride was used for the optimal expression of PKCε in these transfectants.
Transient Transfection of PC12 Cells with PKCε siRNA— PC12 cells (1 × 104/ml) were plated in a 6-well plate. After 24 h, 50 nm PKCε siRNA oligonucleotides (three predesigned Silencer oligonucleotides from Ambion) were transfected into PC12 cells with Lipofectamine 2000 according to the manufacturer's instructions. As a negative control, we used scrambled siRNA that did not exhibit homology to any encoding region but had similar GC content. The efficiency of transfection and knock-out of PKCε was determined by Western immunoblotting.
Quantitation of GSH—Cells from 100-mm Petri dishes were homogenized in 1.5 ml of 4% sulfosalicylic acid and centrifuged at 13,000 × g for 10 min. The protein pellet was dissolved in 0.5 ml of 1 m NaOH, and the protein was quantitated with the dye method (42). From the supernatant, GSH was quantitated using the enzymatic recycling assay employing glutathione reductase (43). GSSG was quantitated in the same way after conjugating GSH with 2-vinylpyridine and was expressed as GSH equivalents as previously described (43).
RESULTS
In order to understand the molecular mechanisms involved in oxidant-induced neurite outgrowth, two representative oxidants previously known to induce neurite outgrowth were selected. X/XO was used as a model for the steady state generation of superoxide radicals, which have been implicated in neuronal injury, and CoCl2 was used as a representative transition metal redox catalyst that can induce protein oxidations in the presence of molecular oxygen. The use of CoCl2 is a pharmacological approach that permits the identification and characterization of proteins that are specifically oxidized at metal-binding sites by a “cage” type reaction shielded from cytosolic GSH (44). Conversely, due to variations in the rate of generation of ROS (superoxide) by X/XO, quantitative changes are difficult to control when using this system. Therefore, unless otherwise mentioned, only the results obtained with CoCl2 are presented. When appropriate, the data obtained with X/XO are presented as well.
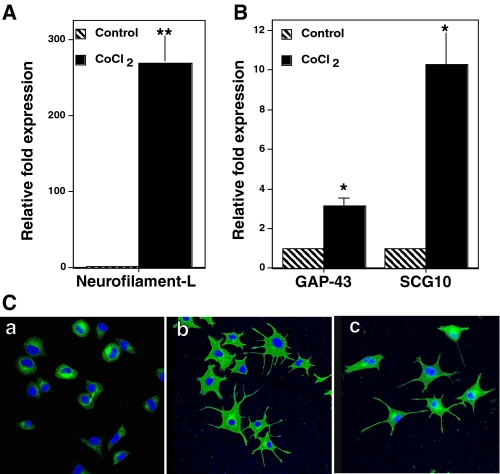
Characterization of Cellular Phenotype Induced by Oxidants—In order to verify that the morphological changes that occurred upon treatment with oxidants were indeed neuronal differentiation, we performed the following experiments. First, we measured the expression of neuronal marker genes (neurofilament-L, GAP-43, and SCG10) by determining their mRNA levels. A severalfold increase in the expression of these neuronal marker genes was observed in PC12 cells treated with CoCl2 or X/XO for 24 h (Fig. 1, A and B). This provides strong evidence that the morphological changes observed with oxidant treatment were indeed due to the neuronal differentiation. Second, we carried out immunofluorescence staining for neuron-specific β-tubulin III in the PC12 cells treated with oxidants. The fluorescence staining intensity increased in the cell bodies as well as the threadlike processes that were induced by oxidants (Fig. 1C). These observations further support the fact that the CoCl2- or X/XO-induced threadlike processes in PC12 cells are indeed neurites.
FIGURE 1.
Induction of neuronal markers in PC12 cells treated with oxidants. A and B, analysis of neuronal marker genes by qRT-PCR. PC12 cells were grown in polylysine-coated 100-mm Petri dishes and treated with 150 μm CoCl2 for 24 h. Total RNA was extracted from the CoCl2-treated cells and control cells and used for qRT-PCR analysis of the mRNAs for neurofilament-L, GAP-43, SCG10, and glyceraldehyde-3-phosphate dehydrogenase. qRT-PCR reading for each sample was normalized to that of glyceraldehyde-3-phosphate dehydrogenase, and the results are shown as the mean ± S.E. for three independent experiments. The values obtained with CoCl2 were compared with the respective control values (which was considered as 1) by the paired t test (*, p < 0.05; **, p < 0.01). C, immunofluorescence staining ofβ-tubulin III. PC12 cells were grown on polylysine-coated culture slides and treated with CoCl2 or the X/XO system for 2 days. Cells were fixed, permeabilized, and incubated with β-tubulin III monoclonal antibody followed by Alexa Fluor 488-conjugated secondary antibody (green). Cells were viewed on a confocal microscope. The nuclei have been stained blue by 4′,6-diamidino-2-phenylindole. a, control untreated cells; b, CoCl2; c, X/XO system.
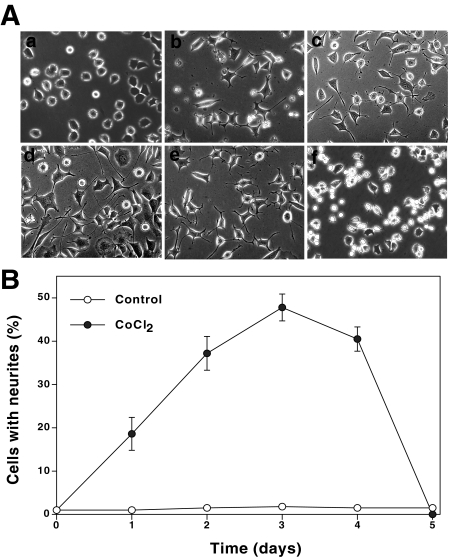
Oxidants Induce Neurite Outgrowth—As shown in Figs. 2 and 3, neurite outgrowth was observed in up to 40–45% of PC12 cells treated with moderate concentrations (50–150 μm) of CoCl2 or low amounts (1 milliunit) of xanthine oxidase in the X/XO system. This is comparable with that of NGF-induced neurite outgrowth in a complete growth medium (high serum). The neurite outgrowth was followed for only 3 or 4 days, since longer treatments with the oxidants caused rounding of cells and cell death (Fig. 2, A and B). In contrast to these moderate concentrations, higher concentrations (>500 μm) of CoCl2 or higher amounts (25 milliunits) of xanthine oxidase induced cell death without the formation of neurites. Conceivably, oxidants induce a concentration-dependent bidirectional response with respect to neuritogenesis.
FIGURE 2.
Time course of induction of morphological changes and neurite outgrowth in PC12 cells treated with CoCl2. A, morphological changes in PC12 cells treated with CoCl2. PC12 cells were seeded at a low density (1 × 104 cells/ml) on polylysine-coated 24-well plates in a complete growth medium. Cells were treated with CoCl2 (150 μm) for 5 days. The morphological changes and neurite outgrowth were followed every day. a, control untreated cells, 1 day; b, CoCl2-treated, 1 day; c, CoCl2-treated, 2 days; d, CoCl2-treated, 3 days; e, CoCl2-treated, 4 days; f, CoCl2-treated 5 days. B, time course of neuritogenesis in CoCl2-treated PC12 cells. Low density cells were treated with CoCl2 (150 μm) for the indicated time periods; cells with neurites were counted and expressed as the percentage of the total cell number. Each value is the mean ± S.E. obtained from three independent experiments.
FIGURE 3.
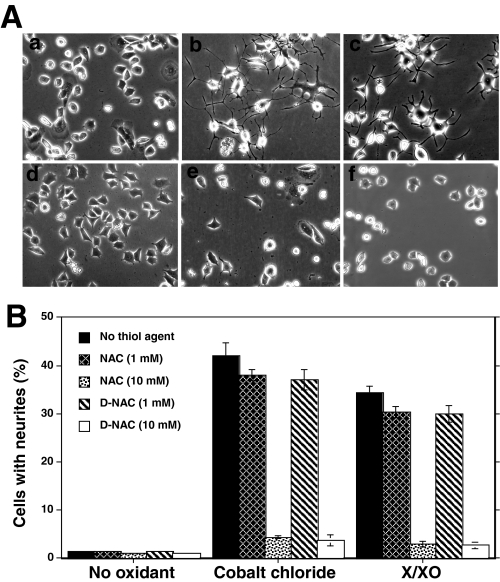
Thiol antioxidants inhibit neurite outgrowth induced by oxidants. A, effect of thiol agents on the morphological changes and neurite outgrowth induced by CoCl2 and X/XO. Neurite outgrowth was induced by treating PC12 cells with oxidants (150 μm CoCl2 or X/XO system) for 3 days in the presence and absence of NAC. a, control untreated cells; b, CoCl2; c, X/XO system; d, NAC alone; e, NAC + CoCl2; f, NAC + X/XO system. B, inhibition of thiol agents on the percentage of cells bearing oxidant-induced neurites. Where indicated, 1 or 10 mm concentrations of thiol agents, NAC or N-acetyl-d-cysteine (D-NAC), were added. The values are expressed as mean ± S.E. The values obtained with thiol agent were compared with the respective values obtained without thiol agents by the paired t test. **, significantly different values (p < 0.01).
