Abstract
Although a clinical connection between pain and depression has long been recognized, how these two conditions interact remains unclear. Here we report that both mechanical allodynia and depression-like behavior were significantly exacerbated after peripheral nerve injury in Wistar-Kyoto (WKY) rats, a genetic variation of Wistar rats with demonstrable depression-like behavior. Administration of melatonin into the anterior cingular cortex contralateral to peripheral nerve injury prevented the exacerbation of mechanical allodynia with a concurrent improvement of depression-like behavior in WKY rats. Moreover, there was a lower plasma melatonin concentration and a lower melatonin receptor expression in the anterior cingular cortex in WKY rats than in Wistar rats. These results suggest that there exists a reciprocal relationship between mechanical allodynia and depression-like behavior and the melotoninergic system in the anterior cingular cortex might play an important role in the interaction between pain and depression.
Keywords: Allodynia, Melatonin, Depression, Anterior cingular cortex, Neuropathic pain, Nerve injury, WKY rat
Introduction
An apparent clinical relationship between pain and depression has long been recognized. Several epidemiological studies demonstrate that pain and depression frequently co-exist in up to 70% of chronic pain cases (Arnow et al, 2006; Bair et al., 2003; Magni et al, 1985; Von Knorring, et al., 1983). Depression has been shown to result in decreased pain threshold and increased analgesic requirement (Jackson, 2003). It is estimated that the occurrence of depression in patients with chronic pain is higher, ranging from 30% to 54%, than that (about 17%) in the general population (Sullivan et al., 1992; Banks, Kerns, 1996; Ferrer-Garcia et al., 2006). Similarly, the presence of a depressive disorder significantly increases the risk of developing chronic pain (Leino, Magni, 1993; Magni et al., 1993; 1994). Patients with a previous history of clinical depression are at least twice as likely to develop a chronic pain condition than patients without depression (Von Korff, Simon, 1996). While antidepressants affect mood changes in chronic pain patients, they do not always improve outcome measures of clinical pain (Littlejohn, Guymer, 2006; Carter 2002). In this regard, several studies have suggested that the effect of antidepressants on chronic pain may not be related to their anti-depression property (Atkinson et al., 1998; Collins et al.2000; Max et al., 1987; 1992; Mico et al., 2006; Sharav et al., 1987). To date, the relationship between pain and depression remains unclear.
A subset of Wistar-Kyoto (WKY) rats, a genetic variation of the Wistar strain (Okamoto and Aoli, 1963, Porsolt et al., 1977; 1978), as well as Flinders sensitive line (FSL) rats (Overstreet et al., 2005), have been used as preclinical models of depression. In comparison with normal Wistar rats, WKY rats demonstrate hormonal, behavioral, and physiological changes that resemble those found in patients with clinical depression. For instance, WKY rats are hypersensitive to stress secondary to the disrupted hypothalamic-pituitary-adrenal and hypothalamic-pituitary-thyroid axes. Moreover, WKY rats exhibit an overall decreased activity, few exploratory behaviors, hypolocomotion, and a high level of behavioral immobility in the forced swimming test (Pare, 1993, 1994, 1996; Armario, 1995). Pharmacologically, desipramine (a tricyclic antidepressant) acutely reverses depression-like behavior such as a prolonged duration of immobility in the forced swimming test in WKY rats (Porsolt et al., 1978; Lopez-Rubalcava, Lucki, 2000; De La Garza, Mahoney 2004).
Previous studies have suggested that common biological pathways and neurotransmitters including serotonin and norepinephrine may be involved in the mechanisms of pain and depression (Schatzberg, 2004; Fishbain,1997) and that certain brain regions such as the anterior cingular cortex (ACC) play a critical role in the integration of mood and nociception (Johansen et al., 2001; Gao et al., 2004; Stacey C. LaGraize, et al., 2004; Frankland and Teixeira, 2005). In addition, melatonin (5-methoxy-N-acetyltryptamine), a pineal neurohormone and a derivative of serotonin, may be critically involved in the regulation of both mood and pain (Sugden, 1983; El-Shenawy et al., 2002). It has been shown that melatonin receptor type 1(MT1)-knockout mice displayed depression-like behavior with altered sensory responses and attention deficits (Weil et al., 2006). Moreover, melatonin has been shown to produce antinociception and enhance morphine analgesia mediated through spinal MT receptors (Tu et al., 2004; Li, 2005). These data suggest that the central melatoninergic system might play an important role in the mechanism of interactions between pain and depression and ACC could be a forebrain region of interest in this process.
Using a preclinical model of combined depression-like behavior and nociceptive behavior (mechanical allodynia) induced by chronic constriction nerve injury (CCI) in WKY rats, we sought to 1) compare the degree of mechanical allodynia after CCI between WKY and Wistar rats and 2) examine the effect of melatonin on both mechanical allodynia and depression-like behavior by its direct administration into ACC.
Results
Exacerbation of depression-like behavior in WKY rats following CCI
The baseline immobility score in the forced swimming test (duration of immobility in seconds during a 5-min period) was nearly three times higher in WKY rats than in Wistar rats (Fig. 1A, B, n=6, P< 0.01). CCI significantly increased the immobility score in the forced swimming test in both WKY and Wistar rats when examined on day 3 after CCI (Fig. 1A,B, each P< 0.05). This increased immobility score reached a plateau on postoperative day 3 in Wistar rats and there were no differences in the immobility score between Wistar-CCI and Wistar-sham rats (Fig. 1B, P> 0.05), suggesting that the surgical incision for either CCI or sham operation may have had some initial impact on the forced swimming test in both Wistar and WKY rats. In contrast, WKY rats had a persistent increase in the immobility score on both postoperative day 3 and 7 (Fig. 1A). Furthermore, the immobility score was consistently higher in WKY-CCI rats than WKY-sham rats and in WKY-CCI rats than Wistar-CCI rats at each testing point after CCI (Fig. 1A, B, P< 0.05).
Fig. 1. Exacerbation of depression-like behavior after CCI.
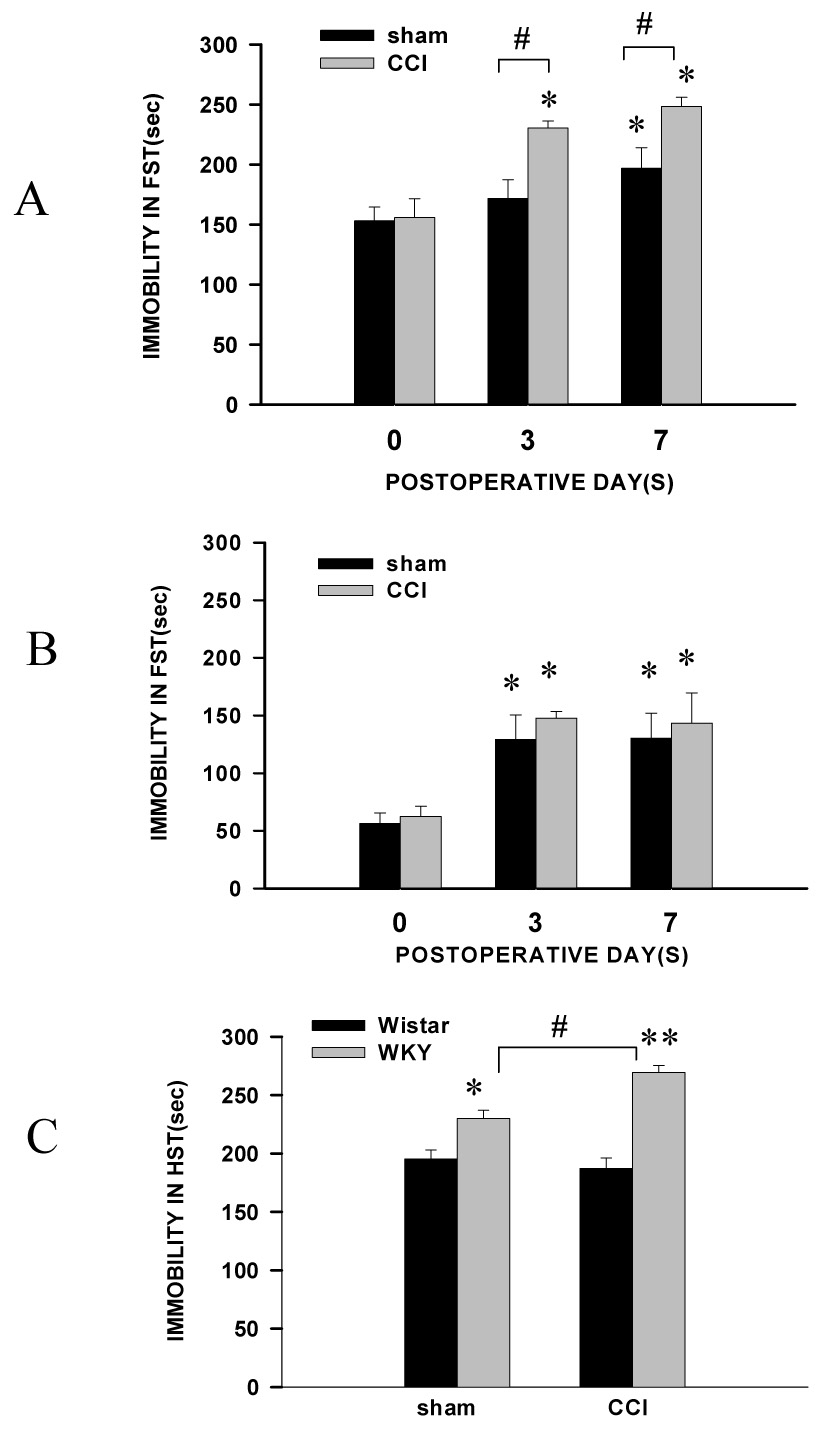
A, B: There were differences in the baseline immobility time in the forced swimming test between WKY (A) and Wistar rats (B). The duration of immobility was increased after CCI in both WKY (A) and Wistar (B) rats. However, the duration of immobility remained significantly higher in WKY rats with CCI than WKY rats with sham operation. C: The immobility time in the horizontal suspension test was longer in WKY rats than Wistar rats with CCI or sham operation, when examined on postoperative day 7. * P <0.05, as compared to the baseline of each corresponding group. # P<0.05 as compared with sham rats of each group. R: Wistar rats; Y: WKY rats.
