Abstract
Most formulations of artificial tears include high-molecular weight hydrophilic polymers (hydrogels) that are usually thought to serve to enhance viscosity and to act as demulcents. A few reports have indicated that application of some of the polymers accelerates healing of wounds in epithelia. Since activation of the epidermal growth factor (EGF) receptor is critical for spontaneous corneal epithelial wound healing, we tested commonly used hydrogels for their ability to activate the EGF receptor and enhance closure of wounds. Five structurally unrelated hydrogels used in artificial tears were found to activate the EGF receptor. Importantly, two of the hydrogels enhanced wound healing in an organ culture model. We propose that the efficacy of hydrogels in treating dry eye may be related to their ability to activate the EGF receptor, and that hydrogels are inexpensive, safe agents to promote healing of wounds in the cornea and possibly in other tissues.
1. Introduction
Application of artificial tears is the most common, and often adequate, therapy for the dry eye group of ocular surface disorders (Johnson and Murphy, 2004). Artificial tears are aqueous solutions of electrolytes, usually containing high-molecular weight hydrophilic polymers (hydrogels). The hydrogel components are typically listed as the “active” ingredients, and they are thought to act by providing viscosity, increased oncotic pressure, and possibly some ill-defined demulcent action. A few reports have indicated that hydrogels, such as methyl cellulose and carboxymethyl cellulose, enhance healing of wounds in the cornea (Garrett et al., 2007; Gaton et al., 1998; Lin and Boehnke, 1999).
The epidermal growth factor receptor is a transmembrane tyrosine kinase receptor, that can be activated by a multitude of diverse mechanisms such as binding to extracellular ligands, phosphorylation by non-receptor tyrosine kinases such as p60src, and through inhibition of tyrosine phosphatases that dephosphorylate the receptor (Bazley and Gullick, 2005; Fischer et al., 2003; Warren and Landgraf, 2006; Wells, 2000). The activated, tyrosine phosphorylated receptor provides binding sites for many signaling molecules, and it triggers numerous intracellular signaling pathways, including phospholipase C-gamma, phosphatidylinositol 3′-kinase, and extracellular signal-regulated kinases 1 and 2 (ERK1/2) (Oda et al., 2005). The EGF receptor is activated upon wounding of the corneal epithelium, and this is absolutely required for the subsequent initiation of migration and healing (Block et al., 2004; Nakamura et al., 2001; Xu et al., 2004; Zieske et al., 2000). Given the importance of EGF receptor activation for motility in corneal epithelial cells after wounding, we examined whether hydrogels used in artificial tears might activate the receptor, and we analyzed their effects on corneal epithelial cell motility in an organ culture model.
2. Materials and methods
2.1. Materials
HCLE cells were kindly provided by Dr. Irene Gipson. Hydroxypropylmethyl cellulose (4000 centipoise, 2% solution), dextran (Average molecular weight 255 kDa) and povidone (embryo tested) were from Sigma-Aldrich, carboxymethyl cellulose (high viscosity), methyl cellulose (4000 centipoise, 2% solution), and propylene glycol were from MP biomedicals, polyvinyl alcohol (86–89% hydrolyzed) and polyethylene glycol 400 were from Alfa Aesar. Antibodies against phospho-ERK1/2, the EGF receptor phosphorylated on tyrosine 1173, and against a C-terminal epitope (to detect total EGF receptor) were from Santa Cruz Biotechnology. Rabbit eyes were from Pel-Freez Biologicals.
2.2. Cell Culture, Cell Treatments and Western-blotting
HCLE cells were grown to confluence in keratinocyte serum-free medium (Gibco-Invitrogen) supplemented with 25 μg/ml bovine pituitary extract, 0.2 ng/ml EGF and 0.3 mM CaCl2. Stimulations were performed after incubating the cells overnight in the same medium without the added EGF and pituitary extract. Cells were stimulated for the indicated times at 37°C, rinsed quickly in ice-cold phosphate-buffered saline (171 mM NaCl, 10.1 mM Na2HPO4, 3.35 mM KCl, 1.84 mM KH2PO4, pH 7.2) and reactions were terminated by addition of SDS-containing sample buffer. Samples were normalized for protein using the BCA reagent (Pierce) and run on 10% polyacrylamide gels in Mini-Protean 3 apparatus (Bio-Rad). Western blotting was performed according to standard procedures, and blots were developed using the SuperSignal® Dura detection kit (Pierce). Equal loading in lanes was verified by staining blots with Ponceau S red (Block et al., 2004). All experiments were performed at least three times with triplicate determinations. To stratify the cells, they were transferred to Dulbecco’s Modified Eagles Medium: F12 1:1, with 10% new-born calf serum and 10 ng/ml EGF, which contains high levels of calcium for three days (Gipson et al., 2003). Stimulations under the stratified conditions were done after starvation overnight with no EGF and 2% new-born calf serum in the same medium.
