Abstract
Surface proteins Shr, Shp, and the ATP-binding cassette (ABC) transporter HtsABC are believed to make up the machinery for heme uptake in Streptococcus pyogenes. Shp transfers its heme to HtsA, the lipoprotein component of HtsABC, providing the only experimentally demonstrated example of direct heme transfer from a surface protein to an ABC transporter in Gram-positive bacteria. To understand the structural basis of heme transfer in this system, the heme-binding domain of Shp (Shp180) was crystallized, and its structure determined to a resolution of 2.1 Å. Shp180 exhibits an immunoglobulin-like β-sandwich fold that has been recently found in other pathogenic bacterial cell surface heme-binding proteins, suggesting that the mechanisms of heme acquisition are conserved. Shp shows minimal amino acid sequence identity to these heme-binding proteins and the structure of Shp180 reveals a unique heme-iron coordination with the axial ligands being two methionines from the same Shp molecule. A negative electrostatic surface of protein structure surrounding the heme pocket may serve as a docking interface for heme transfer from the more basic outer cell wall heme receptor protein Shr. The crystal structure of Shp180 reveals two exogenous, weakly bound hemins, which form a large interface between the two Shp180 molecules in the asymmetric unit. These “extra” hemins form a stacked pair with a structure similar to that observed previously for free hemin dimers in aqueous solution. The propionates of the protein-bound heme coordinate to the iron atoms of the exogenous hemin dimer, contributing to the stability of the protein interface. Gel filtration and analytical ultracentrifugation studies indicate that both full-length Shp and Shp180 are monomeric in dilute aqueous solution.
Keywords: Cell surface protein, heme-binding protein, Streptococcus pyogenes, Shp, heme acquisition
Introduction
The ongoing emergence of antibiotic resistance in pathogenic bacteria has led to interest in resolving critical pathways that can be exploited for new drug design. The heme-uptake system is one such potential target to the dependency of a variety of bacteria on the host for essential iron.1–4 Gram-positive pathogens, such as Streptococcus pyogenes and Staphylococcus aureus, cannot survive on the limited amounts of free iron that are available in their hosts5 and instead use heme-binding proteins to gather iron from host proteins such as human hemoglobin from lysed red blood cells.6–9 These bacteria have evolved transport systems to relay heme through the cell wall and translocate it across the cytoplasmic membrane.
S. pyogenes is a Gram-positive bacterium that causes many human diseases, including streptococcal toxic shock, rheumatic fever, rheumatic heart disease, pharyngitis, and bacteremia.10 S. aureus prefers heme as an iron source, but can use transferring iron for growth.8 S. pyogenes acquires and uses heme as an efficient iron source, but is unable to use iron bound to transferrin.11, 12 The heme acquisition machinery of S. pyogenes consists of Shr, Shp, and the ATP-binding cassette (ABC) transporter HtsABC. Shr is as outer surface protein that can interact with host hemoproteins and bind heme.13 Shp, is another surface protein, and HtsA, is the lipoprotein component of HtsABC, and both bind heme avidly.2, 14 Previous in vitro studies have shown that Shp transfers the reduced (Fe(II)-protoporphyrin IX or heme) or oxidized (Fe(III)-protoporphyrin IX or hemin) cofactor to HtsA.15, 16 The observed rate constant for hemin transfer from Shp to HtsA shows a hyperbolic dependence on apoHtsA concentration, indicating that Shp and apoHtsA form a complex prior to hemin transfer. The limiting first order rate constant for transfer at high protein concentrations is very large (~40 s−1), roughly 105 times greater than that for simple hemin dissociation from holoShp (~0.0003 s−1).16 These kinetic results demonstrate that the heme transfer is direct and activated by formation of a binary holoShp-apoHtsA complex. However, little is known about the structural basis of this rapid heme transfer.
Only a few structures of the proteins involved in bacterial heme acquisition and metabolism have been described and include the Staphylococcus aureus surface proteins IsdH, IsdC, and IsdA.17–19 These S. aureus proteins belong to the NEAT-domain family of proteins and share domain homology with Shr (referred to as S_pyog in the cited reference).20 In addition, crystal structures have also been solved for the heme uptake and metabolism proteins: Serratia marcescens hemophore HasA, Campylobacter jejuni lipoprotein ChaN, Yersinia enterocolitica heme transporter protein HemS, S. aureus lipoprotein IsdE, and Escherichia coli heme oxygenase ChuS.21–25 The heme in IsdA, IsdC, and ChaN is coordinated through a tyrosine-iron linkage, and hydrophobic residues line the heme pocket. Heme bound by the transporter proteins is more exposed to the solvent than that in other heme proteins with catalytic, gas storage, and sensing functions, such as myoglobin, hemoglobin, cytochrome P450s, and guanylyl cyclase. The exposed surfaces are presumed to facilitate rapid heme transfer.
Here we report the first protein structure of a component of the heme-uptake machinery in S. pyogenes. The heme-binding domain of Shp (Shp180) was constructed by removing the N-terminal secretion signal sequence and the C-terminal domain, which presumably attaches to the cell wall. Ran, et al. have shown that Shp180 retains the ability to bind heme and hemin avidly and to transfer these metalloporphyrins rapidly to apoHtsA by the same mechanism as that observed for full-length Shp.26 The structure of Shp180 was determined in its hemin-bound state.
Results
Structure Determination
The structure of Shp180 (Figure 1) was phased using single wavelength anomalous diffraction from a 2.6 Å resolution phasing data set collected at a wavelength near the iron K-edge. The initial model obtained from the phasing data set was refined against a high-resolution data set extending to 2.1 Å (see Table 1 for X-ray crystallization statistics). Two Shp180 molecules are located in the asymmetric unit, each with a single hemin bound to the protein and an exogenous hemin in the crystallization dimer interface. The two Shp180 monomers have a main-chain r.m.s. deviation of 0.76 Å. The largest difference between the two monomers is restricted to six residues following β-strand B8 (Figure 1). When these amino acids are excluded, the main-chain r.m.s. deviation between the monomers is reduced to 0.36 Å, demonstrating nearly identical molecules within the asymmetric unit.27
Figure 1. Shp180 structure.
