Abstract
Prostaglandin D2 is the most abundant prostaglandin in the brain. It has long been described as a modulator of the neuroinflammatory process, but little is known regarding the role of its Gαs-coupled receptor, DP1. Therefore, in this study, the effect of the DP1 receptor on the outcome of cerebral ischemia in wildtype (WT) and DP1 knockout (DP1−/−) C57Bl/6 mice was investigated. Ischemia-reperfusion injury was produced by a 90-min occlusion of the right middle cerebral artery followed by a 4-day reperfusion. Infarct size was 49.0 ± 11.0% larger in DP1−/− mice (n = 11; P < 0.01) than in WT mice (n = 9 per group). However, no differences were detected in the relative cerebral blood flow (CBF) or any of the physiological parameters measured (n = 5 per group) or in the large blood vessel anatomy (n = 3 per group). To further address whether the DP1 protective role in the brain could be extended to neurons, mouse primary corticostriatal neuronal cultures were exposed to the DP1-selective agonist, BW245C, which provided dose-dependent protection against excitotoxicity induced by glutamate. Protection was significant at a dose as low as 0.05 μm. The results indicate that the DP1 receptor is neuroprotective in both in vivo and in vitro paradigms. Development of drugs to stimulate the DP1 receptor in brain could provide a new therapeutic strategy against cerebral ischemia and potentially other neurological conditions.
Keywords: BW245C, focal ischemia, mouse, neuroprotection, prostaglandins, stroke
Introduction
An important feature of the pathophysiological response to ischemic brain injury is the inflammatory cascade triggered in the occluded blood vessels and brain parenchyma. Therefore, an anti-inflammatory strategy may be useful for treating acute stroke, a leading cause of death and disability worldwide for which acute therapeutic management is limited to thrombolysis (Chamorro & Hallenbeck, 2006). Inflammation is at least in part regulated by a family of lipid mediators known as prostaglandins (PGs; Bazan, 2005). PGs are generated through the rate-limiting enzyme cyclooxygenase (COX), which converts arachidonic acid to PGH2. PGH2 is subsequently modified into different prostanoids, including PGE2, PGD2, PGF2α, PGI2, and thromboxane A2. These prostanoids exert their actions via a family of G-protein-coupled receptors; EP (1−4), DP (1−2), FP, IP, and TP, respectively. Each of these receptors differs in its effects on cyclic AMP (cAMP) and/or phosphoinositol turnover and intracellular Ca2+ mobilization.
PGD2 is the most abundant PG in brain (Abdel-Halim et al., 1977; Narumiya et al., 1982). It is synthesized in many organs and has been implicated as a signalling molecule in the mediation or regulation of various biological processes, including platelet aggregation, broncho-constriction, allergic diseases, sleep and wakefulness, seizures, and hypoxia (Masuda et al., 1986; Akarsu et al., 1998; Matsuoka et al., 2000; Monneret et al., 2003; Chen & Bazan, 2005). Two distinct PGD2 synthases (PGDS), the haematopoetic PGDS and the lipocalin PGDS, mediate the last regulatory steps in the biosynthetic pathway of PGD2 production (Urade & Eguchi, 2002; Aritake et al., 2006). PGD2 interacts mainly with two G-protein-coupled receptors; the DP1 receptor stimulates adenylyl cyclase through Gαs, whereas DP2 [initially called chemoattractant receptor T helper type 2 (CRTH2)] inhibits adenylyl cyclase through Gαi and increases intracellular Ca2+ (Malki et al., 2005; Spik et al., 2005). Our earlier studies revealed that the EP2 and EP4 receptors, which enhance cAMP levels, are protective in various toxicity models (Echeverria et al., 2005; Ahmad et al., 2006b), whereas the EP1 receptor, which leads to an increase in intracellular Ca2+, promotes neurotoxicity (Ahmad et al., 2006a).
We hypothesized that the DP1 receptor would be protective in preclinical models of toxicity. Therefore, we investigated the role of the DP1 receptor in transient focal ischemia using wildtype (WT) and DP1 knockout (DP1−/−) mice. To document the protective role of DP1 in neurons, we tested the effect of the DP1-selective agonist, BW245C, on survival of cultured primary neurons following glutamate-induced toxicity. This study provides evidence to help clarify the conflicting results regarding the actions of PGs in the brain.
Materials and methods
Animals
This study was performed in accordance with the NIH guidelines for the use of experimental animals; protocols were approved by the Johns Hopkins Animal Care and Use Committee. C57BL/6 WT and DP1−/− mice were bred in our facility and genotyped by PCR of genomic DNA extracted from tail tissue. Primer pairs were DP1/DP2, which produced a WT band at 370 bp, and DP2/Neo2, which produced a mutant band at 340 bp. Primer sequences were DP1, TCGGTCTTTTATGTGCTCGTG; DP2, GGATCATCTGGATGAAACACC; and Neo2, CCCGTGATATTGCTGAAGAGC. PCR conditions included an initial denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 1 min, 62 °C for 1 min, and 72 °C for 40 s, and a final cycle of 72 °C for 5 min.