Role of Thiol Agents as Reductants and Metal Chelators in the Prevention of Oxidant-induced Neurite Outgrowth—As shown in Fig. 3B, NAC at a low concentration, where it can serve as a precursor for GSH, did not significantly inhibit CoCl2-induced neurite outgrowth. However, NAC substantially inhibited CoCl2-induced neurite outgrowth at high concentrations (10–20 mm), well above those required to serve as a precursor for GSH (Fig. 3, A and B). Furthermore, N-acetyl d-cysteine, which is not a precursor for the synthesis of GSH, also appreciably inhibited CoCl2-induced neurite outgrowth in a manner similar to that observed with NAC (Fig. 3B). Moreover, neurite outgrowth was also prevented by DTT (1 mm), a cell-permeable dithiol agent. These studies suggest that the nonspecific thiol agents inhibit neuritogenesis by acting neither as precursors for GSH nor simply as scavengers of ROS.
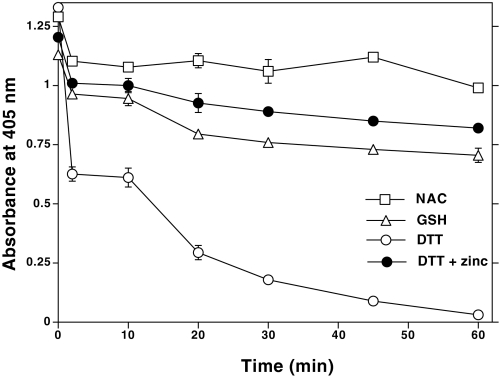
Previous studies have shown that at high concentrations (10–60 mm), NAC acts as a reductant in addition to acting as a scavenger of ROS (45). Additionally, NAC has previously been shown to have heavy-metal chelating properties (46), suggesting that the binding of transition metals to thiolates can cause their oxidation. As shown in Fig. 4, cobalt induced a catalytic oxidation of thiol groups in NAC, GSH, and DTT. Furthermore, cobalt oxidized dithiol (DTT) to a greater extent than monothiols (NAC and GSH), suggesting that vicinal thiols are better oxidized. A 10-fold excess of redox-inert zinc blocked the cobalt-induced oxidation. Conceivably, cobalt, a transition metal, causes the oxidation of thiols, and high concentrations of zinc prevent this oxidation by competing with cobalt for metal-binding thiolates. Therefore, intracellular proteins with zinc-thiolate structures might be good targets for cobalt, where cobalt may displace zinc and induce oxidation of vicinal thiols.
FIGURE 4.
Nonstoichiometric oxidation of thiols by cobalt and its inhibition by excess zinc. In a 96-well microtiter plate, NAC, GSH, or DTT (150 nmol, based on the thiol content) was incubated with CoCl2 (15 nmol) in 150 μl of 20 mm Tris-HCl, pH 7.4, at 30 °C. At the indicated time intervals, 25 μl of this mixture was taken into another 96-well plate to which 100 μl of 1 mm DTNB solution was added. The absorbance of thionitrobenzoic acid was measured at 405 nm, as an indicator of the amount of thiol left remaining after CoCl2-induced oxidation.
We have previously shown that tumor-promoting oxidants can both activate and inactivate PKC (24). Nevertheless, it is not known whether oxidants that induce neuritogenesis (CoCl2 or X/XO) can directly induce the redox modification of PKC. Before addressing this question, it is important to first establish whether or not PKC plays a key role in oxidant-induced neuritogenesis.
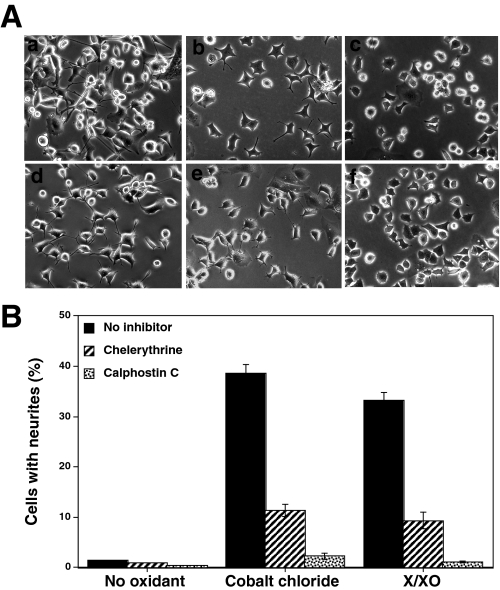
PKC Inhibitors Block Oxidant-induced Neurite Outgrowth— Initially, we used calphostin C and chelerythrine, PKC-specific (but isoenzyme-nonselective) inhibitors, to determine the key role of PKC in oxidant-induced neuritogenesis. As shown in Fig. 5, A and B, both of these cell-permeable PKC inhibitors substantially inhibited CoCl2- and X/XO-induced neuritogenesis. This experiment established that PKC plays an important role in oxidant-induced neuritogenesis. Therefore, more detailed studies were carried out to determine the specific modifications of PKC that occur upon treatment with oxidants.
FIGURE 5.
Inhibition of neurite outgrowth by PKC-specific (but isoenzyme-nonselective) inhibitors. A, effect of PKC-specific inhibitors on oxidant-induced morphological changes. PC12 cells seeded at low density were treated with CoCl2 (150 μm) or the X/XO system along with chelerythrine (2 μm) or calphostin C (200 nm). When calphostin C was used, after adding this inhibitor to the growth medium, cells were exposed to light for 1 h and then incubated at 37 °C in the dark (11). Cells were photographed after 3 days. a, CoCl2 alone; b, CoCl2 + chelerythrine; c, CoCl2 + calphostin C; d, X/XO alone; e, X/XO + chelerythrine; f, X/XO + calphostin C. B, oxidant-induced neurite outgrowth in the presence of PKC-specific inhibitors. Neurite outgrowth was measured 3 days after adding oxidants. The values are the mean ± S.E. of three independent experiments. The values obtained with oxidants in the presence of PKC inhibitors were compared with the respective controls obtained with oxidants alone by the paired t test (*, p < 0.05; **, p < 0.01).
Direct Redox Modification of Purified PKC with CoCl2 and X/XO—It is difficult to determine whether the changes in PKC that occur in intact cells are the result of a direct oxidative modification of PKC induced by cobalt or are indirectly caused by other mechanisms. Therefore, we determined whether CoCl2 induces a direct redox modification of PKC and whether this modification affects its kinase activity and phorbol ester binding. Preliminary studies showed susceptibility of various isolated PKC isoenzymes (α, β, γ, δ, and ε) to cobalt-induced oxidative activation. Because our goal was to determine whether or not CoCl2 oxidizes sulfhydryls in PKC isoenzymes rather than to determine the individual differences in oxidation susceptibility of various PKC isoenzymes, we carried out these studies with a mixture of isoenzymes α, β, and γ, which can be purified in high amounts from rat brains by rapid Ca2+-dependent hydrophobic chromatography (24, 36).
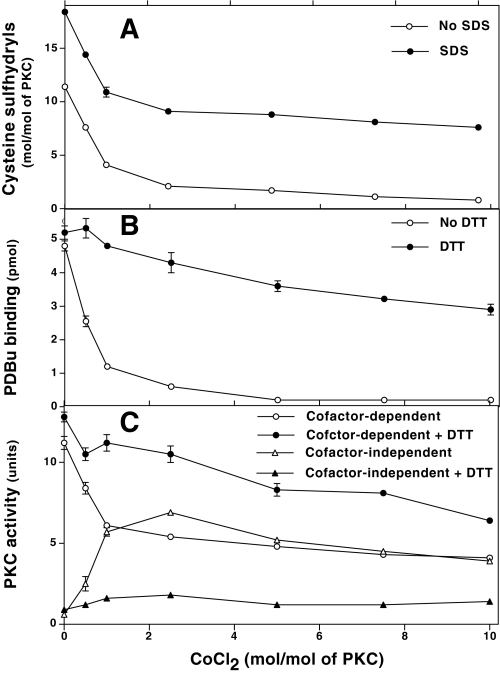
Only 18 sulfhydryls were titrated with DTNB after SDS denaturation of an untreated PKC used as a control. With or without SDS treatment, a biphasic decrease in DTNB-reactive sulfhydryls was observed with increasing concentrations of CoCl2 (Fig. 6A). With a very low amount of CoCl2 (1 mol/mol of PKC), there was a decrease in ∼7 sulfhydryl residues. These results reveal the catalytic nature of this modification. A further increase in cobalt to 10 mol/mol of PKC resulted in a decrease of only 3 additional sulfhydryls. Apparently, 7–8 cysteine sulfhydryls remained unmodified even with higher amounts of CoCl2 (10 mol/mol). Therefore, it is possible that either these cysteine residues may be deeply buried within the protein and thus not accessible for cobalt-induced modification, or they are not readily reacting with DTNB.
FIGURE 6.
CoCl2-induced oxidation of cysteine sulfhydryls in PKC and its relation to loss of phorbol ester binding and gain of cofactor-independent activation of PKC. A highly purified mixture of conventional Ca2+-dependent PKC isoenzymes (0.5 nmol) was incubated with the indicated concentration of CoCl2 for 25 min at 30 °C. Excess CoCl2 was removed by gel filtration. A, quantitation of cysteine sulfhydryls. A major aliquot of the desalted sample was used to quantitate accessible sulfhydryls in PKC with DTNB in a microwell plate. The total sulfhydryls in PKC were determined after the addition of SDS. B, decrease in phorbol ester binding after CoCl2 treatment. Simultaneously, a small aliquot of CoCl2-treated and desalted PKC was diluted in a buffer with and without DTT, and phorbol ester binding was determined using a multiwell filtration method. C, cofactor-independent activation of PKC. An aliquot of desalted sample was initially incubated for 5 min at 30 °C with or without DTT, then PKC activity was determined using histone H1 as a substrate and in the presence or absence of cofactors (Ca2+ and lipids). The values represent means of triplicate estimations.