The stereo image of Shp180 in cartoon representation. The hemin is shown in the ball and stick model. The termini and the β-sheets are labeled.
Table 1. Crystallographic statistics for the refinement of Shp180.
Statistics listed in parentheses are for those in reported in the last refinement shell listed in the resolution range.
| Refined Shp180 Structure | Phasing Data Structure | |
|---|---|---|
| Data Collection | ||
| Resolution range (Å) | 41.2−2.10 (2.16−2.10) | 70.7−2.60 (2.66−2.60) |
| Space Group | P65 | P65 |
| Unit cell dimensions (Å) | a=b=82.3, c=106.5 α=β=90°, γ=120° | a=b=81.7, c=106.8 α=β=90°, γ=120° |
| Total/Unique reflections | 255,892/23,738 | 233,365/12,195 |
| Completeness (%) | 99.6 (95.3) | 96.8 (73.3) |
| Average I/σI | 20.4 (2.3) | 21.8 (2.4) |
| Redundancy | 10.8 (7.1) | 19.2 (5.1) |
| Rmerge | 0.115 | 0.129 |
| Phasing Statistics | (40.9−2.60 Å) | |
| FOMacen/FOMcen | --- | 0.30/0.09 |
| Phasing Poweracen | --- | 0.87 |
| Rcullis | --- | 0.87 |
| Refinement Statistics | ||
| Rwork | 16.3 (22.1) | --- |
| Rfree | 22.2 (30.7) | --- |
| r.m.s.d. bond length (Å) | 0.009 | --- |
| Coordinate error (maximum likelihood, Å) | 0.12 | --- |
| Average protein B-value (Å2) | 28.2 | --- |
| Average hemin B-value (Å2) | 26.0 | --- |
| Average water B-value (Å2) | 39.4 | --- |
| Average ion B-value (Å2) | 52.3 | --- |
| No. of protein molecules/atoms | 2 / 2344 | --- |
| No. of auxiliary molecules | 4 Hemin | --- |
| No. of waters | 323 | --- |
| No. of ions | 9 | --- |
| Ramachandran plot, residues | ||
| In most favored (%) | 94.1 | --- |
| In allowed (%) | 5.9 | --- |
| In generously allowed (%) | 0.0 | |
| In disallowed (%) | 0.0 | |
| Overall G-factor | 0.08 | |
| PDB ID | 2Q7A | --- |
Structure of Shp180
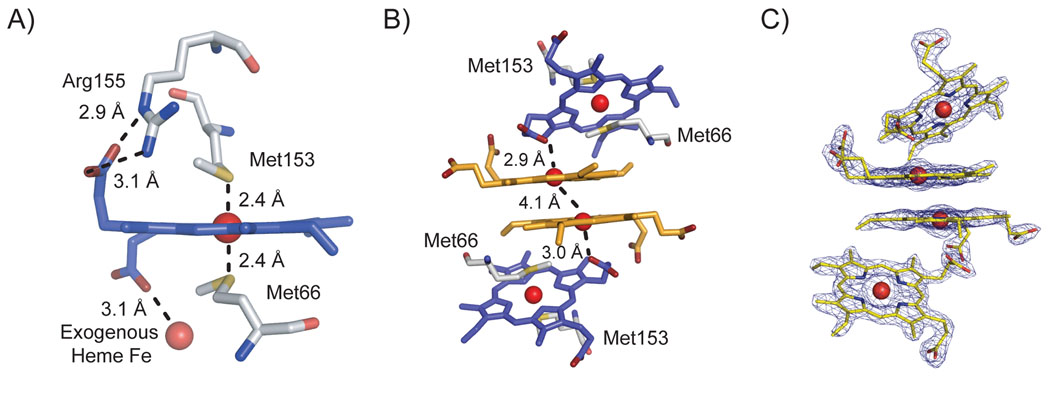
The immunoglobulin-like β-sandwich fold of Shp180 contains 8 β-strands and one α-helix (Figure 1). The 8 β-strands are divided into 3- and 4-stranded anti-parallel β-sheets forming a twisted β-sandwich with the 8th β-strand connecting the two β-sheets. Unlike the heme in the S. aureus or C. jejuni penta-coordinated heme uptake proteins, the heme in Shp is coordinated by two methionines (Figure 2A). Bis-methionyl coordination has only been documented in bacterioferritin, where the thiol ether coordination comes from methionines on separate monomers.28, 29 Shp180 is the first protein to show this arrangement within the same monomer. The bound heme iron is coordinated by Met66 and Met153, which are located on the α-helix and between β-strands B7 and B8, respectively (Figure 1). The sulfur atoms are located 2.4 Å from the iron atom for both Met66 and Met153 (Figure 2A), comparable to the 2.1–2.7 Å distance found between monomers in structures of bis-methionyl bacterioferritin29–31 and to the 2.3 Å average for Fe-S distances in Fe-Cys protein structures (primarily cytochrome P450s) taken from the Protein Data Bank.32, 33 Met66 is more solvent exposed than Met153, which is in agreement with IsdC where the 310-helix portion is more exposed than the beta sheet core.18
Figure 2. Bis-methionyl coordination in Shp180 and hemin stacking in the crystallization interface.
A) Coordination of the protein-bound heme by Met66 and Met153 in Shp180. Distances between the sulfur atom (in yellow) and heme iron (sphere) are listed. Hydrogen bond distances between Arg155 and a propionate are shown and the coordination distance between the propionate and the exogenous heme iron is shown. B) The figure depicts the crystallographic hemin packing between the bound hemes (blue) and the exogenous hemins (orange). Distances between the bound propionate oxygen (red) and the exogenous hemin iron atom (sphere) and the distance between the two exogenous hemin irons are shown. C) Electron density of the protein-bound and exogenous hemins at 1.5σ using the orientation from B). The hemins are displayed in yellow.
