Abstract
The Notch pathway is a conserved cell-to-cell signaling mechanism, in which extracellular signals are transduced into transcriptional outputs through the nuclear effector CSL. CSL is converted from a repressor to an activator through the formation of the CSL-NotchIC-Mastermind ternary complex. The RAM (RBP-J associated molecule) domain of NotchIC avidly interacts with CSL; however, its role in assembly of the CSL-NotchIC-Mastermind ternary complex is not understood. Here we provide a comprehensive thermodynamic, structural, and biochemical analysis of the RAM-CSL interaction for components from both mouse and worm. Our binding data show that RAM and CSL form a high affinity complex in the presence or absence of DNA. Our structural studies reveal a striking distal conformational change in CSL upon RAM binding, which creates a docking site for Mastermind to bind to the complex. Finally, we show that the addition of a RAM peptide in trans facilitates formation of the CSL-NotchIC-Mastermind ternary complex in vitro.
The Notch pathway mediates signaling between neighboring cells and plays important roles in cellular differentiation, proliferation, apoptosis, and stem cell renewal (1, 2). Notch signaling is required during embryonic development and patterning as well as for cell fate decisions during adult homeostasis. Proper regulation of Notch is essential, because errant signaling results in congenital defects, cardiovascular disorders, and cancer (3-5). As first identified and characterized in Drosophila melanogaster and Caenorhabditis elegans (6), the canonical Notch signaling pathway is activated when the cell surface ligand DSL (Delta, Serrate, Lag-2) interacts with the receptor Notch on an adjacent cell (7). This receptor-ligand interaction initiates two proteolytic cleavages of the receptor Notch, whereupon the intracellular domain of Notch (NotchIC)3 is released from the membrane and translocates to the nucleus. Once inside the nucleus, NotchIC interacts with the DNA-binding transcription factor CSL (CBF-1, Su(H), Lag-1), and together they form a ternary complex with the transcriptional coactivator Mastermind to activate transcription from Notch target genes. A detailed description of the conversion of CSL from a repressor to an activator and its interactions with transcriptional coregulators is shown in Fig. 1.
FIGURE 1.
Overview of transcriptional regulation mediated by CSL. Top, prior to pathway activation, CSL engages transcriptional corepressors (CoR), which recruit multiprotein repressor complexes that function to silence Notch target gene transcription through the action of histone deacetylases (HDAC) (32). Right, upon pathway activation, nuclear NotchIC binds to CSL through its RAM (RBP-jk associated molecule) (12) and ANK domain (18), which is thought to displace corepressors from CSL (33, 34). Bottom, the subsequent binding of Mastermind to CSL-NotchIC renders the ternary complex poised to activate transcription (35, 36). The DNA bound CSL-NotchIC-Mastermind complex recruits general transcription factors such as PCAF/GCN5 and CBP/p300 (37, 38), which contain histone acetylase (HAT) domains, to up-regulate transcription of Notch target genes. Left, transcription is terminated by the degradation of NotchIC, which is mediated by an E3 ubiquitin ligase that recognizes the phosphorylated C-terminal PEST domain, leading to disassembly of the activation complex (39).
Previously, using Notch pathway components from C. elegans, we determined crystal structures for DNA-bound CSL and the CSL-NotchIC-Mastermind ternary complex bound to DNA (Fig. 2, A and B) (8, 9). Concurrent with our structural studies of the worm complex, the human ternary complex was also determined (10). These structural studies revealed the overall architecture of CSL and the transcriptionally active ternary complex, which have been remarkably conserved through evolution (11). As shown in Fig. 2, the three domains of CSL, which constitute “core” CSL: the N-terminal domain (NTD), β-trefoil domain (BTD), and the C-terminal domain (CTD), simultaneously mediate interactions with both NotchIC and Mastermind. The RAM and ANK domains of NotchIC interact with the BTD and CTD of CSL, respectively (Fig. 2, B and C). Mastermind adopts an elongated bent helical conformation in the complex, with the N-terminal helical region of Mastermind forming a tripartite interaction with ANK of NotchIC and the CTD of CSL, and the C-terminal helical region of Mastermind interacts with the NTD of CSL (Fig. 2B).
FIGURE 2.
Overview of previously determined CSL structures. Ribbon diagrams for the coregulator-free worm CSL-DNA complex (A) and the worm CSL-NotchIC-Mastermind ternary complex (B) are shown (8, 9). CSL domains NTD, BTD, and CTD are colored cyan, green, and orange, respectively. A β-strand that bridges all three domains is colored magenta. For the ternary complex in B, the NotchIC RAM domain (RAM) and ANK are colored red and yellow, respectively; Mastermind (MM) is colored gray. The DNA is represented as a stick model. C, domain schematics for core CSL, NotchIC, and Mastermind are colored according to ribbon diagrams. D, structural alignment of CSL from the worm and human ternary complex structures (9, 10). Cα backbone representation of worm CSL (2FO1) in tricolor (cyan, green, and orange) overlaid onto human CSL (2F8X) colored gray. Alignment was performed over the NTD, highlighting the interdomain differences in BTD and CTD between the two CSL structures. Worm CSL in the context of the ternary complex structure undergoes large interdomain movements that are not observed in the human CSL structure. Despite these interdomain differences, a loop structure within NTD (henceforth referred to as the NTD loop) is in a similar open conformation in both CSL structures. In the coregulator-free worm CSL-DNA structure, the NTD loop is in a closed conformation. The structure-function explanation for this conformational change is evident, because opening of the NTD loop removes steric hindrances that would otherwise block the C-terminal helix of Mastermind binding to CSL, preventing ternary complex formation. Also shown are the site of RAM (red) binding to the BTD and the location of the NTD loop.
Although these ternary complex structures were a milestone in the field, several unanswered questions remain regarding what role individual domains of NotchIC play in binding to CSL and the subsequent assembly of the active ternary complex. In particular, the function of the NotchIC RAM domain is a point of contention. On one hand, RAM is absolutely conserved in all Notch receptors; and in contrast to ANK, RAM avidly interacts with CSL both in vitro and in vivo (12-14). On the other hand, RAM is dispensable for signaling in cells under nonphysiological conditions when ANK is overexpressed (15-17). Similarly, in vitro, RAM is not necessary for reconstituting the CSL-NotchIC-Mastermind ternary complex when ANK is present in large excess (18), and indeed, the structure of the human CSL-NotchIC-Mastermind ternary complex did not contain RAM (10). Furthermore, there are significant conformational differences within CSL between the two ternary complex structures (Fig. 2D), raising questions as to the significance and contribution of RAM to these observed conformational differences (11).