At the initial stage of cobalt-induced modification, PKC lost phorbol ester binding and exhibited cofactor-independent kinase activity (Fig. 6, B and C). However, with increased modification of cysteine sulfhydryl residues, a decrease in PKC activity was observed. Treatment with DTT (1 mm) restored phorbol ester binding and converted PKC back from a cofactor-independent form to a cofactor-dependent form. This suggests that cobalt induced a redox modification of PKC. Furthermore, in another experiment, the proteolytically derived regulatory domain required lower concentrations of CoCl2 to inhibit its PDBu binding, whereas the isolated catalytic domain required higher concentrations of CoCl2 for the inactivation of its kinase activity (data not shown). Collectively, these observations suggest that the regulatory domain is more sensitive to cobalt than the catalytic domain. The higher sensitivity of the regulatory domain may be due to the presence of the cysteine-rich zinc-thiolate structures in this region of PKC.
Although X/XO induced similar changes in PKC activity and phorbol ester binding, these modifications were only partially reversed by DTT. Therefore, it is possible that superoxide/H2O2 formed from this system might have oxidized other amino acid residues in addition to cysteine residues, causing an irreversible oxidation of PKC. CoCl2 and X/XO, at the concentrations that affected PKC, did not affect protein kinase A, phosphorylase kinase or protein phosphatase 2A, suggesting specificity of the oxidants for PKC isoenzymes.
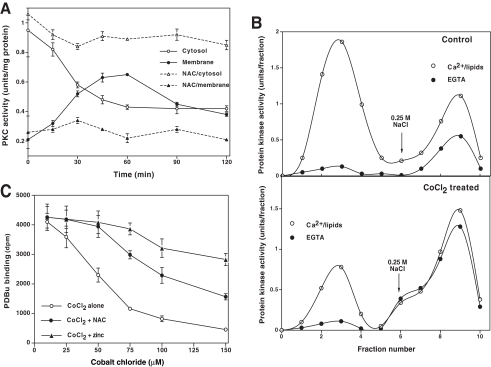
Redox Modification of PKC Occurring in PC12 Cells Treated with Oxidants—In PC12 cells treated with CoCl2, there was an initial decrease in PKC activity in the cytosolic fraction (Fig. 7A) along with a concomitant increase in PKC activity in the detergent-extractable membrane fraction, suggesting a translocation of PKC from the cytosol to membrane. During this time, PKC activity was still dependent upon lipids. Nonetheless, after 60–90 min, PKC converted to a cofactor independent constitutively activated form, which eluted with a high concentration (0.25 m) of NaCl from a DEAE-cellulose column (Fig. 7B). Furthermore, during the activation period, phorbol ester binding within intact cells also decreased, suggesting a modification of the regulatory domain, which binds phorbol esters (Fig. 7C). With prolonged treatment (>90–120 min) with CoCl2, PKC lost some of its activity, and it could only be partially recovered with mercapto agents.
FIGURE 7.
Redox modification of PKC in CoCl2-treated PC12 cells. A, translocation of PKC from cytosol to membrane fraction. Confluent PC12 cells grown in 100-mm Petri dishes were treated with 150 μm CoCl2 for the indicated time periods. PKC activity in the cytosol and detergent-extracted membrane fractions was determined using neurogranin substrate peptide. B, DEAE-cellulose chromatography of native and modified forms of PKC isolated from control PC12 cells (untreated) and cells treated with 150 μm CoCl2 for 90 min. The detergent-soluble cell extract containing total PKC isoenzymes (cytosol and membrane) was applied to a small (0.5-ml) DEAE-cellulose (Whatman DE52) column. The bound PKC isoenzymes (native forms) were eluted with 0.1 m NaCl (peak 1), whereas the modified forms of isoenzymes exhibiting less dependence on cofactors were eluted with 0.25 m NaCl (peak 2). Fractions of 0.25 ml were collected. C, phorbol ester binding to intact PC12 cells treated with CoCl2. Cells were grown in polylysine-coated 6-well plates and treated with the indicated concentrations of CoCl2 for 90 min. Then specific [3H]PDBu binding was determined as described under “Experimental Procedures.” The values represent means of triplicate estimations.
Effect of Thiol Agents and Zinc on the Redox Modification of PKC in PC12 Cells—Since isolated PKC is apparently modified by redox regulation, we tested whether NAC could block and/or reverse the changes occurring in PKC within oxidant-treated PC12 cells. As shown in Fig. 7, A and C, NAC (10 mm) blocked the effect of CoCl2 on both PKC activity and phorbol ester binding. Moreover, when these oxidant-mediated changes in PKC were allowed to occur in the absence of thiol agents, NAC also reversed these changes (data not shown), suggesting that at high concentrations, NAC can also act as a strong reductant. Similarly, the inclusion of zinc chloride (50 μm) in the growth medium also blocked the redox modification of PKC (data not shown) and loss of phorbol ester binding observed with intact PC12 cells (Fig. 7C). Since cobalt and zinc competitively bind to zinc-thiolates, it is likely that the redox inert zinc might have protected this site from oxidation. Nonetheless, the possibility cannot be ruled out that zinc blocked the cellular uptake of cobalt, the details of which are not clearly known in mammalian cells. These studies further support the notion that the oxidants tested in this study directly redox-modified PKC at the zinc-thiolate structures present in its regulatory domain.
Although NAC both blocked and reversed PKC modification induced by CoCl2 in PC12 cells, it was only effective in blocking the PKC modification induced by X/XO. Furthermore, the delayed application of NAC resulted in only a partial recovery of the X/XO-induced changes in PKC once they were allowed to occur. Conversely, zinc neither blocked nor reversed the PKC modifications induced by X/XO. This suggests a subtle difference in the PKC modification induced by X/XO versus that induced by CoCl2.
Effect of Cellular GSH on CoCl2-induced Modification of PKC—Since intracellular GSH protects the cell from oxidative damage, it is possible that GSH might protect PKC from the oxidative modification induced by CoCl2. Alternatively, it is possible that CoCl2 does not directly modify PKC but oxidizes GSH to form GSSG, which can modify PKC by S-glutathiolation, as shown by others (47). To test both possibilities, PC12 cells were initially treated for 24 h with 100 μm l-buthionine-(SR)-sulfoximine, a specific inhibitor of GSH synthesis. Total GSH (reduced and oxidized) decreased more than 90% from 32.6 nmol/mg protein in the control to 2.7 nmol/mg protein in the l-buthionine-(SR)-sulfoximine-treated cells. Nevertheless, CoCl2 modified PKC to the same extent in both the control and GSH-depleted cells (data not shown). Furthermore, CoCl2 (50–150 μm) treatment did not appreciably increase the amount of GSSG in PC12 cells. Even a transient increase in GSSG is likely to have been reversed by glutathione reductase in intact cells. This suggests that CoCl2 might directly modify PKC without the need for an initial formation of GSSG and also that this modification of PKC is not influenced by the cellular status of GSH.
Identification of PKC Isoenzymes That Mediate the Actions of CoCl2—Western immunoblotting analysis revealed the presence of all tested isoenzymes (α, β, γ, δ, ε, and ζ) in the PC12 cell extract. Upon treatment with CoCl2, all isoenzymes translocated from the soluble to particulate fraction. Since a sustained membrane association of PKC isoenzymes (especially conventional isoenzymes) may result in loss of their activity by dephosphorylation (48), it is important to measure their individual kinase activity, in addition to analyzing them by Western immunoblotting. However, when we initially attempted to measure the activity of these isoenzymes in the detergent-extractable particulate fraction by an immunocomplex kinase assay, the immunoprecipitable activity was only a small fraction of that expected from the total activity. This limitation can give potential artifacts in comparing the activity of various translocated PKC isoenzymes by immunocomplex kinase assay. Therefore, we preferred to use isoenzyme-selective inhibitors to identify the specific PKC isoenzyme(s) involved in oxidant-induced neuritogenesis.
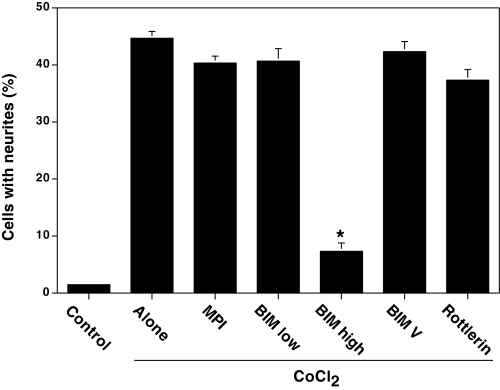
Initially, we used a cell-permeable myristoylated pseudosubstrate inhibitory polypeptide (PKCα and -β, residues 20–28), which preferentially inhibits conventional PKC isoenzymes (α, β, and γ). It did not significantly inhibit CoCl2-induced neurite outgrowth, thus excluding a role of these isoenzymes in this process (Fig. 8). BIM, a cell-permeable PKC-specific inhibitor, did not inhibit CoCl2-induced neurite outgrowth at a concentration of 100 nm. Given the fact that BIM is a highly potent inhibitor for conventional PKC with an IC50 of ∼10 nm (49), the lack of inhibition of CoCl2-induced neuritogenesis despite the use of a 100 nm concentration of BIM further excludes the possible role of the conventional PKC isoenzymes in this process. Since BIM inhibits novel PKC isoenzymes δ and ε with a lower affinity (IC50 ∼ 0.2 μm), we tested BIM at a higher (2 μm) concentration to inhibit these isoenzymes. At this concentration, BIM inhibited neurite outgrowth. In order to exclude the possibility of nonspecific inhibition of other enzymes by BIM at high (2 μm) concentration, we used its inactive analogue BIM V (2 μm) as a negative control. This inactive analogue did not inhibit neurite outgrowth. Since BIM poorly inhibits PKCζ with high IC50 (∼5 μm), it is unlikely that PKCζ plays a key role in oxidant-induced neuritogenesis. Furthermore, oxidants fail to induce neurite outgrowth in PC12 cells pretreated with a high (0.5 μm) concentration of phorbol 12-myristate 13-acetate, which depletes both conventional and novel PKC isoenzymes but not PKCζ (data not shown). This observation further excludes the role of PKCζ in this process. Given these results, PKCε and -δ isoenzymes are most likely the isoenzymes involved in oxidant-induced neurite outgrowth.