2.3. Wounding in Organ Culture
To inflict wounds in rabbit eyes in organ culture, a 7.5 mm diameter mark was made with a trephine, and the epithelium was removed by means of an Algerbrush II (Alger Equipment Co.) and a sharp forceps. The wounded corneas were excised with a 2–3 mm scleral rim and placed on hemispheric supports made from the round end of transfer pipets (Samco Scientific Corporation) in 12-well dishes. The wound was briefly stained with 0.1% fluorescein and photographed. They were subsequently incubated submerged at 37°C in Ham’s F12 Medium:Dulbecco’s Modified Eagle’s medium (1:1). Initial time-course experiments determined the optimal incubation time of the organ cultures to 60 hours. The time-course analysis demonstrated that healing takes place during the whole incubation period, i.e. that the cells are actively covering the wound even at the end of the incubation period. The corneas were then stained with 1% alizarin red in phosphate buffered saline and photographed again. The percentage of healing was calculated using MetaMorph software (Molecular Devices) based on the areas of the wounds before and after incubation for each cornea.
3. Results
3.1 Activation of the EGF Receptor by Hydrogels
HCLE is a human corneal limbal epithelial cell-line that has been immortalized by a three-step process involving abrogation of p16INK4A/Rb and p53 functions, and overexpression of the catalytic subunit of the telomerase holoenzyme. When grown in low Ca2+ concentrations, they grow as monolayers, but stratify upon transfer to high Ca2+. They express a range of keratins and mucins similar to that expressed in native corneal epithelium (Gipson et al., 2003).
Unstratified HCLE cells were incubated with hydrogels that are commonly used in commercially available artificial tears, using concentrations in the ranges of the formulations. To test for activation of the EGF receptor, we immunoblotted extracts with an antibody that recognizes the EGF receptor phosphorylated on tyrosine 1173, which is the major binding site for the adaptor protein shc (Batzer et al., 1994). Of the seven polymers that were tested, five activated the EGF receptor (Fig. 1). Methylcellulose and povidone were most potent, but significant activation was also seen with hydroxypropylmethyl cellulose (also known as hypromellose), carboxymethyl cellulose, and polyvinyl alcohol. Dextran, polyethylene glycol, glycerol, and propylene glycol 400, were inactive. The activating ability of the compounds is not dependent on charge, as most of the compounds are uncharged. Three of the active compounds have a backbone of cellulose, whereas the synthetic compounds polyvinyl alcohol and povidone do not, so there is no obvious structural requirement for the ability to activate the EGF receptor. Other components in artificial tears, which are occasionally listed as “active” ingredients, such as glycerol and propylene glycol did not activate the EGF receptor (Fig. 1). Methyl cellulose, hydroxypropylmethyl cellulose and carboxymethyl cellulose were also tested on stratified HCLE cells and found to activate the EGF receptor (data not shown). The level of activation was lower than that seen in stratified HCLE cells, but it should be noted that the cells are grown under very different conditions in the two states (see Methods), and that a direct comparison is therefore not possible.
Fig. 1.
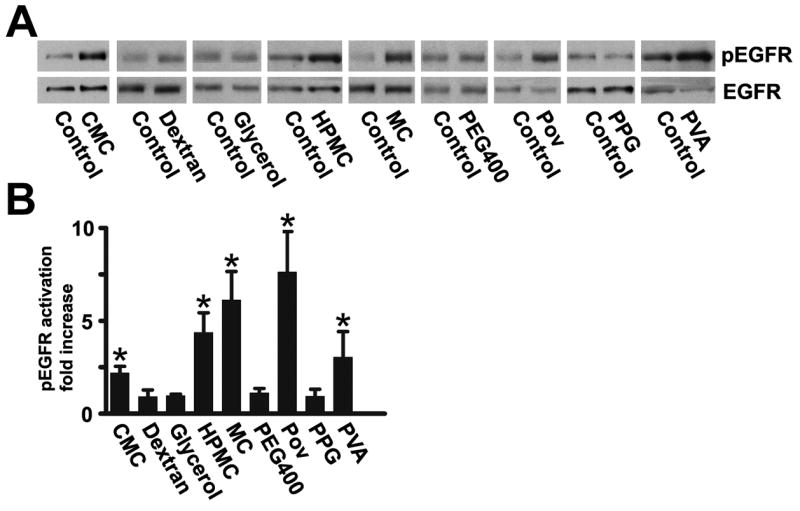
(A) Activation of the EGF receptor by components in artificial tears. HCLE cells were incubated for 40 seconds with the indicated hydrogels and immunblotted with an antibody that recognizes the EGF receptor phosphorylated on tyr-1173 (pEGFR). Blots were stripped and then incubated with an antibody that recognizes the total amount of EGF receptor (EGFR). Equal loading of the lanes of the gels in this and the following figures was verified by staining the blots with Ponceau S Red. Abbreviations: CMC, carboxymethyl cellulose (0.5%); HPMC, hydroxypropylmethyl cellulose (0.25%); MC, methyl cellulose (0.5%); PEG400, polyethylene glycol 400 (1%); POV, povidone (0.6%); PPG, Propylene glycol (1%), PVA, polyvinyl alcohol (1.4%). Glycerol was used at 1%, and dextran at 0.1%. (B) Densitometry of autoradiogram. The values are means of six determinations, and the error bars are standard deviations. CMC, HPMC, MC, POV, and PVA (which are highlighted with asterixes) all yielded statistically significant increases in EGF receptor phosphorylation (p<0.025 or less) according to the two-tailed Mann-Whitney test.