In a horizontal suspension test performed on postoperative day 7, the immobility time was significantly increased in WKY rats with CCI, but not in Wistar rats, as compared with sham-operated WKY or Wistar rats (Fig. 1C, P< 0.05, n=6). This observation was consistent with the results obtained using an open field test in both Wistar and WKY rats. The score reflecting exploratory and locomotor activities (frequency of rearing and crossing during a 5-min period) was significantly lower in WKY rats than Wistar rats on postoperative day 7 (Table 1, n=6, P< 0.05 or 0.01). Collectively, the data indicate that, as compared with Wistar rats, WKY rats showed a consistently lower baseline level of activity and exacerbation of depression-like behavior after CCI.
Table 1.
Open Field Test
| Rearing (# of episodes) | Crossing (# of episodes) | ||
|---|---|---|---|
| Wistar | Sham | 22.6±3.3 | 26±2.0 |
| Wistar | CCI | 11.2±1.5# | 20.6±2.2 |
| WKY | Sham | 4.0±1.4* | 5.2±1.1** |
| WKY | CCI | 3.0±1.0* | 5.2±0.7** |
Numbers represent group mean±standard errors.
P< 0.05
P< 0.01, as compared to each corresponding group of Wistar rats
P< 0.05, as compared to Wistar rats with sham operation.
Exacerbation of mechanical allodynia in WKY rats with depression-like behavior
There were no differences in the baseline mechanical nociceptive threshold between WKY and Wistar rats (Fig. 2A,B, P>0.05, n= 6), nor were there differences in the postoperative mechanical nociceptive threshold in hindpaws contralateral to CCI in either WKY or Wistar rats when compared with corresponding WKY or Wistar rats with sham operation (Fig. 2A, P>0.05, n=6), indicating that the presence of depression-like behavior in WKY rats did not alter the baseline nociceptive response to mechanical stimulation.
Fig. 2. Exacerbation of mechanical allodynia after CCI.
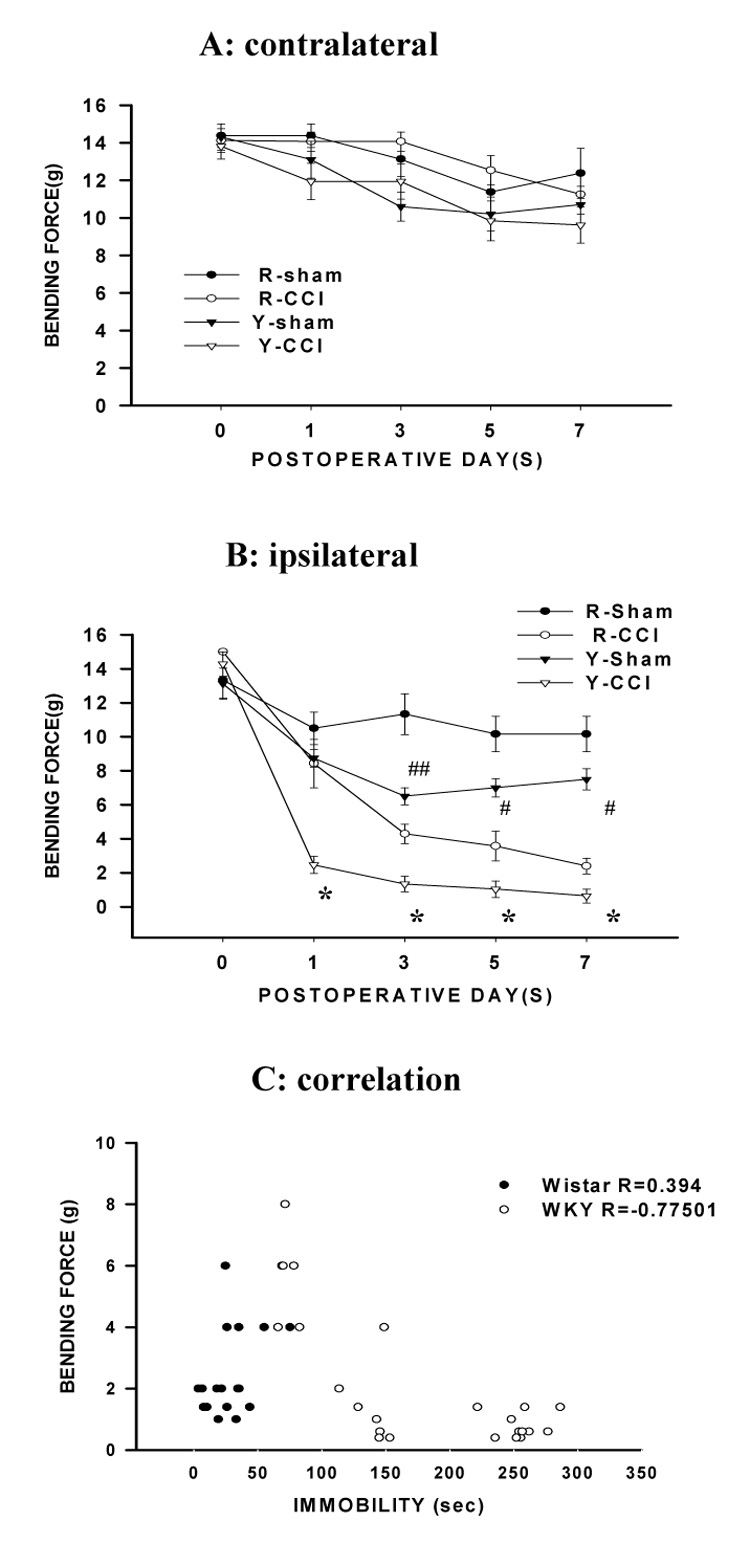
A, B: There were no differences in baseline mechanical nociceptive threshold (in bending force) between WKY and Wistar rats and both WKY and Wistar rats developed mechanical allodynia in the ipsilateral (B) but not contralateral (A) hindpaw after CCI. However, the threshold bending force was lower in WKY rats than in Wistar rats, indicating the exacerbation of mechanical allodynia after CCI in WKY rats. *P<0.05, as compared to Wistar-CCI rats; #P<0.05 or ## P<0.01, as compared to Wistar-sham rats. R-sham and Y-sham: Wistar and WKY rats with sham operation. R-CCI and Y-CCI: Wistar and WKY rats with CCI. C: A longer immobility time in the forced swimming test was inversely correlated with a lower threshold bending force in mechanical allodynia test in WKY rats (R= −0.775) but not in Wistar rats (R=0.394).
While both WKY and Wistar rats showed mechanical allodynia (decreased threshold to von Frey filament stimulation) in the hindpaw ipsilateral to CCI, mechanical allodynia was significantly exacerbated in WKY rats as compared with that in Wistar rats (Fig. 2B). Specifically, (1) the onset of mechanical allodynia after CCI was shorter in WKY rats than Wistar rats such that significant mechanical allodynia developed on postoperative day 1 in WKY rats instead of on day 3 in Wistar rats (Fig. 2B, P< 0.01, n=6); (2) the mechanical nociceptive threshold was significantly lower in WKY rats than Wistar rats on each day of behavioral testing (Fig. 2B, p<0.01, n= 6); and (3) WKY rats also exhibited a significantly lower mechanical nociceptive threshold than Wistar rats after sham operation (Fig. 2B, P< 0.05, n=6). These results indicate that CCI exacerbated mechanical allodynia (both onset and degree) in WKY rats as compared with Wistar rats.
A correlative relationship between mechanical allodynia and depression-like behavior was examined by using the Spearman’s rank analysis. The analysis showed that a longer immobility time in the forced swimming test (in seconds) inversely correlated with a lower mechanical nociceptive threshold (bending force in grams) in WKY rats (Fig. 2C; R= − 0.775, n=25, P< 0.001; Y=−0.0227X+6.2362) but not in Wistar rats (Fig. 2C; R= 0.394, n =17, P> 0.05; Y=0.0304X+1.5994). In this analysis, the data on mechanical allodynia and forced swimming test were pooled from WKY and Wistar rats in several experimental groups (see below) in order to make a meaningful analysis. The data indicates a correlative relationship between the degree of depression-like behavior and mechanical allodynia in WKY rats.
Improvement of mechanical allodynia and depression-like behavior by intra-ACC melatonin
Since depression-like behavior correlated with exacerbated mechanical allodynia after CCI in WKY rats, improving depression-like behavior might be expected to modulate mechanical allodynia in WKY rats. To examine this possibility, melatonin (4 µg) was administered into the ACC contralateral to CCI through an implanted cannula. The injection was given once daily for seven consecutive days after either CCI (WKY-CCI) or sham operation (WKY-sham). The melatonin dose (4 µg) was selected following a pilot dose-response experiment that showed no effect of a lower melatonin dose (2 µg) on depression-like behavior in WKY rats.
This melatonin treatment significantly improved depression-like behavior in both WKY-sham and WKY-CCI rats such that the duration of immobility in the forced swimming test was reduced on day 7, as compared with vehicle-treated WKY-sham and WKY-CCI rats (Fig. 3A, P<0.05, n=6). This treatment also improved the immobility score in the horizontal suspension test in WKY rats (Fig. 3B, P<0.05, n=5). Moreover, mechanical allodynia was significantly improved in these same WKY rats receiving the 7-day melatonin treatment. In particular, 1) intra-ACC melatonin mainly reversed exacerbation of mechanical allodynia in WKY rats (Fig 4A, P< 0.01, n=6) because the mechanical nociceptive threshold did not return back to the baseline in these WKY rats (Fig. 4A) and 2) the same melatonin (4 µg) treatment failed to improve mechanical allodynia in Wistar-CCI rats (Fig. 4C, P> 0.05, n=6), nor did it change the baseline mechanical nociceptive threshold in WKY-sham rats (Fig. 4A, P> 0.05, n=6).
Fig. 3. Effect of melatonin on depression-like behavior.