Details of the heme-binding site
In addition to coordination to the sulfur atoms of Met66 and Met153, the hemin is surrounded by hydrophobic residues, which include Leu62, Val67, Leu93, Val150, Pro152, Val157, and Phe159. Within the same Shp180 monomer, one hemin propionate O atom is hydrogen bonded to N2 and N. of Arg155 (3.1 and 2.9 Å, Figure 2A) and to two water molecules (2.7 Å each), but remains solvent exposed. One propionate in IsdC and IsdA also hydrogen bonds to the backbone and side chain atoms, but remains exposed to the solvent.18, 19 The other O atom of the bound heme propionate is coordinated to the exposed iron of the exogenously bound hemin dimer located at the interface between the two Shp180 molecules. The Fe-O bond lengths for the latter interactions are 3.0 and 2.9 Å for the two Shp180 molecules (Figure 2B). The exogenous hemins are stacked in a dimer and related by a two-fold axis (Figure 2B–C), forming a structure similar to previously reported hemin dimers in aqueous solution.34 The propionate groups of the exogenous hemin dimer form hydrogen bonds with several water molecules and Asn127 (2.7 Å). The pyrrole nitrogen atoms of the hemin dimer are located within 3.6 Å of each partner’s iron atom, and these exogenous heme irons are located 4.1 Å from one another (Figure 2B). The packing of the exogenous hemin dimer and the protein-bound hemin is similar to the structure of β-hematin where the propionate of one hemin coordinates to the iron of another hemin (Figure 2C).35, 36
Excluding the exogenous hemin dimer-protein interface, the surface contact area in the interface between the two Shp180 monomers is only ~276 Å2. The exogenous hemin dimer increases the contact area between the two protein monomers by a factor of ~2 to an area of ~600 Å2 and clearly stabilizes the crystallization dimer interface. Tyr96 hydrogen bonds to the symmetry related Tyr96 at a distance of 2.4 Å near the exogenous hemin dimer, further strengthening the interface.
Analysis of the redox and oligomeric state of Shp and Shp180
Spectroscopic analysis of the Shp180 crystals revealed a peak at 420 nm indicating that the protein is in the oxidized state.16 The crystals also showed a lower than expected ratio between the 420 nm peak (bis-Met coordinated hemichrome) and the 370 nm peak (aqueous hemin) compared to that of oxidized Shp180 in solution, consistent with the presence of an extra “free” hemin in the crystals (Figure 3A).
Figure 3. Spectroscopic, gel-filtration, and ultracentrifugation analysis of Shp180 and Shp.
A) Spectroscopic scan of oxidized Shp180 in solution (Oxi Shp180) and crushed crystals in mother liquor (Crystallized Shp180). An additional scan of the Shp180 crystals corrected for Rayleigh scattering is shown (Corrected Crystallized Shp180). B) Elution of oxidized (Oxi) and reduced (Red) Shp180 and Shp. Molecular weights are listed next to their respective plots. The standard curve used to calculate the molecular weights and correlation coefficient is shown in the upper right. C) Molecular weight distribution results obtained from sedimentation velocity experiments with oxidized Shp180 (blue) and Shp (red), as described in Materials and Methods. Molecular weights peaks are labeled.
Calibrated gel filtration and sedimentation velocity ultracentrifugation results indicate that the reduced and oxidized forms of holoShp180 exist as a monomer in solution, with both experiments yielding molecular weights (MWs) between 14 and 16 kDa (Figure 3B–C). These results are consistent with the calculated MW of 17.2 kDa for heme-bound Shp180. Gel filtration results for full-length oxidized and reduced holoShp suggest a MW of ~40 kDa, which is significantly larger than the calculated value of 25.9 kDa (Figure 3B). In contrast, the sedimentation velocity results gave a smaller than expected S-value (2.3 s), which assuming spherical geometry predicts a MW for full-length Shp of only 20 kDa (Figure 3C). Combined, these results strongly suggest that both Shp and Shp180 are monomeric in aqueous solution and that Shp180 appears to be globular. In contrast, full-length Shp is elongated and rod-like with a larger than expected exclusion volume and frictional coefficient, which is also seen in ultracentrifugation studies of IsdA.37 The larger exclusion volume of the rod shape explains the larger than expected apparent MW for full-length Shp obtained from the gel filtration experiment, and the larger frictional coefficient explains the smaller S-value and MW calculated from the sedimentation velocity experiments.
Structural homology
Shp180 does not share significant amino acid sequence similarity with the heme-binding NEAT domain proteins.20 However, it does share the immunoglobulin-like structural fold associated with S. aureus surface heme-uptake proteins, IsdC,18 IsdA,19 and IsdH.17 Even with sequence identities of 19 and 17%,38 the structural alignment of carbon α-atoms between Shp180 and IsdC and between Shp180 and IsdA show an r.m.s. deviation of 2.0 Å and 2.2 Å, respectively. These values are typical r.m.s. deviations between proteins sharing the same structural fold, but less than 20% sequence identity.27 An overlay of Shp180 and IsdC shows that the most significant differences are mainly confined to their loop regions (Figure 4A). Unlike IsdC, Shp180 does not contain a 310-helical lip distal to the heme, but instead has a longer alpha helix where Met66 coordinates the heme iron (arrow in Figure 4A–C). The heme solvent exposure of Shp180 is 29%, compared to 34% for IsdC and 35% for IsdA. The protein-bound hemin in Shp180 is rotated 90° in relation to the bound hemin in IsdC and IsdA (Figure 4B–C). The small decrease in solvent exposure is likely due to the coordination of the iron by the second axial methionine. There is a large diversity among the amino acid sequences of Shp180 and NEAT domain proteins, and a search through FFAS03,39 a peptide-profile sequence alignment tool, indicates that an amino acid sequence motif for cell surface heme-binding proteins in Gram-positive bacteria is indefinable at present.
Figure 4. Structural comparisons of Shp180 and IsdC proteins.