Time course studies showed that activation by the hydrogels was rapid, and a peak of activity was detected starting at 15 seconds. For illustration, the time course of activation by methylcellulose is presented in Fig. 2A. The receptor activation was accompanied by a peak of ERK1/2 activation after a lag phase of 3.5–5 minutes This is qualitatively similar to that seen after stimulation with ligands of the EGF receptor, which also results in a peak of activation. For comparison purposes, cells were stimulated with HB-EGF for 10 minutes, which shows the maximal level of stimulation that can be archived in the HCLE cells with exogenous ligand.
Fig. 2.
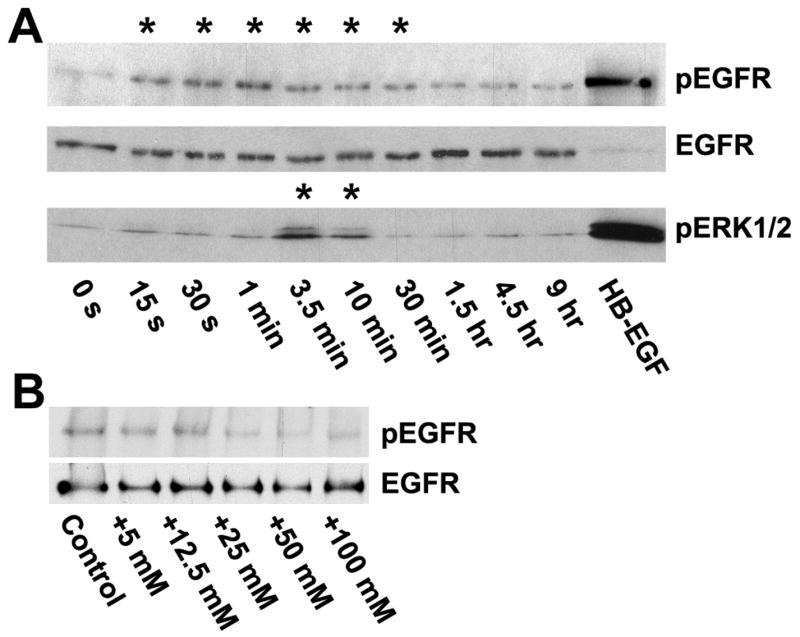
Time and dose-response of EGF receptor activation by methyl cellulose. (A) HCLE cells were incubated for various times with 0.5% methyl cellulose and extracts subjected to Western blotting. The filters were cut and the upper halves were stained with an anti-phospho-EGF receptor (tyr-1173) antibody. They were then stripped and subsequently blotted with an antibody that recognizes the total amount of EGF receptor. In the lane labeled “HB-EGF” the cells were stimulated with 10 ng/ml HB-EGF for 10 minutes. The lower half of the filters was stained with an antibody against phospho-ERK1/2. The signal for pEGFR at the earliest time-point (15 s) was significantly increased (p<0.001) according to the two-tailed Mann-Whitney test), and the last significant (p<0.05) time point was 30 minutes. For clarity, the time-points with statistically enhanced phosphorylation are accentuated with asterixes. The signal from pERK1/2 was significantly increased at 3.5 and 10 minutes (p<0.01) (B) HCLE cells were incubated for 45 seconds with tissue culture medium containing additional amounts of NaCl, as indicated, and immunoblotted with the anti-phospho-EGF receptor antibody. No significant differences were detected.