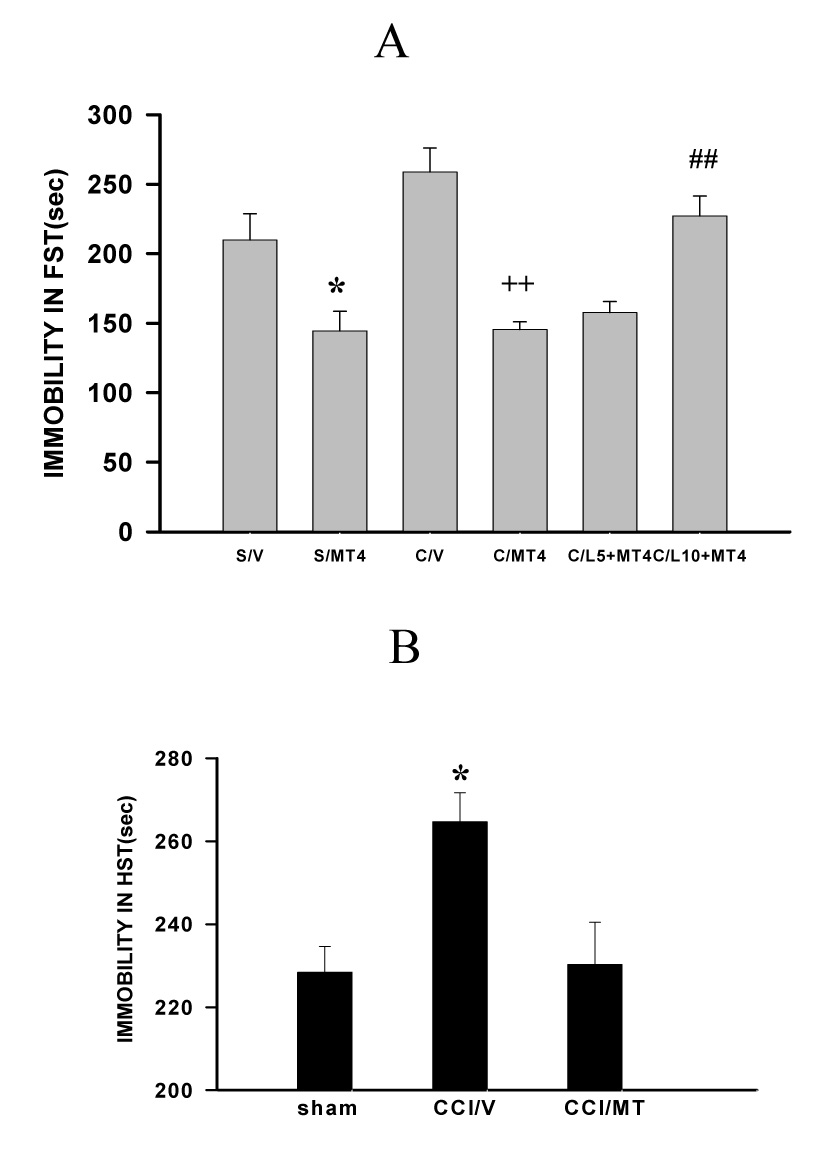
A: When examined on postoperative day 7, the immobility time in the forced swimming test (FST) in WKY rats was significantly improved by melatonin (MT, 4 µg, once daily × 7 days) administered into the ACC contralateral to CCI. The melatonin effect was blocked by the broad MT receptor antagonist luzindole [10 µg but not 5 µg (L10, L5)] injected into the same ACC at 10 min before melatonin (4 µg). *P<0.05, as compared with the sham-vehicle (V) group; ++ P<0.01, as compared with the CCI-vehicle group; ## P<0.01, as compared with the CCI-MT group. B. The same melatonin (MT) treatment also improved the immobility time in the horizontal suspension test (HST) in WKY rat on day 7 after CCI. *P#0.05, as compared with sham and CCI-MT groups.
Fig. 4. Effect of melatonin on exacerbated mechanical allodynia.
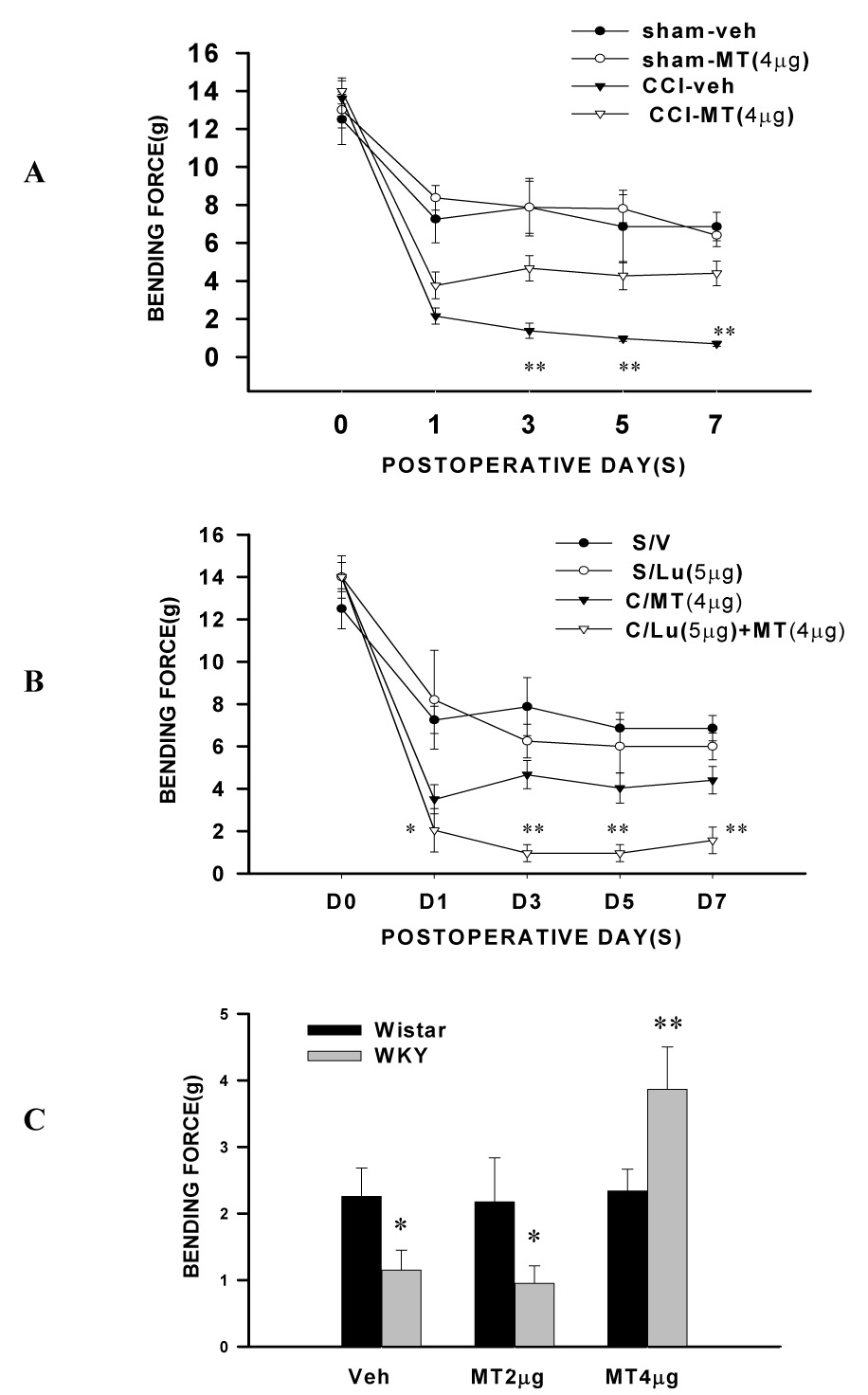
A, B: Exacerbation of mechanical allodynia was significantly improved in WKY rats by administration of melatonin (MT, 4 µg, once daily × 7 days) into the ACC contralateral to CCI. Melatonin alone did not affect the behavioral response in WKY rats with sham operation (P>0.05). ** P< 0.01, as compared with CCI-MT groups. The melatonin effect was blocked by luzindole (Lu) injected into the contralateral ACC at 10 min before melatonin (4 µg). *P<0.05, **P<0.01, as compared with CCI-MT groups. C: The effect of melatonin (MT) was dose-dependent (4 > 2 µg =vehicle) in WKY rats and melatonin (2 or 4 µg) did not change mechanical allodynia in Wistar rats after CCI. * P< 0.05 and ** P<0.01, as compared to Wistar rats.
The above melatonin effects were mediated through MT receptors within ACC because intra-ACC co-administration of melatonin (4 µg) and a broad MT receptor antagonist luzindole (5 or 10 µg; once daily × 7 days) blocked the melatonin effect on both depression-like behavior (Fig. 3, P< 0.05; n=6) and mechanical allodynia in WKY rats (Fig. 4, P< 0.05; n=6; 5 µg luzindole), whereas intra-ACC luzindole alone did not significantly change the baseline mechanical nociceptive threshold in WKY-sham rats (Fig. 4, P> 0.05, n=6). Collectively, these results indicate that intra-ACC melatonin concurrently improved depression-like behavior and attenuated mechanical allodynia in WKY rats through activation of MT receptors.
Lower plasma melatonin concentration and MT receptor expression in WKY rats
Since exogenous melatonin improved depression-like behavior and attenuated mechanical allodynia in WKY rats, it suggests that there might be differences in plasma melatonin concentration and/or MT receptor expression in ACC between WKY and Wistar rats. This possibility was examined using ELISA (plasma melatonin concentration), real-time PCR and Western blot (MT receptor expression). The data showed that the basal plasma melatonin concentration was significantly lower in WKY rats than in Wistar rats (Fig. 5, P< 0.05, n=6). When examined on day 7 after CCI, the plasma melatonin concentration was increased in Wistar rats (Fig. 5, P<0.05) but remained low in WKY rats (Fig. 5, P>0.05, n=6). However, the difference in the ratio of the plasma melatonin concentration between WKY and Wistar rats remained similar before and after CCI (see Fig. 5).
Fig. 5. Plasma melatonin concentration.