In the present study, we endeavored to characterize the CSL-RAM interaction and address the question of whether the isolated RAM domain of NotchIC would trigger any conformational changes in CSL in the absence of ANK and Mastermind, suggesting that allostery is a component of the mechanism that converts CSL from a repressor to an activator. Therefore, we pursued a structural, thermodynamic, and biochemical characterization of the NotchIC RAM domain interaction with CSL, using Notch components from both mouse and worm. Our structural studies afford unprecedented higher resolution models of CSL, which include two crystal forms of worm CSL-RAM complexes bound to DNA and a crystal structure of mouse CSL bound to DNA. Using electrophoretic mobility shift assays (EMSA), we analyzed the contribution of RAM to assembly of the CSL-NotchIC-Mastermind ternary complex. Using isothermal titration calorimetry (ITC), we determined the affinity and energetics of the CSL-RAM binding reaction. Our results reveal that NotchIC RAM binding to the BTD of CSL triggers a distal conformational change in the NTD of CSL; a peptide corresponding to RAM facilitates formation of the CSL-NotchIC-Mastermind ternary complex; and NotchIC RAM binds to core CSL with nanomolar affinity. Taken together, our studies provide molecular snapshots of intermediary Notch pathway transcription complexes and the energetics that underlie their formation, which clarify the role of NotchIC RAM in signaling.
EXPERIMENTAL PROCEDURES
Cloning, Expression, and Protein Purification—The cloning, expression, and purification for the C. elegans orthologs CSL (Lag-1), NotchIC (Lin-12), and Mastermind (Sel-8/Lag-3) were described previously (8, 9). The Mus musculus CSL ortholog, residues 53-474 (core) or residues 203-393 (BTD), was cloned into the pGEX-6P-1 vector; murine Notch1 protein, encoding residues 1744-2113 (RAMANK) and 1827-2113 (ANK), were also cloned into pGEX-6P-1. Transformed bacteria were grown at 37 °C in LB medium, cooled to 20 °C, induced with 0.1 mm isopropyl β-d-thiogalactopyranoside, and grown overnight at 20 °C. The bacteria were harvested by centrifugation, resuspended in phosphate-buffered saline, and frozen. The cell pellets were lysed by sonication, cleared by centrifugation and filtration, and subsequently loaded onto a glutathione-Sepharose column (GE Healthcare). The column was washed with phosphate-buffered saline, and the GST fusion proteins were eluted using reduced glutathione. The elutants were dialyzed, and the GST tag cleaved with Precision Protease (GE Healthcare) per the manufacturer's protocol. A subsequent GST affinity column removed the GST moiety. All of the protein constructs were further purified to homogeneity using ion exchange and size exclusion chromatography. Peptides encoding the RAM domain were generated by cloning mouse Notch1 residues 1744-1771, Lin-12 residues 930-957, and Glp-1 residues 788-817 into a modified pET 28b(+) vector (generous gift of Dr. Christopher Lima). This vector encodes a fragment of SMT3, producing a His-SMT3-RAM fusion protein. The fusion protein was overexpressed as described above. The lysate was run over a nickel affinity column. The column was washed, and fusion protein was eluted with imidazole. The fusion protein was then cleaved to remove His-SMT3 from the RAM moiety using the Ulp1 protease (gift of Dr. Christopher Lima), which leaves only an N-terminal serine residue attached to RAM following cleavage. The RAM peptide was separated from the fusion by size exclusion chromatography in 30% acetonitrile, 0.1% trifluoroacetic acid, dried down in a vacuum centrifuge, and stored at -80 °C.
Crystallization and Data Collection—A 13-mer DNA duplex with TT/AA single-stranded overhangs was cocrystallized with all complexes (8). Mouse CSL-DNA complexes were set up in a 1:1.1 molar ratio, and the Hampton Research Index Screen was used to identify initial crystallization conditions, using an Art Robbins Phoenix Crystallization Robot. The crystals were optimized at 4 °C in 100 mm Bis-Tris, pH 5.5, 27% polyethylene glycol 3350, with and without 100 mm NaCl. The crystals were cryoprotected by soaking in mother liquor solution containing increasing amounts of xylitol and flash frozen in LN2 for transport. The diffraction data were collected at the Advanced Photon Source, beamline 22-ID. The crystals belong to the orthorhombic space group P21212, with unit cell dimensions a = 66.77 Å, b = 95.39 Å, c = 113.70 Å (supplemental Table S1).
Worm CSL-RAM-DNA complex crystals were obtained using similar methods. Two orthorhombic crystal forms were obtained at 4 °C, the C2221 form (a = 62.94 Å, b = 95.97 Å, c = 126.31 Å) was grown using a chemically synthesized RAM peptide corresponding to Lin-12 residues 938-950, and the P212121 (a = 60.153 Å, b = 98.866 Å, c = 126.313 Å) form using the recombinant RAM peptide (930-957). The C2221 crystals were grown in 100 mm Bis-Tris, pH 6.0, 200 mm MgCl2, and 26% polyethylene glycol 3350, cryoprotected in 20% sorbitol, and flash frozen, and data were collected at the National Synchrotron Light Source beamline X6A. The P212121 crystals were grown in 100 mm Bis-Tris, pH 5.5, 100 mm ammonium sulfate, and 21% polyethylene glycol 3350, cryoprotected in 15% ethylene glycol, and flash frozen, and the data were collected at Advanced Photon Source beamline 22-ID. All of the data were integrated and scaled using HKL2000 (19).
Structure Determination, Model Building, and Refinement—The known structures of C. elegans CSL-DNA (Protein Data Bank code 1TTU) and human CSL (Protein Data Bank code 2F8X) were used with Phaser (20) to obtain molecular replacement solutions for the worm CSL-DNA-RAM and mouse CSL-DNA structures. Model bias was reduced through the use of prime-and-switch from RESOLVE (21), and Coot (22) was used for manual rebuilding of models. Refinement of the structures was carried out using Refmac with TLS parameters (23), and composite-omit maps were generated using CNS to further validate models and areas of weak density (24). The quality of the structure was assessed by PROCHECK (25). The mouse CSL-DNA structure is completely resolved, containing all three domains of CSL and the 15-mer DNA duplex, with the exception of two short unresolved regions: amino acids 256-261 of the BTD, and amino acids 198-199 in the NTD. The structure has been refined to an Rwork = 21.9% and Rfree = 25.6% (supplemental Table S1).