The EGF receptor has been reported to be activated by changes in tonicity of the medium (Lezama et al., 2005), and we considered whether the results might simply be due to changes in osmotic pressure by addition of the hydrogels. We note that hydrogels at the concentrations used here give a very modest contribution to osmotic pressure, generally in the order of 0.06–0.6 mOsm/kg (Holly and Esquivel, 1985). When the HCLE cells were stimulated with medium including up to 50 mM NaCl, adding approximately 80 mOsm/kg to the osmotic pressure (Sweeney and Beuchat, 1993), no activation of the EGF receptor was observed (Fig. 2B). Therefore, it is unlikely that the hydrogels activate the receptor simply through an osmotic effect.
3.2 Activation of the EGF Receptor by Commercial Artificial Tears
We tested the ability of artificial tears to activate the EGF receptor by incubation directly on HCLE cells. We noted that addition of artificial tears containing the preservatives benzalkonium chloride or perborate (which presumably is hydrolysed in artificial tears to generate H2O2) resulted in strong activation of the EGF receptor, and we therefore tested these compounds directly on the HCLE cells. As is seen in Fig. 3, both preservatives resulted in strong dose-dependent activation of the EGF receptor. The activation by benzalkonium chloride peaked at about 0.01%, a concentration often used in commercial preparations. Cell damage was obvious at higher concentrations as determined by the Trypan Blue exclusion test (data not shown), which may explain the declines the higher concentrations.
Fig. 3.
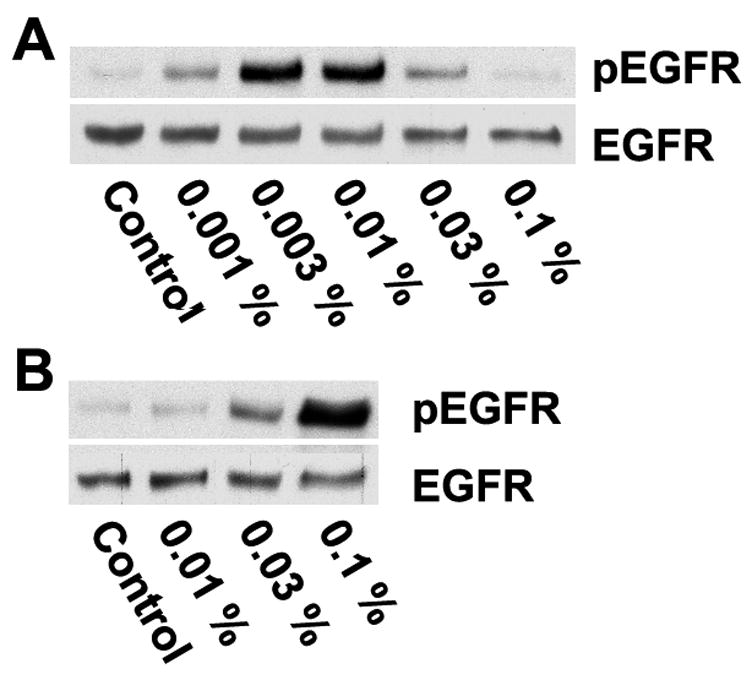
Induction of EGF receptor activation by preservatives in artificial tears. HCLE cells were stimulated for 40 seconds with medium containing the indicated concentrations of (A) benzalkonium chloride or (B) peroxyborate and immunoblotted with an anti-phospho-EGF receptor (tyr-1173) antibody, or an antibody that recognizes total EGF receptor.
Preservative-free tears were subsequently tested. They varied significantly in their ability to activate the EGF receptor, and the three most potent ones, which all contain hydroxypropylmethyl cellulose, are illustrated in Fig. 4A and B. It should be noted that in addition to differences in content of hydrogels, the artificial tears also have very different contents of electrolytes and other small molecular weight compounds, which is likely to influence the results. Many commercial artificial tears are hypotonic (Gilbard, 2005). However, as noted in Fig. 4C, when cells were stimulated with medium diluted with up to two volumes of water, no activation of the EGF receptor was observed, and the effects of the artificial tears are therefore unlikely to be due to a hypotonic shock.
Fig. 4.