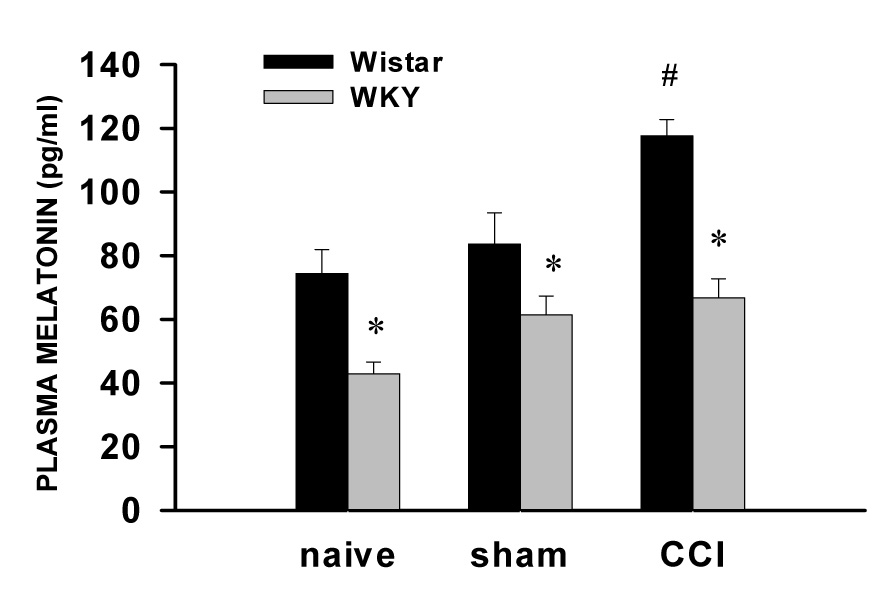
The basal plasma melatonin concentration was lower in WKY rats than in Wistar rats. On day 7 after CCI, the plasma melatonin concentration remained lower in WKY rats but increased in Wistar rats. * P<0.05, as compared with Wistar rats; # P<0.05, as compared with sham or naive Wistar rats.
In addition, the basal MT1 receptor expression in ACC of WKY rats was lower at the mRNA and protein level than that of Wistar rats (Fig. 6, P<0.05, n=6), whereas the basal MT2 receptor expression in ACC was similar between WKY and Wistar rats (Fig. 6, P>0.05; n=6). After CCI, the expression of both MT1and MT2 receptors in the contralateral ACC was downregulated in both WKY and Wistar rats (Fig. 7, P< 0.05, n=6). However, the expression of MT1 and MT2 receptor was significantly lower in WKY rats than in Wistar rats when examined on postoperative day 3 and day 7 (Fig. 7, P< 0.05, n=6). There were no differences in the MT1 and MT2 receptor expression in the contralateral ACC between WKY-sham and WKY-naïve rats (Fig. 6 & Fig. 7, P> 0.05, n=5), indicating a negligible influence of sham operation on the MT receptor expression. These data indicate that WKY rats had a lower plasma melatonin concentration and lower intra-ACC MT receptor expression than Wistar rats both before and after CCI.
Fig. 6. Expression of MT1 and MT2 receptor in ACC.
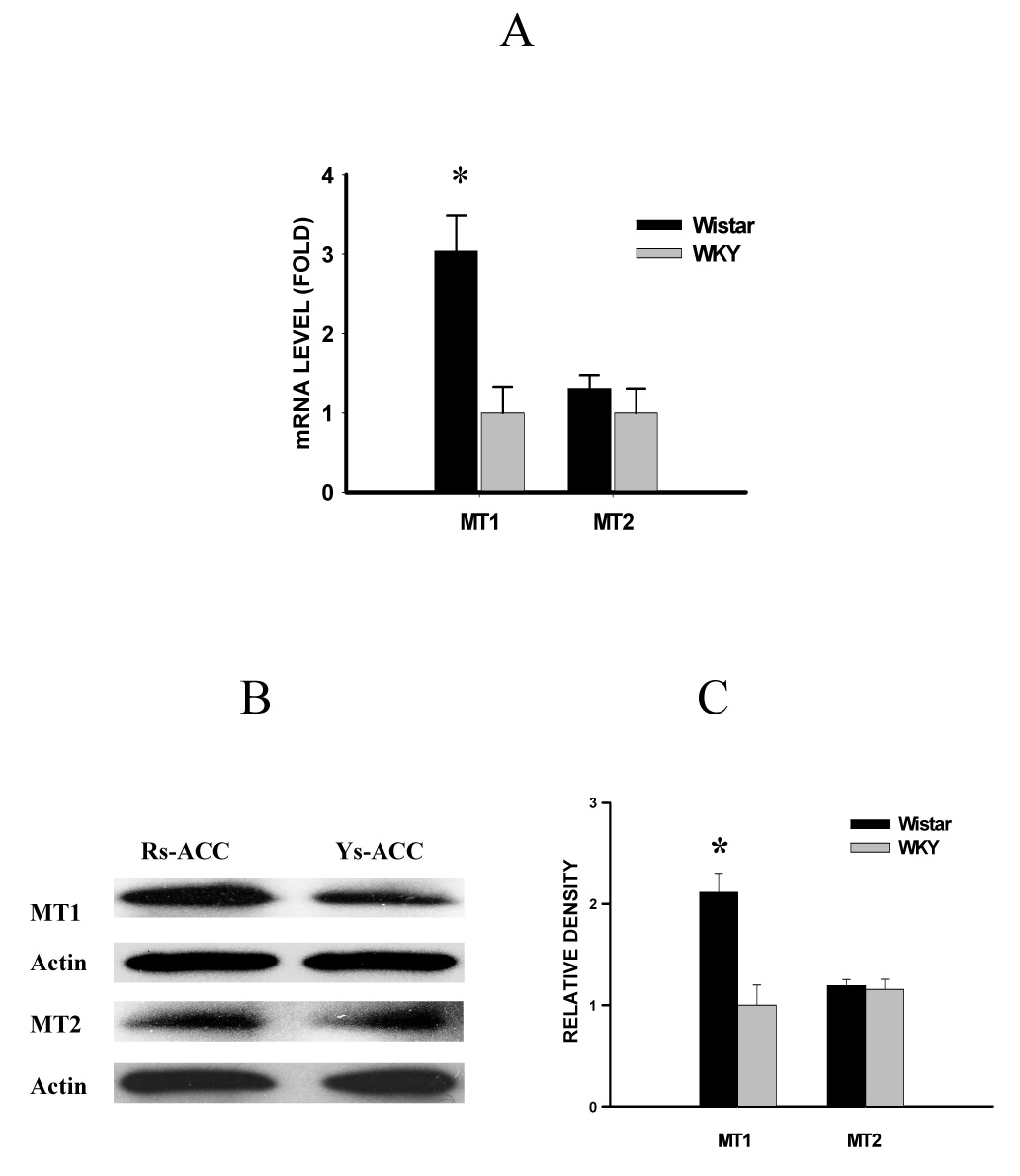
The basal MT1 receptor mRNA (A: real-time PCR) and protein level (B: Western blot; C: statistics) in the contralateral ACC was lower in WKY than in Wistar rats. There were no significant differences in the basal MT2 receptor mRNA and protein level in the contralateral ACC between WKY and Wistar rats. A, C: *P<0.05, as compared to WKY rats. Rs-ACC and Ys-ACC: samples taken from the contralateral ACC of Wistar and WKY rats, respectively.
Fig. 7. Expression of MT1 and MT2 receptor in ACC after CCI.
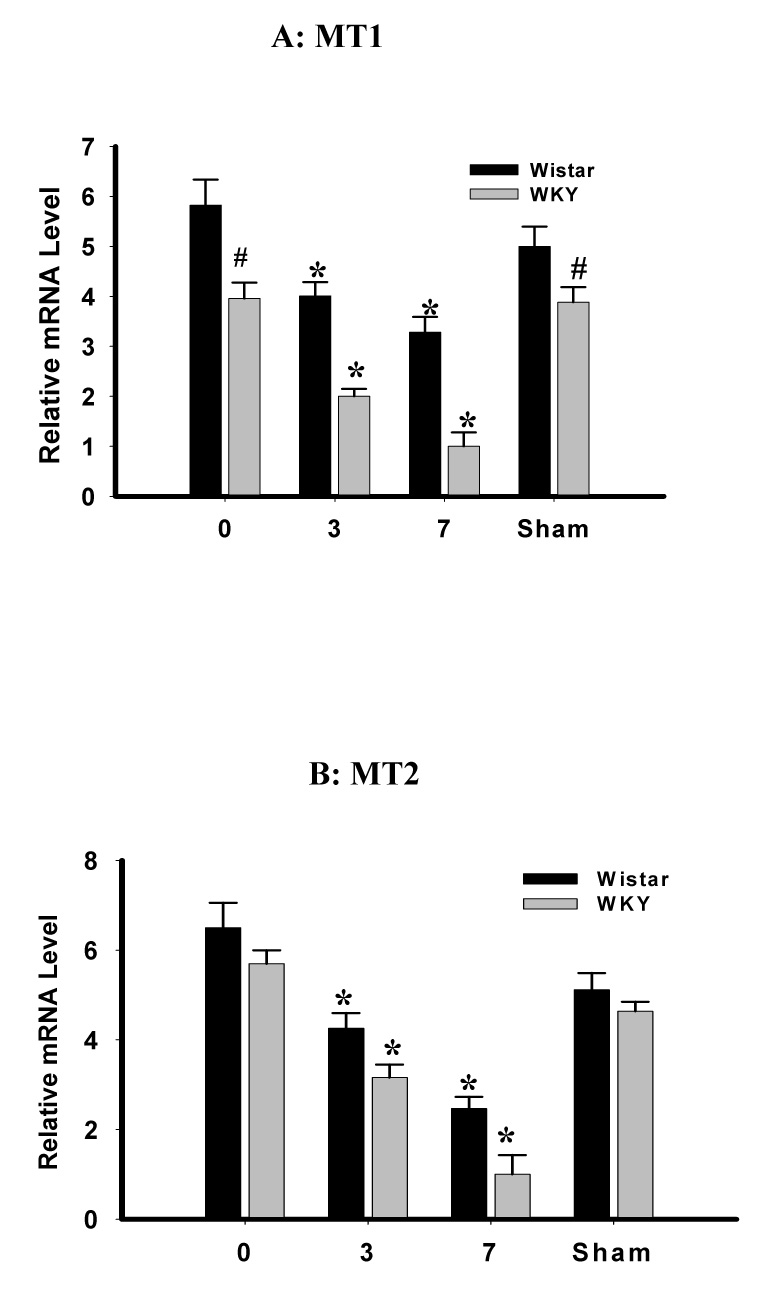
A, B: The MT1 (A) and MT2 (B) receptor expression (real-time PCR) in the contralateral ACC was downregulated after CCI in both WKY and Wistar rats. However, the expression of MT1 and MT2 receptors in WKY rats was significantly lower than that in Wistar rats when examined on postoperative day 3 (MT2) and day 7 (MT1 & MT2). *P<0.05, as compared to day 0 in each corresponding group. # P<0.05, as compared to Wistar rats on the same day.