A) Stereo image of the C. trace between Shp180 (blue) and IsdC (red, PDB ID 2O6P). The arrow points to differences between the longer alpha helix Shp180 where Met66 coordinates one side of the heme and the shorter 310-helical lip of IsdC. B) & C) Heme pockets of Shp180 (B) and IsdC (C). The proteins are shown in blue, the residues that coordinate to the heme are labeled and shown in yellow, and the hemes are in gray. D) & E) Electrostatic surface potential of the Shp180 (D) and IsdC (E) proteins. Electrostatic surface potentials were calculated (keV) in the presence of 140 mM monovalent salt concentration at 310 K. The hemins are shown in yellow for both Shp180 and IsdC.
Surface charge distribution
The electrostatic properties of Shp and IsdC were examined. The calculated isoelectric point (pI)40 of Shp and the heme-binding portion of Shp180 are 5.3 and 4.6, respectively, whereas the calculated pI of Shr is 8.7. Similarly, IsdA has a calculated pI of 9.1, whereas IsdC has a calculated pI of 6.6, and again the two heme transport pathway components have opposite charges under physiological conditions. These observations suggest that favorable electrostatic interactions may facilitate the formation of bimolecular protein complexes to facilitate unimolecular heme transfer. This type of facilitated transfer mechanism is observed for the reaction of holoShp with apoHtsA.16, 26 Interestingly, although both Shp180 and IsdC are acidic proteins, they differ in the electrostatic surface around their heme-binding pockets. Shp180 displays a large electronegative ring around the heme-binding site (Figure 4D). Favorable electrostatic interactions between proteins are often found to drive complex formation in nature41 and the negative ring surround the Shp180 heme pocket may present a potential binding site with the more basic Shr. In contrast, IsdC does not have a negative ring around the bound heme, but shows dispersed positive and negative patches (Figure 4E).
Discussion
We have solved the structure of the heme-binding domain of Shp, a protein that transfers its heme to HtsA. The C37S mutation in Shp180 was used to facilitate crystallization by avoiding potential oxidation of the Cys residue during the crystallization process. The C37S residue is 23.9 Å away from the heme iron atom in the middle of β-strand B1 (Figure 1A) and does not cause an alteration in heme transfer rate or mechanism of Shp180. Truncated Shp180 binds heme, has the same EPR and UV-visible spectral properties as full-length Shp, and retains the ability to rapidly transfer both hemin and heme to apoHtsA by the activated ternary complex mechanism that is observed for the full-length Shp-apoHtsA reactions.26 Thus, the structure of Shp180 provides valuable information for understanding the mechanisms of hemin transfer by the full-length cell surface protein.
Shp180 has the immunoglobulin-like β-sandwich fold that is found in the NEAT domain of these S. aureus IsdA, IsdH, and IsdC. The heme is located in the cavity formed by the single α-helix and β-strands B7 and B8 in Shp180 (Figure 1), an arrangement that is similar to those in IsdA and IsdC. The oligomeric studies revealed that Shp180 has an overall elongated protein structure, also seen in IsdA. In addition, like in the S. aureus proteins, the bound hemin in Shp180 has significant solvent exposure.18 Shp was excluded from the NEAT family by Andrade et al. who referred to Shp as Spy1796.20 The homology in amino acid sequence between Shp180 and the NEAT domain of IsdA or IsdC (17–19% identity) is similar to that between the IsdA and IsdC NEAT domains (19% identity),38 suggesting that Shp is at least a distant member of the NEAT family. Thus, the two distinct heme acquisition systems may have evolved from the same ancestor through divergence; in addition, HtsA shares 40% identity in amino acid sequence with IsdE from S. aureus,15, 24 further supporting the divergence theory. The structural similarities between Shp and IsdA/C and homology between HtsA and IsdE suggest that heme uptake and transfer into S. aureus and S. pyogenes proteins may follow similar molecular mechanisms and that parallel functions exist for the two systems. For example, Shr and IsdB may acquire hemin from hemoglobin, and Shp and IsdA/C may relay hemin from Shr and IsdB to HtsA and IsdE, respectively.
The heme iron of Shp is coordinated to Met66 and Met153; Met66 is more exposed to the solvent than Met153, suggesting that the Met153 side is more important for the affinity of Shp for hemin. Bis-methionine coordination was previously observed between two monomers in bacterioferritin.42 The bis-methionyl coordination in Shp is unique because it is the first example of heme iron corrdination to two methionines in the same protein monomer. In contrast, the heme iron is ligated to a Tyr residue in IsdA, IsdC, and ChaN, a His residue in HemS, and His and Tyr residues in HasA. Single Ala replacements of Met66 and Met153 in full-length Shp cause formation of pentacoordinate hemin-Met complexes and slower rates of heme transfer, and Met153, but not Met66, is critical to maintaining the high affinity of Shp for hemin.26 The structure model of Shp180 supports these observations. Furthermore, bis-methionine coordination in Shp is critical for its rapid hemin transfer to apoHtsA.26 These observations suggest that although the basic mechanism of transfer between receptor proteins in bacterial heme acquisition may be the same, the structural details of iron coordination are not strictly conserved.
The heme and hemin transfer from Shp to HtsA involves the displacement of the two Met ligands in Shp with two ligands from apoHtsA, His229 and Met79 (Lei, et al., unpublished data). After formation of the binary protein complex, Shp-to-HtsA heme and hemin transfers show only one first order kinetic phase, indicating that the displacement of that the the displacement of the first Shp Met ligand is rate-limiting. In either case, the two new coordination bonds are formed at effectively the same time. Thus, the two axial ligands of apoHtsA must be close to the two axial positions of hemin in the holoShp-apoHtsA binary complex, requiring that the HtsA axial side chains easily access to the hemin ring in Shp. The bound hemin in Shp180 has significant exposure to solvent on both Met153 and Met66 sides. Thus, simultaneous ligand displacement by adjacent apoHtsA ligands in a Shp-HtsA complex is structurally feasible by sliding movements of the amino acid side chains of apoHtsA across both sides of the hemin plane. The hemin in HasA, IsdA, and IsdC are also significantly exposed to solvent on both sides of the hemin ring, suggesting that hemin transfer by these proteins may use mechanisms similar to the sliding mechanism proposed here for the reaction of holoShp with apoHtsA.