For both worm CSL-RAM structures, the RAM peptide is well resolved. For the P212121 data, whereas the peptide included residues 930-957, only residues 937-951 were resolved. The P212121 structure was refined to a final Rwork = 22.1% and Rfree = 26.4% (supplemental Table S1). For the C2221 structure, which used the RAM peptide that encoded residues 938-950, all of the residues were resolved. The C2221 structure was refined to an Rwork = 22.3% and Rfree = 27.6% (supplemental Table S1). Pymol was used for creation of all figures involving structures.
Isothermal Titration Calorimetry—ITC experiments were performed using a Microcal VP-ITC microcalorimeter. All of the ITC experiments were performed at 25 °C in a buffer containing 50 mm sodium phosphate, pH 6.5, 150 mm NaCl, and 1 mm Tris(2-carboxyethyl)phosphine. All of the RAM peptides and CSL and NotchIC proteins were either resuspended or dialyzed into a buffer-matched solution. For experiments that contained CSL bound to DNA, two DNA oligonucleotides were annealed resulting in a 19-mer blunt-ended DNA duplex containing one CSL-binding site and purified by size exclusion chromatography in a matched buffer for ITC. The experiments were performed with either core CSL or the BTD of CSL in the microcalorimeter cell (10 μm), with or without the 19-mer DNA (11 μm) and titrated with Notch peptides or RAM-ANK constructs (100 μm). An approximately 60 nm Kd for the interaction of CSL with the 19-mer DNA was determined under our experimental conditions, ensuring that all of our RAM binding to CSL-DNA experiments were performed under stoichiometric CSL-DNA conditions. Protein concentrations were calculated using spectrophotometry at UV280 as well as BCA assay (Pierce). The data were analyzed using the ORIGIN software and fitted to a one-site binding model. The data reported are the sum of at least three individual experiments. The c value (c = Ka[M]N) for all experiments was between 5 and 300.
EMSA—Electrophoretic mobility shift assays were performed using the 19-mer DNA duplex stated above. Protein components were in a binding buffer: 10 mm Tris, pH 8, 150 mm NaCl, 100 μg/ml bovine serum albumin, 1 mm dithiothreitol, 5 mm MgCl2, 0.1% Nonidet P-40, and 5% glycerol. Various protein components were mixed with DNA on ice and then allowed to form complexes at room temperature for 15 min, followed by incubation on ice prior to electrophoresis. The complexes were then separated on a 10% nondenaturing PAGE in 0.5× Tris-borate-EDTA or 1× Tris-glycine at 4 °C and visualized using SYBR-GOLD stain (Invitrogen). Statistical analysis was performed using GraphPad.
RESULTS
Structures of Worm CSL-RAM-DNA and Mouse CSL-DNA Complexes—To obtain high resolution structural data for complexes of core CSL with the RAM domain of NotchIC and characterize any structural changes associated with RAM binding to CSL, we used recombinantly purified CSL and RAM constructs from mouse and worm and crystallized these complexes bound to a short piece of duplex DNA, containing a single CSL binding site. We obtained two orthorhombic crystal forms of worm CSL-RAM complexes bound to DNA that diffracted to 2.2 and 2.4 Å resolution, respectively (Fig. 3A). Despite the significantly different crystal lattices, the two worm CSL-RAM complexes are nearly structurally equivalent, overlaying with a high degree of similarity (RMSD =∼0.8 Å for all Cα atoms) (supplemental Fig. S1). Although we were able to crystallize a mouse CSL-RAM complex bound to DNA, these crystals nominally diffracted to only ∼7Å, which precluded determination of the structure; however, we were able to grow an orthorhombic crystal of the mouse CSL-DNA complex that diffracted to 2.2 Å (Fig. 3B). The data collection, structure determination, and refinement statistics for both worm and mouse CSL structures are summarized under “Experimental Procedures” and supplemental Table S1.
FIGURE 3.
Structures of worm CSL-RAM-DNA and mouse CSL-DNA complexes. Ribbon diagrams corresponding to worm CSL-RAM-DNA (A) and mouse CSL-DNA (B) structures reported here are shown. The domain coloring for CSL is the same as in Fig. 1. The RAM domain in A is represented as a stick model colored by atom. C, Cα overlays of BTD-RAM interactions determined here with BTD-RAM interaction from worm ternary complex structure, highlighting high degree of correspondence. The BTD is colored green, and RAM is colored yellow with hydrophobic tetrapeptide residues (VWMP) displayed as sticks. D, molecular surface representation of BTD-RAM interaction with BTD surface colored according to electrostatics: red, negative; blue, positive; gray, nonpolar. RAM is in a stick representation colored by atom. RAM binds in an extended conformation across a large hydrophobic surface and an electronegative patch on BTD. RAM also forms a β-stranded structure with a large loop on BTD that is also implicated in corepressor binding. All of the nonpolar and polar RAM-BTD interactions as well as the β-structure formed with the BTD loop are maintained in the worm CSL-RAM complexes determined here.
Comparison of our two newly determined complexes of CSL-RAM from worm against previously determined CSL structures reveals similarities to both the original structure of CSL bound to DNA (coregulator-free), as well as the structure of CSL in the context of the worm ternary complex structure. As shown in Fig. 3C, the RAM-BTD interactions for the worm CSL-RAM complexes determined here display a high degree of similarity with the RAM-BTD interactions from the worm ternary complex structure, essentially maintaining equivalent interactions. In contrast, the overall domain arrangements (NTD, BTD, and CTD) in the worm CSL-RAM complexes correspond more closely with the domain arrangements from the coregulator-free structure of CSL, exhibiting very little correlation with the sizeable interdomain movements observed for CSL in the worm ternary complex structure (Fig. 4, C and D). Interestingly, closer inspection of the structural comparisons reveals an additional observation: the conformation of a loop structure within the NTD of the CSL-RAM complexes, which we define as the “NTD loop,” assumes an open conformation strikingly similar to the conformation of the NTD loop when Mastermind is bound to CSL in both worm and human CSL-NotchIC-Mastermind ternary complexes (Fig. 4D). The significance of this observation is described in greater detail in subsequent sections.
FIGURE 4.