Discussion
Using a preclinical model of combined depression-like behavior and peripheral nerve injury, we demonstrated that the presence of depression-like behavior exacerbated mechanical allodynia in WKY rats and that intra-ACC administration of melatonin concurrently attenuated mechanical allodynia and improved depression-like behavior in WKY rats without changing the nociceptive response in Wistar rats. In addition to these behavioral findings, WKY rats exhibited a lower level of plasma melatonin concentration and intra-ACC MT receptor expression as compared with Wistar rats. These results indicate that there exists a reciprocal relationship between depression-like behavior and nociceptive behavior (mechanical allodynia) in WKY rats and that the melatoninergic system within ACC could play a significant role in this relationship.
The present data should be interpreted within the limitation of this preclinical model, because WKY rats have been extensively used as a preclinical model of hereditary depression-like behavior whereas clinical depression is likely to be multi-factorial including both genetic and environmental influences. Of note is that, in a previous study examining the similarities and differences in neuropathic pain-related behavior among eight rat strains (Shir et al., 2001), FSL rats, a rat strain also displaying depression-like behavior (Overstreet et al., 2005), showed less allodynia and hyperalgesia induced by partial nerve ligation as compared to Sprague-Dawley rats. It would be of interest in future studies to examine whether differences in nociceptive behavior from different animal models of depression are due to differences in rat strains (e.g., WKY versus FSL) and/or type of nerve injury (e.g., CCI versus partial nerve ligation). In this study, female WKY and Wistar rats were not included considering complex issues regarding the relationship between sex, depression and pain. Since the clinical data suggests that the relationship between pain and depression appears to be more prevalent in female subjects (Sullivan et al., 1992; Banks, Kerns, 1996; Ferrer-Garcia et al., 2006), future studies should examine whether depression-like behavior would have a stronger influence on nociceptive behavior in female WKY rats.
There are additional methodological considerations in this study. For example, the postoperative immobility time in the forced swimming test was increased in both Wistar and WKY rats, albeit transient in Wistar rats, suggesting that the surgical intervention itself may have had some impact on the rat’s motor function. To examine this issue, the open field test was used in the study to assess exploratory and locomotor activities. In addition, a horizontal suspension test, modified from the tail suspension test (Stéru et al, 1985; Chermat et al. 1986), was used in this experiment in order to provide additional assessment of depression-like behavior. This test also indicated the worsening of depression-like behavior in WKY rats after CCI as compared with Wistar rats and an improvement after intra-ACC melatonin, which is consistent with the results from the forced swimming test. Despite this observation, the validity of this horizontal suspension test has not been independently confirmed. Therefore, the data from this test need to be interpreted with this important caveat. Since WKY rats with sham operation exhibited a more decrease in nociceptive threshold than Wistar rats, it further supports the hypothesis that WKY rats with depression-like behavior would be more sensitive to postoperative mechanical stimulation.
Furthermore, there were some differences in baseline immobility scores in the forced swimming test between different groups of WKY rats. We observed that baseline immobility scores do differ between different batches of WKY rats. Accordingly, the comparisons were made between different groups of WKY rats in each batch. It is also possible that the implantation of a cannula may change the baseline immobility score in the forced swimming test. Since WKY rats in both vehicle and melatonin groups were implanted with the same cannula and the comparisons were made within the same batch of WKY rats, this possibility should not have biased the data analysis. In addition, in a preliminary experiment, we injected the same dose of melatonin (4 µg) into Wistar rats and their immobility score in the forced swimming test did not differ before and after the injection (n=3, data not shown), suggesting that the melatonin effect on immobility scores were selective to WKY rats.
To date, several hypotheses have been proposed regarding the relationship between pain and depression (Fishbain et al., 1997; Dohrenwend et al., 1999; Blackburn-Munro, Blackburn-Munro, 2001). Recent studies have suggested that certain brain regions such as amygdala, ACC, and the prefrontal cortex (Schatzberg, 2004; Zhang et al., 2005; Vogt, 2005) and various neurotransmitters including acetylcholine, GABA, serotonin, norepinephrine, and dopamine (Jasmin et al., 2003; 2004; Schatzberg, 2004; Rabe-Jablonska, Miller, 2005) may play a significant role in the interaction between mood changes and nociception. In addition, the hypothalamic-pituitary-adrenal (HPA) axis and alteration of brain-derived neurotrophic factor have been implicated in the relationship between pain and depression as well (Nibuya et al., 1995; 1999; Duman et al., 1997; Tsigos, Chrousos, 2002; Korszun, 2002; Blackburn-Munro, Blackburn-Munro, 2003; Duric, McCarson, 2005; Walf, Frye, 2005; Gameiro et al., 2006).
In the present study, we examined the role of the melatoninergic activity within ACC in modulation of nociceptive and depression-like behavior in WKY rats. Melatonin is a hormone secreted by the pineal gland, which regulates important biological functions including circadian rhythms, sleep, and mood (Sugden, 1983; Morgan et al., 1994; Vanecek, 1998; Raghavendra et al., 2000; Szymanska et al., 2001; El-Shenawy et al., 2002; von Gall et al., 2002; Zahn et al., 2003; Pandi-Perumal et al., 2006). Melatonin receptors are located in the spinal cord and various brain regions (Vitte et al., 1990; Stankov et al., 1991; Morgan et al., 1994; Zahn et al., 2003). It has been shown that melatonin produces a transient antinociceptive effect in rats and mice (Yu et al., 2000a, b; Tu et al., 2004; Onal et al., 2004), modulates lipopolysaccharide-induced hyperalgesia possibly via its influence on the role of proinflammatory cytokines in sensory neurons (Raghavendra et al., 2000), and interacts with opioid antinociception (Golombek et al., 1991; Raghavendra, Kulkarni, 2000; Pang et al., 2001; Li et al., 2005; Shavali et al., 2005). In clinical studies, melatonin has been reported to reduce cluster headache, irritable bowel syndrome, and fibromyalgia (Leone et al., 1996; Citera et al., 2000; Song et al., 2005), although the relationship between depression and chronic pain was not specifically examined in these clinical reports. Evidence also exists that implicates melatonin for the mood regulation (Brzezinski, 1997). For example, clinical depression is associated with nocturnal and diurnal changes in the plasma melatonin concentration (Halbreich et al., 1981; Wetterberg, 1985; Beck-Friis et al., 1985; Frazer et al., 1986; Szymanska et al., 2001). Recent preclinical studies also indicate that knockout of MT1 receptors increased (Weil et al., 2006), whereas a melatonin analog decreased, the immobility time in the forced swimming test (Overstreet et al., 1998). In addition, clinical trials have been proposed to examine the possible use of a melatonin analog in the depression treatment (Guardila-Lemaitre, 2005; den Boer et al., 2006).
Our data indicate an important role of intra-ACC melatoninergic activity in regulating the interaction between pain and depression, since intra-ACC administration of melatonin improved depression-like behavior and attenuated mechanical allodynia after CCI in WKY rats. Of note is that those rats with an incorrect cannula placement did not show improvement in either immobility score or mechanical allodynia, suggesting a selective effect of melatonin within ACC. This behavioral data is also consistent with a low plasma melatonin level in WKY rats and in patients suffering from abdominal pain, idiopathic pain, neuropathic pain, or cluster headache (Waldenlind et al., 1987; Almay et al., 1987; Roberts-Thompson et al., 1988; von Knorring, Ekselius, 1994; Leone et al., 1995). It should be noted that the role of a specific MT receptor subtype was not examined in this study, because luzindole (MT2 > MT1) is not a highly selective antagonist for an MT receptor subtype. Of interest to note is that there was a difference in the concentration of luzindole required to block the melatonin effect in the forced swimming test (10 µg) and to attenuate mechanical allodynia (5 µg), although the same concentration of melatonin (4 µg) improved both conditions. Future studies using MT receptor subtype-selective antagonists and/or knockout mice may help elucidate this issue. It should be emphasized that the current data indicate a correlation between depression and pain, although the improvement by melatonin of both depressive behavior and pain (nociceptive) behavior lends some support to a functional relationship between depression and pain in this animal model. Accordingly, more studies are needed in this area to further elucidate the relationship between pain and depression, including animal models of induced depressive behaviors and examination of the relationship between a melatonin deficiency state and pain behaviors.
The relationship between pain and clinical depression is an important and under-investigated issue. Although antidepressants have been used in clinical pain management, their mechanisms of action remain unclear. For example, nortriptyline (a tricyclic antidepressant) improved pain in patients with low back pain but without clinical depression (Collins et al.2000). In addition, certain types of antidepressants including selective serotonin reuptake inhibitors (SSRI) and selective norepinephrine reuptake inhibitors (SNRI) failed to produce a better outcome than placebo for chronic pain management in some clinical studies (Mico et al., 2006). Our results provide evidence that this preclinical model of combined nociceptive and depression-like behaviors may be useful to investigate the mechanisms underlying the interaction between pain and depression and to explore new treatment options for this significant clinical issue. Our data also suggest that melatonin may be a useful agent for treating combined symptoms of pain and depression.
Materials and Methods
Animals and CCI model
Adult male WKY and Wistar rats (10–11 weeks old) weighing 250–325 gm were used (Charles River Laboratories, Wilmington, MA) and maintained by the accredited animal facility at the Massachusetts General Hospital. The animal room was artificially lighted from 7 AM to 7 PM and the room temperature was maintained at 22 °C. The food pellets and water were available at libitum and the bedding was changed daily. Rats were housed individually in each cage and the experiments started at least three days after their arrival. The experimental protocol was approved through our Institutional Animal Care and Use Committee. Under pentobarbital anesthesia (50 mg/kg, intraperitoneally), CCI was produced by loosely ligating one side of the common sciatic nerve according to the method of Bennett and Xie (1988). Sham rats were produced following the same surgical procedure except for nerve ligation. Post-operative analgesia was not provided because of the confounding effect of an analgesic on the assessment of mechanical allodynia and depression-like behavior.
Brain cannula implantation and drug injection
A brain cannula was implanted for drug injection at the time of operation for CCI or sham injury. Under the same anesthesia, a rat was securely mounted to a stereotaxic instrument with both Bregma and Lambda line at the horizontal level. A guide cannula (made in our lab using 23 1/2 G needles) with a preset length at 1 mm above the intended brain injection site was embedded into the skull at the site of Bregma 1.6 mm and left 0.8 mm (AP1.6; L0.8, H1.5) according to the rat’s brain atlas by Paxinos and Watson (1986) and secured with dental cement. This guide cannula sealed the tiny hole in the skull made by a 23 1/2 G needle and the small skull incision was closed using one or two stitches with 4-0 silk sutures. Rats exhibiting postoperative abnormal behaviors (e.g., poor eating, grooming) were excluded from the experiment (fewer than 10%).