Like bacterioferritin,42 Shp shows a weak absorbance peak at 370 nm, which is often associated with free hemin, but pyridine hemochromagen and iron analysis show only one heme per Shp monomer. However, when Shp180 was crystallized, a significant increase in the ratio of the peak at 370 nm to that at 420 nm is observed, suggesting that the crystallized protein has acquired additional, “free” hemin. During crystallization, a portion of Shp180 precipitated in the hanging-drops within days, and crystals only formed weeks later. It is likely that the protein precipitation facilitates the release of hemin, which dimerizes34 and then nucleates crystallization of Shp180 dimers. This apparent requirement for exogenous hemin in the crystal packing may explain the slow rate of crystal growth. The interface between the two molecules of Shp180, as observed in the crystal structure, is held together primarily by the exogenous hemin dimer, where the porphyrin rings provide the majority of the contact area, ~334 Å2 of the 600 Å2 interface. This situation is also seen in the periplasmic protein, ChaN, where the cofacial proteinbound hemes account for 380 Å2 of the 680 Å2 dimer interface.22
Gel filtration and ultracentrifugation studies indicate that both Shp180 and Shp are monomers in solution and that the truncated hemin-binding domain is globular, whereas the full-length protein has a rod-like shape. However, full-length Shp could in principle bind exogenous hemin dimers, perhaps after massive red blood cell lysis and hemoglobin denaturation, and then transiently form a dimer on the cell surface of S. pyogenes as a mechanism for sequestering even more heme for iron metabolism. These extra “free” hemins might then be taken up passively by apoHtsA or by apoShp after it is transferred its bound hemin to the membrane transporter. However, at present there is no evidence for these more indirect uptake processes.
The exogenous hemin stacking arrangement in the crystals could also represent a mechanism comparable to β-hematin pigment formation in Plasmodium malarial bacteria.35, 36 This protozoan parasite is able to sequester free heme by stacking rings through the interactions of adjacent propionates, which creates a complex that is relatively inert and incapable of generating radical oxygen species (ROS) that could kill the organism. Similar problems with excess free hemin may occur after massive lysis of red cells and Heinz body formation induced by hemolysin activities during infection.
Shp180 has a high sequence homology (75% identity and 91% similarity) to the N-terminal portion of the Streptococcus equi homolog SeShp.43 S. equi causes strangles, an extremely infectious disease of the upper respiratory tract, in horses.44 Proteins involved in the heme-uptake pathway appear to be conserved in S. pyogenes and S. equi.43 From high sequence identity between the proteins of S. equi and S. pyogenes, it is highly probable that SeShp shares the immunoglobulin-like β-sandwich fold and that the hemebinding portion is located in the N-terminal region with bis-methionyl coordination. Thus, it is highly likely that the same heme uptake and transport mechanisms occur in these closely related pathogens.
Materials and Methods
Cloning and protein purification
Shp180 contains amino acids 30–180 of Shp. The amplification of the truncated DNA product was created by using PCR primers 5′-ACCATGGATAAAGGTCAAATTTATGGATG-3′ and 5′-CGAATTCAAGTAACAAGCTGGGCCAAC-3′ from the shp gene clone pSHP that was originally cloned from strain MGAS5005.2 The truncated Shp180 PCR product was inserted into a pET-21d (Novagen, Madison, WI) plasmid at the NcoI and EcoRI sites, yielding recombinant plasmid pSHP180. In order to improve expression levels, a C37S mutation was incorporated and obtained by site-directed mutagenesis using pSHP180, a QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), and primers 5′-AAAGGTCAAATTTATGGATCTATTATTCAAAGAAATTAT-3′ and 5′-ATAATTTCTTTGAATAATAGATCCATAAATTTGACCTTT-3′, yielding pSHP180C37S. The mutated truncated shp gene was sequenced to confirm the desired mutation.
Shp180 was expressed in Escherichia coli BL21(DE3) containing pSHP180C37S, with the majority of the expressed protein in inclusion bodies. After expression, the cell pellet was resuspended in ice-cold 50 ml Tris-HCl at pH 8.0 and sonicated in one-second bursts for 15 minutes on ice, followed by centrifugation at 20,000 × g for 15 minutes. The Shp180 inclusion body was dissolved in 50 ml 8 M urea. The denatured protein was refolded by diluting 40-fold with 20 mM Tris-HCl at pH 8.0 containing excess hemin (using a 1.5 fold hemin to protein ratio). The refolded heme-bound Shp180 was loaded onto a DEAE column (2.5 × 10 cm), and the column was washed with 100 ml of 20 mM Tris-HCl at pH 8.0 and eluted with a 100 ml linear gradient of 0–0.25 M NaCl at room temperature. Shp180 was adjusted to 0.8 M (NH4)2SO4, 20 mM Tris-HCl at pH 8.0 (Buffer B) and then loaded onto a phenyl sepharose column (1.5 × 6 cm), washed with 100 ml of Buffer B, and eluted with a 100-ml linear gradient of Buffer B to 20 mM Tris-HCl at pH 8.0 (Buffer A) at room temperature. Shp180 was dialyzed against Buffer A and concentrated using Centricon Plus 20 filtration devices (Millipore, Bedford, MA).
Crystallization conditions
Crystals were grown by the hanging-drop vapor-diffusion method with 0.8 M ammonium sulfate and 0.1 M MES at pH 6.0 using 70 mg.mL−1 Shp180 in 20 mM Tris at pH 8.0. Hanging drops consisted of 1 µl of protein solution mixed with 1 µl of reservoir solution. Red diffraction quality crystals grew to ~20 × 50 × 100 µm3 needles within two months at 295 K. Shp180 crystals were cryo-protected in a single step using 0.9 M ammonium sulfate, 30% glycerol, and 0.1 M MES at pH 6.0 and flash-cooled in liquid nitrogen.