FIGURE 8.
Inhibition of CoCl2-induced neurite outgrowth by PKC isoenzyme-selective inhibitors. PC12 cells at a low cell density (1 × 104 cells/ml) were treated with CoCl2 either alone or along with the indicated PKC inhibitor, and neurite outgrowth was determined after 3 days. MPI, myristoylated pseudosubstrate inhibitory polypeptide; BIM low, low concentration of BIM (100 nm); BIM high, high concentration of BIM (2 μm); BIM V, negative control BIMV (2 μm). The data are means ± S.E. of three independent experiments. The values obtained with CoCl2 in the presence of PKC inhibitors were compared with the values obtained with CoCl2 alone (*, p < 0.01, evaluated by Student's paired t test).
In order to dissect the relative contribution of PKCδ and -ε to neuritogenesis, rottlerin, a PKCδ-specific inhibitor was used (50). Rottlerin did not inhibit neuritogenesis at 1 μm concentration (Fig. 8), suggesting that PKCδ is most likely not a key player in this process, but it is important to note that the concentration of rottlerin used is only 5-fold higher than the reported IC50 (0.2 μm) for the inhibition of PKCδ (48). Considering the limitation of cell permeability, it is unlikely that this concentration is high enough to completely inhibit PKCδ. Because of the high mitochondrial toxicity of rottlerin (51), we did not use it at the higher concentrations necessary to optimally inhibit PKCδ in PC12 cells. Although this study supports PKCε as the likely candidate for oxidant-induced neuritogenesis, it cannot exclude a possible role for PKCδ in this process.
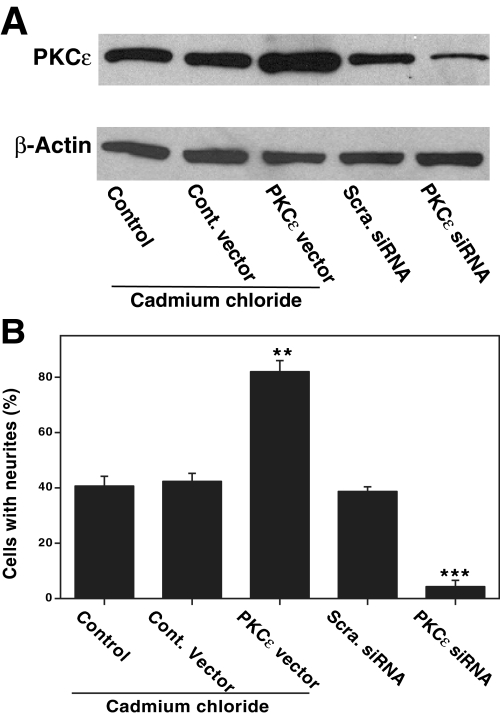
PKCε Levels Correlate with the Extent of Neurite Outgrowth— To further evaluate the role of PKCε in CoCl2-induced neuritogenesis, two different approaches were used to determine whether there is a correlation between the expression of PKCε and the extent of neuritogenesis. In the first approach, we overexpressed PKCε by stable transfection, whereas in the second approach, we suppressed the levels of PKCε by using siRNA.
In PC12 cells stably transfected with a metallothionein-driven PKCε vector, maximal expression of PKCε occurred when 5 μm cadmium chloride was added to the growth medium (Fig. 9A). As shown in Fig. 9B, CoCl2 induced a substantial increase in neurite outgrowth in PC12 cell transfectants optimally overexpressing PKCε compared with that of control cells transfected with an empty vector. These observations support a direct correlation between increased expression of PKCε and oxidant-induced neuritogenesis.
FIGURE 9.
PKCε levels in PC12 cells stably transfected with PKCε vector or transiently transfected with siRNA and the correlation with CoCl2-induced neurite outgrowth. A, Western immunoblotting of PKCε in PC12 cells stably transfected with either control vector or metallothionein-driven expression vector and transiently transfected with either control scrambled siRNA or PKCε siRNA oligonucleotides as described under “Experimental Procedures.” Where indicated, cadmium chloride (5 μm) was added to the growth medium. Immunoblot analysis to determine possible changes in PKCε or β-actin as loading control was carried out. B, neurite outgrowth in the PC12 cells under the conditions present in A. siRNA oligonucleotides were added to cells every 24 h. CoCl2 (150 μm) was added 1 day after the initiation of siRNA treatment, and neurite outgrowth was measured after 3 days. **, values for PKCε vector are statistically different from control vector (paired t test, p < 0.01). ***, values for PKCε siRNA are statistically different from control scrambled siRNA (p < 0.01).
A transient transfection with three predesigned siRNA oligonucleotides resulted in a decrease in PKCε as measured by Western immunoblotting (Fig. 9A). Furthermore, immunoblot analysis revealed that other PKC isoenzymes, such as α, β, γ, and δ, were not affected by the transfection with PKCε siRNA oligonucleotides, demonstrating the specificity involved in the knock-out procedure. The experiments were continued with the PKCε siRNA oligonucleotide that produced the greatest knock-out (a decrease of ∼80% of the control). This decrease in PKCε immunoreactivity caused by siRNA transfection directly correlated with a decrease in neurite outgrowth (Fig. 9B). On the contrary, the negative control scrambled siRNA decreased neither PKCε nor neurite outgrowth. Therefore, these experiments further support a key role for PKCε in neuritogenesis.
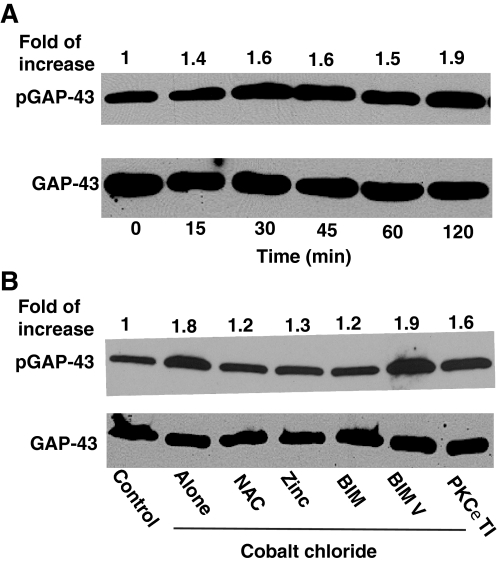
Phosphorylation of GAP-43, a PKC-specific Neuronal Substrate Protein—Although PKC was activated by oxidants using cell extracts in the test tube, it is important to determine whether this activation occurs in intact cells. For this purpose, we determined the degree of phosphorylation of the endogenous protein, GAP-43 at Ser41, which is a highly specific substrate for PKC. In PC12 cells treated with CoCl2, the phosphorylation of GAP-43 increased in parallel with PKC activity (Fig. 10A). NAC and zinc, which inhibited CoCl2-induced oxidative activation of PKC, also inhibited GAP-43 phosphorylation (Fig. 10B). PKC-selective inhibitor BIM at high (2 μm) concentrations, but not at low (100 nm) concentrations, also inhibited phosphorylation of GAP-43. In addition, a PKCε translocation inhibitor moderately inhibited this process. Furthermore, partial knock-out of PKCε by a transfection with its siRNA (but not scrambled siRNA) decreased GAP-43 phosphorylation (data not shown). Therefore, PKC, especially the ε isoenzyme, plays a direct role in the CoCl2-induced phosphorylation of GAP-43. In contrast, the MAPK kinase inhibitor PD98059 did not decrease this phosphorylation, suggesting the lack of a direct role for the MAPK system in the CoCl2-induced phosphorylation of GAP-43 at Ser41.
FIGURE 10.
Induction of phosphorylation of GAP-43 in CoCl2-treated PC12 cells and its inhibition by NAC, zinc, and PKC-specific inhibitors. A, time course of increased phosphorylation of GAP-43 in CoCl2 treated cells. At confluence, PC12 cells were serum-starved overnight and then treated with 150 μm CoCl2 for the indicated time periods. Cell lysates were subjected to Western immunoblotting to determine phosphorylation of GAP-43 at Ser41 using anti-phospho-Ser41-GAP-43 antibodies. Anti-GAP-43 antibodies were used to determine the total (unphosphorylated and phosphorylated) GAP-43 as a loading control. The band density of the phosphorylated GAP-43 was quantitated by densitometry and normalized to the band density of the total GAP-43. B, inhibition of GAP-43 phosphorylation by NAC, zinc, and PKC-specific inhibitors. PC12 cells were initially treated with NAC (10 mm), ZnCl2 (50 μm), BIM (2 μm), or BIM V (2 μm) for 1 h and then treated with 150 μm CoCl2 for an additional 30 min, and the cell lysates were subjected to Western immunoblotting. Cells were transiently permeabilized (82) and treated with 150 μg of PKCε translocation inhibitor (TI) before the addition of CoCl2.