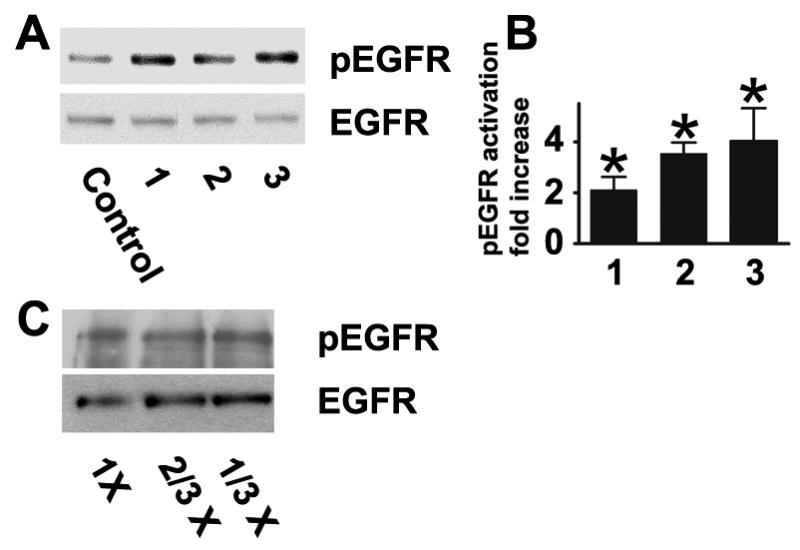
Induction of EGF receptor activation by commercial preservative-free artificial tears. (A) 1, 2, and 3 refer to Bion Tears (Alcon Laboratories, Inc.), Tears Naturale (Alcon Laboratories, Inc.), and Visine Pure Tears Portables (Pfeizer Consumer Healthcare), respectively. The tears were applied to directly to HCLE cells for 40 seconds, and extracts were immunnoblotted with the phospho- and total EGF receptor antibodies. (B). Densitometry of autoradiogram. The values are means of at least six determinations, and the error bars are standard deviations. Stimulations were significant at p<0.0025 or less by the two-tailed Mann-Whitney test (C) HCLE cells were stimulated for 40 seconds with medium or medium diluted to 2/3 or 1/3 concentration with water. No significant differences were detected.
3.3 Enhancement of Wound Healing by Hydrogels
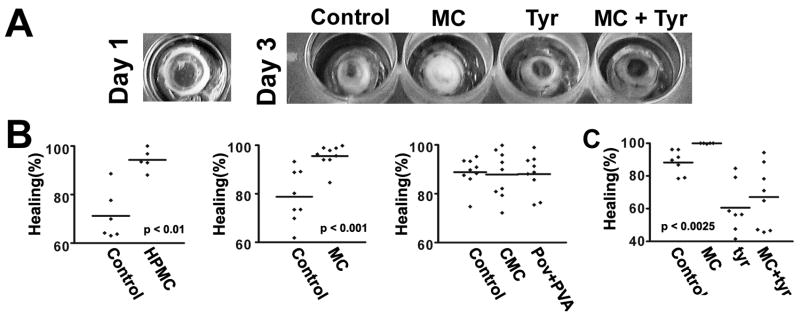
To examine whether hydrogels enhance healing, their effects were studied in organ culture. A central circular area of the corneal epithelium in rabbit eyes was removed, and the eyes were incubated for 60 hours either with 0.25% hydroxypropylmethyl cellulose, 0.5% methyl cellulose, 0.5% carboxymethyl cellulose, or a combination of 1.4% polyvinyl alcohol and 0.6% povidone. The concentrations that were used are representative of those in commercial artificial tears. Polyvinyl alcohol and povidone are usually combined in commercial preparations at the concentrations used. To ensure good contact with the hydrogels, a submerged culture system was used. There was significant variability in the degrees of healing between different eyes, necessitating fairly large sample sizes. As is seen in Fig. 5A, hydroxypropylmethyl cellulose and methyl cellulose increased healing rates significantly, whereas carboxymethyl cellulose, and polyvinyl alcohol/povidone did not. To see if healing was dependent on activation of the EGF receptor, healing was also performed in the presence of tyrphostin AG 1478. The inhibitor inhibited spontaneous healing, in agreement with previous observations (Zieske et al., 2000). In the experiment shown in figure 5B, methyl cellulose enhanced healing very significantly (p<0.0025). However, in the presence of tyrphostin AG 1478 no significant enhancement by methyl cellulose was seen.
Fig. 5.
Hydrogels accelerate healing of wounds in organ culture. Rabbit corneas were wounded as described in Materials and Methods, and allowed to heal for 60 hours in the presence of 0.25% hydroxypropylmethyl cellulose (HPMC), 0.5% methyl cellulose (MC), 0.5% carboxymethyl cellulose (CMC), or a combination of 0.6% povidone and 1.4% polyvinyl alcohol (PVA). The final areas were measured using the MetaMorph program (Molecular Devices Corp.). The graphs show representative results. Each data-point is the value from individual eyes. The p values were determined by the two-tailed Mann-Whitney test, and the lines indicate the means. (A) Left: Picture of wounded eye taken immediately after debriedement and stained with flourescein. The image was digitally enhanced to show the stained area. Right: Representative eyes after healing stained with alizarin red. (B) Healing in the presence of the indicated compounds. Enhanced healing by HPMC and MC were observed in four separate experiments. (C) Healing in the presence of 0.5% methyl cellulose, and 12.5 μM tyrphostin AG 1478 (tyr), where indicated. Tyrphostin AG 1478 reduced the spontaneous and MC-stimulated healing significantly (p<0.007 and p<0.0025, respectively). One out of three similar experiments is shown.