Comparison of CSL structures. The figure shows Cα overlays for mouse and worm CSL structures determined here against previously determined worm and human CSL structures, highlighting differences in domain dispositions and conformation of the NTD loop. CSL domains are colored as in Fig. 1. For comparison, a structurally equivalent Cα atom is denoted as a sphere in the NTD loops. For overlays performed in B and C, the alignment was done over the entire CSL molecule; for overlays in A and D, the alignment was done only over the NTD of CSL, because of the substantial interdomain movements of BTD and CTD about the NTD. A, overlay of the coregulator-free form of worm CSL (apo) in tricolor with worm CSL from the ternary complex (NIC+MM) colored gray, with RMSD greater than 2.4 Å for all Cα atoms; note the open and closed conformations of the NTD loop. B, overlay of coregulator-free mouse CSL (apo) in tricolor with human CSL from the ternary complex (NIC+MM) colored gray, with RMSD of ∼0.8 Å for corresponding Cα atoms; note the open and closed conformations of the NTD loop similar to worm structures in A. C, overlay of coregulator-free worm CSL (apo) in tricolor with worm CSL-RAM colored gray, with RMSD of less than 0.8 Å for corresponding Cα atoms; note the open and closed conformations of the NTD loop. D, overlay of worm CSL-RAM in tricolor with worm CSL from ternary complex (NIC+MM) colored gray, with RMSD greater than 2.4 Å for all Cα atoms; note the similar open conformations of the NTD loop.
Our murine CSL construct reported here is 99% identical to human CSL with only three conservative amino acid changes, and our structure of mouse CSL bound to DNA represents the only structure of mammalian CSL determined in a coregulator-free form (Fig. 3B). Overall, there is a high degree of correspondence when the Cα atoms are overlaid between mouse and human CSL structures with an RMSD of ∼1 Å (Fig. 4B). The primary areas of structural difference between the mouse and human structures are localized to the BTD and the conformation of the NTD loop. For the BTD structural difference, a large loop within BTD that engages RAM is disordered in the mouse CSL structure, but in the human ternary complex structure, even though RAM is not present, the loop adopts an ordered structure similar to what is observed in all of the worm CSL-RAM complexes. For the primary structural difference in NTD, the NTD loop, which is in an open conformation in the human ternary complex structure to accommodate Mastermind binding, is in a closed conformation similar to what is observed for the corresponding NTD loop in the coregulator-free worm CSL-DNA structure (Fig. 4B).
RAM-induced Conformational Changes—As previously shown in the CSL-NotchIC-Mastermind ternary complex structures (Fig. 2B), approximately one-half of the CSL-Mastermind interaction is composed of the C-terminal helix of Mastermind binding across a concave surface formed by the NTD β-sheet (Fig. 2B). Located at one end of the NTD β-sheet is the NTD loop, which is formed by a β-hairpin motif and is in an open conformation in both worm and human ternary complex structures and in a closed conformation in the coregulator-free structure of worm CSL (Fig. 5, A and B). Opening of the NTD loop is a necessary prerequisite for Mastermind binding, to remove steric hindrances with the NTD loop in the closed conformation (Fig. 5). The magnitude of this conformational change, as measured by the shift in Cα positions going from the closed to open conformation, ranges from ∼3-7 Å. For our current structural studies, we wanted to determine the conformation of the NTD loop in the context of our CSL-RAM structures and whether the isolated RAM domain of NotchIC is the trigger for the conformational change in the NTD loop. As shown in Fig. 5 (C and D), both of our worm RAM-CSL complexes, which are in completely different crystal lattices, reveal that the NTD loop adopts the open conformation. Moreover, for our mouse CSL-DNA complex, which does not contain RAM, the NTD loop is in a closed conformation (Fig. 5A). All of the NTD loop structures are well resolved, and their respective conformations are supported by compelling omit map electron density over this region, as shown in supplemental Fig. S2. In addition, it is unlikely that effects from the crystal lattice influence the conformation of the NTD loop, because none of the NTD loop regions participate in crystal lattice contacts. It should also be mentioned that the corresponding NTD loop movements between worm and mouse CSL are analogous, but not identical, which is likely due to the conservative changes in sequence over this region (supplemental Fig. S3). Taken together, our comparison of the CSL complexes determined here with previously determined CSL structures reveals a striking result: in the absence of RAM, the NTD loop of CSL is in a closed conformation, which sterically prevents Mastermind interacting with CSL; upon RAM binding to the BTD of CSL, this interaction triggers a long range conformational change in CSL, in which the NTD loop changes from a closed to an open conformation, providing a docking site for loading the C-terminal helix of Mastermind.
FIGURE 5.
Analysis of NTD loop conformations. The figure shows detailed structural comparisons of the NTD loop from CSL proteins in the coregulator-free, complexed with RAM, and ternary complex forms represented in cross-eyed stereo pairs, and emphasizes the steric clash of Mastermind with the closed conformation of the NTD loop. The NTD of CSL and the C-terminal helix of Mastermind are depicted as ribbon diagrams and colored cyan and black, respectively. A, comparison of NTD loop conformations from the coregulator-free structure of mouse CSL (cyan loop) and human CSL from the ternary complex (gray loop). Residues Ile131-Gln139 and Ile91-Gln99 in the NTD loop for mouse and human CSL, respectively, are represented as sticks and colored by atom type. The side chains of Gln136 and Leu59 from mouse CSL and human Mastermind, respectively, are colored red to represent the putative steric clash between the NTD loop in the closed conformation with Mastermind. For B-D, worm CSL residues I292-Q300, which correspond to the NTD loop, are drawn as sticks and colored by atom. The side chains of Arg299 and Leu99 from worm CSL and Mastermind, respectively, are colored red to indicate potential steric clashes. B, comparison of NTD loop conformations for coregulator-free structure of worm CSL (cyan loop) with worm CSL from the ternary complex (gray loop). C, comparison of NTD loop conformations for coregulator-free structure of worm CSL (cyan loop) with worm CSL from RAM complex (magenta loop). D, comparison of NTD loop conformations for worm CSL-RAM complex (magenta loop) with worm CSL from the ternary complex (gray loop).