Structure Determination
X-ray diffraction data used for initial phasing were collected at the Life Sciences Collaborative Access Team (LS-CAT) Sector 21ID-D beamline at the Advanced Photon Source (Argonne National Laboratories, Argonne, IL). Data were collected at a wavelength of 1.6531 Å at 100K and diffraction images were integrated and scaled to a resolution of 2.6 Å using HKL2000.45 The iron substructure of Shp180 was determined using HySS46 and Shp180 was phased using autoSHARP,47 which uses multiple auxiliary programs from the CCP4 suite.48 The starting backbone model was partially built (~75% of the two chains in the asymmetric unit) with ARP/wARP49 and the remaining model and side-chains were built manually in COOT50 and refined in REFMAC5.51 A higher resolution X-ray diffraction data set was collected at the General Medicine and Cancer Institutes Collaborative Access Team (GM/CA-CAT) Sector 23ID-D beamline at the Advanced Photon Source. Data were collected at a wavelength of 0.9793 Å at 100K and diffraction images were integrated and scaled to a resolution of 2.1 Å using HKL2000.45 The 2.1 Å Shp180 structure was determined using molecular replacement with the previous 2.6 Å Shp180 structure in MolRep.52 The model was manually fitted in COOT50 and refined in REFMAC5.51
Spectroscopic analysis, ultracentrifugation, and size-exclusion chromatography
Before the proteins were analyzed by gel-filtration chromatography, the reduced (containing iron(II)) and oxidized (containing iron(III)) forms were verified spectroscopically for peaks at 428 nm and 420 nm, respectively.16 Spectroscopic scans of Shp180 crystals were made using an ND-1000 Spectrophotometer (NanoDrop, Wilmington, DE) from crushed crystals in 2 µl of mother liquor. Spectroscopic scans of the Shp180 crystals were manually corrected for Rayleigh scattering as follows:
| Eqn. 1 |
where x is the linear and y are the exponential scattering coefficients. From the Shp180 crystal spectroscopic scans, x and y were estimated to be 2.4 × 107 and 3.45, respectively. The reduced and oxidized form of Shp180 and Shp were analyzed on a calibrated gel filtration Superdex 75 PC 3.2/30 column (GE Healthcare, Piscataway, NJ) in 20 mM Tris at pH 8.0. The reduced and oxidized form of Shp180 and the oxidized form of Shp were run using 150 µM protein. The reduced form of Shp was run at 40 µM. Flow rate was kept at 0.04 ml•min−1 for the size-exclusion experiments. Sedimentation velocity experiments were performed using oxidized Shp180 and Shp at 150 µM in 20 mM Tris at pH 8.0, 25° C in a Beckman Model XLA centrifuge at a rotor speed of 60,000 rpm. Data were analyzed for S value and MW distributions (assuming spherical geometry, Vbar=0.722 cm3•g−1) using Ultrascan 9.0 (University of Texas at San Antonio, San Antonio, TX).
Surface area and root mean squared deviation calculations
The solvent accessible surface area of the Shp180 heme and crystallographic dimer crystallization packing surface areas were calculated using AREAIMOL.53 The percentage of heme surface area exposure was calculated using 825 Å2 as the free heme area.18 Root mean squared (r.m.s.) deviations between selected protein structures were calculated in VMD.54 The structural alignment involved aligning 98 Shp180 residues out of 122 (~80%) that were aligned against 123 residues of IsdC and 100 Shp180 residues out of 122 (~82%) that were aligned against 121 residues of IsdA. Electrostatic surface potentials of Shp180 and IsdC were calculated using APBS.55 Figures were prepared in PyMOL.56
Protein Data Bank accession code
The atomic coordinates of Shp180 were deposited in the RCSB Protein Data Bank32 under the accession number 2Q7A.
Acknowledgements
This work was supported by the National Institutes of Health Grants 5 T32 GM08349 (R.A.), K22 AI057347 (B.L.), R01 GM035469 (J.S.O.), and R01 HL47020 (J.S.O.), the National Center for Research Resources Grant P20 RR-020185 (B.L.), the National Institutes of Health Protein Structure Initiative U54 GM0749011 (E.B. & G.N.P.), the Montana State University Agricultural Experimental Station (B.L.), and the Robert A. Welch Foundation Grant C0612. GM/CA-CAT has been funded in whole or in part with Federal funds from the National Cancer Institute (Y1-CO-1020) and the National Institute of General Medical Science (Y1-GM-1104). Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor for the support of this research program (Grant 085P1000817). Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under contract No. DEAC02- 06CH11357 (GM/CA-CAT) and No. DE-AC02–06CH11357 (LS-CAT). We are appreciative of the access to crystallization equipment and X-ray facilities at the Center for Eukaryotic Structural Genomics (CESG) and to the ND-1000 Spectrophotometer in the laboratory of Dr. Judith E. Kimble at the University of Wisconsin-Madison. Thanks to Dr. Craig A. Bingman for crystallographic support (CESG), Ryan M. Bannen (Dept. of Biochemistry, University of Wisconsin-Madison) for helping with the calibrated gel filtration experiments, and Elena J. Levin and Christopher M. Bianchetti (Dept. of Biochemistry, University of Wisconsin-Madison) for useful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O. Passage of heme-iron across the envelope of Staphylococcus aureus. Science. 2003;299:906–909. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- 2.Lei B, Smoot LM, Menning HM, Voyich JM, Kala SV, Deleo FR, Reid SD, Musser JM. Identification and characterization of a novel heme-associated cell surface protein made by Streptococcus pyogenes. Infect Immun. 2002;70:4494–4500. doi: 10.1128/IAI.70.8.4494-4500.