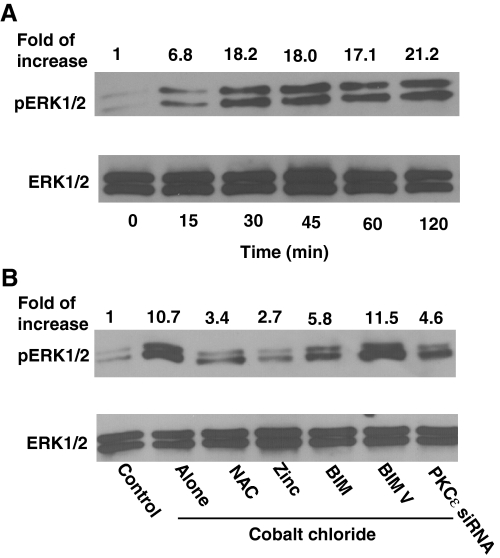
The Role of PKC in the Activation of MAPK ERK1/2 in Neuritogenesis—Within minutes of treatment with CoCl2, an activation of ERK1/2 occurred, as measured by Western immunoblotting with phosphospecific MAPK antibodies (Fig. 11A). This activation persisted for at least 120 min. Similar results were obtained with the X/XO system (data not shown). Both NAC and zinc blocked the CoCl2-induced activation of ERK1/2 (Fig. 11B). BIM at high (2 μm) concentrations, but not at low (100 nm) concentrations, blocked this activation as well. A knock-out of PKCε decreased CoCl2-induced activation of ERK1/2. Although a PKCε translocation inhibitor was also used in this experiment, the results obtained were inconsistent. Collectively, all of these data suggest that PKC, particularly the ε isoenzyme, plays a key role in the CoCl2-induced activation of ERK1/2. MAPK kinase inhibitor PD98059 did not affect the CoCl2-induced activation of PKC. Nonetheless, it substantially decreased CoCl2-induced activation of ERK1/2 and neuritogenesis (data not shown). Therefore, PKC may function as an upstream signaling enzyme that couples the direct action of oxidants to a downstream MAPK pathway leading to neuritogenesis.
FIGURE 11.
Activation of ERK1/2 in CoCl2-treated PC12 cells and its inhibition by NAC, zinc, and PKC-specific inhibitors. A, time course of activation of ERK1/2 in PC12 cells treated with CoCl2. Overnight serum-starved PC12 cells were treated with 150 μm CoCl2 for the indicated time periods. Cell lysates were subjected to Western immunoblotting to determine the activation of ERK1/2 by using anti-phospho-ERK1/2 (pThr202/Tyr204) antibodies. Anti-ERK1/2 antibodies were used to determine the total (unphosphorylated and phosphorylated) ERK1/2 (loading control). The band density of the phosphorylated ERK1/2 was quantitated by densitometry and normalized to the band density of the total ERK1/2 present in the same lane. B, inhibition of activation of ERK1/2 by NAC, zinc, and PKC-specific inhibitors. Conditions described in the legend to Fig. 10B were used. In addition, PC12 cells were pretreated with PKCε siRNA for 24 h and then treated with 150 μm CoCl2 for 30 min.
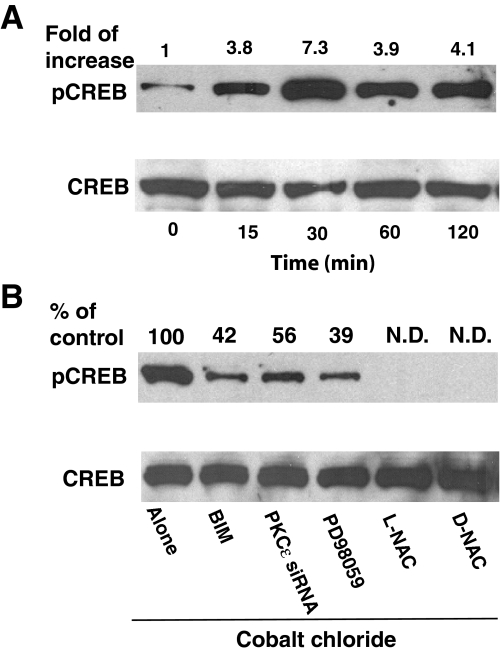
Activating Phosphorylation of CREB and the Role of the PKCε-ERK Pathway—In order to identify the downstream mechanisms involved in oxidant-induced neuritogenesis, we determined whether the PKCε-ERK pathway, activated in response to sublethal oxidative stress, could stimulate activating phosphorylation of CREB at Ser133. CoCl2 strongly stimulated the phosphorylation of CREB with a peak at 30 min of stimulation (Fig. 12A). Incubation with X/XO also induced the phosphorylation of CREB (data not shown). Both pretreatment with high (2 μm) concentrations of bisindolylmaleimide to inhibit PKCε and transfection with PKCε siRNA resulted in a partial inhibition of the phosphorylation of CREB (Fig. 12B). Furthermore, blocking of the ERK pathway by pretreatment with PD98059 also resulted in a partial inhibition of CREB phosphorylation. In contrast, antioxidants N-acetyl-l-cysteine and N-acetyl-d-cysteine completely inhibited the phosphorylation of CREB. Therefore, this study cannot rule out the possibility of a partial contribution of other protein kinases (such as p38 MAPK), activated in response to sublethal oxidative stress, in the phosphorylation of CREB. This study does suggest, however, that the PKCε-ERK pathway is, at least in part, involved in the activating phosphorylation of CREB at Ser133.
FIGURE 12.
Activating phosphorylation of CREB in CoCl2-treated PC12 cells. A, time course of increase in phosphorylation of CREB in CoCl2-treated PC12 cells. Serum-starved PC12 cells were treated with CoCl2 (150 μm) for the indicated time periods. Phosphorylation of CREB at Ser133 was determined by Western immunoblotting using anti-phospho-Ser133-CREB antibodies. Phospho-CREB band staining was corrected based on the band intensity of CREB (total) present in same lane and expressed as relative -fold increase compared with that of control. B, inhibition of phosphorylation of CREB by inhibitors of PKCε and ERK pathway as well as by thiol antioxidants. PC12 cells were initially treated with BIM (2 μm), PD98059 (50 μm), NAC (L-NAC), or N-acetyl-d-cysteine (D-NAC) (10 mm) for 1 h and then treated with 150 μm CoCl2 for an additional 30 min. In addition, PC12 cells were transfected with PKCε siRNA (50 nm) for 24 h and then treated with CoCl2. The phosphorylation observed with CoCl2 in the absence of inhibitors (control) was considered as 100%. N.D., not detectable.
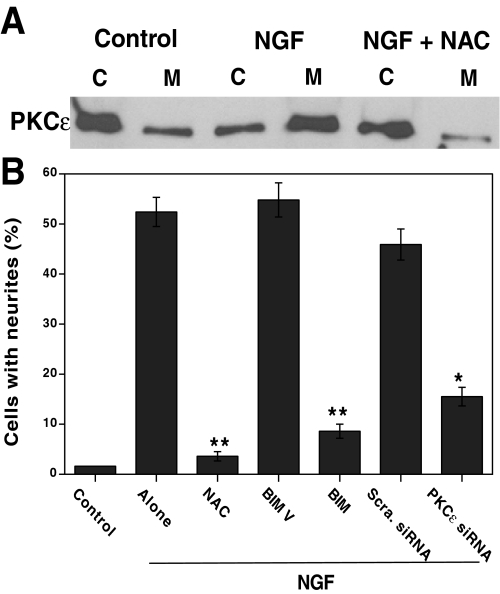
Role of Redox Activation of PKCε in NGF-induced Neurite Outgrowth—In order to assess the biological significance of the neuritogenesis induced via oxidative activation of PKCε by exogenous oxidants, we examined whether the small amounts of ROS generated during NGF signaling induce oxidative activation of PKCε (13–15). This hypothesis was tested using the same PC12 cell model system. Western immunoblotting showed a cytosol to membrane translocation of PKCε in the NGF-treated PKC cells, suggesting its activation (Fig. 13A). NAC blocked both PKCε translocation and NGF-induced neuritogenesis, suggesting a possible role of ROS in these processes. Furthermore, both high (2 μm) concentrations of BIM used to inhibit PKCε and knock-out of PKCε by siRNA partially inhibited NGF-induced neurite outgrowth (Fig. 13B). Conversely, controls exposed to BIM V (2 μm) or scrambled siRNA did not exhibit decreased neuritogenesis. This suggests that oxidative activation of PKCε, at least in part, plays a critical role in NGF-induced neuritogenesis.
FIGURE 13.
Role of oxidants in NGF-induced translocation of PKCε and neurite outgrowth. A, thiol antioxidant (NAC) inhibits NGF-induced cytosol to membrane translocation of PKCε. Serum-starved PC12 cells were treated with NGF (50 ng/ml) in the presence and absence of NAC (10 mm) for 15 min, and both cytosol (C) and detergent-solubilized membrane (M) fractions were prepared and subjected to Western immunoblotting as described under “Experimental Procedures.” B, effect of thiol antioxidant and PKCε inhibitors on NGF-induced neurite outgrowth. PC12 cells were initially treated with either NAC (10 mm) or PKC inhibitor BIM (2 μm) for 1 h or PKCε siRNA (50 nm) for 1 day and then treated with NGF. Neurite outgrowth was measured after 3 days. Differences between treatment groups were assessed by paired t test. **, p < 0.01; NAC is significantly lower than its control “alone” (no NAC). **, p < 0.01; BIM is significantly lower than its control BIM V. *, p < 0.05; PKCε siRNA is significantly lower than its control scrambled siRNA.