Role of RAM in Ternary Complex Assembly—We next sought to examine the functional significance of the RAM-induced conformational changes observed in our structural studies. Using EMSA and our recombinantly purified components, we analyzed the contribution of RAM to assembly of the CSL-NotchIC-Mastermind ternary complex on a DNA substrate. As shown in Fig. 6, for both mouse and worm components, a RAM peptide added in trans facilitates formation of a ternary complex composed of CSL, ANK, and Mastermind. It was previously shown for reconstitution of the human CSL-NotchIC-Mastermind ternary complex (18) that the ternary complex forms in the absence of RAM but does so inefficiently and requires ANK in large excess. We observe similar results for our mouse components but show that in the presence of an exogenous RAM peptide, the ternary complex forms more efficiently than complexes formed only in the presence of ANK (Fig. 6, C and D). Interestingly, at all concentrations at which we were able to analyze binding, assembly of the worm ternary complex does not occur without RAM; however, the addition of the RAM peptide with ANK allows for ternary complex formation (Fig. 6A). Taken together, these results suggest that RAM has an additional role in ternary complex formation independent of targeting NotchIC to CSL in the nucleus and that RAM binding to CSL actively facilitates ternary complex formation.
FIGURE 6.
EMSA analysis of ternary complex assembly. The figure shows the contribution of RAM peptide to formation of the CSL-NotchIC-Mastermind ternary complex. Components of each binding reaction are denoted above each gel. EMSAs showing additional controls are included in the supplemental data (supplemental Fig. S4). A, assembly of worm ternary complex. The concentration of CSL is 1 μm, and the concentrations of all other components (RAMANK, RAM, ANK, Mastermind, and DNA) are 10 μm. For lanes 7-9, increasing concentrations of ANK 10, 20, and 30 μm, respectively, were utilized in the binding reaction. The worm ternary complex does not form without RAM (lanes 7-9); however, the addition of an exogenous RAM peptide allows for the ternary complex to form (lane 4). B, assembly of mouse ternary complex. The concentration of DNA is 1.5 μm; the concentrations of CSL, RAMANK, and ANK are 1.0 μm, except for lanes 7-9, in which the concentrations of ANK are 0, 2.5, and 5.0 μm, respectively. The concentration of Mastermind and RAM are 10 μm. The mouse ternary complex forms with or without RAM (lanes 4, 6, 8, and 9). C, efficiency of mouse ternary complex formation with and without exogenous RAM peptide. For all lanes, the concentration of CSL, Mastermind, RAM, and DNA are 1, 10, 10, and 1.5 μm, respectively. Increasing concentrations of ANK (0.025, 0.1, 0.5, and 2.0 μm) are included in lanes 2-5 and 7-10. The addition of the RAM peptide (lanes 2-5) increases the efficiency of ternary complex formation, as compared with ternary complex formation without RAM peptide (lanes 7-10). D, quantitation of mouse ternary complex formation. The data points were generated from integration of band intensities in C from three independent experiments. The bar graph shows the percentages of ternary complex formation (y axis) as a function of ANK concentration (x axis). A control peptide, consisting of a scrambled RAM sequence, had no effect on ternary complex formation (data not shown).
Thermodynamics of the CSL-RAM Interaction—Subsequently, we sought to determine the thermodynamic parameters that underlie CSL-RAM complex formation. We performed ITC binding studies with our purified recombinant constructs of CSL and RAM, in which CSL was titrated with RAM peptide at 25 °C. As shown in Table 1, the interaction of core CSL with RAM is an enthalpically driven reaction with a 1:1 stoichiometry and disassociation constants for mouse and worm CSL of ∼30 nm and ∼2 μm, respectively (supplemental Fig. S5). Moreover, to assess any potential differences in the binding reaction when CSL is bound to DNA, we performed additional calorimetry experiments, in which CSL was bound to a 19-mer of duplex DNA containing a single CSL-binding site. Overall, the affinity and free energy of binding for the CSL-RAM interaction is unchanged whether CSL is free in solution or bound to a cognate DNA (Table 1). Interestingly, closer examination of the mouse CSL-RAM thermodynamic parameters in the presence and absence of DNA reveals different enthalpic/entropic contributions to the reaction but similar free energies of binding (Table 1). The approximate 2 kcal/mol enthalpy/entropy compensation between the two reactions may reflect a favorable preordering of mouse CSL when bound to DNA prior to interacting with RAM. A similar compensation may also occur with the worm components, but the compensatory effect is not as prominent and may reflect fundamental thermodynamic differences between worm and mammalian CSL.
TABLE 1.
Calorimetric data for RAM and RAMANK binding to CSL
The values are the means of at least three independent experiments, and the errors represent the standard deviations of multiple experiments.
| CSL | Ligand | K | Kd | Δ G° | Δ H° | -T Δ S° |
|---|---|---|---|---|---|---|
| m−1 | μm | kcal/mol | kcal/mol | kcal/mol | ||
| mBTD | mRAM | 5.15 (±0.11) × 107 | 0.032 | −10.2 ± 0.05 | −15.5 ± 0.2 | 5.3 ± 0.1 |
| mCSL | mRAM | 5.15 (±2.1) × 107 | 0.022 | −10.4 ± 0.3 | −17.1 ± 0.3 | 6.6 ± 0.1 |
| mCSL + DNA | mRAM | 2.94 (±0.13) × 107 | 0.034 | −10.1 ± 0.002 | −15.0 ± 0.3 | 4.8 ± 0.3 |
| mCSL + DNA | wRAM (Lin12) | 3.35 (±1.1) × 107 | 0.032 | −10.2 ± 0.2 | −15.2 ± 1.6 | 5.0 ± 1.8 |
| mCSL + DNA | wRAM (Glp1) | 1.32 (±0.20) × 107 | 0.077 | −9.7 ± 0.09 | −15.1 ± 0.19 | 5.4 ± 0.10 |
| mCSL + DNA | mRAMANK | 7.47 (±2.5) × 107 | 0.014 | −10.7 ± 0.2 | −19.7 ± 2.3 | 9.02 ± 2.5 |
| wCSL | wRAM (Lin12) | 3.20 (±0.22) × 105 | 3.13 | −7.4 ± 0.02 | −15.6 ± 0.7 | 8.1 ± 0.7 |
| wCSL + DNA | wRAM (Lin12) | 4.87 (±1.6) × 105 | 2.05 | −7.8 ± 0.1 | −15.5 ± 3.5 | 7.8 ± 3.4 |
| wCSL + DNA | wRAM (G1p1) | 6.43 (±1.0) × 105 | 1.58 | −7.9 ± 0.1 | −16.9 ± 0.02 | 8.9 ± 0.12 |
We then pursued a molecular explanation for the observed differences in binding for mouse and worm CSL-RAM complexes. Because of the high degree of sequence conservation and essentially identical tertiary structures for worm and mammalian CSL, the greater than 50-fold difference in affinity for RAM was unexpected. Initially, we examined, as the source of the difference, the interaction of worm CSL with the RAM domain from Glp-1, the other Notch receptor paralog in C. elegans; however, the affinity and thermodynamic parameters of the worm CSL interaction with Glp-1 RAM were essentially identical to those measured for Lin-12 RAM (Table 1). Next, we performed cross-species calorimetric binding studies, in which mouse CSL was titrated with worm RAM and vice-versa. Whereas mouse CSL binds to both worm RAM peptides with essentially identical thermodynamic parameters (Table 1), titration of worm CSL with mouse RAM resulted in no measurable heat changes during the reaction. Additionally, we tried calorimetry experiments at different temperatures and buffers; however, in all cases a change in heat upon worm CSL-mouse RAM complexation was unmeasurable, which is not necessarily indicative that the binding reaction does not occur. We therefore turned to EMSA to characterize the worm CSL-mouse RAM interaction. As shown in supplemental Fig. S6A, worm CSL titrated with either worm or mouse RAM results in an apparent Kd of ∼1 μm, which is comparable with the ∼2 μm disassociation constant measured by ITC. Additionally, we performed EMSAs for mouse CSL with mouse RAM and worm RAM (supplemental Fig. S6B), as well as for mouse CSL and worm CSL with mouse and worm RAMANK (described below), to validate the complementarity of the two approaches for measuring binding constants. As expected, the binding constants measured by ITC and EMSA are equivalent within experimental error (Table 1 and supplemental Fig. S6). Taken together, these results suggest that the difference in binding affinity of mouse CSL versus worm CSL for RAM is not due to the RAM paralog or ortholog used in the reaction but rather is intrinsic to the species-specific CSL protein.