Transient ischemia protocol
Transient focal cerebral ischemia was induced by middle cerebral artery (MCA) occlusion (MCAO) with an intraluminal filament technique as described (Ahmad et al., 2006b). Briefly, adult male mice (20−28 g) were placed under halothane anaesthesia. Body temperature was maintained at 37.0 ± 0.5 °C. Relative cerebral blood flow (CBF) was monitored by laser-Doppler flowmetry (Moor instruments, Devon, England) over the parietal cortex supplied by the MCA. Occlusion of the MCA was accomplished with a 7−0 Ethilon nylon monofilament (Ethicon, Somerville, NJ) coated with flexible silicone and confirmed by a decrease in CBF. During the 90-min occlusion, anaesthesia was discontinued, and the animals were transferred to a humidity and temperature-controlled chamber. Then the mice were again anaesthetized, and the filament was withdrawn. The mice were returned to the chamber for approximately 2 h before being returned to their cages.
Assessment of neurological score
Neurological function was measured in each mouse at day 4 after reperfusion according to the following graded scoring system, as described previously (Li et al., 2004); 0, no deficit; 1, forelimb weakness and torso turning to the ipsilateral side when held by tail; 2, circling to affected side; 3, unable to bear weight on affected side; and 4, no spontaneous locomotor activity or barrel rolling.
Quantification of infarct volume
After 4 days of reperfusion, the mice were anaesthetized deeply with halothane, and the brains were harvested and sliced coronally into five 2-mm-thick sections, which were incubated with 1% 2,3,5-triphenyl-tetrazolium chloride (TTC) in saline for 30 min at 37 °C. The area of brain infarct, identified by the lack of TTC staining, was measured on the rostral and caudal surfaces of each slice and numerically integrated across the thickness of the slice to obtain an estimate of infarct volume (Sigma Scan Pro, Systat, Port Richmond, CA). Volumes from all five slices were summed to calculate total infarct volume over the entire hemisphere, expressed as a percentage of the volume of the contralateral hemisphere. Infarct volume was corrected for swelling by comparing the volumes in the ipsilateral and contralateral hemispheres. The corrected volume of the infarcted hemisphere was calculated as corrected volume of infarcted hemisphere = volume of contralateral hemisphere – (volume of ipsilateral hemisphere – volume of infarct) (Doré et al., 2003).
Measurement of body temperature, blood gases, and mean arterial blood pressure
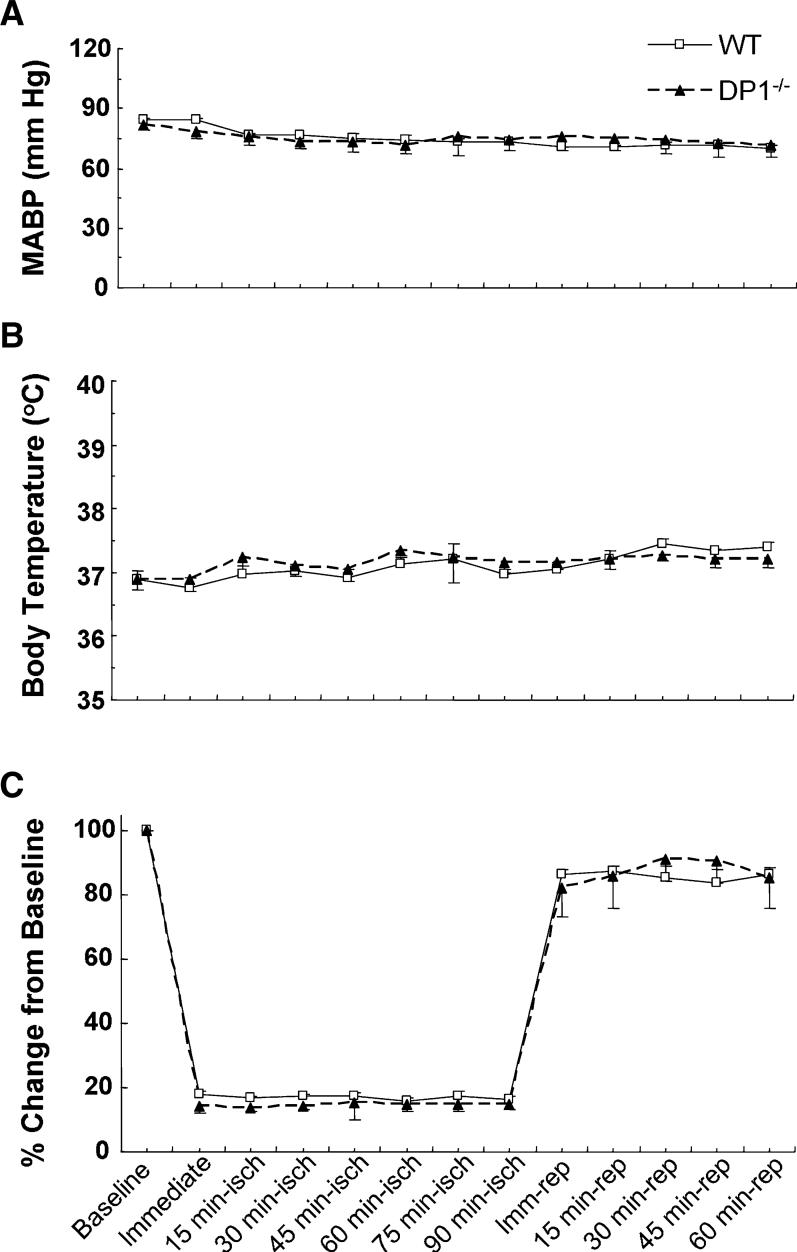
Body temperature was determined with a rectal probe in a separate cohort of animals (n = 5) at baseline and at 15-min intervals for 90 min of ischemia and 60 min of reperfusion. The femoral artery was cannulated for measurement of arterial blood gases and mean arterial blood pressure (MABP), which were measured at the same time points.