DISCUSSION
ROS are produced as part of the inflammatory response to spinal cord and traumatic brain injuries, and the enzymes directly and specifically influenced by them are molecular targets for new therapies to promote neuritogenesis. Various studies have indicated that CoCl2, X/XO, and hyperoxia can promote neuritogenesis in PC12 cells through the induction of ROS (10, 52). In addition, the transitional metal manganese has been shown to increase neuritogenesis (53). Furthermore, the free radical-trapping agent α-phenyl-N-tert-butylnitron has been reported to induce neurite outgrowth by a redox mechanism (54), and NGF-induced neuritogenesis has been reported to require the generation of ROS (13–15). Therefore, it is possible that ROS are common signals for neurite outgrowth in PC12 cells.
Oxidants induce a bidirectional response in PC12 cells; at lower concentrations, they induce neuritogenesis, whereas at higher concentrations, they induce cell death (10, 52). Similarly, oxidants induce an activation of PKC at lower concentrations and an inactivation of PKC at higher concentrations (24). PKC activation induces a wide variety of cellular processes, including cell growth, adhesion, and motility, whereas PKC inactivation triggers cell death (23). The bidirectional regulation of this kinase is well suited for explaining the paradoxical effects of oxidants. In addition to PKC, various specific molecular targets that are directly modified and either inactivated or activated by oxidants have been identified, including protein-tyrosine phosphatase, Ras, and transcriptional factors c-Jun and p53 (55–58). In contrast, some targets, such as MAPK, are indirectly activated by oxidants (59). Although these targets are very important in oxidant-induced cellular regulation, it is not clear at present whether they also respond bidirectionally to oxidants.
Molecular targets for oxidant-induced neuritogenesis should have the following five properties. First, the target should have structural features allowing for the specific and bidirectional response to oxidants; second, the target should explain how such oxidations occur in cells with high (millimolar) concentrations of GSH; third, the target should translocate to organelles where critical events necessary for neuritogenesis occur; fourth, the target should regulate growth-associated proteins, which are intimately involved in cytoskeletal reorganization; finally, the target should protect the cells from oxidant-induced cell death in order to facilitate neuritogenesis. Our present studies reveal that PKC isoenzymes appear to possess all five of these criteria, and the experimental evidence to support this notion is discussed below.
First, in order to characterize PKC as an appropriate molecular target for oxidants, we assessed the molecular basis for the specific and bidirectional response of PKC to oxidants in relation to the opposing cellular effects (cell death and neuritogenesis) elicited by oxidants. Studies conducted with both modal monothiols and dithiols indicate that cobalt and zinc compete for thiolates. The PKC zinc-thiolate motif is coordinated by a CXXC sequence in which two cysteine residues are separated by two other amino acids (25, 60). This is a highly redox-active center that is present in the thioredoxin family of proteins (61). These vicinal thiol residues can be readily oxidized to disulfides with the assistance of a transition metal present in the cell or in solution. Thus, the binding of redox-inert zinc prevents the oxidation of the two vicinal cysteine residues. Redox-active cobalt, by replacing redox-inert zinc, catalyzes the oxidation of these vicinal sulfhydryls. Since this is a catalytic reaction, a small amount of intracellular free cobalt is sufficient to oxidize proteins with accessible zinc-thiolate motifs. Therefore, PKC, by having zinc-thiolate motifs, is an appropriate target for cobalt.
Once cobalt induces the oxidation of cysteine residues in the regulatory domain, this domain can no longer bind the zinc that is necessary to support the conformation responsible for the binding of diacylglycerol and phorbol esters. This modification therefore results in loss of phorbol ester binding. The collapse of zinc fingers is most likely to perturb the conformation of the regulatory domain, which in turn relieves the inhibitory effect of the pseudosubstrate region (present in the regulatory domain) on the C-terminal catalytic region. This results in the generation of a cofactor-independent constitutively activated form of PKC. At high concentrations, in addition to binding to the regulatory domain, cobalt may also bind to the low affinity sites consisting of cysteine residues within the catalytic domain and cause their sulfhydryl oxidation, thus leading to the inactivation of PKC. This modification is reversed with DTT. Previous studies have revealed that the catalytic domain has two pairs of vicinal sulfhydryls, which upon oxidation by selenite form two disulfide bonds, resulting in a loss of kinase activity (36). Besides inducing thiol-reversible inactivation, at higher concentrations cobalt also induces thiol-irreversible inactivation of PKC. Higher concentrations of oxidants have been previously shown to induce irreversible oxidation of other amino acids, such as histidine, tryptophan, and methionine, in addition to cysteine (44). This may occur at the PKC sites to which cobalt binds with low affinity. Therefore, cobalt, in a concentration-dependent fashion, either modifies only the regulatory domain that activates PKC or additionally modifies the catalytic domain that inactivates PKC.
That PKC, with its zinc-thiolate motif, is an appropriate target for cobalt is further supported by the fact that other enzymes we have tested in this study, such as protein kinase A, phosphorylase kinase, and protein phosphatase 2A, all lack the zinc-thiolate motif and were not affected by cobalt. Because other protein kinases (except Raf and protein kinase D) and protein phosphatases lack zinc-thiolate motifs, they are unlikely candidates for the action of cobalt at low concentrations. Nevertheless, this study cannot exclude the possibility of “nonkinase” proteins with a C1 domain with zinc-thiolate motifs as targets for oxidants. For example, chimaerins, with cysteine-rich C1 domains similar to those of PKC isoenzymes (62), may also be targets for oxidation. Additionally, other proteins, such as metallothioneine, that contain zinc-thiolate motifs (63) may also be targets for this modification. Moreover, it is possible that cobalt at higher concentrations may nonspecifically react with cysteine residues in some proteins. Nevertheless, it is unlikely that the bidirectional response that is seen in PKC will also be seen in these specific or nonspecific targets.
Second, as an additional criterion for a molecular target for oxidants, we assessed how PKC oxidative changes occur in the cell with millimolar concentrations of GSH. Although in the test tube or in crude cell extracts, GSH at millimolar concentrations inhibits the CoCl2-induced redox modification of PKC, it does not inhibit this modification in intact PC12 cells. Since PKC is loosely associated with the membrane in resting cells (64), redox regulation of PKC might occur at the interface between the membrane and hydrophobic regions of the protein shielded from cytosolic GSH. Therefore, we may deduce that the CoCl2-induced oxidation of PKC occurs within the vicinity of the enzyme and is not due to globally generated free radicals, which were previously shown in PC12 cells treated with high (>300 μm) concentrations of CoCl2 (65).
Third, to further support PKC as a molecular target for oxidants, we assessed the translocation of PKC from the soluble to particulate fraction. There is a possibility that the cobalt-induced oxidative modification of PKC triggers the membrane translocation (stabilization) of PKC. Another possibility is that cobalt might have initially activated phospholipases, resulting in the generation of lipid second messengers, which subsequently caused the membrane association of PKC. Previous studies have shown an activation of phospholipases A2, C, and D by oxidants (66, 67).
Although oxidants induced membrane translocation of various isoenzymes, the present studies carried out with isoenzyme-specific PKC inhibitors and overexpression or knock-out of PKCε strongly suggest a role of PKCε in oxidant-induced neuritogenesis. Previous studies have demonstrated a specific role for PKCε in NGF-induced neuritogenesis (20–22). There are also studies that support a role of PKCδ in this process as well (68). Moreover, some studies suggest an active role of both PKCδ and -ε isoenzymes in NGF-induced neurite outgrowth (69). Therefore, it is possible that both isoenzymes may be required for oxidant-induced neuritogenesis. After membrane association, PKC-binding proteins might protect ε and δ isoenzymes. The sustained activation of these isoenzymes facilitates the phosphorylation of membrane-bound growth-associated proteins.
Fourth, we assessed the ability of PKC to phosphorylate and regulate the growth-associated protein GAP-43, which is intimately involved in neuritogenesis. We measured PKC activity in relation to GAP-43 phosphorylation by employing a peptide corresponding to the phosphorylation domain of neurogranin, which shares homology with the phosphorylation domain of GAP-43. In addition, we measured GAP-43 phosphorylation in intact PC12 cells. This measurement of GAP-43 phosphorylation has two benefits. First, GAP-43 is a highly specific substrate for PKC (32), and its phosphorylation in intact cells reveals a true activation state of the enzyme. Second, GAP-43 phosphorylation is also a component of the actin cytoskeletal reorganization, which is needed for neuritogenesis (33). One of the intriguing observations in this study is that CoCl2 induced a rapid phosphorylation of GAP-43 in PC12 cells, which is blocked by NAC and various PKC inhibitors. Conceivably, PKC, especially the ε isoenzyme, is a molecular target for the oxidant-induced regulation of GAP-43, which influences the structure of the actin cytoskeleton.