For injection, a small (30 G) needle was inserted through the guide cannula at a preset depth from the skull surface (H2.5 for ACC). A drug solution (1 µl) was gently injected through the inserted needle. After the injection, the needle was retained for 10 min. At the end of each experiment and for tissue harvest, the injection site was examined according to the rat’s atlas. The correct injection site was defined as the needle tip being located inside the boundary of the ACC as illustrated in figure 8. Rats with an incorrect injection site (a needle tip outside of the ACC or in contact with the boarder of the ACC) were excluded from the data analysis (Fig. 8).
Fig. 8. Location of injection sites within ACC.
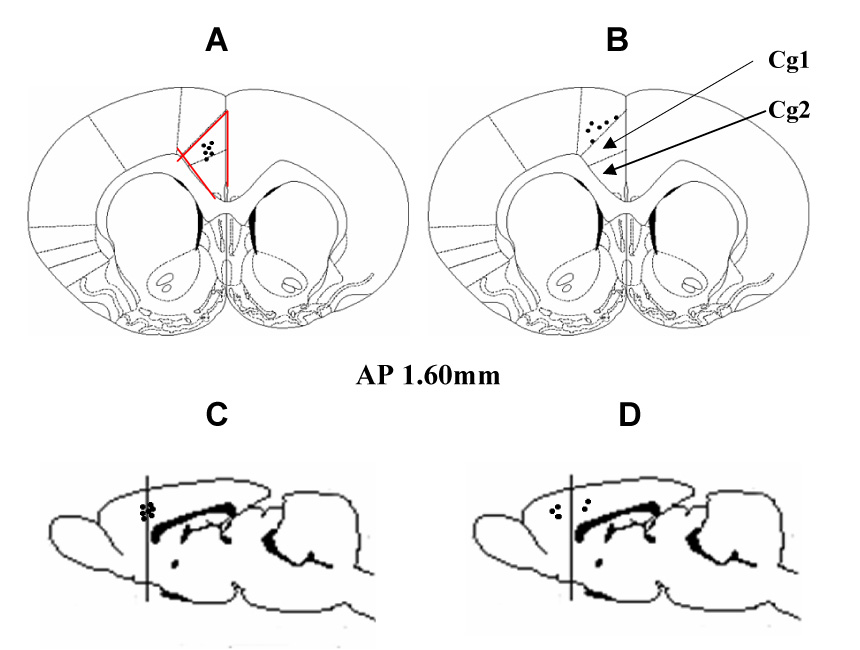
The outlined area shows the ACC (Cg1 and Cg2) region (AP 1.60 mm). A, C: Black dots represent correct sites of needle placement inside ACC. B, D: Black dots indicate incorrect needle placements.
Melatonin and luzindole (a broad MT receptor antagonist; MT2 > MT1) were purchased from Sigma (St. Louis, MO) and dissolved in 5% ethanol diluted in artificial cerebral spinal fluid (CSF) containing (in mM) NaCl 126, KCl 2.5, NaHCO3 26, CaCl2 2, MgCl2 2, NaH2PO4 1.25, glucose 10 (pH 7.35). The dose range for each agent was chosen according to previous studies (Yu et al., 2000b; Tu et al., 2004) and our pilot experiment. When a combination of luzindole and melatonin was indicated, luzindole was given 10 min before the melatonin administration.
Forced swimming test
The forced swim test was performed based on the original method described by Porsolt et al (1977; 1978). One day prior to the test, a rat was placed for conditioning in a clear plastic tank (45×35×60 cm) containing 30 cm of water (24±0.5 °C) for 5 min (pretest session). Twenty-four hours later, the rat was tested under the same condition for 5 min (test session). Both WKY and Wistar rats underwent the same test procedure. Following each session, the rat was removed from the water tank, dried with a towel, placed in a warm cage for 15 min, and then returned to the home cage. Each test session was recorded with a stopwatch. A rat was judged to be non-swimming when its hind legs were no longer moving and the rat was hunched forward (a floating position). The total duration of immobility (non-swimming) within a 5-min session was recorded as immobility scores (in seconds) and compared among groups (Detke et al., 1995; Lopez-Rubalcava and Lucki, 2000). All sessions were observed by the same experimenter blinded to the group assignment to minimize between-experimenter and between-session variations. All test sessions took place between 2 PM and 5 PM.
Horizontal suspension test
To provide additional assessment of depression-like behavior, a horizontal body suspension test was used in both WKY and Wistar rats. This horizontal suspension test was modified from the tail suspension test that has been used to assess the effect of antidepressants on depressive behaviors as compared with antipsychotics or anxiolytics (Stéru et al, 1985; Chermat et al. 1986). The rat’s body was supported by a fabric splint and placed in a horizontal position. This method avoided the hemodynamic stress from being hung by a tail and minimized a potential confounding factor from postoperative limb pain (discomfort). The rat was suspended for 6 min during which the animal showed periods of movement and immobility. The duration of immobility was recorded with a stopwatch as discussed in the forced swimming test. A rat was considered to be immobile when no movement of torso and legs (no struggling, jerks, and body torsions) were observed.
Open field test
Open field test was used to examine exploratory and locomotor activities (Wesierska et al., 2006; Pertsov, 2006; Bellermann et al., 2006; Dias et al., 2006). This test was performed under room lights in an open box (57 × 57 cm with a 43 cm tall wall) marked with peripheral and internal squares (Bellermann et al., 2006; Dias et al., 2006; Pertsov, 2006). During a 5-min session, the rat’s locomotor activity was recorded as the number of crossings into peripheral and internal squares, whereas exploratory activities were assessed as the episodes of rearing against the field wall.
Mechanical allodynia test
Rats were habituated to the test environment once daily (a 60 min session) for two consecutive days before the baseline testing. Each rat was placed on a metal mesh floor, covered with a plastic box (15×15×18 cm), and allowed to settle down for 30 min before the test. Von Frey filaments were used to determine the lowest mechanical threshold required for a brisk paw withdrawal according to the method (using an ascending and descending order of filaments) described by Tal and Bennett (1994) and in our previous report (Mao et al., 1997). A filament was applied perpendicularly to the rat’s plantar area and a 10 second interval was used between two applications. A positive response was defined as at least one clear withdrawal response out of five applications. The cutoff force was 20 gm. All tests were conducted between 2 PM and 5 PM.
Statistical analysis
Data from the above tests were analyzed by using non-parametric Friedman repeated measures ANOVA on rank across various time points to detect overall differences in each group followed by the Newman-Keuls’ test. For comparisons between two groups, the Mann-Whitney rank sum test was used. For all data analysis, differences were considered to be statistically significant at the level of α=0.05. To examine a correlative relationship between mechanical allodynia and depression-like behavior, the Spearman’s correlation coefficient analysis was used to determine the relationship between the immobility score (forced swimming test) and threshold bending force (mechanical allodynia) in WKY and Wistar rats.
ELISA
Blood samples were taken from a jugular vein between 2 PM and 5 PM to minimize natural variation of the serum melatonin level. Samples from individual rats were kept at −80 °C and wrapped with aluminum foil until assay for the photo protection. The melatonin ELISA kit (catalog #RE 540 21) was purchased from IBL (Immuno-Biological Laboratories, Minneapolis, MN). The standard curve was generated using the reference standard set supplied in the kit. Samples were measured according to the instructions provided by the manufacturer. Fifty µL of the standard or the extracted sample from each rat were first put into individual plate wells, followed by adding 50 µL each of melatonin biotin and antiserum. The plate was well shaken, sealed with adhesive foil, and incubated for 20 h at 4 °C under a dark condition. Next day, each well was washed three times with the assay buffer and added with 150 µL of an enzyme conjugate supplied in the kit. The plate was sealed again and incubated for 120 min at room temperature in a shaker. Each well was again washed three times with the assay buffer and added with 200 µL of para-nitrophenyl phosphates (PNPP) substrate solution. The plate was again incubated for 30 min at room temperature in a shaker. The reaction was stopped after 50 µL of a PNPP stop solution was added into the wells. The absorbance values were assayed using a microplate reader (Bio-TeK Instruments Winooski, VT) at the wavelength of 405 nm. The serum melatonin concentration was calculated based on the standard curve and presented as pg/ml.
Real-time PCR
Rats were decapitated under pentobarbital anesthesia after the last behavioral test, ipsilateral and contralateral ACC (to CCI) was separately harvested and rapidly frozen on dry ice, stored at −80 °C for later assay. Total RNA was isolated from tissue samples with Trizol Reagent® (Invitrogen, Carlsbad, CA). After the DNase treatment (Promega, Madison, WI), RNA was reverse-transcribed to first strand cDNA using primers and M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA). Real-time quantitative PCR was performed using an Invitrogen SYBR green kit on an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA). The primers for MT1 were: 5’-TGCTACATTTGCCACA GTCTC-3’ (forward) and 5’-GACCTATGAAGTTGAGTGGGG-3’ (reverse); for MT2: 5’-TACATCAGCCTCATCTGGCTT-3’ (forward) and 5’-CACAAACAC TGCGAAC ATGGT-3’ (reverse); and for GAPDH (control): 5’-TTCACCACCA TG GAGAAGGC-3’ (forward) and 5’-GGCATGGACTGTGGTCATGA-3’(reverse). PCR amplification began with a 10 min preincubation period at 95 °C, followed by 43 cycles of denaturation at 94 °C for 30 s, annealing at 57 °C (MT1, MT2) or 55 °C (GAPDH) for 30 s, and elongation at 72 °C for 1 min. All experiments were performed in triplicates.