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Field LH, Headley VL, Payne SM, Berry LJ. Influence of iron on growth, morphology, outer membrane protein composition, and synthesis of siderophores in Campylobacter jejuni. Infect Immun. 1986;54:126–132. doi: 10.1128/iai.54.1.126-132.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wooldridge KG, Williams PH. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg ED. Iron and infection. Microbiol Rev. 1978;42:45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto BR, Verweij-van Vught AM, MacLaren DM. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol. 1992;18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- 7.Rouault TA. Microbiology. Pathogenic bacteria prefer heme. Science. 2004;305:1577–1578. doi: 10.1126/science.1102975. [DOI] [PubMed] [Google Scholar]
- 8.Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. Iron-source preference of Staphylococcus aureus infections. Science. 2004;305:1626–1628. doi: 10.1126/science.1099930. [DOI] [PubMed] [Google Scholar]
- 9.Stojiljkovic I, Perkins-Balding D. Processing of heme and heme-containing proteins by bacteria. DNA Cell Biol. 2002;21:281–295. doi: 10.1089/104454902753759708. [DOI] [PubMed] [Google Scholar]
- 10.Musser JM, Krause RM. The revival of group A streptococcal disease with a commentary on staphylococcal toxic shock syndrome. In: Krause RM, Fauci A, editors. Emerging Infections. San Diego, Calif: Academic Press; 1998. [Google Scholar]
- 11.Francis RT, Jr, Booth JW, Becker RR. Uptake of iron from hemoglobin and the haptoglobin-hemoglobin complex by hemolytic bacteria. Int J Biochem. 1985;17:767–773. doi: 10.1016/0020-711x(85)90262-9. [DOI] [PubMed] [Google Scholar]
- 12.Eichenbaum Z, Muller E, Morse SA, Scott JR. Acquisition of iron from host proteins by the group A streptococcus. Infect Immun. 1996;64:5428–5429. doi: 10.1128/iai.64.12.5428-5429.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates CS, Montanez GE, Woods CR, Vincent RM, Eichenbaum Z. Identification and characterization of a Streptococcus pyogenes operon involved in binding of hemoproteins and acquisition of iron. Infect Immun. 2003;71:1042–1055. doi: 10.1128/IAI.71.3.1042-1055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei B, Liu M, Voyich JM, Prater CI, Kala SV, DeLeo FR, Musser JM. Identification and characterization of HtsA, a second heme-binding protein made by Streptococcus pyogenes. Infect Immun. 2003;71:5962–5969. doi: 10.1128/IAI.71.10.5962-5969.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M, Lei B. Heme transfer from streptococcal cell surface protein Shp to HtsA of transporter HtsABC. Infect Immun. 2005;73:5086–5092. doi: 10.1128/IAI.73.8.5086-5092.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nygaard TK, Blouin GC, Liu M, Fukumura M, Olson JS, Fabian M, Dooley DM, Lei B. The mechanism of direct heme transfer from the streptococcal cell surface protein Shp to HtsA of the HtsABC transporter. J Biol Chem. 2006;281:20761–20771. doi: 10.1074/jbc.M601832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilpa RM, Fadeev EA, Villareal VA, Wong ML, Phillips M, Clubb RT. Solution structure of the NEAT (NEAr Transporter) domain from IsdH/HarA: the human hemoglobin receptor in Staphylococcus aureus. J Mol Biol. 2006;360:435–447. doi: 10.1016/j.jmb.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Sharp KH, Schneider S, Cockayne A, Paoli M. Crystal Structure of the Heme-IsdC Complex, the Central Conduit of the Isd Iron/Heme Uptake System in Staphylococcus aureus. J Biol Chem. 2007;282:10625–10631. doi: 10.1074/jbc.M700234200. [DOI] [PubMed] [Google Scholar]
- 19.Grigg JC, Vermeiren CL, Heinrichs DE, Murphy ME. Haem recognition by a Staphylococcus aureus NEAT domain. Mol Microbiol. 2007;63:139–149. doi: 10.1111/j.1365-2958.2006.05502.x. [DOI] [PubMed] [Google Scholar]
- 20.Andrade MA, Ciccarelli FD, Perez-Iratxeta C, Bork P. NEAT: a domain duplicated in genes near the components of a putative Fe3+ siderophore transporter from Gram-positive pathogenic bacteria. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-9-research0047. RESEARCH0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnoux P, Haser R, Izadi N, Lecroisey A, Delepierre M, Wandersman C, Czjzek M. The crystal structure of HasA, a hemophore secreted by Serratia marcescens. Nat Struct Biol. 1999;6:516–520. doi: 10.1038/9281. [DOI] [PubMed] [Google Scholar]
- 22.Chan ACK, Lelj-Garolla B, Rosell FI, Peterson KA, Mauk AG, Murphy MEP. Cofacial Heme Binding is Linked to Dimerization by a Bacterial Heme Transport Protein. J. Mol. Biol. 2006;362:1108–1119. doi: 10.1016/j.jmb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Schneider S, Sharp KH, Barker PD, Paoli M. An induced fit conformational change underlies the binding mechanism of the heme transport proteobacteria-protein HemS. J Biol Chem. 2006;281:32606–32610. doi: 10.1074/jbc.M607516200. [DOI] [PubMed] [Google Scholar]
- 24.Grigg JC, Vermeiren CL, Heinrichs DE, Murphy ME. Heme coordination by staphylococcus aureus IsdE. J Biol Chem. 2007 doi: 10.1074/jbc.M704602200. [DOI] [PubMed] [Google Scholar]
- 25.Suits MD, Jaffer N, Jia Z. Structure of the Escherichia coli O157:H7 heme oxygenase ChuS in complex with heme and enzymatic inactivation by mutation of the heme coordinating residue His-193. J Biol Chem. 2006;281:36776–36782. doi: 10.1074/jbc.M607684200. [DOI] [PubMed] [Google Scholar]