Previous studies have shown a conformationally hidden actin-binding motif in PKCε that is unique to this individual member of the PKC family (70). This motif becomes exposed upon the activation of this isoenzyme and functions as a dominant localization signal. Other studies have revealed a colocalization of PKCε with F-actin at the cortical cytoskeleton, which is important for neurite outgrowth (71). Interestingly, GAP-43 also binds to actin (32). Furthermore, both GAP-43 and PKC are translocated to the plasma membrane during neuritogenesis (72). Therefore, a colocalization of PKCε and GAP-43 with actin and the plasma membrane may bring PKCε into close proximity of its phosphorylation substrate GAP-43. Although isolated GAP-43 is phosphorylated by various PKC isoenzymes in the test tube (73), the colocalization of PKCε and GAP-43 makes PKCε the most likely isoenzyme for the phosphorylation of GAP-43 in intact PC12 cells.
Finally, PKC is a likely molecular target for oxidants, because it protects cells from oxidant-induced cell death. Cellular adaptation to environmental oxidants is an important limiting factor for neuritogenesis. Previous studies have shown that an overexpression of PKCε in PC12 cells induces an increase in expression of Bcl-2, an antiapoptotic protein (74). Moreover, Bcl-2 has previously been shown to rescue PC12 cells from hyperoxia-induced cell death, and such an adaptation to oxidative stress facilitates the induction of a neuronal phenotype (75). Besides playing a central role in neuritogenesis, ERK1 and -2, activated in response to PKC oxidative activation, protect cells from oxidants. ERK-activated p90RSK phosphorylates and activates CREB, which in turn induces the transcriptional activation of cell survival proteins that have a cAMP-response element in their promoter region (4, 5). In this context, it is interesting to note that thioredoxin, a protein-disulfide reductase, has a cAMP-response element in its promoter region, and its induction plays a key role in neuroprotection (76, 77). If the inactivated state of PKCε, caused by disulfide formation in the catalytic domain, persists for a sufficiently long period of time, cell death by the ceramide-mediated pathway may result (78). Therefore, thioredoxin, with its high affinity for the PKC catalytic domain (79, 80), may reduce oxidant-induced disulfides and thus regenerate PKC activity, which in turn protects cells from death. At the same time, thioredoxin, with its weak affinity for the regulatory domain, fails to reverse the redox modification of cysteines in this domain, and as a result PKC retains its oxidant-induced constitutive activity. Given the fact that PKCε is a cell survival enzyme in various cell types, including PC12 cells (26, 81), its overexpression and activation may protect cells from oxidant-induced cell death, which in turn facilitates neuritogenesis.
Although exogenously administered oxidants cause the activation of PKCε and downstream pathways, it is important to consider the fact that this oxidative regulation of PKCε is also observed in PC12 cells treated with NGF. The limited amount of ROS produced in response to NGF signaling is sufficient to induce the activation of the PKC pathway, which, in cooperation with other mediators of NGF signaling, induces neuritogenesis. Therefore, the oxidative regulation observed in this study has biological significance in neuritogenesis in addition to its role in cellular response to exogenously administered oxidants.
This study has demonstrated that oxidants at sublethal doses induce neuritogenesis via a PKC pathway. Since a variety of other agents (e.g. polyphenolic compounds and drugs) induce ROS, it will be interesting to determine whether sublethal doses of these agents can induce neurite outgrowth as well. Understanding the mechanisms involved in compensatory axonal growth will help in the development of pharmacological agents for recovery after neuronal injuries and for reversal of age-related loss of neuronal plasticity.
Acknowledgments
We thank Elizabeth Hogg, Simcha N. Gottlieb, Nanda K. Kappa, and Harry Ma for assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant NS 046538 (to T. H. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ROS, reactive oxygen species; PKC, protein kinase C; NGF, nerve growth factor; X/XO, xanthine/xanthine oxidase; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; NAC, N-acetyl-l-cysteine; DTNB, 5,5-dithiobis (2-nitrobenzoic acid); PDBu, phorbol 12,13-dibutyrate; DTT, dithiothreitol; BIM, bisindolylmaleimide; CREB, cAMP-response element-binding protein; qRT, quantitative reverse transcription; siRNA, small interfering RNA.
References
- 1.Schwab, M. E. (2002) Science 295 1029-1031 [DOI] [PubMed] [Google Scholar]
- 2.Markus, A., Patel, T. D., and Snider, W. D. (2002) Curr. Opin. Neurobiol. 12 523-531 [DOI] [PubMed] [Google Scholar]
- 3.Greene, L. A., and Tischler, A. S. (1976) Proc. Natl. Acad. Sci. U. S. A. 73 2424-2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaudry, D., Stork, P. J. S., Lazarovici, P., and Eliden, L. E. (2002) Science 296 1648-1649 [DOI] [PubMed] [Google Scholar]
- 5.Gerdin, M. J., and Eiden, L. E. (2007) Sci. STKE 2007, pe15. [DOI] [PMC free article] [PubMed]
- 6.Klushman, I., and Schwab, M. E. (1997) Brain Res. 762 173-184 [DOI] [PubMed] [Google Scholar]
- 7.Hausmann, O. N. (2003) Spinal Cord 41 369-378 [DOI] [PubMed] [Google Scholar]
- 8.Moskovitz, J., Yim M. B., and Chock, P. B. (2002) Arch. Biochem. Biophys. 397 354-359 [DOI] [PubMed] [Google Scholar]
- 9.Wagner, S., Hussain, M. Z., Hunt, T. K., Bacic, B., and Becker, H. D. (2004) Wound Repair Regen. 12 368-373 [DOI] [PubMed] [Google Scholar]
- 10.Katoh, S., Mitsui, Y., Kitani, K., and Suzuki, T. (1997) Biochem. Biophys. Res. Commun. 241 347-351 [DOI] [PubMed] [Google Scholar]
- 11.Gopalakrishna, R., Chen, Z.-H., and Gundimeda, U. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 12233-12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae, Y. S., Kang, S. W., Seo, M. S., Baines, I. C., Tekle, E., Chock, P. B., and Rhee, S. G. (1997) J. Biol. Chem. 272 217-221 [PubMed] [Google Scholar]
- 13.Peunova, N., and Enikolopov, G. (1995) Nature 375 68-73 [DOI] [PubMed] [Google Scholar]
- 14.Suzukawa, K., Miura, K., Mitsushita, J., Resau, J., Hirose, K., Crystal, R., and Kamata, T. (2000) J. Biol. Chem. 275 13175-13178 [DOI] [PubMed] [Google Scholar]
- 15.Kamata, H., Tanaka, C., Yagisawa, H., Matsuda, S., Gotoh, Y., Nishida, E., and Hirata, H. (1996) J. Biol. Chem. 271 33018-33025 [DOI] [PubMed] [Google Scholar]
- 16.Neet, K. E., and Campenot, R. B. (2001) Cell. Mol. Life. Sci. 58 1021-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang, L., Sawada, T., Decker, S. J., and Saltiel, A. R. (1995) J. Biol. Chem. 270 13585-13588 [DOI] [PubMed] [Google Scholar]
- 18.Obermeier, A., Bradshaw, H. A., Seedorf, K., Choidas, A., Schlessinger, J., and Ullrich, A. (1994) EMBO J. 13 1585-1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonni, A., Ginty, D. D., Dudek, H., and Greenberg, M. E. (1995) Mol. Cell Neurosci. 6 168-183 [DOI] [PubMed] [Google Scholar]
- 20.Fagerstrom, S., Pahlman, S., Gestblom, C., and Nanberg, E. (1996) Cell Growth Differ. 7 775-785 [PubMed] [Google Scholar]
- 21.Hundle, B., McMahon, T., Dadgar, J., and Messing, R. O. (1995) J. Biol. Chem. 270 30134-30140 [DOI] [PubMed] [Google Scholar]
- 22.Brodie, C., Bogi, K., Acs, P., Lazarovici, P., Petrovics, G., Anderson, W. B., and Blumberg, P. M. (1999) Cell Growth Differ. 10 183-191 [PubMed] [Google Scholar]
- 23.Gopalakrishna, R., and Jaken, S. (200) Free Radic. Biol. Med. 28 1349-1361 [DOI] [PubMed] [Google Scholar]
- 24.Gopalakrishna, R., and Anderson, W. B. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 6758-6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazanietz, M. G., Wang, S., Milne, G. W., Lewin, N. E., Liu, H. L., and Blumberg, P. M. (1995) J. Biol. Chem. 270 21852-21859 [DOI] [PubMed] [Google Scholar]