Western blot
ACC tissue samples (see above) were homogenized in a homogenization buffer (20mM Tris-HCl, pH 7.5, 150mM NaCl, 1mM EDTA, 1mM EGTA, 2.5mM Sodium pyrophosphate, 1mMβ-Glycerophosphate, 1mM Sodium Orthovanadate, 0.01% Tirton-X100, 0.01% NP-40) containing a mixture of proteinase inhibitors (Sigma). The tissue homogenates were centrifuged at 12,000 rpm × 20 min at 4 °C. Protein samples (100 µg) were loaded and separated on SDS-PAGE gel (12%) and transferred to polyvinylidene difluoride filters (Millipore, Bedford, MA). The filters were blocked with 5% non-fat dry milk and subsequently incubated overnight at 4 °C with a primary antibody (MEL-1A-R, sc-13186; MEL-1B-R, sc-13177, goat polyclonal, 1:100, Santa Cruz), and 1 hr at room temperature with an appropriate HRP-conjugated secondary antibody (Donkey anti-goat 1:4000; Santa Cruz). ECL solution was used for visualization and the blots were exposed onto a hyperfilm (Amersham) for 1–10 min. The blots were then incubated in a stripping buffer (67.5 mM Tris, pH 6.8, 2% SDS, and 0.7% β-mercaptoethanol) for 30 min at 50 °C and reprobed with a monoclonal rabbit anti-β-actin antibody (1:12,000; Alpha Diagnostic International, San Antonio) as loading control. Samples were probed in triplicates. The density of each specific band was measured with Adobe PhotoShop and normalized against a corresponding β-actin loading control. Differences in normalized band density among different groups were compared using one-way ANOVA followed by post hoc Newman–Keuls' tests.
Acknowledgment
This work was supported by US PHS RO1 grants NS45681 and DE18538.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almay BG, von Knorring L, Wetterberg L. Melatonin in serum and urine in patients with idiopathic pain syndromes. Psychiatry Res. 1987;22:179–191. doi: 10.1016/0165-1781(87)90033-3. [DOI] [PubMed] [Google Scholar]
- Armario A, Gavalda A, Marti J. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology. 1995;20:879–890. doi: 10.1016/0306-4530(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, Kraemer HC, Dea R, Robinson R, Hayward C. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68:262–268. doi: 10.1097/01.psy.0000204851.15499.fc. [DOI] [PubMed] [Google Scholar]
- Atkinson JH, Slater MA, Williams RA, Zisook S, Patterson TL, Grant I, Wahlgren DR, Abramson I, Garfin SR. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain. 1998;76:287–296. doi: 10.1016/S0304-3959(98)00064-5. [DOI] [PubMed] [Google Scholar]
- Banks S, Kerns R. Explaining high rates of depression in chronic pain. A diathesis-stress framework. Psycho Bull. 1996;119:95–110. [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and Pain Comorbidity; a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Beck-Friis J, Kjellman BF, Aperia B, Unden F, von Rosen D, Ljunggren JG, Wetterberg L. Serum melatonin in relation to clinical variables in patients with major depressive disorder and a hypothesis of a low melatonin syndrome. Acta Psychiatr Scand. 1985;71:319–330. doi: 10.1111/j.1600-0447.1985.tb02531.x. [DOI] [PubMed] [Google Scholar]
- Bellermann M, Tse AD, Misiaszek JE, Fouad K. Adaptations in the walking pattern of spinal cord injured rats. J Neurotrauma. 2006;23:897–907. doi: 10.1089/neu.2006.23.897. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Blackburn-Munro RE. Chronic pain, chronic stress and depression: Coincidence or consequence? J Neuroendocrinol. 2001;13:1009–1023. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Blackburn-Munro R. Pain in the brain: are hormones to blame? Trends Endocrinol Metab. 2003;14:20–27. doi: 10.1016/s1043-2760(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Brzezinski A. Melatonin in humans. New Eng J Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- Carter GT. Antidepressants in pain management. Curr Opin Investig Drugs. 2002;3:454–458. [PubMed] [Google Scholar]
- Chermat R, Thierry B, Mico JA, Steru L, Simon P. Adaptation of the tail suspension test to the rat. J Pharmacol. 1986;17:348–350. [PubMed] [Google Scholar]
- Citera G, Arias MA, Maldonado-Cocco JA, Lazaro MA, Rosemffet MG, Brusco LI, Scheines EJ, Cardinalli DP. The effect of melatonin in patients with fibromyalgia: a pilot study. Clin Rheumatol. 2000;19:9–13. doi: 10.1007/s100670050003. [DOI] [PubMed] [Google Scholar]
- Collins SL, Moore RA, McQuay HJ, Wiffen P. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20:449–458. doi: 10.1016/s0885-3924(00)00218-9. [DOI] [PubMed] [Google Scholar]
- De La Garza R, Mahoney JJ. A distinct neurochemical profile in WKY rats at baseline and in response to acute stress: implications for animal models of anxiety and depression. Brain Research. 2004;1021:209–218. doi: 10.1016/j.brainres.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Den Boer JA, Bosker FJ, Meesters Y. Clinical efficacy of agomelatine in depression: the evidence. Int Clin Psychopharmacol Suppl. 2006;1:S21–S24. doi: 10.1097/01.yic.0000195661.37267.86. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;21:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Dias FR, Carey RJ, Carrera MP. Conditioned locomotion induced by unilateral intrastriatal administration of apomorphine: D(2) receptor activation is critical but not the expression of unconditioned response. Brain Res. 2006;1083:85–95. doi: 10.1016/j.brainres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP, Raphael KG, Marbach JJ, Gallagher RM. Why is depression comorbid with chronic myofascial face pain? A family study test of alternative hypotheses. Pain. 1999;83:183–192. doi: 10.1016/s0304-3959(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duric V, McCarson KE. Hippocampal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression is decreased in rat models of pain and stress. Neuroscience. 2005;133:999–1006. doi: 10.1016/j.neuroscience.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Ferrer-Garcia MD, Wernicke JF, Detke MJ, Iyengar S. The depression-pain complex: overlap between the two problems and implications for neuropathic pain. In: Campbell JN, Basbaum AI, Dray A, Dubner R, Dworkin RH, Sang CN, editors. Emerging Strategies for the Treatment of Neuropathic Pain. Seattle: IASP Press; 2006. pp. 307–325. [Google Scholar]
- Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- El-Shenawy SM, Abdel-Salam OM, Baiuomy AR, El-Batran S, Arbid MS. Studies on the anti-inflammatory and anti-nociceptive effects of melatonin in the rat. Pharmacol Res. 2002;46:235–243. doi: 10.1016/s1043-6618(02)00094-4. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Teixeira CM. A pain in the ACC. Molecular Pain. 2005;1:14–16. doi: 10.1186/1744-8069-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A, Brown R, Kocsis J, Caroff S, Amsterdam J, Winokur A, Sweeney J, Stokes P. Patterns of melatonin rhythms in depression. J Neural Transm Suppl. 1986;21:269–290. [PubMed] [Google Scholar]
- Gameiro GH, da Silva Andrade A, Nouer DF, Ferraz de Arruda Veiga MC. How may stressful experiences contribute to the development of temporomandibular disorders? Clin Oral Investig. 2006;10:261–268. doi: 10.1007/s00784-006-0064-1. [DOI] [PubMed] [Google Scholar]
- Gao JY, Ren WH, Zhang YQ, Zhao ZQ. Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain. 2004;110:343–353. doi: 10.1016/j.pain.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Escolar E, Burin LJ, De Brito Sanchez MG, Cardinali DP. Time-dependent melatonin analgesia in mice: inhibition by opiate or benzodiazepine antagonism. Eur J Pharmacol. 1991;194:25–30. doi: 10.1016/0014-2999(91)90119-b. [DOI] [PubMed] [Google Scholar]
- Guardiola-Lemaitre B. Melatoninergic receptor agonists and antagonists: pharmacological aspects and therapeutic perspective. Ann Pharm Fr. 2005;63:385–400. doi: 10.1016/s0003-4509(05)82308-9. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Weinberg U, Stewart J, Klein DF, Weitzman ED, Quitkin FM. An inverse correlation between serum levels of desmethylimipramine and melatonin-like immunoreactivity in DMI-responsive depressives. Psychiatry Res. 1981;4:109–113. doi: 10.1016/0165-1781(81)90014-7. [DOI] [PubMed] [Google Scholar]
- Jackson KC, 2nd, St Onge EL. Antidepressant pharmacotherapy: considerations for the pain clinician. Pain Pract. 2003;3:135–143. doi: 10.1046/j.1533-2500.2003.03020.x. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–320. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulated cortex. Proc. Natl Acad Sci U. S. A. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korszun A. Facial pain, depression and stress - connections and directions. J Oral Pathol Med. 2002;31:615–619. doi: 10.1034/j.1600-0714.2002.00091.x. [DOI] [PubMed] [Google Scholar]
- Leino P, Magni G. Depression and distress symptoms as predictors of low back pain, neck-shoulder pain, and other musculoskeletal morbidity: a 10 year follow-up of metal industry employees. Pain. 1993;53:89–94. doi: 10.1016/0304-3959(93)90060-3. [DOI] [PubMed] [Google Scholar]
- Leone M, D'Amico D, Moschiano F, Fraschini F, Bussone G. Melatonin versus placebo in the prophylaxis of cluster headache: a double-blind pilot study with parallel groups. Cephalalgia. 1996;16:494–496. doi: 10.1046/j.1468-2982.1996.1607494.x. [DOI] [PubMed] [Google Scholar]
- Leone M, Lucini V, D'Amico D, Moschiano F, Maltempo C, Fraschini F, Bussone G. Twenty-four-hour melatonin and cortisol plasma levels in relation to timing of cluster headache. Cephalalgia. 1995;15:224–229. doi: 10.1046/j.1468-2982.1995.015003224.x. [DOI] [PubMed] [Google Scholar]
- Li SR, Wang T, Wang R, Dai X, Chen Q, Li RD. Melatonin enhances antinociceptive effects of delta-, but not mu-opioid agonist in mice. Brain Res. 2005;1043:132–138. doi: 10.1016/j.brainres.2005.02.067. [DOI] [PubMed] [Google Scholar]
- Littlejohn GO, Guymer EK. Fibromyalgia syndrome: which antidepressant drug should we choose? Curr Pharm Des. 2006;12:3–9. [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Magni G, Schifano F, De Leo D. Pain as a symptom in elderly depressed patients. Eur Arch Psychiatry Neurol Sci. 1985;235:143–145. doi: 10.1007/BF00380984. [DOI] [PubMed] [Google Scholar]
- Magni G, Marchetti M, Moreschi C, Merskey H, Luchini SR. Chronic musculoskeletal pain and depression symptoms in the National Health and Nutrition Examination. I. Epidemiologic follow-up study. Pain. 1993;53:163–168. doi: 10.1016/0304-3959(93)90076-2. [DOI] [PubMed] [Google Scholar]