Previous experiments by Lubman et al. (26) used biochemical methods and ITC to characterize the interaction of the isolated BTD of CSL with NotchIC from mouse. From their studies, they concluded that RAM is necessary and sufficient for the interaction of NotchIC with the BTD of CSL and that other domains of NotchIC, such as ANK and PPD, do not participate in the interaction with BTD. Overall, the results from Lubman et al. are very consistent with our studies of core CSL with RAM; however, because Lubman et al. focused only on the BTD of CSL, we were keen to determine whether other domains of NotchIC participate with RAM for binding to core CSL, because ANK makes extensive interactions with the CTD of CSL in the CSL-NotchIC-Mastermind ternary complex structures. For mouse, the free energy of binding and the disassociation constant for the interaction of mouse RAMANK with mouse CSL is, within error, only marginally distinguishable from the complex formed by mouse RAM with mouse CSL (Table 1). An additional ITC experiment, in which mouse CSL was titrated with mouse ANK, produced no measurable heat (data not shown). Because of buffer restraints for ITC, which were incompatible with our worm RAMANK construct, we again turned to EMSA to analyze and compare the interaction of worm CSL with worm RAM and RAMANK. As shown in supplemental Fig. S6C, worm CSL forms a complex with worm RAMANK, as well as mouse RAMANK, with an apparent Kd of ∼1 μm, which is comparable with the disassociation constants measured by ITC and EMSA for worm CSL with worm RAM or mouse RAM. For completeness, we also performed EMSAs for mouse CSL with mouse RAMANK and worm RAMANK (supplemental Fig. S6D), and as expected, the disassociation constants estimated by EMSA were very consistent with those determined by ITC. Thus, as similarly observed by Lubman et al. for the isolated BTD of CSL, within our experimental system of NotchIC and core CSL bound to DNA, the RAM domain of NotchIC solely facilitates the initial interaction with CSL, with ANK interacting very weakly or not at all with CSL in the absence of Mastermind.
DISCUSSION
Canonical Notch signaling ultimately results in changes in gene expression, which initiate transcriptional programs that are manifested at the cellular level. The widespread role of Notch signaling in development and adult homeostasis, as well as its pathogenic role when misregulated in human disease, underscores the importance of a molecular understanding of transcriptional regulation in the Notch pathway. The transcription factor CSL plays a central role in transducing Notch signals into transcriptional outputs, and formation of the CSL-NotchIC-Mastermind ternary complex is essential to up-regulating transcription from Notch target genes. Although the previously determined structures of the human and worm CSL-NotchIC-Mastermind ternary complex structures illuminated the molecular details of an active Notch pathway transcription complex, our knowledge of the exact sequence of molecular events leading to the formation of the active ternary complex is incomplete. In particular, the role of the conserved RAM domain of NotchIC, which likely targets and forms the initial interaction of NotchIC with CSL in the nucleus, remains poorly understood. To bridge this gap in our understanding, our study focused on elucidating the function of RAM in ternary complex assembly.
Our analyses of Notch pathway components from disparate organisms afford us the opportunity to identify elements of function that are conserved through evolution from those aspects of function that are organism specific. Our structural studies of worm CSL-RAM complexes reveal that these structures share similarities with both the coregulator-free and ternary complex forms of CSL, lending support to the notion that these CSL-RAM structures are intermediary complexes toward formation of the active ternary complex structure. Although the RAM interaction with the BTD of CSL is equivalent between the CSL-RAM complexes determined here and the CSL-RAM interaction described in the worm ternary complex structure (Fig. 3C), the overall relative domain arrangements of NTD, BTD, and CTD are different between these complex structures (Fig. 4D). The disposition of domains for the CSL-RAM complex more closely corresponds to the coregulator-free form of worm CSL (Fig. 4C). Thus, RAM is not the trigger for the large interdomain conformational changes observed for CSL in the CSL-NotchIC-Mastermind ternary complex structure. Despite this, the conformation of the NTD loop in the CSL-RAM structures is more similar to the open conformation observed in the worm ternary complex structure and not the closed conformation observed in the coregulator-free worm CSL structure (Fig. 5, B-D).
The opening and closing of the NTD loop in response to bound coregulators appears to be conserved in mammals (Fig. 5A). The mouse structure of CSL determined here, which does not contain RAM, is overall very similar to the structure of CSL from the human ternary complex (Fig. 4B), with one important difference: the conformation of the NTD loop is in a closed conformation, similar to the coregulator-free worm CSL structure (Fig. 5, compare A and B). In addition, our EMSA analysis of ternary complex assembly from both mouse and worm demonstrate that RAM can act in trans, facilitating formation of the ternary complex (Fig. 6). Taken together, our studies reveal a provocative new role for the RAM domain of NotchIC: the binding of RAM to the BTD of CSL induces a long range allosteric change in the NTD, the function of which is to create a binding surface for the C-terminal helix of Mastermind.