Cerebral vessel diameter
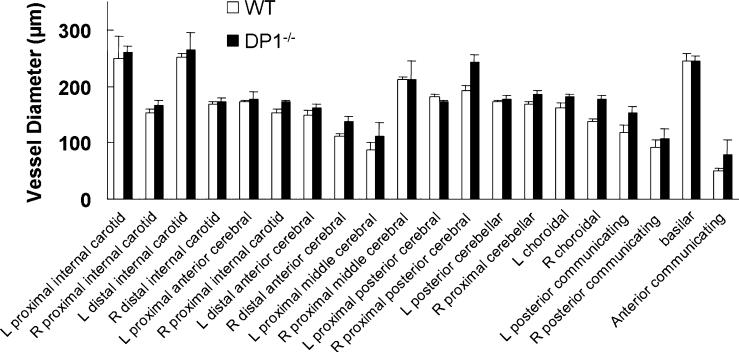
To compare the gross cerebral vessel anatomy in WT and DP1−/− mice, three naïve mice of each genotype were anaesthetized deeply and perfused via the heart left ventricle with saline followed by black latex. Then their brains were harvested with the circle of Willis intact. The brains were placed in 10% formalin for 24 h before examination with Metavue software (Meta Imaging Series Software, Downing-town, PA).
Primary neuronal cultures
All materials used for cell culture were obtained from Invitrogen (Carlsbad, CA), unless otherwise stated. Corticostriatal neuronal cells were isolated from E17 embryos of timed pregnant mice. Cortical neurons were plated at a density of 0.5 × 106 cells per well in B27 supplemented, HEPES-buffered, high glucose Neurobasal medium, as described previously (Doré et al., 1999). Cells were incubated at 37 °C in a 95% air and 5% CO2 humidified atmosphere until the day of experiment. Half of the initial medium was removed at day 4 and replaced with fresh medium. After 14 days in culture, neurons were incubated in fresh medium with or without BW245C (Cayman Chemical Co, Ann Arbor, MI) or vehicle and 30 μm glutamate (Sigma, St Louis, MO). All experiments were conducted in the B27 minus anti-oxidant supplemented Neurobasal medium. Cell survival was measured with the MTT colourimetric assay (Zhuang et al., 2003). Experiments were repeated with at least three separate batches of cultures.
Statistical analysis
Data are expressed as means ± SEM. Multiple comparisons were analysed by the anova and Tukey's test, with significance set at P < 0.05.
Results
Comparison of anatomical and physiological parameters in WT and DP1−/− mice
In terms of the large blood-vessel anatomy in the brains, no significant difference between WT and DP1−/− mice was detected (Fig. 1). Considering the potential effect of DP1 on the vasculature, we monitored physiological parameters at baseline, during MCAO ischemia, and 1 h after reperfusion in a cohort of five mice. The pH, PaCO2, PaO2, and mean arterial blood pressure (MABP) were not significantly different between WT and DP1−/− mice at baseline (Table 1, Fig. 2A), and remained unchanged during the course of MCAO and reperfusion. Core body temperature was also similar in the WT and DP1−/− mice and remained unchanged during the course of the experiment (Fig. 2B).
Fig. 1.
Genetic deletion of the DP1 receptor did not affect cerebral vasculature. Macroscopic analysis of cerebral arterial vasculature revealed no differences between DP1−/− and WT mice.
Table 1.