4. Discussion
In this study, hydrogels that are commonly used in artificial tears were found to activate the EGF receptor. To the best of our knowledge the observation that hydrogels can activate a signaling receptor is novel. Since there is no structural similarity between these compounds and the natural ligands for the receptor, one may wonder how activation occurs. Hydrogels adhere to the glycocalyx of cells thorough a variety of non-specific interactions, and binding of hydrogels could therefore perturb the function of membrane proteins at many levels (Peppas and Sahlin, 1996; Salamat-Miller et al., 2005; Sudhakar et al., 2006). For instance, binding to the EGF receptor could result in a shift in its dimerization status with other members of the EGF receptor family, influence the recycling dynamics of the receptor, or interfere with binding to proteins that inhibit its activity (Odintsova et al., 2000; Odintsova et al., 2003). We have previously found that the EGF receptor is transactivated by ligand binding in HCLE cells by wounding or by hepatocyte growth factor (Block et al., 2004; Spix et al., 2007). However, when we tested methylcellulose, we found no evidence that it activates the EGF receptor by this type of mechanism (data not shown).
Hydroxypropylmethyl cellulose and methyl cellulose clearly promote healing of wounds in the epithelium in organ culture. The fact that tyrphostin AG 1478 blocks the effect of hydrogels on corneal epithelial cell migration points to the importance of EGF receptor signaling in healing and says that hydrogels act upstream of EGF receptor activation. However, enhancement of migration by EGF and HB-EGF has been difficult to detect in our organ culture system, although it should be recognized that small effects are difficult to measure because of the variability of the responses of individual eyes. Therefore it is likely that the hydrogels have effects in addition to stimulating the EGF receptor. The corneal epithelium contains numerous growth factor signaling systems that impact on cell motility (Klenkler and Sheardown, 2004; Wilson et al., 2003), so it is possible that the hydrogels have effects on other receptors. In addition, carboxymethyl cellulose binds to the extracellular matrix and enhances adhesion of corneal epithelial cells, so an effect on the matrix could also contribute to increased cell motility and wound healing (Garrett et al., 2007).
The time-course of stimulation with EGF, which has been studied in great detail (Blagoev et al., 2004; Kholodenko et al., 1999; Olsen et al., 2006; Schoeberl et al., 2002), is similar to that seen with the hydrogels: a rapid peak followed by a decline to very low levels. However, occupancy of a very small fraction of the EGF receptors, 1% or less, is sufficient to elicit a full biological response (Teramura et al., 2006, and references therein). We therefore expect that the prolonged effect on reepitheliazation is due to continuous low levels of EGF receptor stimulation, which would be difficult to detect in the immunoblots.
Two frequently used preservatives in commercial artificial tears activate the EGF receptor strongly. Benzalkonium chloride may activate the EGF receptor by binding to G proteins or through some generalized membrane perturbation (Patarca et al., 2000). Sodium perborate is hydrolyzed to H2O2 in the aqueous environment of artificial tears, which is known to activate the EGF receptor (Aslan and Ozben, 2003). However, the use of either of these to enhance EGF receptor activation clinically seems dubious. Benzalkonium chloride has severe adverse effects when applied to eyes (Noecker, 2001), and H202 is also toxic and is rapidly deactivated by catalase in the tear film (Riley and Wilson, 1993).
A very large number of studies have indicated that EGF accelerates healing of wounds in the cornea in a variety of different animal models and in humans (for reviews see Baldwin and Marshall, 2002; Imanishi et al., 2000; Klenkler and Sheardown, 2004; Lu et al., 2001; for reviews see Schultz et al., 1994). A key to success is to apply constant stimulation either by frequent applications or using some continuous release device (Brown et al., 1988; Hori et al., 2007; Sheardown et al., 1993) Soluble hydrogels may reside on the cell surface for hours (Garrett et al., 2007; Lee et al., 2000; Peppas and Sahlin, 1996; Salamat-Miller et al., 2005; Sudhakar et al., 2006), and even more prolonged contact may be achieved when applied with continuous release devices. Hydrogels are considered safe (Fu et al., 2005), and our studies suggest that particularly hydroxypropylmethyl cellulose and methyl cellulose may be used to promote wound healing. Furthermore, activation of the EGF receptor may in part explain the usefulness of artificial tears in treatment of dry eye.