Two other points should also be addressed; first, the RAM induced change in the NTD loop creates approximately one-half of the Mastermind-binding site in the CSL-NotchIC-Mastermind ternary complex. The molecular events that create the second half of the Mastermind-binding site, which is formed between the CTD of CSL and the ANK domain of NotchIC, are not understood at the molecular level and beyond the scope of the current study. Second, although our structural analyses strongly suggest a long range conformational change in the NTD loop triggered by RAM, our structures do not definitively point to an explanation for how this allosteric change is transmitted through the protein. However, an interesting area of future study will be the contribution of the absolutely conserved charged residue pair Asp336-Arg338 in worm CSL (Asp160-Arg162 in mouse), which participates in polar interactions with the NTD loop, and, at least in the worm CSL structures, makes substantially different interactions with the NTD loop when it is either in the open or closed conformation.
Our binding studies of CSL-RAM complexes from both worm and mouse reveal that CSL forms a high affinity complex with RAM and a nanomolar disassociation constant (Table 1). However, our binding studies revealed one important difference between CSL orthologs, i.e. there is an approximate 50-fold (2 kcal/mol) difference in the affinity for binding to RAM; the affinity of mouse CSL for RAM is greater than the affinity of worm CSL for RAM. Moreover, this difference is not due to the RAM ortholog or paralog but rather intrinsic to the species-specific CSL molecule. Our EMSA experiments reveal additional differences between mouse and worm CSL in assembly of the ternary complex, i.e. the mouse ternary complex can be formed in the absence of RAM, albeit inefficiently, but formation of the worm ternary complex, at least in vitro, is absolutely dependent on RAM (Fig. 6). Interestingly, the mouse NTD loop structure is less well ordered, as judged by its corresponding electron density (supplemental Fig. S2), than for the worm NTD loops. We speculate that the mammalian NTD loop is more dynamic, which could account for the formation of the mammalian ternary complex in vitro in the absence of RAM. Although the biological ramifications of these differences are unclear, these data suggest that at the thermodynamic level there is a fundamental difference between mouse and worm CSL with regards to how these molecules interact with RAM.
Our binding studies of CSL-RAM complexes additionally demonstrate that the affinity of CSL for RAM is unchanged whether CSL is free in solution or bound to DNA (Table 1). Because of the properties of linked equilibria (27), this implies that the affinity for DNA by CSL or CSL-RAM is equivalent. Although a detailed binding study of the interaction of CSL with DNA is not available, these results do suggests that there is no energetic preference for CSL interacting with NotchIC in the nucleoplasm or while CSL is bound to DNA. Moreover, this observation lends support to emergent models of CSL-mediated transcription complexes forming prior to binding at Notch target genes (28, 29), which challenges the dogmatic view of CSL statically bound to DNA. Certainly, it will be of interest in future studies to characterize the thermodynamic parameters of CSL-DNA and CSL-corepressor complexes, which will bear significantly upon these new mechanisms.
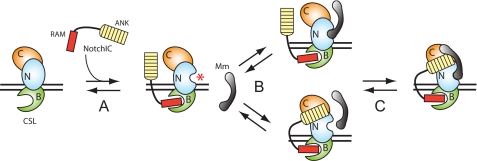
In conclusion, we envision a revised model describing the molecular events that assemble the active CSL-NotchIC-Mastermind transcription complex (Fig. 7). Upon pathway activation, RAM targets and initiates the interaction of NotchIC with CSL both free in the nucleoplasm and bound to target gene DNA. Our results here show that RAM binding to the BTD of CSL produces an allosteric change in the NTD, the function of which is to create one-half of the docking site required for Mastermind to bind to the complex. Results from others have shown that the requirement for RAM can be obviated when ANK is overexpressed (15-18). Because the Notch signal is not amplified and limiting concentrations of NotchIC must compete with abundant corepressors in the nucleus for CSL, we speculate that RAM makes NotchIC a more effective competitor for CSL binding under physiological conditions. Following RAM binding, the subsequent steps required for complete assembly of the CSL-NotchIC-Mastermind ternary complex are still unresolved. We put forward two possibilities (Fig. 7): first, RAM binding to CSL allows for the C-terminal helix of Mastermind to interact with the complex followed by the interaction of ANK and the N-terminal helix of Mastermind with CTD; and second, RAM binding to CSL triggers the NTD conformational change, and subsequently ANK binds to the CTD, forming a complete docking site for Mastermind to interact with the complex. We favor the second possibility, because tethering of ANK to CSL through the RAM-BTD interaction would substantially increase the local concentration of ANK (30). Although we were not able to observe an interaction between ANK and CSL, the CSL-ANK interaction may be very weak and not detectable under our experimental conditions for ITC. Consistent with this hypothesis, using FRET the Blacklow laboratory has recently reported a very weak but measurable affinity between CSL and ANK (31).
FIGURE 7.
Revised model of ternary complex assembly. The figure diagrams the sequence of events leading to formation of transcriptionally active ternary complex. CSL is drawn bound to DNA with all three functional domains, NTD (N), BTD (B), and CTD (C), which are colored cyan, green, and orange, respectively. The RAM and ANK domains of NotchIC are colored red and yellow, respectively. Mastermind (Mm) is colored gray. A, upon pathway activation, RAM binding to CSL both targets NotchIC to CSL and triggers an allosteric change in the NTD of CSL, which is denoted by a red asterisk. B, two possibilities exist: Mastermind (Mm) interacts with the complex to direct ANK binding to CSL (top) or ANK interacts with the CTD of CSL (bottom), creating the complete Mastermind docking site. The second scenario is more likely, because of the tethering of ANK to CSL through RAM, which would dramatically increase the local concentration of ANK (30). C, either case leads to formation of CSL-NotchIC-Mastermind ternary complexes, occupying sites at Notch target genes.
Supplementary Material
Acknowledgments
We thank Zhenyu Yuan, Andrew Russell, and Xiaomei Chai from the Kovall laboratory for assistance with protein purification and X-ray data collection. We are grateful to Tom Thompson and Andrew Herr for constructive criticism of the manuscript and biophysical data. We also thank the staffs at beamlines National Synchrotron Light Source X6A and Advanced Photon Source SER-CAT 22-ID.
The atomic coordinates and structure factors (codes 3BRD, 3BRF, and 3BRG) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported, in whole or in part, by National Institutes of Health Grant CA120199. This work was also supported by a grant from the Leukemia Research Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and supplemental Figs. S1-S6.