Effect of MCAO on physiological parameters in WT and DP1−/− mice
| Wildtype Mice |
DP1−/− Mice |
|||||
|---|---|---|---|---|---|---|
| Parameter | Baseline | 1 h MCAO | 1 h reperfusion | Baseline | 1 h MCAO | 1 h reperfusion |
| pH | 7.36 ± 0.02 | 7.34 ± 0.01 | 7.35 ± 0.01 | 7.35 ± 0.01 | 7.30 ± 0.02 | 7.31 ± 0.02 |
| PaCO2 | 38.5 ± 1.1 | 40.5 ± 1.2 | 39.0 ± 1.1 | 42.3 ± 1.3 | 39.8 ± 1.8 | 42.5 ± 1.7 |
| PaO2 | 105 ± 3 | 131 ± 3 | 107 ± 2 | 115 ± 7 | 122 ± 5 | 113 ± 5 |
Fig. 2.
DP1 receptor deletion did not affect physiological parameters. (A) Mean arterial blood pressure (MABP), (B) core body temperature, and (C) relative cerebral blood flow (CBF) were recorded at baseline, at induction of ischemia, and at 15-min intervals during ischemia and 1 h of reperfusion. Change in CBF was recorded as a per cent of baseline.
Laser-Doppler flowmetry showed that relative CBF in WT and DP1−/− mice decreased by a similar value to 16.4 ± 1.2% and 14.9 ± 2.5% of baseline, respectively, during MCAO (Fig. 2C). Furthermore, the relative CBF was not significantly different between the two mouse genotypes at any time during ischemia or 1 h of reperfusion.
Effect of DP1 receptor deletion on neurological score and ischemic brain injury
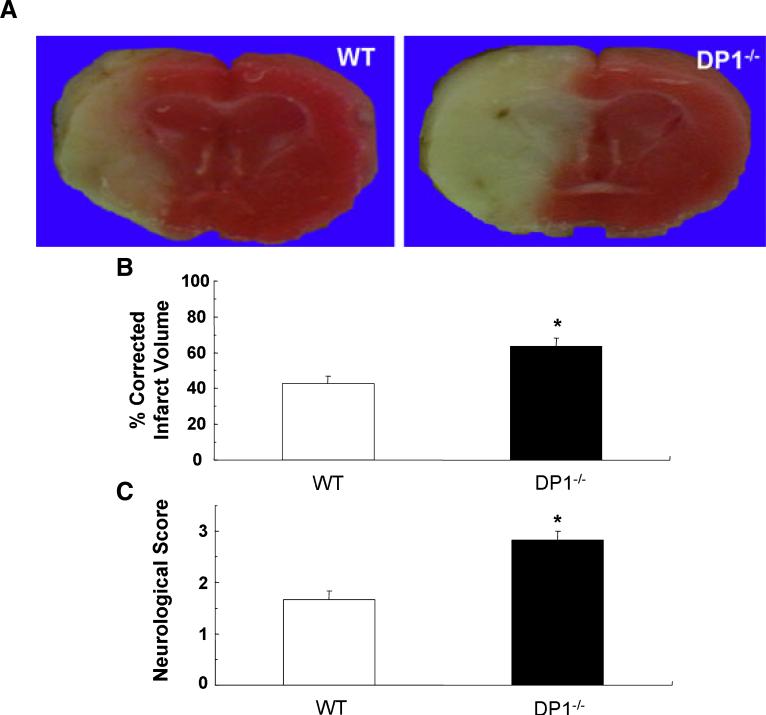
In a cohort of mice separate from those used to measure physiological parameters, we examined whether the DP1 receptor is protective in the focal ischemia model by comparing neurological scores and infarct volumes of WT (n = 9) and DP1−/− (n = 11) mice (Fig. 3). The per cent corrected infarct volume was 49.0 ± 11.0% (P< 0.01) larger in DP1−/− than in WT mice. In addition, the neurological scores were significantly higher in the DP1−/− mice than in the WT mice (P < 0.01).
Fig. 3.
Genetic deletion of the DP1 receptor enhanced ischemic brain injury and neurological dysfunction after transient ischemia. (A) Photographs of infarcted brain slices from WT and DP1−/− mice. (B) Per cent corrected hemispheric infarct volume was significantly larger in DP1−/− mice than in WT mice after 90 min of ischemia and 4 days of reperfusion (*P < 0.01 vs. WT). (C) Neurological scores were assessed 4 days after ischemia. Scores were significantly higher in DP1−/− mice than in WT mice, indicating greater neurological dysfunction (*P < 0.01 vs. WT).