- 26.Ran Y, Zhu H, Liu M, Fabian M, Olson JS, Aranda R, Phillips GN, Jr, Dooley DM, Lei B. Bis-Methionine Ligation to Heme Iron in the Streptococcal Cell Surface Protein Shp Facilitates Rapid Hemin Transfer to HtsA of the HtsABC Transporter. J Biol Chem. 2007 doi: 10.1074/jbc.M705967200. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chothia C, Lesk AM. The relation between the divergence of sequence and structure in proteins. Embo J. 1986;5:823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheesman MR, Thomson AJ, Greenwood C, Moore GR, Kadir F. Bis-methionine axial ligation of haem in bacterioferritin from Pseudomonas aeruginosa. Nature. 1990;346:771–773. doi: 10.1038/346771a0. [DOI] [PubMed] [Google Scholar]
- 29.Cobessi D, Huang LS, Ban M, Pon NG, Daldal F, Berry EA. The 2.6 A resolution structure of Rhodobacter capsulatus bacterioferritin with metal-free dinuclear site and heme iron in a crystallographic 'special position'. Acta Crystallogr D Biol Crystallogr. 2002;58:29–38. doi: 10.1107/s0907444901017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frolow F, Kalb AJ, Yariv J. Structure of a unique twofold symmetric haem-binding site. Nat Struct Biol. 1994;1:453–460. doi: 10.1038/nsb0794-453. [DOI] [PubMed] [Google Scholar]
- 31.Swartz L, Kuchinskas M, Li H, Poulos TL, Lanzilotta WN. Redox-dependent structural changes in the Azotobacter vinelandii bacterioferritin: new insights into the ferroxidase and iron transport mechanism. Biochemistry. 2006;45:4421–4428. doi: 10.1021/bi060146w. [DOI] [PubMed] [Google Scholar]
- 32.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harding MM. Small revisions to predicted distances around metal sites in proteins. Acta Crystallogr D Biol Crystallogr. 2006;62:678–682. doi: 10.1107/S0907444906014594. [DOI] [PubMed] [Google Scholar]
- 34.de Villiers KA, Kaschula CH, Egan TJ, Marques HM. Speciation and structure of ferriprotoporphyrin IX in aqueous solution: spectroscopic and diffusion measurements demonstrate dimerization, but not muoxo dimer formation. J Biol Inorg Chem. 2007;12:101–117. doi: 10.1007/s00775-006-0170-1. [DOI] [PubMed] [Google Scholar]
- 35.Carney CK, Schrimpe AC, Halfpenny K, Harry RS, Miller CM, Broncel M, Sewell SL, Schaff JE, Deol R, Carter MD, Wright DW. The basis of the immunomodulatory activity of malaria pigment (hemozoin) J Biol Inorg Chem. 2006;11:917–929. doi: 10.1007/s00775-006-0147-0. [DOI] [PubMed] [Google Scholar]
- 36.Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. The structure of malaria pigment beta-haematin. Nature. 2000;404:307–310. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]
- 37.Vermeiren CL, Pluym M, Mack J, Heinrichs DE, Stillman MJ. Characterization of the heme binding properties of Staphylococcus aureus IsdA. Biochemistry. 2006;45:12867–12875. doi: 10.1021/bi0607711. [DOI] [PubMed] [Google Scholar]
- 38.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaroszewski L, Rychlewski L, Li Z, Li W, Godzik A. FFAS03: a server for profile--profile sequence alignments. Nucleic Acids Res. 2005;33:W284–W288. doi: 10.1093/nar/gki418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. Protein Identification and Analysis Tools on the ExPASy Server. In: Walker JM, editor. The Proteomics Protocols Handbook. Humana Press; 2005. [Google Scholar]
- 41.Shaul Y, Schreiber G. Exploring the charge space of protein-protein association: a proteomic study. Proteins. 2005;60:341–352. doi: 10.1002/prot.20489. [DOI] [PubMed] [Google Scholar]
- 42.Andrews SC, Le Brun NE, Barynin V, Thomson AJ, Moore GR, Guest JR, Harrison PM. Site-directed replacement of the coaxial heme ligands of bacterioferritin generates heme-free variants. J Biol Chem. 1995;270:23268–23274. doi: 10.1074/jbc.270.40.23268. [DOI] [PubMed] [Google Scholar]
- 43.Nygaard TK, Liu M, McClure MJ, Lei B. Identification and characterization of the heme-binding proteins SeShp and SeHtsA of Streptococcus equi subspecies equi. BMC Microbiol. 2006;6:82. doi: 10.1186/1471-2180-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Timoney JF. The pathogenic equine streptococci. Vet Res. 2004;35:397–409. doi: 10.1051/vetres:2004025. [DOI] [PubMed] [Google Scholar]
- 45.Otwinowski Z, Minor W. Processing of X-Ray Diffraction Data Collected in Oscillation Mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 46.Grosse-Kunstleve RW, Adams PD. Substructure search procedures for macromolecular structures. Acta Crystallogr D Biol Crystallogr. 2003;59:1966–1973. doi: 10.1107/s0907444903018043. [DOI] [PubMed] [Google Scholar]
- 47.de la Fortelle E, Bricogne G. Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods in Enzymology. 1997;276:472–494. doi: 10.1016/S0076-6879(97)76073-7. [DOI] [PubMed] [Google Scholar]
- 48.Project CC. The CCP4 Suite: Programs for Protein Crystallography. Acta Cryst. 1994;D50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 49.Perrakis A, Morris R, Lamzin VS. Automated protein model building combined with iterative structure refinement. Nat Struct Biol. 1999;6:458–463. doi: 10.1038/8263. [DOI] [PubMed] [Google Scholar]
- 50.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 51.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 52.Vagin A, Teplyakov A. MOLREP: an automated program for molecular replacement. J. Appl. Cryst. 1997;30:1022–1025. [Google Scholar]
- 53.Lee B, Richards FM. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971;55:379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- 54.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. 27–28. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 55.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci U S A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeLano WL. The PyMOL Molecular Graphics System. Palo Alto CA, USA: DeLano Scientific; 2002. [Google Scholar]