Footnotes
The abbreviations used are: NotchIC, intracellular domain of Notch; NTD, N-terminal domain; BTD, β-trefoil domain; CTD, C-terminal domain; EMSA, electrophoretic mobility shift assay(s); ITC, isothermal titration calorimetry; GST, glutathione S-transferase; RMSD, root mean square deviation; ANK, ankyrin repeat; RAM, RBP-J associated molecule.
References
- 1.Artavanis-Tsakonas, S., Rand, M. D., and Lake, R. J. (1999) Science 284 770-776 [DOI] [PubMed] [Google Scholar]
- 2.Bonde, J., Hess, D. A., and Nolta, J. A. (2004) Curr. Opin. Hematol. 11 392-398 [DOI] [PubMed] [Google Scholar]
- 3.Gridley, T. (2003) Hum. Mol. Genet. 12 R9-R13 [DOI] [PubMed] [Google Scholar]
- 4.Garg, V., Muth, A. N., Ransom, J. F., Schluterman, M. K., Barnes, R., King, I. N., Grossfeld, P. D., and Srivastava, D. (2005) Nature 437 270-274 [DOI] [PubMed] [Google Scholar]
- 5.Leong, K. G., and Karsan, A. (2006) Blood 107 2223-2233 [DOI] [PubMed] [Google Scholar]
- 6.Greenwald, I. (1998) Genes Dev. 12 1751-1762 [DOI] [PubMed] [Google Scholar]
- 7.Bray, S. J. (2006) Nat. Rev. Mol. Cell Biol. 7 678-689 [DOI] [PubMed] [Google Scholar]
- 8.Kovall, R. A., and Hendrickson, W. A. (2004) EMBO J. 23 3441-3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson, J. J., and Kovall, R. A. (2006) Cell 124 985-996 [DOI] [PubMed] [Google Scholar]
- 10.Nam, Y., Sliz, P., Song, L., Aster, J. C., and Blacklow, S. C. (2006) Cell 124 973-983 [DOI] [PubMed] [Google Scholar]
- 11.Kovall, R. A. (2007) Curr. Opin. Struct. Biol. 17 117-127 [DOI] [PubMed] [Google Scholar]
- 12.Tamura, K., Taniguchi, Y., Minoguchi, S., Sakai, T., Tun, T., Furukawa, T., and Honjo, T. (1995) Curr. Biol. 5 1416-1423 [DOI] [PubMed] [Google Scholar]
- 13.Roehl, H., Bosenberg, M., Blelloch, R., and Kimble, J. (1996) EMBO J. 15 7002-7012 [PMC free article] [PubMed] [Google Scholar]
- 14.Jarriault, S., Brou, C., Logeat, F., Schroeter, E. H., Kopan, R., and Israel, A. (1995) Nature 377 355-358 [DOI] [PubMed] [Google Scholar]
- 15.Kurooka, H., Kuroda, K., and Honjo, T. (1998) Nucleic Acids Res. 26 5448-5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffries, S., Robbins, D. J., and Capobianco, A. J. (2002) Mol. Cell Biol. 22 3927-3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aster, J. C., Xu, L., Karnell, F. G., Patriub, V., Pui, J. C., and Pear, W. S. (2000) Mol. Cell Biol. 20 7505-7515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nam, Y., Weng, A. P., Aster, J. C., and Blacklow, S. C. (2003) J. Biol. Chem. 278 21232-21239 [DOI] [PubMed] [Google Scholar]
- 19.Otwinowski, Z., and Minor, W. (1997) Method Enzymol. 276 307-326 [DOI] [PubMed] [Google Scholar]
- 20.Read, R. J. (2001) Acta Crystallogr. D Biol. Crystallogr. 57 1373-1382 [DOI] [PubMed] [Google Scholar]
- 21.Terwilliger, T. C. (2000) Acta Crystallogr. D Biol. Crystallogr. 56 965-972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emsley, P., and Cowtan, K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60 2126-2132 [DOI] [PubMed] [Google Scholar]
- 23.Winn, M. D., Isupov, M. N., and Murshudov, G. N. (2001) Acta Crystallogr. D Biol. Crystallogr. 57 122-133 [DOI] [PubMed] [Google Scholar]
- 24.Brunger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T., and Warren, G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54 905-921 [DOI] [PubMed] [Google Scholar]
- 25.Laskowski, R. A., Macarthur, M. W., Moss, D. S., and Thornton, J. M. (1993) J. Appl. Crystallogr. 26 283-291 [Google Scholar]
- 26.Lubman, O. Y., Ilagan, M. X., Kopan, R., and Barrick, D. (2007) J. Mol. Biol. 365 577-589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wyman, J., and Gill, S. J. (1990) Binding and Linkage: Functional Chemistry of Biological Macromolecules, University Science Books, Mill Valley, CA
- 28.Krejci, A., and Bray, S. (2007) Genes Dev. 21 1322-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nam, Y., Sliz, P., Pear, W. S., Aster, J. C., and Blacklow, S. C. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 2103-2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertagna, A., Toptygin, D., Brand, L., and Barrick, D. (2008) Biochem. Soc. Trans. 36 157-166 [DOI] [PubMed] [Google Scholar]
- 31.Del Bianco, C., Aster, J. C., and Blacklow, S. C. (2008) J. Mol. Biol. 376 131-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai, E. C. (2002) EMBO Rep. 3 840-845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kao, H. Y., Ordentlich, P., Koyano-Nakagawa, N., Tang, Z., Downes, M., Kintner, C. R., Evans, R. M., and Kadesch, T. (1998) Genes Dev. 12 2269-2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou, S., Fujimuro, M., Hsieh, J. J., Chen, L., Miyamoto, A., Weinmaster, G., and Hayward, S. D. (2000) Mol. Cell Biol. 20 2400-2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petcherski, A. G., and Kimble, J. (2000) Nature 405 364-368 [DOI] [PubMed] [Google Scholar]
- 36.Wu, L., Aster, J. C., Blacklow, S. C., Lake, R., Artavanis-Tsakonas, S., and Griffin, J. D. (2000) Nat. Genet. 26 484-489 [DOI] [PubMed] [Google Scholar]
- 37.Fryer, C. J., Lamar, E., Turbachova, I., Kintner, C., and Jones, K. A. (2002) Genes Dev. 16 1397-1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallberg, A. E., Pedersen, K., Lendahl, U., and Roeder, R. G. (2002) Mol. Cell Biol. 22 7812-7819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fryer, C. J., White, J. B., and Jones, K. A. (2004) Mol. Cell 16 509-520 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.