Neuroprotective effect of DP1 agonist, BW245C, against glutamate toxicity
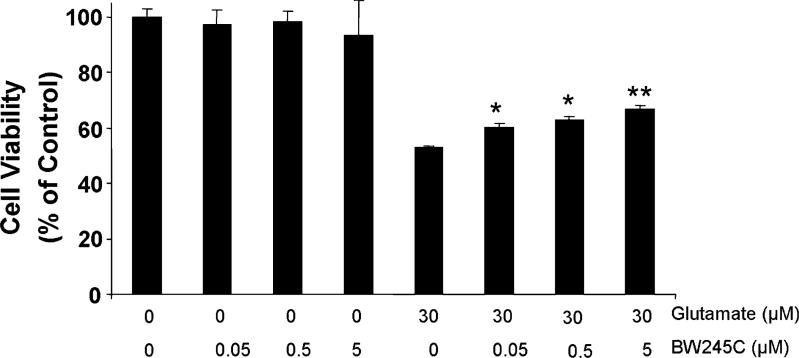
To determine whether DP1 receptor activity contributes to protection against excitotoxic insult in neurons, primary neuronal cultures were exposed to glutamate (30 μm) and the DP1 agonist, BW245C. BW245C was chosen mainly because it has no detectable affinity to DP2, as determined in mouse, rat, guinea pig, and human (Shichijo et al., 2003; Liu et al., 2005). Glutamate decreased the viability of neuronal cell cultures by approximately 50%. BW245C significantly reduced the toxic effect of glutamate in a dose-dependent manner (Fig. 4), with 0.05−5.0 μm having a significant protective effect.
Fig. 4.
The DP1-selective agonist, BW245C, provides neuroprotection against glutamate-induced toxicity. Cultured mouse corticostriatal neurons were treated with BW245C (0, 0.05, 0.5, and 5 μm) in the presence or absence of 30 μm glutamate for 24 h before being assessed for cell viability. Experiments were repeated three times with similar results. *P < 0.05 and **P < 0.01 compared with the group treated with glutamate alone.
Discussion
This study was designed to investigate whether the PGD2 DP1 receptor has a protective role in focal cerebral ischemia. We found that genetic deletion of the mouse DP1 receptor increased the size of the MCAO-induced infarct. In parallel, MCAO caused significantly greater dysfunction in DP1−/− mice than in WT mice. Moreover, treatment of primary corticostriatal neuronal cultures with a highly selective DP1 agonist, BW245C, significantly and dose-dependently enhanced neuronal survival in the presence of glutamate, illustrating that DP1 protects neurons specifically. These data suggest that DP1 plays a role in decreasing brain injury in a paradigm of transient cerebral ischemia, and that its activation can reduce neuronal death in vitro.
PGD2 is the most abundant prostaglandin in the brain (Abdel-Halim et al., 1977) and has been shown to increase following ischemia (Kempski et al., 1987). It is well recognized to have roles in inflammation and other cell functions (Flower et al., 1976). Previously published studies have suggested that the PGD2 receptor DP1 is expressed in leptomeninges, choroid plexus, and eye tissues (Oida et al., 1997; Wright et al., 1999; Mizoguchi et al., 2001). DP1 expression has also been observed in grey matter in hippocampus, brainstem, thalamus, and cerebral cortex (Liang et al., 2005), the latter being one of the main brain regions affected after transient focal cerebral ischemia. Because recent reports have presented conflicting results regarding the therapeutic potential of prostaglandins and cyclooxygenase (COX) inhibitors in preclinical and clinical models, it is now essential to understand the roles of the respective PG receptors that mediate most of the downstream effects.
Prostanoid receptors IP, DP1, EP2, and EP4 are coupled to Gαs, whereas receptors EP3 and DP2 couple to Gαi. As a working hypothesis, we have proposed that the prostanoid receptors that bind to Gαs, which activates adenylyl cyclase to increase intracellular cAMP, are neuroprotective (Boie et al., 1995). In inflammatory cells, cAMP accumulation is generally associated with inhibition of effector cell function. Our previous in vitro and in vivo work indicated that EP2 and EP4 receptors protect neurons against oxidative stress and cell death following exposure to β–amyloid 1−42 fragment, excitotoxicity, and ischemic stroke (Ahmad et al., 2005; Echeverria et al., 2005; Ahmad et al., 2006b). A previous study also supported this concept that stimulation of the DP1 receptor in neurons derived from E18 rat hippocampus increased neuronal survival at least partially because of an enhancement in cAMP levels (Liang et al., 2005). PGD2 binds mainly to the DP1 and DP2 receptors, which have opposing effects on cAMP production (Crider et al., 1999; Sharif et al., 2000; Liang et al., 2005). Our current findings present the first evidence that DP1 can be protective in mouse ischemic neuronal injury.
It has been suggested that the plasticity of the posterior communicating artery impacts the development of ischemia after bilateral common carotid artery occlusion or posterior cerebral artery occlusion (Kitagawa et al., 1998). Therefore, to verify that there were no differences in large vessel anatomy that might cause a disparity in stroke outcome between the two groups, we measured the large vessel diameters in DP1−/− and WT mice. No clear detectable differences were observed. It also has been reported that the primary injury that occurs at the time of the traumatic or cerebrovascular event prevents secondary injury by controlling the abnormal physiology encountered after brain injury (Littlejohns & Bader, 2005). In our study, precise maintenance of physiological parameters, particularly blood pressure and blood gases, was used to assure the similarity of ischemic insult in the two genotypic groups of mice.