Acknowledgments
We wish to acknowledge the assistance of Jennifer Koury in preparing cultures of HCLE cells. This work was supported by the National Institutes of Health Grants EY013463 and EY08098, and grants from Research to Prevent Blindness and The Eye and Ear Foundation (Pittsburgh, PA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aslan M, Ozben T. Oxidants in receptor tyrosine kinase signal transduction pathways. Antioxid Redox Signal. 2003;5:781–8. doi: 10.1089/152308603770380089. [DOI] [PubMed] [Google Scholar]
- Baldwin HC, Marshall J. Growth factors in corneal wound healing following refractive surgery: A review. Acta Ophthalmol Scand. 2002;80:238–47. doi: 10.1034/j.1600-0420.2002.800303.x. [DOI] [PubMed] [Google Scholar]
- Batzer AG, Rotin D, Urena JM, Skolnik EY, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994;14:5192–201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazley LA, Gullick WJ. The epidermal growth factor receptor family. Endocr Relat Cancer. 2005;12(Suppl 1):S17–27. doi: 10.1677/erc.1.01032. [DOI] [PubMed] [Google Scholar]
- Blagoev B, Ong SE, Kratchmarova I, Mann M. Temporal analysis of phosphotyrosine-dependent signaling networks by quantitative proteomics. Nat Biotechnol. 2004;22:1139–45. doi: 10.1038/nbt1005. [DOI] [PubMed] [Google Scholar]
- Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004;279:24307–12. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- Brown GL, Curtsinger LJ, White M, Mitchell RO, Pietsch J, Nordquist R, von Fraunhofer A, Schultz GS. Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann Surg. 1988;208:788–94. doi: 10.1097/00000658-198812000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31:1203–8. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- Fu X, Li X, Cheng B, Chen W, Sheng Z. Engineered growth factors and cutaneous wound healing: success and possible questions in the past 10 years. Wound Repair Regen. 2005;13:122–30. doi: 10.1111/j.1067-1927.2005.130202.x. [DOI] [PubMed] [Google Scholar]
- Garrett Q, Simmons PA, Xu S, Vehige J, Zhao Z, Ehrmann K, Willcox M. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2007;48:1559–67. doi: 10.1167/iovs.06-0848. [DOI] [PubMed] [Google Scholar]
- Gaton DD, Stiebel-Kalish H, Loya N, Weinberger D, Kashtan Y, Solomon A. The effect of tear substitute and silicone oil on re-epithelisation of the cornea. Eye. 1998;12 (Pt 1):141–4. doi: 10.1038/eye.1998.24. [DOI] [PubMed] [Google Scholar]
- Gilbard JP. The scientific context and basis of the pharmacologic management of dry eyes. Ophthalmol Clin North Am. 2005;18:475–84. v. doi: 10.1016/j.ohc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- Holly FJ, Esquivel ED. Colloid osmotic pressure of artificial tears. J Ocul Pharmacol. 1985;1:327–36. doi: 10.1089/jop.1985.1.327. [DOI] [PubMed] [Google Scholar]
- Hori K, Sotozono C, Hamuro J, Yamasaki K, Kimura Y, Ozeki M, Tabata Y, Kinoshita S. Controlled-release of epidermal growth factor from cationized gelatin hydrogel enhances corneal epithelial wound healing. J Control Release. 2007;118:169–76. doi: 10.1016/j.jconrel.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19:113–29. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- Johnson ME, Murphy PJ. Changes in the tear film and ocular surface from dry eye syndrome. Prog Retin Eye Res. 2004;23:449–74. doi: 10.1016/j.preteyeres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Kholodenko BN, Demin OV, Moehren G, Hoek JB. Quantification of short term signaling by the epidermal growth factor receptor. J Biol Chem. 1999;274:30169–81. doi: 10.1074/jbc.274.42.30169. [DOI] [PubMed] [Google Scholar]
- Klenkler B, Sheardown H. Growth factors in the anterior segment: role in tissue maintenance, wound healing and ocular pathology. Exp Eye Res. 2004;79:677–88. doi: 10.1016/j.exer.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: the next generation. J Pharm Sci. 2000;89:850–66. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Lezama R, Diaz-Tellez A, Ramos-Mandujano G, Oropeza L, Pasantes-Morales H. Epidermal growth factor receptor is a common element in the signaling pathways activated by cell volume changes in isosmotic, hyposmotic or hyperosmotic conditions. Neurochem Res. 2005;30:1589–97. doi: 10.1007/s11064-005-8837-5. [DOI] [PubMed] [Google Scholar]