- 26.Nishizuka, Y. (1992) Science 258 607-613 [DOI] [PubMed] [Google Scholar]
- 27.Griner, E. M., and Kazanietz, M. G. (2007) Nat. Rev. Cancer 7 281-294 [DOI] [PubMed] [Google Scholar]
- 28.Newton, A. C. (1997) Curr. Opin. Cell Biol. 9 161-167 [DOI] [PubMed] [Google Scholar]
- 29.Skene, J. H. P. (1989) Annu. Rev. Neurosci. 12 127-156 [DOI] [PubMed] [Google Scholar]
- 30.Hughes-Davis, E. J., Cogen, J. P., Jakowec, M. W., Cheng, H. W., Grenningloh, G., Meshul, C. K., and McNeill, T. H. (2005) J. Neurosci. 135 1231-1239 [DOI] [PubMed] [Google Scholar]
- 31.Mobarak, C. D., Anderson, K. D., Morin, M., Beckel-Mitchener, A., Rogers, S. L., Furneaux, H., King, P., and Perrone-Bizzozero, N. I. (2000) Mol. Biol. Cell 11 3191-3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coggins, P., and Zwiers, H. (1989) J. Neurochem. 53 1895-1901 [DOI] [PubMed] [Google Scholar]
- 33.He, Q., Dent, E. W., and Meiri, K. F. (1997) J. Neurosci. 17 3515-3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogita, K., Miyamoto, S., Yamaguchi, K., Koide, H., Fujisawa, N., Kikkawa, U., Sahara, S., Fukami, Y., and Nishizuka, Y. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 1592-1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saido, T. C., Mizuno, K., Konno, Y., Osada, S., Ohno, S., and Suzuki, K. (1992) Biochem. 21 482-490 [DOI] [PubMed] [Google Scholar]
- 36.Gopalakrishna, R., Gundimeda, U., and Chen, Z. H. (1997) Arch. Biochem. Biophys. 348 25-36 [DOI] [PubMed] [Google Scholar]
- 37.Kosaka, Y., Ogita, K., Ase, K., Nomura, H., Kikkawa, U., and Nishizuka, Y. (1988) Biochem. Biophys. Res. Commun. 151 973-981 [DOI] [PubMed] [Google Scholar]
- 38.Gopalakrishna, R., Chen, Z. H., Gundimeda, U., Wilson, J. C., and Anderson, W. B. (1992) Anal. Biochem. 206 24-35 [DOI] [PubMed] [Google Scholar]
- 39.Chen, S.-J., Klann, E., Gower, M. C., Powell, C. M., Sessoms, J. S., and Sweatt, J. D. (1993) Biochemistry 32 1032-1039 [DOI] [PubMed] [Google Scholar]
- 40.Gopalakrishna, R., Gundimeda, U., Anderson, W. B., Colburn, N. H., and Slaga, T. J. (1999) Arch. Biochem. Biophys. 363 246-258 [DOI] [PubMed] [Google Scholar]
- 41.Olah, Z., Lehel, C., Jakab, G., and Anderson, W. B. (1994) Anal. Biochem. 221 94-102 [DOI] [PubMed] [Google Scholar]
- 42.Bradford, M. M. (1976) Anal. Biochem. 72 248-256 [DOI] [PubMed] [Google Scholar]
- 43.Baker, M. A., Cerniglia, G. J., and Zaman, A. (1990) Anal. Biochem. 190 360-365 [DOI] [PubMed] [Google Scholar]
- 44.Stadtman, E. R., and Oliver, C. N. (1991) J. Biol. Chem. 266 2005-2008 [PubMed] [Google Scholar]
- 45.Yan, C. Y. I., Ferrari, G., and Greene, L. A. (1995) J. Biol. Chem. 270 26827-26832 [DOI] [PubMed] [Google Scholar]
- 46.Lorber, A., Baumgartner, W. A., Bovy, R. A., Chang, C. C., and Hollcraft, R. (1973) J. Clin. Pharmacol. 13 332-336 [DOI] [PubMed] [Google Scholar]
- 47.Ward, N. E., Stewart, J. R., Ioannides, C. G., and O'Brian, C. A. (2000) Biochemistry 39 10319-10329 [DOI] [PubMed] [Google Scholar]
- 48.Parker, P. J. (2003) Methods Mol. Biol. 233 159-162 [DOI] [PubMed] [Google Scholar]
- 49.Martiny-Baron, G., Kazanietz, M. G., Mischak, H., Blumberg, P. M., Kochs, G., Hug, H., Marme, D., and Schachtele, C. (1993) J. Biol. Chem. 268 9194-9197 [PubMed] [Google Scholar]
- 50.Gschwendt, M., Muller, H.-J., Kielbasssa, K., Zhang, R., Kittstein, W., Rincke, G., and Marks, F. (1994) Biochem. Biophys. Res. Commun. 199 93-98 [DOI] [PubMed] [Google Scholar]
- 51.Soltoff, S. P. (2001) J. Biol. Chem. 276 37986-37992 [DOI] [PubMed] [Google Scholar]
- 52.Kotake-Nara, E., and Saida, K. (2006) Sci. World J. 6 176-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin, W. H., Higgins, D., Pacheco, M., Aletta, J., Perini, S., Marcucci, K. A., and Roth, J. A. (1993) J. Neurosci. Res. 34 546-561 [DOI] [PubMed] [Google Scholar]
- 54.Tsuji, M., Inanami, O., and Kuwabara, M. (2001) J. Biol. Chem. 276 32779-32785 [DOI] [PubMed] [Google Scholar]
- 55.Barrett, W. C., DeGnore, J. P., Keng, Y. F., Zhang, Z. Y., Yim, M. B., and Chock, P. B. (1999) J. Biol. Chem. 274 34543-34546 [DOI] [PubMed] [Google Scholar]
- 56.Mallis, R. J., Buss, J. E., and Thomas, J. E. (2001) Biochem. J. 355 145-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xanthoudakis, S., Miao, G., and Curran, T. (1992) EMBO J. 11 3323-3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun, X. Z., Vinci, C., Makamura, L., Han, S., Tran, D., Nguyen, J., Hamann, M., Grazzini, S., Sheppard, S., Gutova, M., Zhou, F., Thomas, J., and Momand, J. (2003) Antioxid. Redox Signal. 5 655-665 [DOI] [PubMed] [Google Scholar]
- 59.Guyton, K. Z., Liu, Y., Gorospe, M., Xu, Q., and Holbrook, N. J. (1996) J. Biol. Chem. 271 4138-4142 [DOI] [PubMed] [Google Scholar]
- 60.Kikkawa, U., Ogita, K., Ono, Y., Asaoka, Y., Shearman, M., Fujii, T., Ase, K., Sekiguchi, K., and Nishizuka, Y. (1987) FEBS Lett. 223 212-216 [DOI] [PubMed] [Google Scholar]
- 61.Lillig, C. H., and Holmgren, A. (2007) Antioxid. Redox Signal. 9 25-47 [DOI] [PubMed] [Google Scholar]
- 62.Yang, C., and Kazanietz, M. G. (2007) Biochem. J. 403 1-12 [DOI] [PubMed] [Google Scholar]
- 63.Maret, W., and Vallee, B. L. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 3478-3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gopalakrishna, R., Barsky, S. H., Thomas, T. P., and Anderson, W. B. (1986) J. Biol. Chem. 261 16438-16445 [PubMed] [Google Scholar]
- 65.Zou, W., Yan, M., Xu, W., Huo, H., Sun, L., Zheng, Z., and Liu, X. (2001) J. Neurosci. Res. 64 646-653 [DOI] [PubMed] [Google Scholar]
- 66.Salgo, M. G., Corongiu, F. P., and Sevanian, A. (1992) Biochim. Biophys. Acta 1127 131-140 [DOI] [PubMed] [Google Scholar]
- 67.Ito, Y., Nakashima, S., and Nozawa, Y. (1997) J. Neurochem. 69 729-736 [DOI] [PubMed] [Google Scholar]
- 68.O'Driscoll, K. R., Teng, K. K., Fabbro, D., Greene, L. A., and Weinstein, I. B. (1995) Mol. Cell. Biol. 6 449-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burry, R. W. (1998) J. Neurosci. Res. 53 214-222 [DOI] [PubMed] [Google Scholar]
- 70.Prekeris, R., Hernandez, R. M., Mayhew, M. W., White, M. K., and Terrian, D. M. (1998) J. Biol. Chem. 273 26790-26798 [DOI] [PubMed] [Google Scholar]
- 71.Zeidman, R., Troller, U., Raghunath, A., Pahlman, S., and Larsson, C. (2002) Mol. Biol. Cell 13 12-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Hooff, C. O. M., Holthuis, J. C. M., Oestreicher, A. B., Boonstra, J., De Graan, P. N. E., and Gispen, W. H. (1989) J. Cell Biol. 108 1115-1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oehrlein, S. A., Parker, P. J., and Herget, T. (1996) Biochem. J. 317 219-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weinreb, O., Bar-Am, O., Amit, T., Chillag-Talmor, O., and Youdim, M. B. (2004) FASEB J. 18 1471-1473 [DOI] [PubMed] [Google Scholar]
- 75.Katoh, S., Mitsui, Y., Kitani, K., and Suzuki, T. (1999) Biochem. J. 338 465-470 [PMC free article] [PubMed] [Google Scholar]
- 76.Masutani, H., Bai, J., Kim, Y. C., and Yodoi, J. (2004) Mol. Neurobiol. 29 229-242 [DOI] [PubMed] [Google Scholar]
- 77.Chiueh, C. C., Andoh, T., and Chock, P. B. (2005) Ann. N. Y. Acad. Sci. 1042 403-418 [DOI] [PubMed] [Google Scholar]
- 78.Hannun, Y. A. (1996) Science 274 1855-1859 [DOI] [PubMed] [Google Scholar]
- 79.Watson, J. A., Rumsby, M. G., and Wolowacz, R. G. (1999) Biochem. J. 343 301-305 [PMC free article] [PubMed] [Google Scholar]
- 80.Witte, S., Villaba, M., Bi, K., Liu, Y., Isakov, N., and Altman, A. (2000) J. Biol. Chem. 275 1902-1909 [DOI] [PubMed] [Google Scholar]
- 81.Uemura, K., Aki, T., Yamaguchi, K., and Yoshida, K. (2003) Life Sci. 21 1595-1607 [DOI] [PubMed] [Google Scholar]
- 82.Johnson, J. A., Gray, M. O., Chen, C.-H., and Mochly-Rosen, D. (1996) J. Biol. Chem. 271 24962-24966 [DOI] [PubMed] [Google Scholar]