PGD2 has been suggested to regulate hypothermia (Ueno et al., 1982) under various physiological and pathological conditions. In fact, several reports have suggested that hypothermia plays a protective role in ischemic brain injury (Prandini et al., 2005; Taniguchi et al., 2005). With our experimental protocol, we observed no differences in body temperature between WT and DP1−/− mice at baseline. Nonetheless, to ensure that potential changes in temperature did not affect the stroke outcome, we maintained the body temperature with a temperature-controlled chamber after surgery. Thus, every effort was made to ensure that induced insults were similar between the wildtype and knockout mice.
It is known that induction of prostanoids by inflammation can affect the CBF (Iadecola et al., 2001). Furthermore, cerebral microcirculation can be modified by prostanoid activation of coagulation/fibrinolysis processes, vascular reactivity, permeability of endothelial cells, leucocyte adhesion, transmigration into the inflamed tissue, and modulation of local immune cell function (Kontos et al., 1981). Koch et al. (2005) reported that DP receptor agonists affect systemic and regional haemodynamics in rats, and Whittle et al. (1983) found BW245C to have anti-thrombotic activities. The BW compounds interact with both the FP and DP receptors, but because they have a greater affinity for the DP receptors, the latter association is more likely to occur (Liang et al., 2005). Spatz et al. (1994) reported that exogenous PGD2 dose-dependently enhanced the production of PGF2α, thromboxane B2, and PGE2, which can act variously as vasoconstrictors and/or vasodilators. However, in our study, we found no gross differences between DP1−/− and WT mice in regard to CBF, blood pressure, or blood gases during ischemia or up to 1 h of reperfusion. Further work would be required to examine possible subtle blood flow changes in the various brain blood vessels and capillaries.
Glutamate exicitotoxicity is also associated with oxidative stress in neurodegenerative disorders (Coyle & Puttfarcken, 1993). In cerebral ischemic injury, glutamate accumulates in the extracellular fluid (Dirnagl et al., 1999) and causes excessive activation of NMDA and non-NMDA receptors. This overactivation of the receptors results in accumulation of intracellular sodium, calcium, and fluid, which leads to oedema formation. To confirm further the protective effect of DP receptors in neurons, and to confirm whether the potent damaging effect of DP1 receptor deletion in this study was caused by a cascade of excitotoxic events, we investigated the effect of BW245C on glutamate-induced toxicity in mouse primary neuronal cultures. In the presence of glutamate, BW245C significantly protected neuronal cells. In our preliminary work, the commercially available receptor antagonist, BWA868C, did not have a significant effect on glutamate toxicity outcome in mouse corticostriatal neuronal cells at DIV 14 (data not shown); alternative selective G-protein-coupled receptor drugs should be developed and tested before reaching a conclusion. However, all together, the results indicate that DP1 most likely does contribute to the prevention of excitotoxicity cascades in neurons.
Our data provide evidence that the DP1 receptor has a protective effect in cerebral ischemic brain injury and in cultured neurons. Additional investigation with drugs that can either stimulate or antagonize the DP1 receptors is now required to explore the mechanisms of action. Such drugs could lead the way toward the design of new therapeutic avenues for the treatment of acute stroke and possibly other neurodegenerative conditions.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health, NS046400 and AG022971 (SD). We thank Claire Levine for her assistance in preparing this manuscript.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- CBF
cerebral blood flow
- MCAO
middle cerebral artery occlusion
- PG
prostaglandin.