- Lin CP, Boehnke M. Influences of methylcellulose on corneal epithelial wound healing. J Ocul Pharmacol Ther. 1999;15:59–63. doi: 10.1089/jop.1999.15.59. [DOI] [PubMed] [Google Scholar]
- Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood) 2001;226:653–64. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Sotozono C, Kinoshita S. The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp Eye Res. 2001;72:511–7. doi: 10.1006/exer.2000.0979. [DOI] [PubMed] [Google Scholar]
- Noecker R. Effects of common ophthalmic preservatives on ocular health. Adv Ther. 2001;18:205–15. doi: 10.1007/BF02853166. [DOI] [PubMed] [Google Scholar]
- Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. 2005;1:2005 0010. doi: 10.1038/msb4100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odintsova E, Sugiura T, Berditchevski F. Attenuation of EGF receptor signaling by a metastasis suppressor, the tetraspanin CD82/KAI-1. Curr Biol. 2000;10:1009–12. doi: 10.1016/s0960-9822(00)00652-7. [DOI] [PubMed] [Google Scholar]
- Odintsova E, Voortman J, Gilbert E, Berditchevski F. Tetraspanin CD82 regulates compartmentalisation and ligand-induced dimerization of EGFR. J Cell Sci. 2003;116:4557–66. doi: 10.1242/jcs.00793. [DOI] [PubMed] [Google Scholar]
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–48. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Patarca R, Rosenzwei JA, Zuniga AA, Fletcher MA. Benzalkonium salts: effects on G protein-mediated processes and surface membranes. Crit Rev Oncog. 2000;11:255–305. [PubMed] [Google Scholar]
- Peppas NA, Sahlin JJ. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials. 1996;17:1553–61. doi: 10.1016/0142-9612(95)00307-x. [DOI] [PubMed] [Google Scholar]
- Riley MV, Wilson G. Topical hydrogen peroxide and the safety of ocular tissues. Clao J. 1993;19:186–90. [PubMed] [Google Scholar]
- Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57:1666–91. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Schoeberl B, Eichler-Jonsson C, Gilles ED, Muller G. Computational modeling of the dynamics of the MAP kinase cascade activated by surface and internalized EGF receptors. Nat Biotechnol. 2002;20:370–5. doi: 10.1038/nbt0402-370. [DOI] [PubMed] [Google Scholar]
- Schultz G, Khaw PT, Oxford K, MaCauley S, Van Setten G, Chegini N. Growth factors and ocular wound healing. Eye. 1994;8 (Pt 2):184–7. doi: 10.1038/eye.1994.43. [DOI] [PubMed] [Google Scholar]
- Sheardown H, Wedge C, Chou L, Apel R, Rootman DS, Cheng YL. Continuous epidermal growth factor delivery in corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 1993;34:3593–600. [PubMed] [Google Scholar]
- Spix JK, Chay EY, Block ER, Klarlund JK. Hepatocyte growth factor induces epithelial cell motility through transactivation of the epidermal growth factor receptor. Exp Cell Res. 2007;313:3319–25. doi: 10.1016/j.yexcr.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery--a promising option for orally less efficient drugs. J Control Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Sweeney TE, Beuchat CA. Limitations of methods of osmometry: measuring the osmolality of biological fluids. Am J Physiol. 1993;264:R469–80. doi: 10.1152/ajpregu.1993.264.3.R469. [DOI] [PubMed] [Google Scholar]
- Teramura Y, Ichinose J, Takagi H, Nishida K, Yanagida T, Sako Y. Single-molecule analysis of epidermal growth factor binding on the surface of living cells. Embo J. 2006;25:4215–22. doi: 10.1038/sj.emboj.7601308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CM, Landgraf R. Signaling through ERBB receptors: multiple layers of diversity and control. Cell Signal. 2006;18:923–33. doi: 10.1016/j.cellsig.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Wells A. Tumor invasion: role of growth factor-induced cell motility. Adv Cancer Res. 2000;78:31–101. doi: 10.1016/s0065-230x(08)61023-4. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Netto M, Ambrosio R., Jr Corneal cells: chatty in development, homeostasis, wound healing, and disease. Am J Ophthalmol. 2003;136:530–6. doi: 10.1016/s0002-9394(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Xu KP, Ding Y, Ling J, Dong Z, Yu FS. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:813–20. doi: 10.1167/iovs.03-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest Ophthalmol Vis Sci. 2000;41:1346–55. [PubMed] [Google Scholar]