- Magni G, Moreschi C, Rigatti-Luchini S, Merskey H. Prospective study on the relationship between depression symptoms and chronic musculoskeletal pain. Pain. 1994;56:289–297. doi: 10.1016/0304-3959(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Zhu J, Lu J, Mayer DJ. The inhibition of nitric oxide-activated poly(ADP-ribose) synthetase attenuates transsynaptic alteration of spinal cord dorsal horn neurons and neuropathic pain in the rat. Pain. 1997;72:355–366. doi: 10.1016/s0304-3959(97)00063-8. [DOI] [PubMed] [Google Scholar]
- Max MB, Culnane M, Schafer SC. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987;37:589–596. doi: 10.1212/wnl.37.4.589. [DOI] [PubMed] [Google Scholar]
- Max MB, Lynch S, Muir J. Effects of desipramine, amitriptyline and fluoxetine on pain in diabetic neuropathy. New Eng J Med. 1992;326:1250–1256. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- Mico JA, Ardid D, Berrocoso E, Eschalier A. Antidepressants and pain. Trends Pharmacol Sci. 2006;27:348–354. doi: 10.1016/j.tips.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Morgan PJ, Barrett P, Howell HE, Helliwell R. Melatonin receptor: localization, molecular pharmacology and physiological significance. Neurochem Int. 1994;24:101–146. doi: 10.1016/0197-0186(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Takahashi M, Russell DS, Duman RS. Repeated stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci Lett. 1999;267:81–84. doi: 10.1016/s0304-3940(99)00335-3. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Aoli K. Development pf a straom of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- Onal SA, Inalkac S, Kutlu S, Kelestimur H. Intrathecal melatonin increases the mechanical nociceptive threshold in the rat. Agri. 2004;16:35–40. [PubMed] [Google Scholar]
- Overstreet DH, Pucilowski O, Retton MC, Delagrange P, Guardiola-Lemaitre B. Effects of melatonin receptor ligands on swim test immobility. Neuroreport. 1998;9:249–253. doi: 10.1097/00001756-199801260-00014. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders sensitive line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: nature’s most versatile biological signal? FEBS Journal. 2006;273:2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- Pang CS, Tsang SF, Yang JC. Effects of melatonin, morphine and diazepam on formalin-induced nociception in mice. Life Sci. 2001;68:943–951. doi: 10.1016/s0024-3205(00)00996-6. [DOI] [PubMed] [Google Scholar]
- Pare WP. Passive-avoidance behavior in Wistar-Kyoto (WKY), Wistar and Fischer-344 rats. Physiol Behav. 1993;54:845–852. doi: 10.1016/0031-9384(93)90291-m. [DOI] [PubMed] [Google Scholar]
- Pare WP. Open field, learned helplessness, defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Pare WP. Enhanced retrieval of unpleasant memories influenced by shock controllability, shock sequence and rat strain. Biol Psychiatry. 1996;39:808–813. doi: 10.1016/0006-3223(95)00220-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th Ed. New York: Academic Press; 1997. [Google Scholar]
- Pertsov SS. The behavior of rats in response to changes in the light regime and administration of melatonin. Neurosci Behav Physiol. 2006;36:767–772. doi: 10.1007/s11055-006-0086-9. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioral despair in rats: a new model sensitive to antidepressant treatment. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair’ in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol. 1978;51:291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- Rabe-Jablonska J, Miller A. Links between pain and depression. Psychiatr Pol. 2005;39:7–20. [PubMed] [Google Scholar]
- Raghavendra V, Agrewala JN, Kulkarni SK. Melatonin reversal of lipopolysacharides-induced thermal and behavioral hyperalgesia in mice. Eur J Pharmacol. 2000;395:15–21. doi: 10.1016/s0014-2999(00)00196-5. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Kulkarni SK. Possible mechanisms of action in melatonin reversal of morphine tolerance and dependence in mice. Eur J Pharmacol. 2000;409:279–289. doi: 10.1016/s0014-2999(00)00849-9. [DOI] [PubMed] [Google Scholar]
- Roberts-Thompson IC, Knight RE, Kennaway DJ, Pannall PR. Circadian rhythms in patients with abdominal pain syndromes. Aust NZ J Med. 1988;18:569–574. doi: 10.1111/j.1445-5994.1988.tb00126.x. [DOI] [PubMed] [Google Scholar]
- Schatzberg Alan F. Depresion and physical symptoms: The mind-body connection. J Clin Psychiatry. 2004;65:867–876. doi: 10.4088/jcp.v65n0621. [DOI] [PubMed] [Google Scholar]
- Shavali S, Ho B, Govitrapong P, Sawlom S, Ajjimaporn A, Klongpanichapak S, Ebadi M. Melatonin exerts its analgesic actions not by binding to opioid receptor subtypes but by increasing the release of beta-endorphin an endogenous opioid. Brain Res Bull. 2005;64:471–479. doi: 10.1016/j.brainresbull.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sharav Y, Singer E, Schmidt E, Dionne RA, Dubner R. The analgesic effect of amitriptyline on chronic facial pain. Pain. 1987;31:199–209. doi: 10.1016/0304-3959(87)90036-4. [DOI] [PubMed] [Google Scholar]
- Shir Y, Zeltser R, Vatine JJ, Carmi G, Belfer I, Zangen A, Overstreet DH, Raber P, Zeltzer Z. Correlation of intact sensibility and neuropathic pain-related behaviors in eight inbred and outbred rat strains and selection lines. Pain. 2001;90:75–82. doi: 10.1016/s0304-3959(00)00388-2. [DOI] [PubMed] [Google Scholar]
- Song GH, Leng PH, Gwee KA, Moochhala SM, Ho KY. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut. 2005;54:1402–1407. doi: 10.1136/gut.2004.062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGraize Stacey C, Labuda Christopher J, Rutledge Margaret A, Jackson Raymond L, Fuchs Perry N. Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Experimental Neurology. 2004;188:139–148. doi: 10.1016/j.expneurol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Stankov B, Cozzi B, Lucini V, Capsoni S, Fauteck J, Fumagalli P, Fraschini F. Localization and characterization of melatonin binding sites in the brain of the rabbit (Oryctolagus cuniculus) by autoradiography and in vitro ligand-receptor binding. Neurosci Lett. 1991;133:68–72. doi: 10.1016/0304-3940(91)90059-3. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Sugden D. Psychopharmacological effects of melatonin in mouse and rat. J Pharmacol Exp Ther. 1983;227:587–591. [PubMed] [Google Scholar]
- Sullivan MJ, Reesor K, Mikail S, Fisher R. The treatment of depression in chronic low back pain: review and recommendations. Pain. 1992;50:5–13. doi: 10.1016/0304-3959(92)90107-M. [DOI] [PubMed] [Google Scholar]
- Szymanska A, Rabe-Jablonska J, Karasek M. Diurnal profile of melatonin concentrations in patients with major depression: relationship to the clinical manifestation and antidepressant treatment. Neuro Endocrinol Lett. 2001;22:192–198. [PubMed] [Google Scholar]
- Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57:275–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Tu Y, Sun RQ, Willis WD. Effects of intrathecal injections of melatonin analogs on capsaicin-induced secondary mechanical allodynia and hyperalgesia in rats. Pain. 2004;109:340–350. doi: 10.1016/j.pain.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Vanecek J. Cellular mechanisms of melatonin action. Physiol Rev. 1998;78:687–721. doi: 10.1152/physrev.1998.78.3.687. [DOI] [PubMed] [Google Scholar]
- Vitte PA, Harthe C, Pevet P, Claustrat B. Brain autoradiographic study in the golden hamster after intracarotid injection of [14C]melatonin. Neurosci Lett. 1990;110:1–5. doi: 10.1016/0304-3940(90)90777-7. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulated gyrus. Nature Reviews Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309:151–162. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- Von Knorring L, Perris C, Eisemann M, Erisksson U, Perris H. Pain as a symptom in depressive disorders. I. Relationship to diagnostic subgroup and depressive symptomatology. Pain. 1983;15:19–26. [Google Scholar]
- Von Knorring L, Ekselius L. Idiopathic pain and depression. Qual Life Res. 1994;3 Suppl 1:S57–S68. doi: 10.1007/BF00433378. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Simon G. The relationship between pain and depression. Br J Psychiatry. 1996 Suppl 30:101–108. [PubMed] [Google Scholar]
- Waldenlind E, Gustafsson SA, Ekbom K, Wetterberg L. Circadian secretion of cortisol and melatonin in cluster headache during active cluster periods and remission. J. Neurol. Neurosurg. Psychiatry. 1987;50:207–213. doi: 10.1136/jnnp.50.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30:1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Hotchkiss AK, Gatien ML, Pieke-Dahl S, Nelson RJ. Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res Bull. 2006;68:425–429. doi: 10.1016/j.brainresbull.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Wesierska M, Klinowska HD, Adamska I, Fresko I, Sadowska J, Albrecht J. Cognitive flexibility but not cognitive coordination is affected in rats with toxic liver failure. Behav Brain Res. 2006;171:70–77. doi: 10.1016/j.bbr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Wetterberg L. Melatonin and affective disorders. Ciba Found Symp. 1985;17:253–265. doi: 10.1002/9780470720981.ch15. [DOI] [PubMed] [Google Scholar]
- Yu CX, Zhu CB, Xu SF, Cao XD, Wu GC. Selective MT(2) melatonin receptor antagonist blocks melatonin-induced antinociception in rats. Neurosci Lett. 2000a;24(282):161–164. doi: 10.1016/s0304-3940(00)00883-1. [DOI] [PubMed] [Google Scholar]
- Yu CX, Zhu CB, Xu SF, Cao XD, Wu GC. The analgesic effects of peripheral and central administration of melatonin in rats. Eur J Pharmacol. 2000b;403:49–53. doi: 10.1016/s0014-2999(00)00421-0. [DOI] [PubMed] [Google Scholar]
- Zahn PK, Lansmann T, Berger E, Speckmann EJ, Musshoff U. Gene expression and functional characterization of melatonin receptors in the spinal cord of the rat: implications for pain modulation. J Pineal Res. 2003;35:24–31. doi: 10.1034/j.1600-079x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang Y, Zhao ZQ. Anterior cingulate cortex contributes to the descending facilitatory modulation of pain via dorsal reticular nucleus. Eur J Neurosci. 2005;22:1141–1148. doi: 10.1111/j.1460-9568.2005.04302.x. [DOI] [PubMed] [Google Scholar]