References
- Abdel-Halim MS, Hamberg M, Sjoquist B, Anggard E. Identification of prostaglandin D2 as a major prostaglandin in homogenates of rat brain. Prostaglandins. 1977;14:633–643. doi: 10.1016/0090-6980(77)90190-3. [DOI] [PubMed] [Google Scholar]
- Ahmad AS, Ahmad M, de Brum-Fernandes AJ, Doré S. Prostaglandin EP4 receptor agonist protects against acute neurotoxicity. Brain Res. 2005;1066:71–77. doi: 10.1016/j.brainres.2005.10.068. [DOI] [PubMed] [Google Scholar]
- Ahmad AS, Saleem S, Ahmad M, Doré S. Prostaglandin EP1 receptor contributes to excitotoxicity and focal ischemic brain damage. Toxicol. Sci. 2006a;89:265–270. doi: 10.1093/toxsci/kfj022. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Saleem S, Zhuang H, Ahmad AS, Echeverria V, Sapirstein A, Doré S. 1-HydroxyPGE1 reduces infarction volume in mouse transient cerebral ischemia. Eur. J. Neurosci. 2006b;23:35–42. doi: 10.1111/j.1460-9568.2005.04540.x. [DOI] [PubMed] [Google Scholar]
- Akarsu ES, Keskil S, Kaymaz M, Uysal S, Ceviker N, Ataoglu O, Baykaner K. Antiischemic effect of ZK-118.182 in rabbits: a comparative study with iloprost. Meth. Find. Exp. Clin. Pharmacol. 1998;20:339–342. doi: 10.1358/mf.1998.20.4.469482. [DOI] [PubMed] [Google Scholar]
- Aritake K, Kado Y, Inoue T, Miyano M, Urade Y. Structural and functional characterization of HQL-79, an orally active, selective inhibitor for human hematopoietic prostaglandin D synthase. J. Biol. Chem. 2006;281:15277–15286. doi: 10.1074/jbc.M506431200. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Lipid signaling in neural plasticity, brain repair, and neuroprotection. Mol. Neurobiol. 2005;32:89–103. doi: 10.1385/MN:32:1:089. [DOI] [PubMed] [Google Scholar]
- Boie Y, Sawyer N, Slipetz DM, Metters KM, Abramovitz M. Molecular cloning and characterization of the human prostanoid DP receptor. J. Biol. Chem. 1995;270:18910–18916. doi: 10.1074/jbc.270.32.18910. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Bazan NG. Lipid signaling: sleep, synaptic plasticity, and neuroprotection. Prostaglandins Other Lipid Mediat. 2005;77:65–76. doi: 10.1016/j.prostaglandins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Crider JY, Griffin BW, Sharif NA. Prostaglandin DP receptors positively coupled to adenylyl cyclase in embryonic bovine tracheal (EBTr) cells: pharmacological characterization using agonists and antagonists. Br. J. Pharmacol. 1999;127:204–210. doi: 10.1038/sj.bjp.0702490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. TINS. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Doré S, Otsuka T, Mito T, Sugo N, Hand T, Wu L, Hurn PD, Traystman RJ, Andreasson K. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann. Neurol. 2003;54:155–162. doi: 10.1002/ana.10612. [DOI] [PubMed] [Google Scholar]
- Doré S, Takahashi M, Ferris CD, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc. Natl Acad. Sci. USA. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V, Clerman A, Doré S. Stimulation of PGE2 receptors EP2 and EP4 protects cultured neurons against oxidative stress and cell death following β-amyloid exposure. Eur. J. Neurosci. 2005;22:2199–2206. doi: 10.1111/j.1460-9568.2005.04427.x. [DOI] [PubMed] [Google Scholar]
- Flower RJ, Harvey EA, Kingston WP. Inflammatory effects of prostaglandin D2 in rat and human skin. Br. J. Pharmacol. 1976;56:229–233. doi: 10.1111/j.1476-5381.1976.tb07446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, Morham S, Ross ME. Reduced susceptibility to ischemic brain injury and N-methyl-d-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc. Natl Acad. Sci. USA. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempski O, Shohami E, von Lubitz D, Hallenbeck JM, Feuerstein G. Postischemic production of eicosanoids in gerbil brain. Stroke. 1987;18:111–119. doi: 10.1161/01.str.18.1.111. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J. Cereb. Blood Flow Metab. 1998;18:570–579. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Koch KA, Wessale JL, Moreland R, Reinhart GA, Cox BF. Effects of BW245C, a prostaglandin DP receptor agonist, on systemic and regional haemodynamics in the anaesthetized rat. Clin. Exp. Pharmacol. Physiol. 2005;32:931–935. doi: 10.1111/j.1440-1681.2005.04287.x. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Ellis EF, Dietrich WD, Povlishock JT. Prostaglandins in physiological and in certain pathological responses of the cerebral circulation. Fed. Proc. 1981;40:2326–2330. [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp. Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Liang X, Wu L, Hand T, Andreasson K. Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J. Neurochem. 2005;92:477–486. doi: 10.1111/j.1471-4159.2004.02870.x. [DOI] [PubMed] [Google Scholar]
- Littlejohns L, Bader MK. Prevention of secondary brain injury: targeting technology. AACN Clin. Issues. 2005;16:501–514. doi: 10.1097/00044067-200510000-00007. [DOI] [PubMed] [Google Scholar]
- Liu F, Gonzalo JA, Manning S, O'Connell LE, Fedyk ER, Burke KE, Elder AM, Pulido JC, Cao W, Tayber O, Qiu Y, Ghosh S, Ocain TD, Hodge MR, Suzuki-Yagawa Y. Pharmacological characterization of guinea pig chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2). Prostaglandins Other Lipid Mediat. 2005;76:133–147. doi: 10.1016/j.prostaglandins.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Malki S, Nef S, Notarnicola C, Thevenet L, Gasca S, Mejean C, Berta P, Poulat F, Boizet-Bonhoure B. Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. EMBO J. 2005;24:1798–1809. doi: 10.1038/sj.emboj.7600660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y, Ochi Y, Karasawa T, Hatano N, Kadokawa T, Shimizu M. Protective effect of prostaglandins D2, E1 and I2 against cerebral hypoxia/anoxia in mice. Naunyn Schmiedebergs Arch. Pharmacol. 1986;334:282–289. doi: 10.1007/BF00508783. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y, Eguchi N, Urade Y, Yoshida N, Kimura K, Mizoguchi A, Honda Y, Nagai H, Narumiya S. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Eguchi N, Kimura K, Kiyohara Y, Qu WM, Huang ZL, Mochizuki T, Lazarus M, Kobayashi T, Kaneko T, Narumiya S, Urade Y, Hayaishi O. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proc. Natl Acad. Sci. USA. 2001;98:11674–11679. doi: 10.1073/pnas.201398898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneret G, Cossette C, Gravel S, Rokach J, Powell WS. 15R-methyl-prostaglandin D2 is a potent and selective CRTH2/DP2 receptor agonist in human eosinophils. J. Pharmacol. Exp. Ther. 2003;304:349–355. doi: 10.1124/jpet.102.042937. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Ogorochi T, Nakao K, Hayaishi O. Prostaglandin D2 in rat brain, spinal cord and pituitary: basal level and regional distribution. Life Sci. 1982;31:2093–2103. doi: 10.1016/0024-3205(82)90101-1. [DOI] [PubMed] [Google Scholar]
- Oida H, Hirata M, Sugimoto Y, Ushikubi F, Ohishi H, Mizuno N, Ichikawa A, Narumiya S. Expression of messenger RNA for the prostaglandin D receptor in the leptomeninges of the mouse brain. FEBS Lett. 1997;417:53–56. doi: 10.1016/s0014-5793(97)01253-2. [DOI] [PubMed] [Google Scholar]
- Prandini MN, Neves Filho A, Lapa AJ, Stavale JN. Mild hypothermia reduces polymorphonuclear leukocytes infiltration in induced brain inflammation. Arq. Neuropsiquiatr. 2005;63:779–784. doi: 10.1590/s0004-282x2005000500012. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Williams GW, Davis TL. Pharmacology and autoradiography of human DP prostanoid receptors using [(3)H]-BWA868C, a DP receptor-selective antagonist radioligand. Br. J. Pharmacol. 2000;131:1025–1038. doi: 10.1038/sj.bjp.0703686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichijo M, Sugimoto H, Nagao K, Inbe H, Encinas JA, Takeshita K, Bacon KB, Gantner F. Chemoattractant receptor-homologous molecule expressed on Th2 cells activation in vivo increases blood leukocyte counts and its blockade abrogates 13,14-dihydro-15-keto-prostaglandin D2-induced eosinophilia in rats. J. Pharmacol. Exp. Ther. 2003;307:518–525. doi: 10.1124/jpet.103.055442. [DOI] [PubMed] [Google Scholar]
- Spatz M, Stanimirovic DB, Uematsu S, McCarron RM. Vasoactive peptides and prostaglandin D2 in human cerebromicrovascular endothelium. J. Auton. Nerv. Syst. 1994;49(Suppl):S123–S127. doi: 10.1016/0165-1838(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Spik I, Brenuchon C, Angeli V, Staumont D, Fleury S, Capron M, Trottein F, Dombrowicz D. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J. Immunol. 2005;174:3703–3708. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Morikawa E, Mori T, Matsui T. Neuroprotective efficacy of selective brain hypothermia induced by a novel external cooling device on permanent cerebral ischemia in rats. Neurol. Res. 2005;27:613–619. doi: 10.1179/016164105X22110. [DOI] [PubMed] [Google Scholar]
- Ueno R, Narumiya S, Ogorochi T, Nakayama T, Ishikawa Y, Hayaishi O. Role of prostaglandin D2 in the hypothermia of rats caused by bacterial lipopolysaccharide. Proc. Natl Acad. Sci. USA. 1982;79:6093–6097. doi: 10.1073/pnas.79.19.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urade Y, Eguchi N. Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostaglandins Other Lipid Mediat. 2002;68−69:375–382. doi: 10.1016/s0090-6980(02)00042-4. [DOI] [PubMed] [Google Scholar]
- Whittle BJ, Moncada S, Mullane K, Vane JR. Platelet and cardiovascular activity of the hydantoin BW245C, a potent prostaglandin analogue. Prostaglandins. 1983;25:205–223. doi: 10.1016/0090-6980(83)90105-3. [DOI] [PubMed] [Google Scholar]
- Wright DH, Nantel F, Metters KM, Ford-Hutchinson AW. A novel biological role for prostaglandin D2 is suggested by distribution studies of the rat DP prostanoid receptor. Eur. J. Pharmacol. 1999;377:101–115. doi: 10.1016/s0014-2999(99)00358-1. [DOI] [PubMed] [Google Scholar]
- Zhuang H, Pin S, Li X, Doré S. Regulation of heme oxygenase expression by cyclopentenone prostaglandins. Exp. Biol. Med. 2003;228:499–505. doi: 10.1177/15353702-0322805-13. [DOI] [PubMed] [Google Scholar]